Abstract
Apoptosis plays a crucial role in clearing old or critically compromised cells, and actively maintains epithelial homeostasis and epithelial morphogenesis during embryo development. But how is the apoptotic signaling pathway able to orchestrate such complex and dynamic multi-cellular morphological events at the tissue scale? In this review we collected the most updated knowledge regarding how apoptosis controls different cytoskeletal components. We describe how apoptosis can control epithelial homeostasis though epithelial extrusion, a highly orchestrated process based on high- order actomyosin structures and on the coordination between the apoptotic and the neighboring cells. Finally, we describe how the synergy among forces generated by multiple apoptotic cells can shape epithelia in embryo development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple mechanisms of cell death have been described, from accidental, such as necrosis, to programmed, such as apoptosis, including unconventional types of cell death, such as pyroptosis [1]. Apoptosis is currently the most investigated cell death mechanism, playing crucial roles in the development and tissue homeostasis, as well as in defence from pathogens. It was first defined in 1972 by Kerr, Wyllie, and Currie [2], although some signs of the existence of this process had been reported by Karl Vogt and Walther Flemming in the nineteenth century [3]. Since then, apoptosis has been investigated extensively, providing a detailed picture of the signaling pathways involved, the morphological cell events, as well as its role in development and tissue homeostasis.
The most general definition of apoptosis is an energy-dependent, irreversible and highly coordinated program that leads to dying cells being dismantled without releasing the cytoplasm content, or the organelles and nucleus, into the extracellular space. Various stimuli can trigger apoptosis and are transduced by the intrinsic, extrinsic and perforin/granzyme-dependent pathways. All the apoptotic pathways converge in caspases-3 and -7 activation, which leads to the execution phase of apoptosis [4]. Caspases are proteases that cleave specifically after an aspartate, which coevolve with their targets and triggered their activation, inactivation or change of function [5]. There are several recognized targets of caspases in human cells and their cleavage is involved in most of the molecular and morphologic events related to apoptosis.
The morphological events associated with apoptosis were identified in the original studies. However, their complexity, regulation and implications in development and tissue homeostasis have only been investigated recently. This review reports on the most recent knowledge of the features and regulation of apoptotic morphological events. The focus is on their role in embryo development and in epithelial extrusion, the latter being fundamental in the maintenance of epithelial homeostasis.
Cell morphological changes during apoptosis
One distinctive feature of apoptosis, compared to passive cell death, is the execution of energy-consuming mechanical processes, such as shrinking of the cell, blebbing, nuclear fragmentation and formation of apoptotic bodies [6]. The efficiency of these processes is critical for dismantling the apoptotic cell without loss of cytoplasm content in the extracellular space.
Cell shrinkage
Apoptotic cells show a significant loss of cell size, termed as cell shrinkage, which can vary between 15 and 85% depending on the cell type and on the experimental approach used to detect it [7]. Although cell shrinkage is one of the most studied morphological changes of the apoptotic cell, there is still no standard explanation. Dehydration affects several systems and can be determined by K+ or Cl− efflux [8, 9], cytoplasm acidification [10], efflux of small organic osmolytes, such as taurine [11], and by water exit through aquaporins [12]. However, it cannot be ruled out that some apoptotic bodies are released before the final cell disassembly, thereby causing a temporary reduction in volume [7, 13].
Cell blebbing
Blebbing is one of the most specific hallmarks of apoptosis [6]. During this process, acto-myosin contraction leads to detachment of the plasma membrane from the actin cortex, generating weak spots. In each spot, hydrostatic pressure leads to the formation of a protrusion with a spherical morphology (i.e., bleb) which contains cytosol or organelles. This phase of apoptosis is associated with a significant increase in phosphorylation of the myosin light chain subunit that eventually leads to an increase in myosin heavy chain activity. The consequent massive actomyosin contraction causes the detachment of the cell from the substrate, acquisition of a spherical morphology, and formation of a great number of blebs on the entire surface of the apoptotic cell [14]. Blebs are highly dynamic protrusions that continuously expand and retract.
