Abstract
Massive neutrophil infiltration is an early key event in infectious inflammation, accompanied by chemotactic leukotriene (LT)B4 generation. LTB4 biosynthesis is mediated by 5-lipoxygenase (5-LOX), but which pathogenic factors cause 5-LOX activation during bacterial infections is elusive. Here, we reveal staphylococcal exotoxins as 5-LOX activators. Conditioned medium of wild-type Staphylococcus aureus but not of exotoxin-deficient strains induced 5-LOX activation in transfected HEK293 cells. Two different staphylococcal exotoxins mimicked the effects of S. aureus-conditioned medium: (1) the pore-forming toxin α-hemolysin and (2) amphipathic α-helical phenol-soluble modulin (PSM) peptides. Interestingly, in human neutrophils, 5-LOX activation was exclusively evoked by PSMs, which was prevented by the selective FPR2/ALX receptor antagonist WRW4. 5-LOX activation by PSMs was confirmed in vivo as LT formation in infected paws of mice was impaired in response to PSM-deficient S. aureus. Conclusively, exotoxins from S. aureus are potent pathogenic factors that activate 5-LOX and induce LT formation in neutrophils.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a complex biological immune response to harmful stimuli including bacteria or fungi to regain homeostasis. Neutrophils are powerful effector cells at sites of inflammation and bacterial invasion that combat the infectious threat by phagocytosis, reactive oxygen species generation, degranulation, formation of antimicrobial neutrophil extracellular traps, but also by formation of pro-inflammatory cytokines and lipid mediators (LM), e.g., leukotrienes (LTs) [1, 2]. LTs are formed in a fine-tuned regulated process via the 5-lipoxygenase (5-LOX) pathway from arachidonic acid (AA). Upon neutrophil stimulation, 5-LOX is activated by elevated intracellular Ca2+ levels ([Ca2+]i) and subsequent Ca2+-binding at the N-terminal C2-like domain along with serine phosphorylations of 5-LOX [3]. These events induce 5-LOX translocation from the cytosol to the nuclear membrane, where 5-LOX forms the LT biosynthetic complex with the 5-LOX-activating protein (FLAP) [4, 5].
Recently, we showed that macrophages biosynthesize substantial amounts of lipid mediators (LM) including LTs when exposed to pathogenic bacteria such as Staphylococcus (S.) aureus or Escherichia (E.) coli [6], and others observed LT biosynthesis in neutrophils exposed to Salmonella enterica serovar Typhimurium [7]. However, the molecular mechanisms of how these bacteria elicit LT formation are not completely understood. Several processes are conceivable, including phagocytosis, activation of pattern-recognition receptors, and release of cytolytic toxins [8]. Phagocytosis is a central microbicidal function of neutrophils and macrophages, and LTs are known to stimulate this process [9, 10]. However, whether pathogen internalization causes LT formation is yet unknown. Pattern-recognition receptors such as toll-like receptors (TLR) detect microbial molecular pattern like lipopolysaccharides (LPS), peptidoglycan, and CpG-DNA [11]. While LPS fails to stimulate LT formation, it can prime leukocytes for increased LT production upon exposure to bacterial N-formylated oligopeptides [12].
Bacteria initiate inflammation by the release of cytolytic exotoxins, so-called pore-forming toxins (PFT), known as strong virulent factors that damage cellular membranes of the host and elicit diverse inflammatory processes independent of phagocytosis or physical host–pathogen interaction, but rather by assembly of pores into the membrane [13]. S. aureus releases two types of β-barrel PFTs. The archetype of PFTs is α-hemolysin that is secreted as monomer, acts via the ADAM10 receptor, and associates as a homo-heptamer pore within the cellular membrane. The other type of PFTs is bi-component exotoxins such as leukocidins and γ-hemolysin that also bind to G protein-coupled receptors (GPCRs) and form hetero-oligomeric pores [14]. In addition, amphipathic and alpha-helical peptides, designated as phenol-soluble modulins (PSMs), are highly virulent exotoxins with key functions in the pathogenesis of S. aureus infections [15]. The expression of virulent exotoxins of S. aureus is regulated by a complex network including the accessory gene regulator (Agr) system and DNA-binding proteins, such as SarA [16]. Knockout of both global regulators is characterized by a dramatic reduction of virulence factor expression and loss of pathogenicity [17].
Whether bacterial exotoxins can activate 5-LOX is poorly investigated. It was reported before that the bacterial exotoxins streptolysin O, alveolysin, and theta toxin can increase LT formation in granulocytes [18] and α-hemolysin from S. aureus was proposed as inducer of LT biosynthesis [19]. Based on our recent discovery that pathogenic bacteria induce formation of LT in human cells [6], we here aimed to unveil how pathogenic bacteria stimulate human host cells for LT biosynthesis and to uncover the mechanism underlying 5-LOX activation. Our data indicate that pathogenic S. aureus secrete various exotoxins that activate the 5-LOX pathway. Thus, we found that PSMs activate 5-LOX and LT formation in neutrophils, which could be prevented by an antagonist of formyl-peptide receptor-2 (FPR2/ALX), a proposed receptor for PSMs [20]. Conclusively, our data suggest that exotoxins, in particular PSMs, play a prominent role in progression of S. aureus-induced infectious inflammation by stimulating LT formation.
Results
Pathogenic bacteria or bacteria-conditioned medium induce 5-LOX translocation in non-immunocompetent HEK293 cells
To exclude immune-relevant functions and components such as phagocytosis for bacteria-induced 5-LOX activation, we first used non-immunocompetent HEK293 cells stably transfected with human 5-LOX and FLAP (HEK_5-LOX/FLAP), a suitable model to study cellular mechanisms of 5-LOX activation [21]. This experiment allowed us to rule out the contribution of phagocytosis as HEK293 cells lack expression of major phagocytic receptors such as FcgRs (receptor for Fc fraction of immunoglobulins) and complement receptor 3 (CR3) [22, 23]. Stimulation of these cells with pathogenic E. coli (serotype O6:K2:H1; ratio E. coli:HEK cells = 50:1) induced translocation of intranuclear 5-LOX and co-localization with FLAP at the nuclear membrane 30 min upon exposure (Fig. 1a). Besides Gram-negative E. coli, the Gram-positive S. aureus (LS1 strain) elicited 5-LOX translocation in HEK cells as well, however, in a delayed manner compared to E. coli, that is, 90 min upon exposure. In contrast, the non-pathogenic E. coli strain BL21 (DE3) and the non-virulent Staphylococcus carnosus TM300 [24, 25] failed to induce 5-LOX translocation (Fig. 1a). Secreted soluble exotoxins are potent virulence factors of bacteria [8] and these factors might constitute relevant stimuli for 5-LOX activation. Thus, HEK_5-LOX/FLAP cells were incubated with sterile-filtered bacteria-conditioned medium (BCM, free of bacteria and cell debris) and 5-LOX translocation was assessed. BCM derived from pathogenic S. aureus or E. coli-induced 5-LOX translocation within 10–30 min of exposure, respectively (Fig. 1b). As expected, BCM of the non-pathogenic E. coli BL21 or S. carnosus TM300 did not induce 5-LOX translocation, not even after prolonged incubation (90 min, Fig. 1b). These data suggest that factors released by pathogenic bacteria cause 5-LOX translocation, which is an essential step for LT biosynthesis [4].
Pathogenic bacteria or bacteria-conditioned medium induce 5-LOX translocation in non-immunocompetent HEK293 cells. Subcellular localization of 5-LOX was determined by immunofluorescence microscopy in HEK293 cells stably expressing human 5-LOX (stained in red) and FLAP (green). a HEK_5-LOX/FLAP cells were incubated with intact pathogenic E. coli (O6:K2:H1), S. aureus (LS1), non-pathogenic E. coli BL21 (DE3), or S. carnosus (TM300) using an MOI of 1:50 (HEK293 cells vs. bacteria) for indicated time periods. b Bacteria-conditioned medium (BCM) was collected by sterile filtration of overnight cultures of E. coli [O6:K2:H1 or BL21 (DE3)], S. aureus (LS1), and S. carnosus (TM300) followed by stimulation of HEK_5-LOX/FLAP cells in PBS+/+ with 10% of BCM. Microscopic images are given as overlay of 5-LOX and FLAP staining. Results shown are representative for at least three independent experiments
Exotoxins secreted by S. aureus induce 5-LOX activation
Exotoxins are virulence factors secreted by bacteria that can cause host cell permeabilization and damage, induce ion imbalances, and activate a variety of pathways in the target cell [26]. The PFT α-hemolysin is one of the most potent exotoxins formed by S. aureus [14] and appeared as potential candidate factor capable of activating 5-LOX. Treatment of HEK_5-LOX/FLAP cells with isolated α-hemolysin at 1 and 10 µg/mL clearly induced 5-LOX translocation and 5-LOX/FLAP co-localization within 30 min (Fig. 2a). In contrast, with Ca2+-ionophore A23187, a commonly used stimulus for 5-LOX that rapidly elevates intracellular Ca2+ levels [21], 5-LOX/FLAP co-localization was achieved already after 10 min (Fig. 2a).