Blebs are formed during the entire execution phase of apoptosis until cell dismantling into apoptotic bodies. There are two main phases: initially only small cell surface bulges form; while in the later stage, larger dynamic blebs appear that contain fragments of organelles [15].
The function of plasma membrane blebbing during apoptosis is not clear. Although it may simply be a by-product of massive myosin contraction leading to substrate detachment and the formation of apoptotic bodies, a recent report showed that during blebbing, a damage-associated molecular signature is released. Following such molecular cues, professional phagocytic cells can be attracted to the apoptotic site and contribute to clearing the apoptotic bodies [16].
Other cell protrusions
Although blebs are by far the most frequent type of protrusions found in apoptotic cells, other types of protrusion have been described in specific cell types [17]. Microtubule spikes were observed in A431 epithelial cells as long microtubule-rich rigid protrusions forming 1 h after classical blebbing. These structures form independently of myosin contraction, and instead exploit the polymerization of microtubules [18]. Apoptopodia are protrusions corresponding to the stage preceding the release of apoptotic bodies. They are string-like structures connecting the future apoptotic bodies to the rest of the cell [19]. Beaded apoptopodia are another type of apoptopodia-like protrusions recently observed in apoptotic human THP-1 monocytic cells and primary human monocytes. These long and thin protrusions segment and form a long chain of blebs that then disassemble into apoptotic bodies [20].
Apoptotic bodies
The last stage of apoptosis coincides with the most dramatic morphological event: cell body disassembly into several apoptotic bodies [17]. These membrane-bound vesicles contain cytoplasm, organelles and nuclear fragments and are released from the dying cell into the extracellular space. Among the different types of extracellular vesicles, apoptotic bodies are the largest (typically 1–5 μm in diameter) and can be easily separated from cultured cells, tissues and blood-derived samples [21]. Their main function is to subdivide the apoptotic cell body into several parts that can then be phagocytosed by other cells. For this reason, apoptotic bodies emit “eat-me” signals that facilitate the interaction and subsequent phagocytosis [22]. Apoptotic body clearance needs to be efficient for tissue homeostasis to take place: if their phagocytosis fails, they undergo secondary necrosis which then causes inflammation and tissue damage [23].
Despite the importance of apoptotic bodies in the clearance of apoptotic cells, the mechanism leading to their formation still remains unclear. Whereas in beaded apoptopodia, an abscission process has been proposed to explain fragmentation in multiple vesicles [20], non-cell autonomous mechanisms have been suggested to explain apoptotic body separation, such has shear stress [19] or forces generated by neighboring cells in compact tissues as in epithelia [24]. However, such mechanisms are restricted to specific contexts and do not seem to justify the robustness and universality of cell body fragmentation in apoptotic bodies during apoptosis.
The role and regulation of cytoskeleton during apoptosis
In animal cells, the cytoskeleton is the main structure involved in the generation of mechanical forces. However, the various components of cytoskeleton play different roles in apoptosis (Fig. 1).
Cytoskeleton rearrangements during apoptosis. This figure shows how the three types of cytoskeleton are reorganized during apoptosis and their functional role in such process. The actomyosin cytoskeleton starts an intense contractile activity which leads to plasma-membrane blebbing and nuclear fragmentation. The intermediate filament cytoskeleton is completely disassembled. The microtubule cytoskeleton is initially disassembled due to proteolysis of centriole. It then reassembles itself in the apoptotic microtubule network (AMN), which protects plasma membrane proteins from caspase proteolytic activity
Intermediate filaments
Intermediate filaments are disassembled, relieving cells from their structural constraints. Keratin 18 is typically expressed in epithelial cells and is cleaved by caspases in two sites, causing the dismantling of the keratin 18 cytoskeleton and the formation of several aggregates devoid of structural functions [25]. In mesenchymal cells, caspases also cleave vimentin, causing disassembly of vimentin cytoskeleton in later phases of apoptosis, coinciding with nuclear fragmentation [26]. Nuclear lamin is also cleaved by caspases and such cleavage is required for nuclear shrinking and chromatin condensation [27].