Exotoxins secreted by S. aureus induce 5-LOX activation. Subcellular 5-LOX distribution was detected by immunofluorescence microscopy in HEK293 cells stably expressing human 5-LOX (stained in red) and FLAP (green). a HEK_5-LOX/FLAP cells were incubated with 0.5 µM A23187 or α-hemolysin at given concentrations for the indicated time periods. b Bacteria-conditioned medium (BCM) was collected by sterile filtration of overnight cultures of S. aureus strains (LS1, LS1∆hla, or LS1∆agr/sarA). HEK_5-LOX/FLAP cells were stimulated in PBS+/+ containing 10% of BCM or unconditioned medium for the indicated periods. Microscopic images are given as overlay of 5-LOX and FLAP staining. Results shown are representative for at least three independent experiments
To confirm that exotoxins such as α-hemolysin represent the secreted factors of bacteria that induce 5-LOX translocation, we took advantage of S. aureus mutant strains (LS1 derivatives) that are deficient in exotoxin formation [17]. Of interest, treatment of HEK_5-LOX/FLAP cells with BCM of the mutant strain LS1∆hla, unable to form α-hemolysin, still induced 5-LOX translocation (Fig. 2b), suggesting that other exotoxins are operative as well. In fact, BCM of S. aureus LS1∆agr/sarA, a non-virulent S. aureus strain that is deficient in exotoxin formation [17] failed to evoke 5-LOX translocation (Fig. 2b). Together, these data suggest that secreted factors, such as α-hemolysin but also additional exotoxins, mediate bacteria-induced 5-LOX activation in non-immunocompetent HEK cells.
Signaling pathways mediating bacteria-induced 5-LOX translocation in HEK293 cells
The p38 MAPK pathway in eukaryotic cells mediates signal transduction in response to distinct PFTs [27, 28], and is also involved in cell stress-induced 5-LOX translocation via phosphorylation of 5-LOX at Ser271 by p38 MAPK-dependent MK2 [29]. To study the involvement of p38 MAPK in bacteria-elicited 5-LOX translocation, HEK_5-LOX/FLAP cells were pretreated with the p38 MAPK inhibitors skepinone-L or SB203580 [30] and stimulated with BCM of S. aureus (LS1). 5-LOX translocation was evident 30 min upon exposure to the bacteria, independent on pharmacological intervention with p38 MAPK (Fig. 3a). In addition, the 5-LOX_S271A mutant, where the phosphorylation site Ser271 was replaced by alanine, still translocated to the nuclear envelope upon exposure to S. aureus-BCM comparable to wild-type 5-LOX (Fig. 3b, c). Therefore, S. aureus-induced 5-LOX activation is seemingly independent of the p38 MAPK pathway and phosphorylation of 5-LOX at Ser271.
Signaling pathways mediating bacteria-induced 5-LOX translocation in HEK293 cells. Subcellular localization of 5-LOX was determined by immunofluorescence microscopy. a HEK293 cells stably expressing 5-LOX (stained in red) and FLAP (green) were pre-incubated with 0.1% DMSO (vehicle), with the p38-MAPK inhibitors skepinone-L (0.3 µM) or SB203580 (1 µM) for 10 min at 37 °C and stimulated with 10% BCM derived from S. aureus (LS1) for the indicated periods. b HEK293 cells stably co-expressing FLAP with the 5-LOX_S271A mutant were stimulated as described in a. Microscopic images are given as overlay of 5-LOX and FLAP staining. c Stable expression of 5-LOX, 5-LOX_S271A, and FLAP in HEK293 cells was verified by immunoblotting. Images are representative for three independently collected cell lysates. Intracellular Ca2+ concentration of Fura-2AM-labeled HEK293 cells was monitored upon stimulation with d 10 µg/mL α-hemolysin or with e 10% of unconditioned medium (vehicle) or BCM of S. aureus strains (LS1, LS1∆hla or LS1∆agr/sarA) under continuous fluorescence reading. 2 µM ionomycin was used as positive control. Results are given as mean ± SEM. #p < 0.05, ##p < 0.01, and ###p < 0.001 LS1 or ionomycin vs. vehicle control or *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the corresponding LS1 wild-type strain using one-way ANOVA with Tukey multiple comparison post hoc test. f 5-LOX translocation was detected by immunofluorescence microscopy in HEK293 cells stably expressing 5-LOX (red) and FLAP (green) after 10 min of pre-incubation with or without 10 mM EDTA in PBS+/+ followed by incubation for 90 min with 10 µg/mL α-hemolysin or 10% BCM of S. aureus (LS1). Microscopic images are given as overlay of 5-LOX and FLAP staining. Results shown are representative for at least three independent experiments
5-LOX activity and translocation depends on elevation of [Ca2+]i mediated by Ca2+ binding to the N-terminal C2-like domain of the enzyme [31]. To study whether bacterial stimuli can elevate [Ca2+]i, HEK293 cells were stained with the Ca2+-sensitive dye Fura-2AM and suspended cells were exposed to α-hemolysin or ionomycin, respectively. α-Hemolysin (10 µg/mL) caused a continuous Ca2+ influx up to 90 min, whereas ionomycin (positive control) induced a rapid increase in [Ca2+]i that reached a maximum after few seconds (Fig. 3d). Interestingly, α-hemolysin at 1 µg/mL did not induce Ca2+ influx (not shown), although 5-LOX translocation occurred at this concentration. Experimental conditions might be causative for this discrepancy as adherent cells behave different versus cells in suspension. In addition, a moderate increase of [Ca2+]i within 90 min was observed for the vehicle control, which can be explained by slight cytotoxic effects of Fura-2AM and inadequate cell-culture conditions during the measurement.
Next, [Ca2+]i in HEK_5-LOX/FLAP cells were monitored upon treatment with BCM of the S. aureus strains LS1, LS1∆hla, and LS1∆agr/sarA that exhibit complete, impaired, or abolished exotoxin release, respectively [17]. Elevated [Ca2+]i was evident immediately after the addition of BCM derived from LS1 but also by BCM obtained from the LS1∆hla strain, suggesting that other exotoxins than α-hemolysin may increase [Ca2+]i. In contrast, the BCM derived from LS1∆agr/sarA failed to elevate [Ca2+]i (Fig. 3e), which may explain the failure of this BCM to cause 5-LOX translocation (see Fig. 2b). Moreover, chelation of extracellular Ca2+ by 10 mM EDTA prevented 5-LOX/FLAP co-localization in HEK cells treated with either 10 µg/mL α-hemolysin or BCM of LS1 (Fig. 3f). These data suggest that 5-LOX activation/translocation by staphylococcal exotoxins is mediated by elevation of [Ca2+]i regardless of p38 MAPK pathway and phosphorylation of Ser271.
S. aureus-derived exotoxins induce 5-LOX activation in human neutrophils accompanied by elevation of [Ca2+]i
Encouraged by the finding that exotoxins induce 5-LOX translocation in non-immunocompetent HEK293 cells, we asked whether these factors may activate 5-LOX also in relevant immune cells that combat bacteria. Neutrophils play a central role in innate immunity that kill bacteria, express abundant 5-LOX, and possess high capacities to produce LTs [12, 32]. We incubated human neutrophils with BCM of S. aureus and its mutant strains, and analyzed 5-LOX-derived LMs. BCMs derived from LS1 significantly induced the formation of the major 5-LOX products such as 5-HETE, LTB4 and its all-trans isomers, and 20-OH-LTB4 compared to incubations with non-conditioned medium (Fig. 4a). Of interest, the BCM from LS1∆hla induced 5-LOX product formation as well, and α-hemolysin up to concentrations of 100 µg/mL caused only moderate 5-LOX activity in neutrophils (Fig. 4a). BCM from the non-virulent LS1∆agr/sarA did not induce 5-LOX product formation (Fig. 4a), suggesting that also in neutrophils, certain exotoxins, but not α-hemolysin, efficiently activate 5-LOX. Moreover, other 5-LOX- or cyclooxygenase (COX)-derived LM produced from AA, eicosapentaenoic acid (EPA), or docosahexaenoic acid (DHA) was generated by neutrophils exposed to BCM from LS1, and deletion of α-hemolysin had minor impact on LM induction, whereas BCM from the LS1∆agr/sarA strain was virtually inactive (Fig. 4b). In agreement with the results from above, BCM from LS1 and LS1∆hla caused rapid increase in [Ca2+]i, whereas the LS1∆agr/sarA strain and α-hemolysin failed in this respect (Fig. 4c). Along these lines, 5-LOX translocation in neutrophils was induced by BCM derived from LS1 and LS1∆hla, but not by BCM from LS1∆agr/sarA and by α-hemolysin (Fig. 4d). Therefore, staphylococcal exotoxins increase [Ca2+]i and activate 5-LOX in neutrophils, but they appear to be distinct from α-hemolysin that is clearly operative in HEK293 cells in this respect.