Microtubules
Despite an initial disassembly, the microtubule cytoskeleton plays a crucial role in several specific events during apoptosis. Its disassembly is triggered by caspase-mediated cleavage of proteins composing the centriole, followed by destabilization of this structure preventing de novo microtubule polymerization [28]. However, before cell body disassembly into apoptotic bodies, the microtubule cytoskeleton is re-formed beneath the plasma membrane in the apoptotic microtubule network (AMN) [29]. During this phase, the organization of the microtubule does not depend on conventional gamma-tubulin ring complex nucleation. AMN maintains membrane integrity, specifically by determining a caspase-free zone that protects transmembrane proteins from proteolytic cleavage. According to this interpretation, AMN is necessary to preserve plasma membrane functionality and to prevent the early occurrence of secondary necrosis [30]. In addition, non-centrosomal bundles of densely packed, dynamic microtubules have been observed and described in the cytoplasm of apoptotic cells. These bundles carry condensed chromatin toward the cell surface, mediate chromatin condensation in blebs, and sustain the formation of microtubule-spikes [18].
Acto-myosin
The dominant cytoskeleton component in the morphological events observed during apoptosis is the actomyosin cytoskeleton. During apoptosis, the actomyosin cytoskeleton actively rearranges cell morphology, causing nuclear fragmentation, membrane blebbing and apoptotic bodies [16, 28, 31]. The actomyosin cytoskeleton is involved in nuclear fragmentation in combination with nuclear lamina degradation. In fact, cleavage of the nuclear lamins A/C and B1 weakens the nuclear envelope, which is then crushed by actomyosin contraction [32]. Blebs emerge on the cellular surface via the detachment of the plasma membrane from the cortical cytoskeletal network. Once detached, the plasma membrane rapidly expands sustained by the high hydrostatic pressure generated by the massive actomyosin contraction occurring during apoptosis [33]. Non-muscular myosin activity is regulated by phosphorylation of the myosin light chain (MLC) subunit that in turn activates the ATPase activity of the heavy subunit. In the execution phase of apoptosis, a sharp increase in MLC phosphorylation leads to an acute activation of actomyosin cytoskeleton contraction [14].
Regulation of acto-myosin contraction
Several molecular regulators are involved in the increase in MLC phosphorylation. The members of the Rho-activated kinase (RaK) family, such as Rho-associated protein kinase 1 (ROCK1), myotonic dystrophy kinase-related CDC42-binding kinase alpha (MRCKα) and MRCKβ, are all kinases that are able to phosphorylate and activate MLC [31, 34, 35]. RaKs are large multidomain proteins, whose kinase activity is restricted in time and space thanks to the presence of inhibitory and localizing domains, respectively. Caspases release their full kinase activity by cleavage and separation from the inhibitory and localization domains [34, 36]. As a result of such constitutive activation, MLC pools beneath the plasma membrane are acutely phosphorylated, causing cortical acto-myosin contraction and plasma membrane blebbing. Interfering with this regulation, by inhibiting either RaKs or myosin ATPase activity, blocks membrane blebbing, nuclear disintegration and the formation apoptotic bodies [16, 31, 32].
RaKs are not the only enzymes controlling the acto-myosin contraction during apoptosis. Myosin light chain kinase (MLCK) is another MLC kinase not belonging to the RaK family but activated by ROCK through phosphorylation. Interestingly, MLCK can also be activated by caspase-mediated proteolytic cleavage, and its pharmacological inhibition prevents plasma membrane blebbing [14].
Furthermore, the phosphorylation of MLC during apoptosis can also be promoted by inhibition of the negative regulators, such as MLC phosphatase. Constitutively activated RaKs phosphorylate myosin phosphatase by targeting subunit 1 (MYPT1) on threonine 696. This causes its dissociation from MLC, thus further sustaining its phosphorylation. MYPT1 itself is a substrate of caspase-mediate cleavage, which irreversibly prevents its phosphatase activity [37].
The morphological events observed during apoptosis rely on the integrity and functionality of the actomyosin cytoskeleton. However, this assumption does not mean that in certain models and in the final stages of cell disintegration, the actomyosin cytoskeleton is not also disassembled. For instance, it has been reported that actin is cleaved by caspases thereby causing actomyosin cytoskeleton dismantling in neutrophils and in myofibroblasts [38, 39]. However, in most of the other cellular systems, actin shows resistance to caspase-mediated degradation. Although a definitive explanation of such actin resistance to caspase-mediated proteolysis is still lacking, it has been proposed that in most cell types there are certain factors preventing caspases from reaching the cleavage site on actin [40].
The role of epithelial extrusion in tissue homeostasis
Through exposure to the external environment, epithelial cell layers are constantly subjected to a wide spectrum of harmful physical, chemical and biological agents. Consequently, they evolved with a very fast turnover, so as to eliminate cells before the accumulation of damage has a negative impact on the barrier function or leads to the development of cancer.
Both cell division and the removal of dying cells within the epithelial layer are challenging: (1) both events have to avoid the formation of gaps that would undermine the barrier function; (2) each epithelial cell is connected to neighboring cells and to the basal membrane by multiple cell adhesions; and (3) cell division and cell death have to be balanced to maintain epithelial functionality.
The maintenance of cell–cell contacts is so critical that epithelial cells have evolved different modalities of cell division depending on the aspect ratio, e.g., columnar vs cuboidal [41]. Consequently, epithelial cell division is carried out by maintaining cell–cell adhesions with their neighbors and by forming new cell–cell adhesions in the interface between the two daughter cells during cytokinetic ring constriction [42].
Likewise, elderly or critically damaged cells are eliminated from the epithelial layer, while maintaining epithelial structure and integrity. This coordinated process of elimination is universally defined as epithelial extrusion. Different modalities of epithelial extrusion have been described, such as apical (when cells are extruded from the apical side and expelled in the external environment), basal (when cells are extruded toward the basal membrane and may be eaten by other cells), lateral (when cells are enveloped by neighbors and apoptotic corps are extruded laterally) [43], apoptotic (when apoptosis is part of the extrusion process) or live (when cells are extruded when they are still alive and may undergo anoikis, a specialized type of apoptosis caused by the absence of adhesion) [44]. The mechanism of cell extrusion selected by the epithelial cell depends on several factors, such as the type of extrusion stimulation, the level of cell density, the number of layers, the type of epithelium, organ, organism, stage of development, and the presence of topological defects [45,46,47].
Irrespectively of the triggering event and the means by which it occurs, epithelial extrusion must respect some basic rules: (1) elimination of the dying cell has to be carried out without the formation of a gap; (2) extrusion relies on the execution of potent and highly dynamic morphological changes; (3) it depends on cooperation between the dying cell and its neighbors. These features make epithelial extrusion one of the most interesting examples of the emerging properties of epithelial communities, where coordination among cells is fundamental to carry out the safe elimination of dying or excessive numbers of cells.
In cancer, this homeostatic equilibrium is disrupted and an increasing amount of evidence shows that oncogenic transformation hijacks the epithelial extrusion machinery promoting abnormal growth [44]. The expression of mutated K-Ras (G12V) in the MDCK epithelial layer promotes live basal epithelial extrusion, via autophagic degradation of S1P [48]. In another model, the colorectal adenocarcinoma Caco-2 cell line, the expression of mutated H-Ras (G12V) promotes an incomplete form of apical extrusion where the extruded cells remain alive and loosely attached to the apical side of the epithelium [49]. Both these mechanisms can lead to an ectopic proliferation of oncogenic transformed cells and the invasion of the neighboring tissues.