S. aureus-derived exotoxins induce 5-LOX activation in human neutrophils accompanied by elevation of [Ca2+]i. a 5-LOX product formation of human neutrophils was detected upon stimulation with 1% unconditioned medium vs. 1% BCM of S. aureus (LS1, LS1∆hla or LS1∆agr/sarA) or 100 µg/mL α-hemolysin vs. vehicle (water) for 10 min at 37 °C. Data are expressed in pg/5 × 106 cells as mean ± SEM of three different donors. #p < 0.05, ##p < 0.01 and ###p < 0.001 LS1 or α-hemolysin vs. vehicle control or *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the corresponding LS1 wild-type strain using one-way ANOVA with Tukey multiple comparison post hoc test. b Lipid mediator formation of neutrophils shown in a as percentage of LS1 wild type in a heat map. c Fura-2AM-labeled neutrophils were stimulated with 2 µM ionomycin, 100 µg/mL α-hemolysin, 1% unconditioned medium, or 1% BCM of S. aureus (LS1, LS1∆hla, or LS1∆agr/sarA) for 10 min at 37 °C and intracellular Ca2+ concentrations were monitored by continuous fluorescence reading. Data are given as mean ± SEM, n = 4 donors; #p < 0.05, ##p < 0.01 and ###p < 0.001 LS1 or ionomycin vs. vehicle control or *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the corresponding LS1 wild-type strain using one-way ANOVA with Tukey multiple comparison post-hoc test. d 5-LOX translocation was monitored by immunofluorescence microscopy in neutrophils stimulated with 1% unconditioned medium or 1% BCM of S. aureus (LS1, LS1∆hla or LS1∆agr/sarA), or 100 µg/mL α-hemolysin for 10 min at 37 °C. Microscopic images are given as overlay of 5-LOX (red) and FLAP (green) staining and are representative for three independent experiments. e LM formation of human neutrophils was assessed after pre-incubation with 300 nM MK886, 3 µM zileuton, or vehicle (0.1% DMSO) prior to stimulation with 1% BCM of S. aureus (LS1) for 10 min at 37 °C. Data are expressed in percentage of vehicle control as mean ± SEM of three different donors. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. vehicle control using two-tailed Student’s t test. f LM formation of neutrophils shown in e as percentage of vehicle control. g Neutrophils were treated with 1% BCM of S. aureus (LS1, LS1∆hla, and LS1∆agr/sarA) or unconditioned medium (vehicle) for 10 min at 37 °C followed by automatic cell counting including trypan blue staining. Viability is presented as percentage of viable cells vs. total cell number. h Lactate dehydrogenase (LDH) release of neutrophils was measured upon stimulation with 1% BCM of S. aureus (LS1, LS1∆hla, LS1∆agr/sarA) or 1% Triton X-100 for 10 min at 37 °C. Values are presented as percentage of Triton X-100 treatment and as mean ± SEM, n = 3. #p < 0.05, ##p < 0.01 and ###p < 0.001 LS1 vs. vehicle control or *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the corresponding LS1 wild-type strain using one-way ANOVA with Tukey multiple comparison post-hoc test
To exclude cytotoxic effects of S. aureus-derived exotoxins within the incubation period, we analyzed if the BCM of LS1, LS1Δhla, and LS1∆agr/sarA strains could affect neutrophil viability. Only marginal detrimental effect of these BCMs on neutrophil integrity/viability was observed, as monitored by trypan-blue measurement and LDH release (Fig. 4g, h). Moreover, treatment of neutrophils with the FLAP inhibitor MK886 (or the 5-LOX inhibitor zileuton) and subsequent stimulation with BCM of LS1 completely abrogated 5-LOX product formation (Fig. 4e, f). Since FLAP inhibition requires cellular integrity and causes suppression of LT formation only in intact cells, we conclude that 5-LOX product biosynthesis occurred within the vital cell and is not an artificial event in potentially formed cell lysates. Note that both MK886 and zileuton reduced only 5-LOX product formation, whereas the formation of COX- and 12/15-LOX-derived LM mediators was instead increased (Fig. 4f), possibly due to substrate shunting.
Phenol-soluble modulins released from S. aureus induce 5-LOX activation in neutrophils
Since BCMs from LS1 and LS1∆hla, but not α-hemolysin or the BCM from the LS1∆agr/sarA mutant were able to induce 5-LOX activation, we aimed to reveal the potential activator(s) released by S. aureus. S. aureus expresses bi-component leukotoxins such as the PFTs and amphipathic α-helical phenol-soluble modulin (PSM) peptides [15]. We determined 5-LOX translocation and product formation as well as Ca2+ influx in neutrophils upon stimulation by distinct BCMs of S. aureus wild-type and mutant strains. Both S. aureus strains, LS1, and the methicillin-resistant strain, USA300, induced the formation of the 5-LOX products 5-HETE, t-LTB4, LTB4, and 20-OH-LTB4 versus unconditioned medium (Fig. 5a). However, BCM from the LS1Δpsmαβ strain, lacking PSM formation, showed diminished capacity to generate these 5-LOX products. The BCM of LS1Δpsmαβ under these conditions did not compromise viability and integrity of neutrophils (trypan blue analysis and LDH release, data not shown). Interestingly, the BCM derived from USA300Δpvl (deficient in Panton-Valentine leukocidin expression) induced comparable amounts of 5-LOX products in neutrophils versus USA300 strain (Fig. 5a). These data suggest that PSMs exclusively activate neutrophils to induce 5-LOX product formation. Remarkably, deficiency of PSM expression particularly affected the release of AA as 5-LOX substrate, compared to other fatty acids such as EPA, DHA, and docosapentaenoic acid (DPA) (Fig. 5b). Strikingly, isolated PSMα1 and PSMα3 induced concentration-dependently (10 µg/mL and 1 µg/mL) 5-LOX product formation in neutrophils (Fig. 5c), and the isolated PSMα3 increased [Ca2+]i. In fact, the BCM of LS1Δpsmαβ showed reduced capacities to elevate [Ca2+]i in neutrophils compared to the LS1 strain (Fig. 5e) and the addition of 10 mM EDTA impaired predominantly the AA-derived LM formation. Note that the LS1-induced DHA-release in neutrophils was barely influenced by EDTA (Fig. 5f). In line with this, 5-LOX translocation in neutrophils was evident upon stimulation with BCMs of LS1, whereas BCM of LS1Δpsmαβ failed (Fig. 5g). Stimulation with isolated PSMα1 and PSMα3 elicited 5-LOX translocation in neutrophils (Fig. 5g). Note that for immunofluorescence analysis, neutrophils had to adhere to the coverslip surface, which explains the subcellular localization of 5-LOX in resting neutrophils within the nucleus as suggested before [33]. Finally, we asked whether PSMs could also activate 5-LOX in HEK_5-LOX/FLAP cells, and indeed, PSMs were capable to induce 5-LOX translocation to the nuclear membrane in these cells as well (Fig. 5g). However, in contrast to neutrophils, BCM of LS1Δpsmαβ still caused 5-LOX translocation in HEK_5-LOX/FLAP cells, presumably because other exotoxins such as α-hemolysin may substitute for PSMs (Fig. 5g). Furthermore, 5-LOX product formation in PSM-stimulated HEK_5-LOX/FLAP cells was observed, albeit exogenously added AA was needed for substantial 5-LOX product biosynthesis (Fig. S1).