Cytoskeletal rearrangements in the apoptotic epithelial extrusion
Apoptotic epithelial extrusion exploits the mechanical forces exerted by the actomyosin cytoskeleton in both the extruding cells and the neighbors (Fig. 2a). Inhibition of actomyosin contraction in the epithelium can completely block the extrusion process [34]. Even though the complexity of actomyosin cytoskeleton rearrangements in epithelial extrusion is not clear, recent reports have shed light on a number of factors [34, 50,51,52].
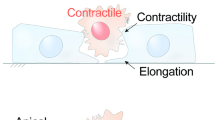
The phases of apoptotic epithelial extrusion. a Epithelial extrusion is a dynamic and highly coordinated process involving both the apoptotic cell and its neighbors. (I) It starts from the commitment to apoptosis from an epithelial cell. (II) The first morphological event is the contraction of the apical actomyosin belt. (III) Several patches of polymerized actin become visible and (IV) eventually coalesce into an extrusion apical actin ring (EAAR). (V) The basal actomyosin ring, formed by the apoptotic cell itself and by its neighbors, contracts until the apoptotic cell no longer has contact with the basal membrane. (VI) Junctions between the neighboring cells are established while the extruding cell is moved upwards until (VII) its complete expulsion. This stepwise process has three main phases: (1) apical contraction, (2) basal contraction, (3) establishment of new lateral cell junctions among neighbors. b Representation of the EAAR as an apical actomyosin ring, pulling several acto-myosin cables in a centripetal fashion. c The basal actomyosin ring is composed of the acto-myosin cytoskeleton from the apoptotic cell itself and from the neighbors. To close the basal side, the dying cell and its neighbors must coordinate with each other. d Once the basal side is closed, the extruding cell has to be expelled from the apical side as well. Simultaneously, new adhesions among the neighboring cells have to be established to complete the gap closure
Epithelial extrusion is highly coordinated over time and can be subdivided into three phases.
(1) Cell-autonomous phase The apoptotic cell exerts potent actomyosin activity and drives apical contractility through the assembly of an extrusion apical actin ring (EAAR). This first phase was recently revealed by two reports definitively proving that the apoptotic cell itself does not act as a passive player in epithelial extrusion, but instead plays a crucial role in the generation of forces [34, 50]. The first morphological event is a rapid burst of contraction of the apoptotic cell that pulls cell–cell borders toward the centre and squeezes its own nucleus [34]. This event is the result of a general, constitutive and irreversible activation of non-muscular myosin that eventually leads to the assembly of an EAAR [34, 50].
The stages of EAAR formation are well characterized. The first event is the thickening and contraction of lateral cortical actin at the apical side of the cell. After a few minutes, some patches of cortical actin, characterized by increased thickness, emerge and then are pulled toward the center. Once the actin patches reach the centre of the cell, they coalesce and form a mature EAAR. This structure is like a highly dynamic donut-like assembly of actin filaments and non-muscular myosin, with a predefined 2-μm diameter. Despite being at the center of the cell, the EAAR is structurally connected with cell–cell or cell–matrix adhesions, through several actin bundles. Mechanically, the EAAR acts as a generator of centripetal forces that continuously pull the actin filaments that make up the bundles (Fig. 2b).
EAAR itself is a dynamic structure, continuously regenerated by actin filaments of bundles that are integrated into the EAAR structure, as proven by FRAP experiments [34]. The role of cell–cell or cell–matrix adhesions, which are mechanically connected with the actomyosin cytoskeleton, is crucial. The EAAR pulls all the associated actin bundles that are gradually shortened, also dragging the focal adhesion to which they are connected [34]. The pulling action of the EAAR restricts the apical side of the apoptotic cell, which is compensated by the migration of the apical side of the neighboring cells, which becomes elongated toward the apoptotic cell itself [50]. In addition, while it is becoming smaller in diameter, the apical surface of the apoptotic cell, which has no cell–cell or cell–matrix adhesions, starts to dynamically produce blebs, some of which also contain part of the nucleus [34].