Phenol-soluble modulins released from S. aureus induce 5-LOX activation in neutrophils. a 5-LOX product formation of human neutrophils was assessed upon stimulation with 1% unconditioned medium vs. 1% BCM of S. aureus (LS1, LS1∆psmαβ, USA300 or USA300∆pvl) for 10 min at 37 °C. Data are expressed in pg/5 × 106 cells as mean ± SEM, n = 3 donors. #p < 0.05, ##p < 0.01 and ###p < 0.001 wild-type strains vs. vehicle control or *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the corresponding S. aureus wild-type strain using one-way ANOVA with Tukey multiple comparison post-hoc test. b LM formation of neutrophils shown in a as percentage of LS1. c LM formation of human neutrophils was assessed upon stimulation with PSMα1 or PSMα3 (10 µg/mL or 1 µg/mL) vs. vehicle control (water) for 10 min at 37 °C. Data are expressed in pg/5 × 106 cells as mean ± SEM, n = 3 donors *p < 0.05, **p < 0.01, ***p < 0.001 vs. vehicle using one-way ANOVA with Tukey multiple comparison post hoc test. d Principal component analysis (PCA) of lipid mediators (red) biosynthesized by neutrophils upon stimulation with isolated bacterial toxins (alpha-toxin = α-hemolysin), LS1 BCM (del = Δ) or unconditioned medium (vehicle) (blue). e Fura-2AM-labeled neutrophils were stimulated by 2 µM ionomycin, 10 µg/mL PSMα3, 1% unconditioned medium or 1% BCM of S. aureus (LS1, LS1∆psmαβ, or LS1∆agr/sarA) for 10 min at 37 °C and intracellular Ca2+ concentrations were monitored by continuous fluorescence reading. Data are mean ± SEM, n = 7 donors; #p < 0.05, ##p < 0.01, and ###p < 0.001 LS1 or ionomycin vs. vehicle control or *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the corresponding LS1 wild-type strain using one-way ANOVA with Tukey multiple comparison post hoc test. f LM formation of neutrophils was assessed after pretreatment with 10 mM EDTA for 10 min and subsequent stimulation with 10 µg/mL PSMα3 for 10 min at 37 °C. Data are expressed in % of vehicle control. g 5-LOX translocation was examined by immunofluorescence microscopy in neutrophils and HEK_5-LOX/FLAP cells stimulated with 10% (HEK) or 1% (neutrophils) unconditioned medium or BCM of S. aureus (LS1 or LS1∆psmαβ) and 10 µg/mL PSMα1 or PSMα3 for 10 min at 37 °C. Microscopic images are given as overlay of 5-LOX (red) and FLAP (green) staining and are representative for three independent donors (neutrophils) or experiments (HEK)
Principal component analysis of neutrophil stimulation by 1% LS1 mutant BCMs, 100 µg/mL α-hemolysin, or 10 µg/mL PSMα3 revealed a distinct clustering of PSM-containing stimuli that mainly induced 5-LOX product formation (Fig. 5d). In contrast, a clear tendency towards COX and 12-LOX products was observed in the absence of PSMs, although total amounts of these mediators were minor compared to 5-LOX products.
In vivo experiments corroborate PSMs as 5-LOX activators
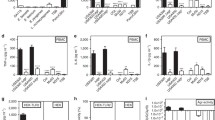
To study exotoxin-induced LT formation in vivo, mice hind paws were subcutaneously infected by S. aureus (USA300) (2 × 107 CFU) and LM formation was monitored over a time course of 48 h in paw tissue homogenates. A rapid increase in 5-LOX product formation (5-HETE, all isomers of LTB4) could be detected already 6 h upon S. aureus infection, followed by a temporal decrease at 24 h and a rebound up to 48 h. This observation was in sharp contrast to homogenates of non-infected paws where only basal levels of 5-LOX products were found over the entire time course. Furthermore, thromboxane B2 (TXB2) and prostaglandin E2 (PGE2) were analyzed as COX-derived products. TXB2 was also elevated in S. aureus-infected paws, whereas PGE2 was detected in both, in infected and non-infected paws over the entire time period, which suggested basal PGE2 formation independent of bacterial infection (Fig. 6a). Interestingly, infection with S. aureus mutant strain USA300Δpsmαβ revealed impaired 5-LOX product formation in the infected hind paws compared to the USA300 at 48 h post S. aureus infection, which confirms the impact of exotoxins in S. aureus-mediated LM formation and supports PSMs as potential inducer of LT formation. Interestingly, mutant strains that lack PSM production (USA300Δpsmαβ) showed comparable levels of TXB2 and PGE2 as the wild type, which substantiate the assumption of exotoxin-independent biosynthesis (Fig. 6b).
In vivo experiments corroborate PSMs as 5-LOX activators. a 5-LOX products in murine paws at different time points upon infection with S. aureus USA300. b 5-LOX products in murine paws 48 h post-infection with S. aureus and mutant strains (USA300, USA300∆psmαβ). 5-LOX products were analyzed by UPLC-MS/MS. Unstimulated controls were obtained by simultaneous injection of sterile PBS into the opposite paw of each mouse. Data are expressed as mean ± SEM in pg, n = 4 mice/group. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the corresponding USA300 wild-type strain using one-way ANOVA with Tukey multiple comparison post hoc test
PSM-induced 5-LOX activation in neutrophils is prevented by blockade of the FPR2/ALX receptor
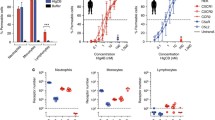
It was reported that PSMs affect inflammatory processes including neutrophil recruitment by sensing the human formyl-peptide receptor 2 (FPR2/ALX) [20]. However, induction of LTB4 formation by these exotoxins has not been shown so far. To examine a putative involvement of FPR2/ALX in PSM-induced LTB4 formation, we first determined the expression of this receptor in human leukocytes. Contrary to monocytes and monocyte-derived M1 and M2 macrophages, neutrophils expressed remarkable levels of FPR2/ALX (Fig. 7a). Next, we pre-incubated human neutrophils with the FPR2/ALX receptor antagonist WRW4 for 10 min prior to stimulation with PSMα3 (10 µg/mL). PSM-induced 5-LOX activation, represented by formation of 5-HETE, LTB4, t-LTB4-isomers, and 20-OH-LTB4, was concentration-dependently inhibited by WRW4 with IC50 values of ~ 1 µM (Fig. 7b). Importantly, when Ca2+-ionophore A23187 was applied as 5-LOX activator, 5-LOX product formation was hardly reduced by WRW4, which confirms the involvement of FPR2/ALX in PSM-induced but not in A23187-induced 5-LOX activation and LT formation (Fig. 7c). To substantiate specific 5-LOX activation by PSMs via FPR2/ALX, N-formyl-methionine-leucyl-phenylalanine (fMLF), a well-known FPR1 receptor agonist, was applied. LT formation in neutrophils was barely stimulated by fMLF compared to PSMs, and WRW4 failed to impair fMLF-evoked LT biosynthesis (Fig. 7d). Furthermore, the chemotaxis inhibiting protein of S. aureus (CHIPS) that selectively blocks FPR1 [34] did not impact PSM-induced LT formation, whereas biosynthesis of LTs in fMLF-challenged neutrophils was attenuated by CHIPS (Fig. 7e). The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met-NH2 (WKYMV-D-Met) specifically activates neutrophils via FPR2/ALX [35]. Accordingly, WKYMV-D-Met stimulated 5-LOX product formation in a concentration-dependent manner with comparable magnitude to PSMs (Fig. 7f), while the negative control WKYMV-L-Met failed in this respect (not shown). Together, using specific agonists and antagonists for FPR1 and FPR2/ALX receptors, 5-LOX activation and LT biosynthesis in neutrophils is mediated by FPR2/ALX.
PSM-induced 5-LOX activation in neutrophils is mediated by the FPR2/ALX receptor. a Expression of FPR2/ALX in human leukocytes was analyzed by immunoblotting. Images are representative for lysates of three independent donors for each cell type. 5-LOX product formation in neutrophils was determined in the presence of 0.1% DMSO (vehicle) or WRW4 at the indicated concentrations (b) or at 10 µM (c, d) upon stimulation with b 10 µg/mL PSMα3, c 2.5 µM A23187 or d 1 µM fMLF. e 5-LOX product formation in neutrophils was determined in the presence of 0.1% DMSO (vehicle) or 1 µg/mL chemotaxis inhibiting protein of S. aureus (CHIPS) upon stimulation with 10 µg/mL PSMα3 or 1 µM fMLF. f 5-LOX product formation in human neutrophils was assessed upon stimulation with WKYMVm and PSMα3 at 10 and 100 nM for 10 min at 37 °C. Data are expressed in pg/5 × 106 cells as mean ± SEM, n = 3–4 donors
Discussion
Here, we show that exotoxins secreted from pathogenic bacteria are capable of inducing 5-LOX activation in human neutrophils that are early effectors of the innate immune defense, but also in transfected non-immunocompetent HEK293 cells. Activation of 5-LOX is reflected by its translocation to the nuclear envelope where FLAP resides, which correlated with elevation of [Ca2+]i. In neutrophils that possess high capacities to generate LT upon appropriate challenge [3], exotoxin-triggered events coincided with abundant 5-LOX product biosynthesis. Intriguingly, experiments with isolated exotoxins and with exotoxin-deficient S. aureus strains revealed α-hemolysin and PSMs as 5-LOX activators in HEK cells, while in neutrophils, only PSMs were operative. Together, our data reveal specific staphylococcal exotoxins as pathophysiological relevant agents that may contribute to the inflammatory response during bacterial infection through 5-LOX-mediated formation of LTs in neutrophils.
We recently showed 5-LOX activation along with LT formation in human macrophages upon exposure to pathogenic S. aureus or E. coli [6]. Here, we aimed at identifying the respective factors and mechanisms responsible for 5-LOX activation. We found that non-immunocompetent HEK293 cells, stably transfected to express 5-LOX and FLAP as key enzymes in LT biosynthesis [21], are susceptible to pathogenic bacteria as well. These cells are unable to phagocytose pathogens and are deficient in toll-like receptor 4 (TLR4) expression [23], which excludes LPS of Gram-negative E. coli as stimulatory principle. Moreover, since cell-free BCM were sufficient to induce 5-LOX activation, direct physical interaction between bacteria and the host cell is not required. Therefore, we suggest that a mechanism apart from phagocytosis and pattern-recognition receptor such as TLR binding is operative. Nevertheless, pathogenicity of the bacteria was a prerequisite, since the non-pathogenic E. coli BL21 (DE3) and S. carnosus TM300 and their BCM failed to stimulate 5-LOX translocation in HEK_5-LOX/FLAP cells. Our data imply that factors, which are secreted by pathogenic bacteria, are responsible for 5-LOX activation.
Staphylococcus aureus is a harmful pathogen causing hospital- and community-associated infections that range from moderately skin infection to severe and life-threatening pneumonia, endocarditis, and osteomyelitis [36,37,38]. As soon as S. aureus invades the host, a struggle for survival starts on both sides of the host–pathogen interplay. Thus, neutrophils are key innate immune cells and are armed with various defense mechanisms, to recognize, engulf, and kill invading S. aureus [32]. On the other hand, S. aureus expresses a wide array of virulence factors capable of damaging host cells and of evading from host´s immune responses, e.g., by releasing chemotaxis inhibitory protein of staphylococci (CHIPS) that bind to formyl-peptide and C5a receptors on neutrophils [34, 39]. Neutrophils possess high capacities to generate LTs, and we thus asked whether pathogenic bacteria are capable of activating 5-LOX also in this cell type, and which virulence factors are causative. Our data show that S. aureus secretes factors that induce 5-LOX activation in neutrophils, which is independent of physical host–pathogen interaction. More specifically, we were able to distinguish between different S. aureus exotoxins and revealed the amphipathic α-helical PSM peptides as potent stimuli for 5-LOX activation in neutrophils.
The virulence potential of S. aureus is defined by the capacity to produce different toxins that damage host cell membranes [14]. Indeed, the S. aureus mutant strain (Δagr/sarA) that lacks the accessory gene regulator (agr) system and the DNA-binding protein SarA with impaired exotoxin formation [40, 41] did not induce 5-LOX translocation in HEK_5-LOX/FLAP cells and in neutrophils. Among different S. aureus exotoxins, the archetype of PFTs is α-hemolysin, released as monomers that assemble to a homo-heptamer pore [42]. Isolated α-hemolysin induced 5-LOX translocation in HEK_5-LOX/FLAP cells, which supports α-hemolysin as 5-LOX activator. However, the S. aureus mutant lacking α-hemolysin expression (LS1Δhla) still induced 5-LOX translocation, which suggested additional bioactive molecules in this respect.
The exotoxins may induce 5-LOX activation by at least two potential routes: (1) 5-LOX phosphorylation at Ser271 by p38 MAPK-dependent MK-2/3 [43] and (2) by elevation of [Ca2+]i. Our data show that inhibition of p38 MAPK by either skepinone-L or SB203580 as well as mutation of Ser271 in 5-LOX to Ala failed to prevent S. aureus-induced 5-LOX translocation, thus excluding phosphorylation as major underlying mechanism. Ca2+-binding upon elevation of [Ca2+]i stimulates 5-LOX translocation and membrane association [3]. In fact, S. aureus-conditioned medium and α-hemolysin induced Ca2+-influx in HEK293 cells, whereas the non-virulent S. aureus strain LS1Δagr/sarA failed to increase [Ca2+]i, and chelation of extracellular Ca2+ by EDTA prevented α-hemolysin-induced 5-LOX translocation. Together, various staphylococcal exotoxins secreted from pathogenic strains are capable of increasing [Ca2+]i in HEK293 cells and accompanied 5-LOX activation.
In contrast to 5-LOX/FLAP-expressing HEK293 cells that hardly form 5-LOX products from endogenous substrate [21], neutrophils possess high capacities to generate LTs from endogenous AA. We found that BCM of S. aureus-induced Ca2+-mediated 5-LOX product biosynthesis in neutrophils, displayed by the marked generation of 5-HETE and LTB4, along with 5-LOX translocation. In addition, in neutrophils, the S. aureus strain LS1Δagr/sarA, deficient in exotoxin generation, failed to induce 5-LOX translocation and product formation, and to increase [Ca2+]i, suggesting a critical role of exotoxins for 5-LOX activation in these cells as well. Intriguingly, in contrast to HEK293 cells, in neutrophils, α-hemolysin failed to elevate [Ca2+]i and to induce 5-LOX translocation, and caused only minute formation of 5-LOX products. Along these lines, BCM from LS1Δhla, deficient in α-hemolysin, showed comparable stimulatory effects on Ca2+, 5-LOX translocation, and product formation as the wild-type strain. Monomeric α-hemolysin mediates its action through ADAM-10 as membrane receptor [44], and it was reported before that neutrophils are fairly resistant to α-hemolysin-induced cytolysis due to low ADAM-10 expression [45], a reasonable explanation for the low susceptibility of neutrophils for 5-LOX activation.
Apart from α-hemolysin, S. aureus releases other exotoxins such as bi-component β-barrel PFTs including PVL, and amphipathic α-helical PSM [14]. Experiments with isolated PSMα1 and PSMα3 as well as by exploiting PSM-deficient S. aureus mutant strains revealed PSMs as relevant factors of S. aureus capable of increasing [Ca2+]i and of 5-LOX activation, whereas PVL was inactive in this respect. The role of PSM could be confirmed in vivo, as infection of mouse paws with S. aureus resulted in a strong increase in LT formation at an early stage of acute inflammation, while infection by the PSM-deficient S. aureus USA300∆psmαβ caused only moderate LTB4 formation. These in vivo data link S. aureus exotoxins and specifically PSMs to LT biosynthesis in inflammatory processes. Note that COX-derived LM biosynthesis seems to be elicited via an alternative mechanism, as the formation of TXB2 and PGE2 was still produced upon infection with exotoxin-deficient S. aureus strains.
PSMs are small amphipathic peptides that can act receptor-independently and elicit cytotoxicity based on their detergent-like properties and high lipid affinity [46]. Recently, the human formyl-peptide receptor 2 (FPR2/ALX) was shown to sense PSMs and thus initiates the pro-inflammatory response by Ca2+ mobilization and leukocyte chemotaxis. Inhibition of this receptor blocked PSM-mediated leukocyte infiltration [20] and the increased chemotaxis was unrelated to elevated levels of tumor necrosis factor α (TNFα) or IL-1β [46]. Here, we applied non-toxic PSM concentrations (1 and 10 µg/mL) that have been used in comparable experimental settings with human neutrophils by others [47, 48]. We suggest that PSM-induced LTB4 formation in neutrophils is mediated by the FPR2/ALX, as pharmacologic antagonism abolished LT formation. Our data thus may link PSMs to induction of chemotaxis, namely, by eliciting the formation of LTB4, one of the most potent chemoattractant in humans [49, 50], through signaling via the FPR2/ALX receptor. Interestingly in HEK cells, PSMs potentially induce a 5-LOX activation through an alternative and receptor-independent route [46], as FPR2/ALX receptor is lacking in these non-immune cells. This receptor exhibits also a binding site for the pro-resolving lipoxin A4 (LXA4), which is transcellularly generated from AA by 5-LOX and 15-LOX [51]. Thus, FPR2/ALX is a potential key receptor on the neutrophil surface that on one hand may induce chemotaxis and leukocyte infiltration by PSM binding, but also counteracts and resolves the inflammatory process by LXA4 ligation. Although cell damage and cytolysis are paramount properties of PSMs even after phagocytosis [52], we show that PSM-induced LT formation is a rapid neutrophil response that requires cellular integrity before PSMs severely damage the host cell.
In summary, S. aureus releases PSMs as potent toxins that induce 5-LOX-mediated LT formation in neutrophils probably at an early stage of bacterial infection. PSM-induced LTB4 biosynthesis could be blocked by FPR2/ALX receptor antagonism and is regulated by Ca2+. Our results support the critical role of PSMs in the pathogenesis of S. aureus as well as efforts to establish anti-PSM treatment as potential strategy to treat S. aureus infections.