(2) Closure of the basal surface The basal surface is closed in a coordinated fashion between the apoptotic cell and the neighbors. While in the cell-autonomous phase, neighboring cells are passive characters guided by the forces generated by the apoptotic cell; in this second phase, they play a crucial role in the final removal of dying cells, as well as in gap closure and maintenance of the epithelial barrier.
The main morphological event in this phase is the formation of a multicellular actomyosin ring at the basal surface of the neighboring cells that gradually restricts itself until it completely occupies the basal area previously occupied by the apoptotic cell [24] (Fig. 2c). Actomyosin activity is required in neighboring cells to assemble the actomyosin ring and for the execution of epithelial extrusion [24]. Interestingly, an actomyosin ring is assembled at the basal side also in the extruding cell itself, suggesting that the extruding cell also plays a role in this phase [24, 34, 50].
(3) Completion of extrusion The final step consists in the expulsion of the dying cell and in the establishment of novel adhesions between neighboring cells, closing the gap at the apical side as well (Fig. 2a). This third phase has been investigated the least. Once the basal side closure has been completed, neighboring cells possibly continue their mechanical role by squeezing the dying cell toward the apical side. Simultaneously, neighboring cells come in contact with their respective lateral surface where they can establish new cell–cell adhesions (Fig. 2d). The importance of this third phase is proven by silencing or genetic ablation of E-Cadherin, the main mediator of anchoring junctions in epithelia [52]. Reduction or elimination of E-Cadherin expression can completely abrogate gap closure, causing leakiness in the epithelial layer, letting pathogens get in, and inflaming the underlying tissue [51].
The molecular mechanisms controlling apoptotic epithelial extrusion
Signal transduction within the apoptotic cell, among the neighboring cells, as well as signal communication between apoptotic and neighboring cells has a crucial role in coordinating cell extrusion.
Epithelial extrusion can be induced through both intrinsic (e.g., by exposing the cells to etoposide, a topoisomerase II inhibitor) and extrinsic (e.g., by treating cells with TNF-related apoptosis-inducing ligand (TRAIL) in combination with the protein synthesis inhibitor cycloheximide) apoptotic pathways [53]. Other stimuli known to induce apoptotic epithelial extrusion are growth factor deprivation [34] and topological defects [47]. Epithelial extrusion induced through the intrinsic pathways requires mitochondrial membrane permeabilization, since both Bcl-2 over-expression and Bax and Bak knockdown are able to block the extrusion process. However, since these signaling proteins are not involved in the extrinsic pathway, neither over-expression of Bcl-2 nor Bax/Bak knockdown are able to impede extrusion induced by extrinsic apoptosis stimuli [53]. Also the pro-apoptotic, Bcl-2 family member, tBid, is involved in epithelial extrusion, since its inhibition blocks apoptotic epithelial extrusion due to growth factor deprivation [34]. Both extrinsic and intrinsic pathways converge in the apoptotic execution pathway, which represents the signaling backbone of epithelial extrusion (Fig. 3a). Accordingly, inhibition of caspases with zVAD-FMK completely abrogates apoptotic epithelial extrusion, causing intra-epithelial secondary necrosis [34, 47, 53].
Signaling mechanisms involved in the execution of apoptotic epithelial extrusion. a Epithelial extrusion can be activated by both extrinsic and intrinsic pathways of apoptosis that converge in the activation of executioner caspases 3 and 7. Proteolytic activation of Rho-activates kinases (RaKs) such as ROCK1 or MRCKα leads to hyperphosphorylation of the regulatory subunit of myosin. The subsequent hypercontraction of the actomyosin cytoskeleton is responsible for the cell-autonomous morphological events observed during epithelial extrusion. b Coordination between the dying cell and its neighbors is fundamental for the successful execution of epithelial extrusion. Two pathways appear to be able to communicate the apoptotic status to the neighbors. Forces generated by hypercontractility of actomyosin cytoskeleton are transmitted to the neighboring cells through cell–cell junctions and activate contractility in neighboring cells by means of Coronin-1B. The apoptotic cell is also able to communicate its status by producing S1P which is detected by S1P2 receptor on neighboring cells, which in turn activate actomyosin contraction though P115GEF, RhoA and ROCK
Once activated, caspases are responsible for triggering all the cell-autonomous morphological events observed during epithelial extrusion. Caspase-3 activates, through proteolytic cleavage, the myosin regulators of the RaK family, such as ROCK1, MRCKα and MRCKβ [31, 34, 35] (Fig. 3a). The proteolytic activation of these three proteins induces EAAR assembly and is necessary for successfully executing epithelial extrusion [34, 51, 52].