Materials and methods
Materials
Unless defined otherwise, bovine serum albumin, calcium chloride, potassium hydrogen phosphate, magnesium sulfate, glucose, and EDTA were purchased from AppliChem (Darmstadt, Germany). MK886 was from Cayman Chemical (Biomol, Hamburg, Germany). Ethanol, methanol, Fura-2AM, and paraformaldehyde solution were from Fisher Scientific (Schwerte, Germany). CHIPS was from HycultBiotech (Uden, The Netherlands). Dulbecco’s modified Eagle’s high-glucose medium with glutamine (DMEM), geneticin, penicillin–streptomycin-solution, and trypsin–EDTA were from GE Healthcare Life Science (Freiburg, Germany). Alexa Fluor 488 goat anti-rabbit, Alexa Fluor 555 goat anti-mouse, hygromycin B, lipofectamine LTX Reagent Plus, and non-immune goat serum were from Invitrogen (Darmstadt, Germany). IRDye 800CW goat anti-rabbit and IRDye 680LT goat anti-mouse IgG secondary antibodies were from Li-Cor Biotechnology (Bad Homburg, Germany). WKYMVm peptide was from Tocris Bioscience (Ellisville, MI, USA). DMSO and HEPES sodium salt were from Merck (Darmstadt, Germany); SDS, tris, and Triton X-100 from Roth GmbH (Karlsruhe, Germany); zileuton was from Sequoia Research Products (Oxford, UK); and Dulbecco’s Buffer Substance (PBS) was purchased from SERVA Electrophoresis (Heidelberg, Germany). UPLC solvents, hexane, methylformiate, acetic acid, acrylamide, WRW4, potassium chloride, and magnesium chloride were from VWR (Darmstadt, Germany). A23187, dextran, fetal calf serum (FCS), phenylmethanesulfonyl fluoride, soybean trypsin inhibitor, leupeptin, α-hemolysin, fMLF, and all the other chemicals were purchased from Sigma-Aldrich (Taufkirchen, Germany). PSMα1 and PSMα3 were synthesized as described [53] or ordered by Genosphere Biotechnologies (Stuttgart, Germany). E. coli (Migula-ATCC19138) Castellani and Chalmers ATCC® serotype O6:K2:H1 was purchased from LGC Standards GmbH (ATCC) (Wesel, Germany) and BL21 (DE3) competent E. coli was from New England Biolabs GmbH (Frankfurt a. M., Germany). The Δpsmαβ mutant of strain LS1 was generated by transduction using ϕ11 lysates of RN4220-307 (Δpsmα1-4:tetM) and RN4220-308 (Δpsmβ1-2:tetM) [54]. Transductants were verified by PCR using oligonucleotides flanking the resistance cassettes.
Cell isolation and cultivation
Experiments with human blood were approved by the ethical review committee of the university hospital Jena, Germany (license number: 4968-11/16). Peripheral human blood from healthy fasted donors (adult males and females, 18–65 years) was obtained as leukocyte concentrate from the University Hospital. Neutrophil isolation was performed as described [55]. Briefly, dextrane-sedimented blood preparations were centrifuged on lymphocyte separation medium followed by hypotonic erythrocyte hemolysis. Remaining neutrophils were washed twice with ice-cold PBS and diluted as depicted for each experiment. Monocytes and monocyte-derived M1 and M2 macrophages for western blot analysis were prepared as published previously [6, 56].
HEK_5-LOX/FLAP cells were cultured in monolayers in DMEM supplemented with 10% FCS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C and 5% CO2. Transfection of HEK293 cells and generation of cell lines stably expressing 5-LOX and FLAP were performed with lipofectamine LTX Reagent Plus according to the vendor’s instructions using pcDNA3.1 plasmids as described [21]. Cells expressing 5-LOX and FLAP or 5-LOXS_271A and FLAP were selected by 200 µg/mL hygromycin B and 400 µg/mL geneticin, respectively. The construct for the 5-LOX_S271A mutant (pcDNA3.1_5-LOX_S271A) was generated and provided by Dr. Oliver Werz [43].
Bacteria-conditioned medium (BCM) preparation
50 mL NB-medium was inoculated with E. coli and cultured at 37 °C. 10 µL of the overnight culture was transferred into fresh medium and cultured at 37 °C until OD600 of 1 was reached. S. aureus strains and S. carnosus were cultivated in BHI medium over night at 37 °C. Bacteria cultures were then centrifuged at 3000g at room temperature for 15 min and either resuspended in the corresponding stimulation buffer or supernatants were sterile-filtered using 0.22 µm PVDF syringe filters (Roth GmbH, Karlsruhe, Germany) to obtain bacteria-conditioned medium (BCM). The absence of bacteria cells was verified by incubation of the BCM for 16 h at 37 °C. Note that OD600 of 1 was considered as 1 × 109 intact bacteria for both E. coli and S. aureus as well as S. carnosus strains, respectively. Infection was performed at a multiplicity of infection ratio (MOI) of 1:50 (HEK vs. bacteria).
Immunofluorescence microscopy (IF)
5-LOX translocation was investigated using immunofluorescence microscopy. Trypsinized HEK293 cells were seeded onto poly-d-lysine-coated glass coverslips (Kleinfeld Labortechnik, Gehrden, Germany) 2 days prior to conducting the experiments and cultured in DMEM medium as described before. Freshly isolated neutrophils were resuspended in PBS containing 1 mM Ca2+ and 0.5 mM Mg2+ (PBS+/+) and 5 × 106 cells were seeded onto glass coverslips 30 min before stimulation. Unstimulated samples were kept without bacteria, BCM, or isolated exotoxins for either the longest or shortest incubation time to exclude effects of the stimulation buffer on 5-LOX translocation. Stimulation with intact bacteria, BCM, α-hemolysin, or PSMs was performed in PBS+/+ for the indicated periods at 37 °C and stopped by paraformaldehyde fixation (4%, 15 min) followed by permeabilization with ice-cold 100% acetone for 5 min following treatment with Triton X-100 (0.25% in PBS) for 10 min at room temperature. Slides were subsequently incubated with primary antibodies diluted in non-immune goat serum overnight at 4 °C. Slides were then incubated with Alexa Fluor 488 goat anti-rabbit (1:1000) and Alexa Fluor 555 goat anti-mouse (1:1000) fluorophore-labeled secondary antibodies for 30 min in the dark and fixed in DAPI-containing ProLong Diamond Antifade Mountant (Invitrogen, Darmstadt, Germany) on glass slides. A Zeiss Axiovert 200 M microscope and a Plan-APOCHROMAT 40×/1.3 Oil DIC (UV)Vis–IR 0.17/∞ objective (Carl Zeiss, Jena, Germany) were used to visualize the cells followed by image acquisition with an AxioCam MR camera (Carl Zeiss). Microscopic images of 20 µm (HEK) or 15 µm (neutrophils) size are given as overlay of 5-LOX (red) and FLAP (green) staining and are representative for three independent experiments.
Analysis of intracellular Ca2+-concentration
To determine the effect of physiological stimuli on the intracellular Ca2+ concentration, 5 × 107 neutrophils were resuspended in 20 mM HEPES buffer pH 7.4 containing 135 mM NaCl, 5 mM KCl, 1 mM MgSO4, 0.4 mM KH2PO4, and 5.5 mM glucose (KREPS–HEPES buffer) and stained with 2 µM Fura-2AM for 45 min (37 °C, 5% CO2) in the dark. After centrifugation (1000 rpm, 5 min, RT), pelleted cells were suspended in KREBS–HEPES buffer supplemented with 1 mM CaCl2 and 0.1% bovine serum albumin (BSA) to a final cell density of 5 × 106 cells/mL. 100 µL of the cell suspension were seeded onto black 96-well microplates PS F-bottom (Greiner bio-one, Frickenhausen, Germany) and stimulated as indicated, followed by continuous measurement of fluorescence emission λem at 510 nm after excitation λex at 340 nm and 380 nm, respectively. Ratios of the emissions were calculated and 1% Triton X-100 treatment was set to 1 in terms of normalization. HEK293 cells were seeded on cell culture flasks 2 days prior to the experiment and labeled with 2 µM Fura-2AM in KREBS–HEPES buffer for 30 min (37 °C, 5% CO2) in the dark. Subsequently, cells were gently washed from the flask and the assay was performed as described using 0.1 × 106 HEK293 cells/well.
Western blot analysis
Stable expression of 5-LOX and FLAP was verified by immunoblotting. HEK cells were harvested by trypsinization and centrifuged at 1200 rpm (10 min, 4 °C). Cell pellets were lysed for 15 min on ice in 20 mM Tris buffer pH 7.4 containing 150 mM NaCl, 2 mM EDTA, and 1% Triton X-100 after the addition of 1 mM phenylmethanesulfonyl fluoride, 10 µg/mL leupeptin, and 10 µg/mL soybean trypsin inhibitor. SDS-PAGE separation on a 16% polyacrylamide gel was followed by wet tank blotting onto a nitrocellulose membrane and overnight incubation in mouse anti-5-LOX (1:1000), rabbit anti-FLAP (1:1000) or mouse anti-β-actin (Santa Cruz Biotechnology Inc., Heidelberg, Germany) primary antibodies, respectively. Membranes were stained with IRDye 800CW goat anti-rabbit or IRDye 680LT goat anti-mouse IgG secondary antibodies and detected by Odyssey infrared imager (Li-Cor Biosciences, Lincoln, NE, USA).
To determine the expression of FPR2/ALX, leukocytes were lysed in 20 mM Tris buffer pH 7.4 containing 150 mM NaCl, 2 mM EDTA, and 1% Triton X-100 after the addition of 1 mM phenylmethanesulfonyl fluoride, 10 µg/mL leupeptin, and 10 µg/mL soybean trypsin inhibitor, followed by SDS-PAGE separation on a 12% polyacrylamide gel, wet tank blotting, and antibody staining as described above utilizing rabbit anit-FPRL1/FPR2 (1:1000, Novus Biologicals, Abingdon, UK) and mouse anti-β-actin as primary antibodies.
Determination of LM formation in human neutrophils and mouse paw homogenates
To assay lipid mediator formation in freshly isolated neutrophils, 5 × 106 cells/mL in PBS buffer containing 1 mM CaCl2 and 0.1% glucose were stimulated with isolated bacterial toxins, 1% of BCM or untreated medium or 2.5 µM A23187 for 10 min at 37 °C. As indicated, cells were pretreated with inhibitors or vehicle control for 10 min at 37 °C. Reactions were terminated with 2 volumes of ice-cold methanol and 0.66 ng d8-5(S)-HETE, 31.2 ng d8-AA, 0.71 ng d4-PGE2, 0.72 ng d5-LXA4, 0.76 ng d5-RvD2, and 0.68 ng d4-LTB4 (Cayman Chemical, Biomol, Hamburg, Germany) were added as internal standards. Acidified samples were subjected to Waters Sep-Pak® Vac 6cc extraction columns (Waters, Milford, MA, USA) and washed with MilliQ water and n-hexane prior to elution with methylformiate. Subsequently, nitrogen-dried samples were solved in 50% methanol and analyzed with UPLC–MS/MS (Acquity™ UPLC system, Waters, Milford, MA, USA; QTRAP 5500 Mass Spectrometer, ABSciex, Darmstadt, Germany; Turbo V™ Source and electrospray ionization). Briefly, analytes were separated on an ACQUITY UPLC® BEH C18 column (1.7 µm, 2.1 × 100 mm; Waters, Eschborn, Germany) at 50 °C and 0.3 mL/min flow using MilliQ water (A) and methanol (B) both acidified with 0.01% acetic acid, starting with 42% of B, increasing up to 86% at 12.5 min followed by isocratic elution at 98% until 15.5 min. Transitions (m/z) were used in a negative ion mode (ion spray voltage 4000 V and heater temperature 500 °C) to detect the indicated lipids as previously described [6].
In vivo experiments
The murine footpad infection models were conducted in accordance with the recommendation and guidelines of the German Society for Laboratory Animal Science 22-2684-04-02-003/15 (Thuringia, Jena). C57BL/6 mice (Charles River) were kept under pathogen-free conditions. Footpad infection was performed as described by [57]. Briefly, C57BL/6 mice were inoculated subcutaneously with 2 × 107 CFU of S. aureus USA300 (in 50 μL PBS 1%) into the right hind footpad. The left hind footpad was inoculated with 50 µL of PBS 1%. Groups of 4 mice were employed per group/experiment. Mice infected with S. aureus USA300 were sacrificed at 2, 6, 9, 24, and 48 h post-infection. Mice infected with USA300∆psmαβ were sacrificed at 48 h after infection. All foot pad tissues were homogenized and stored at − 80 °C until further analysis. Homogenates were subjected to solid phase extraction as described above. LM formation was normalized to 1 µg total protein within the homogenates.
Analysis of cytotoxicity
To evaluate the cytotoxic effect of BCM on human neutrophils, lactate dehydrogenase (LDH) release was measured by CytoTox-ONE™ Homogenous Membrane Integrity assay (Promega GmbH, Mannheim, Germany) according to the vendor’s instructions. Briefly, 5 × 106 cells/mL in PBS buffer containing 1 mM CaCl2 and 0.1% glucose was incubated with 1% BCM or Triton X-100 (1%, positive control for total cell lysis) for 10 min at 37 °C and subsequently centrifuged at 350 g (5 min, 4 °C). Supernatants were collected and diluted to appropriate LDH concentrations for fluorometric detection at 490 nm. In addition, 5 × 106 cells/mL in PBS buffer containing 1 mM CaCl2 and 0.1% glucose were treated with 1% BCM as described above and stained with trypan blue prior to automatic cell counting using Vi-CELL™ XR (Beckmann Coulter GmbH, Krefeld, Germany). Viability is presented as percentage of viable cells to total cell number.
Statistics
If not stated otherwise, microscopic images are representative for n = 3 independent experiments. Remaining results are presented as mean and standard error of the mean (SEM) of n experiments on different days or with different donors. Statistical evaluation was performed as indicated for each figure using SigmaPlot 13.0 software (Systat Software Inc., San Jose). p values < 0.05 were considered as significant. Concrete p values are indicated for 0.05 ≤ p ≤ 0.20. Total amounts of lipid mediators were logarithmized (log10) to assure normal distribution of the values for statistical analysis. Significant differences were tested either between vehicle and wild-type strains or positive controls (e.g., ionomycin), respectively, or between wild-type strains and the corresponding mutant strains. Principal component analysis (PCA) of lipid mediator formation upon stimulation with BCMs of S. aureus LS1 (wild type, Δhla, Δpsmαβ, and Δagr/sarA), isolated α-hemolysin or PSMα3, and vehicle control (unconditioned medium), respectively, was performed using OriginPro 2015G software. Free AA, DHA, EPA, and ω3 DPA were excluded for PCA to focus on mediators that are biosynthesized during incubation. The IC50 values were calculated from four different concentrations in semi-logarithmic graphs applying GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA).
References
Nauseef WM, Borregaard N (2014) Neutrophils at work. Nat Immunol 15:602–611. https://doi.org/10.1038/ni.2921
de Oliveira S, Rosowski EE, Huttenlocher A (2016) Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16:378–391. https://doi.org/10.1038/nri.2016.49
Rådmark O, Werz O, Steinhilber D, Samuelsson B (2015) 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta 1851:331–339. https://doi.org/10.1016/j.bbalip.2014.08.012
Dixon RA, Diehl RE, Opas E, Rands E, Vickers PJ et al (1990) Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature 343:282–284. https://doi.org/10.1038/343282a0
Gerstmeier J, Weinigel C, Rummler S, Radmark O, Werz O et al (2016) Time-resolved in situ assembly of the leukotriene-synthetic 5-lipoxygenase/5-lipoxygenase-activating protein complex in blood leukocytes. FASEB J 30:276–285. https://doi.org/10.1096/fj.15-278010
Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M et al (2018) Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun 9:59. https://doi.org/10.1038/s41467-017-02538-5
Golenkina EA, Galkina SI, Romanova JM, Lazarenko MI, Sud’ina GF (2011) Involvement of red blood cells in the regulation of leukotriene synthesis in polymorphonuclear leucocytes upon interaction with Salmonella typhimurium. APMIS 119:635–642. https://doi.org/10.1111/j.1600-0463.2011.02786.x
Wu HJ, Wang AH, Jennings MP (2008) Discovery of virulence factors of pathogenic bacteria. Curr Opin Chem Biol 12:93–101. https://doi.org/10.1016/j.cbpa.2008.01.023
Peters-Golden M, Henderson WR Jr (2007) Leukotrienes. N Engl J Med 357:1841–1854. https://doi.org/10.1056/nejmra071371
Mancuso P, Nana-Sinkam P, Peters-Golden M (2001) Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun 69:2011–2016. https://doi.org/10.1128/IAI.69.4.2011-2016.2001
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. https://doi.org/10.1038/ni.1863
Surette ME, Palmantier R, Gosselin J, Borgeat P (1993) Lipopolysaccharides prime whole human blood and isolated neutrophils for the increased synthesis of 5-lipoxygenase products by enhancing arachidonic acid availability: involvement of the CD14 antigen. J Exp Med 178:1347–1355
Bischofberger M, Iacovache I, van der Goot FG (2012) Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 12:266–275. https://doi.org/10.1016/j.chom.2012.08.005
DuMont AL, Torres VJ (2014) Cell targeting by the Staphylococcus aureus pore-forming toxins: it’s not just about lipids. Trends Microbiol 22:21–27. https://doi.org/10.1016/j.tim.2013.10.004
Peschel A, Otto M (2013) Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol 11:667–673. https://doi.org/10.1038/nrmicro3110
Bronner S, Monteil H, Prevost G (2004) Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol Rev 28:183–200. https://doi.org/10.1016/j.femsre.2003.09.003
Tuchscherr L, Bischoff M, Lattar SM, Noto Llana M, Pfortner H et al (2015) Sigma factor SigB is crucial to mediate Staphylococcus aureus adaptation during chronic infections. PLoS Pathog 11:e1004870. https://doi.org/10.1371/journal.ppat.1004870
Bremm KD, Konig W, Pfeiffer P, Rauschen I, Theobald K et al (1985) Effect of thiol-activated toxins (streptolysin O, alveolysin, and theta toxin) on the generation of leukotrienes and leukotriene-inducing and -metabolizing enzymes from human polymorphonuclear granulocytes. Infect Immun 50:844–851
Suttorp N, Seeger W, Zucker-Reimann J, Roka L, Bhakdi S (1987) Mechanism of leukotriene generation in polymorphonuclear leukocytes by staphylococcal alpha-toxin. Infect Immun 55:104–110
Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M et al (2010) Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7:463–473. https://doi.org/10.1016/j.chom.2010.05.012
Gerstmeier J, Weinigel C, Barz D, Werz O, Garscha U (2014) An experimental cell-based model for studying the cell biology and molecular pharmacology of 5-lipoxygenase-activating protein in leukotriene biosynthesis. Biochim Biophys Acta 1840:2961–2969. https://doi.org/10.1016/j.bbagen.2014.05.016
Licona-Limon I, Garay-Canales CA, Munoz-Paleta O, Ortega E (2015) CD13 mediates phagocytosis in human monocytic cells. J Leukoc Biol 98:85–98. https://doi.org/10.1189/jlb.2A0914-458R
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P et al (2015) Proteomics. Tissue-based map of the human proteome. Science 347:1260419. https://doi.org/10.1126/science.1260419
Chart H, Smith HR, La Ragione RM, Woodward MJ (2000) An investigation into the pathogenic properties of Escherichia coli strains BLR, BL21, DH5alpha and EQ1. J Appl Microbiol 89:1048–1058
Gotz F, Schumacher B (1987) Improvements of protoplast transformation in Staphylococcus carnosus. FEMS Microbiol Lett 40:285–288. https://doi.org/10.1016/0378-1097(87)90391-0
Los FC, Randis TM, Aroian RV, Ratner AJ (2013) Role of pore-forming t oxins in bacterial infectious diseases. Microbiol Mol Biol Rev 77:173–207. https://doi.org/10.1128/MMBR.00052-12
Porta H, Cancino-Rodezno A, Soberon M, Bravo A (2011) Role of MAPK p38 in the cellular responses to pore-forming toxins. Peptides 32:601–606. https://doi.org/10.1016/j.peptides.2010.06.012
Kramer S, Sellge G, Lorentz A, Krueger D, Schemann M et al (2008) Selective activation of human intestinal mast cells by Escherichia coli hemolysin. J Immunol 181:1438–1445
Werz O, Klemm J, Samuelsson B, Radmark O (2000) 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc Natl Acad Sci USA 97:5261–5266. https://doi.org/10.1073/pnas.050588997
Koeberle SC, Romir J, Fischer S, Koeberle A, Schattel V et al (2011) Skepinone-L is a selective p38 mitogen-activated protein kinase inhibitor. Nat Chem Biol 8:141–143. https://doi.org/10.1038/nchembio.761
Hammarberg T, Provost P, Persson B, Radmark O (2000) The N-terminal domain of 5-lipoxygenase binds calcium and mediates calcium stimulation of enzyme activity. J Biol Chem 275:38787–38793. https://doi.org/10.1074/jbc.M006136200
Mantovani A, Cassatella MA, Costantini C, Jaillon S (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11:519–531. https://doi.org/10.1038/nri3024
Brock TG, McNish RW, Bailie MB, PetersGolden M (1997) Rapid import of cytosolic 5-lipoxygenase into the nucleus of neutrophils after in vivo recruitment and in vitro adherence. J Biol Chem 272:8276–8280. https://doi.org/10.1074/jbc.272.13.8276
de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ et al (2004) Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199:687–695. https://doi.org/10.1084/jem.20031636
Christophe T, Karlsson A, Dugave C, Rabiet MJ, Boulay F et al (2001) The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J Biol Chem 276:21585–21593. https://doi.org/10.1074/jbc.M007769200
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339:520–532. https://doi.org/10.1056/NEJM199808203390806
DeLeo FR, Otto M, Kreiswirth BN, Chambers HF (2010) Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. https://doi.org/10.1016/S0140-6736(09)61999-1
Kavanagh N, Ryan EJ, Widaa A, Sexton G, Fennell J et al (2018) Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin Microbiol Rev. https://doi.org/10.1128/CMR.00084-17
Foster TJ (2005) Immune evasion by staphylococci. Nat Rev Microbiol 3:948–958. https://doi.org/10.1038/nrmicro1289
Arya R, Princy SA (2013) An insight into pleiotropic regulators Agr and Sar: molecular probes paving the new way for antivirulent therapy. Future Microbiol 8:1339–1353. https://doi.org/10.2217/fmb.13.92
Cheung AL, Eberhardt KJ, Chung E, Yeaman MR, Sullam PM et al (1994) Diminished virulence of a sar-/agr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Invest 94:1815–1822. https://doi.org/10.1172/JCI117530
Dal Peraro M, van der Goot FG (2016) Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol 14:77–92. https://doi.org/10.1038/nrmicro.2015.3
Werz O, Szellas D, Steinhilber D, Radmark O (2002) Arachidonic acid promotes phosphorylation of 5-lipoxygenase at Ser-271 by MAPK-activated protein kinase 2 (MK2). J Biol Chem 277:14793–14800. https://doi.org/10.1074/jbc.M111945200
Wilke GA, Bubeck Wardenburg J (2010) Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci USA 107:13473–13478. https://doi.org/10.1073/pnas.1001815107
Nygaard TK, Pallister KB, Zurek OW, Voyich JM (2013) The impact of alpha-toxin on host cell plasma membrane permeability and cytokine expression during human blood infection by CA-MRSA USA300. J Leukoc Biol 94:971–979. https://doi.org/10.1189/jlb.0213080
Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY et al (2007) Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13:1510–1514. https://doi.org/10.1038/nm1656
Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR et al (2010) Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog 6:e1001133. https://doi.org/10.1371/journal.ppat.1001133
Li L, Pian Y, Chen S, Hao H, Zheng Y et al (2016) Phenol-soluble modulin alpha4 mediates Staphylococcus aureus-associated vascular leakage by stimulating heparin-binding protein release from neutrophils. Sci Rep 6:29373. https://doi.org/10.1038/srep29373
Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W et al (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498:371–375. https://doi.org/10.1038/nature12175
Kienle K, Lammermann T (2016) Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev 273:76–93. https://doi.org/10.1111/imr.12458
Chiang N, Arita M, Serhan CN (2005) Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids 73:163–177. https://doi.org/10.1016/j.plefa.2005.05.003
Surewaard BG, de Haas CJ, Vervoort F, Rigby KM, DeLeo FR et al (2013) Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol 15:1427–1437. https://doi.org/10.1111/cmi.12130
Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D et al (2010) Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 6:e1000715. https://doi.org/10.1371/journal.ppat.1000715
Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C et al (2012) The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog 8:e1003016. https://doi.org/10.1371/journal.ppat.1003016
Boyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 97:77–89
Garscha U, Romp E, Pace S, Rossi A, Temml V et al (2017) Pharmacological profile and efficiency in vivo of diflapolin, the first dual inhibitor of 5-lipoxygenase-activating protein and soluble epoxide hydrolase. Sci Rep 7:9398. https://doi.org/10.1038/s41598-017-09795-w
Nippe N, Varga G, Holzinger D, Loffler B, Medina E et al (2011) Subcutaneous infection with S. aureus in mice reveals association of resistance with influx of neutrophils and Th2 response. J Invest Dermatol 131:125–132. https://doi.org/10.1038/jid.2010.282
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (GA2101/2-1, SFB1127 ChemBioSys, and SFB 1278 Polytarget) and SFB-TR156 to C.W., E.R. received a stipend from Landesgraduierten-Akademie Jena. V.A. was supported by the International Leibniz Research School for Microbial and Biomolecular Interactions (ILRS). L.T. and B.L. were supported by the Leibniz Science Campus InfectoOptics SAS-2015-HKI-LWC.
Author information
Authors and Affiliations
Contributions
ER, VA, JF, AS, and CW performed the experiments; ER and UG performed data analysis; ER, OW, and UG designed the study and all authors contributed to the discussion and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Romp, E., Arakandy, V., Fischer, J. et al. Exotoxins from Staphylococcus aureus activate 5-lipoxygenase and induce leukotriene biosynthesis. Cell. Mol. Life Sci. 77, 3841–3858 (2020). https://doi.org/10.1007/s00018-019-03393-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03393-x