ROCK1 or MRCKα inhibition or knockdown blocks epithelial extrusion in several cell types, forcing the dying cells to complete apoptosis in the epithelium and then causing the accumulation of apoptotic remains in the interstitial space [34]. Conversely, the expression of a cleaved form of these kinases induces EAAR assembly end epithelial extrusion [34, 51, 52]. Less explored, but potentially relevant in the context of myosin activation during epithelial extrusion, is the caspase-dependent activation of MLCK [54] and inactivation of MYPT1 [37].
Successful accomplishment of epithelial extrusion strictly relies on the coordination between the apoptotic cell and the neighbors. When looking for signaling cues allowing such communication, Rosenblatt and colleagues found that the apoptotic cell produces and secretes Sphingosine-1-phosphate (S1P) that binds to a G-protein-coupled receptor, S1P receptor 2 (S1P2), on the neighboring cells [55] (Fig. 3b). Indeed, disrupting this signal by Sphingosine kinase (SphK) inhibitors, silencing of S1P2 or neutralizing S1P with an inhibitor, blocks epithelial extrusion and produces holes in the epithelial layer [55]. To induce apical extrusion, the dying cell must produce S1P at the basal side. Rosenblatt and colleagues found that adenomatous polyposis coli (APC) is key to correctly localizing S1P production, and APC knockdown is sufficient to invert the direction of extrusion [56].
However, there is still no direct explanation of how S1P can be produced and localized specifically at the basolateral membrane of the extruding cells. Possible hypotheses regard a specific localization of SphK1 or SphK2, local inhibition of S1P phosphatases or localized activation/expression of S1P membrane transporters [57]. Further studies are needed to clarify this point.
Once the presence of an apoptotic cell has been detected, the neighboring cells have to reorganize their own actomyosin cytoskeleton to form a basal ring that gradually closes the basal surface of the extruding cell. Rosenblatt and colleagues found that S1P2 in neighboring cells binds S1P and then activates Rho via p115 RhoGEF, thereby inducing acto-myosin recruitment and contraction at baso-lateral intracellular junctions [58].
Interestingly, the regulation of microtubule dynamics plays a crucial role in localizing p115 RhoGEF at the basal side in both the dying cell and in the neighbors (Fig. 3b). Microtubules show a reorientation toward the basal side during apical epithelial extrusion and their disruption with nocodazole or taxol is sufficient to promote basal cell extrusion [58]. Although microtubule dynamics seems to play a role in both the extruding and the neighboring cells, mosaic silencing of the microtubule regulator APC shows that APC controls apical extrusion in a cell-autonomous fashion [56].
Yap and colleagues proposed a model based on mechanosensing through E-Cadherin in adherent junctions [52]. The extruding cell and the neighbors are mechanically connected through E-cadherin, which transmits the forces generated by hypercontraction of actomyosin cytoskeleton in the apoptotic cell. In fact, knockdown of E-cadherin hampers epithelial extrusion [52, 55]. E-cadherin adhesions under mechanical tension attract coronin 1B (Coro1B) which reorganizes actin filaments into co-aligned perijunctional bundles, rich in non-muscular myosin and which can contract the extruding cell (Fig. 3b). In the absence of Coro1B, neighboring cells fail to recruit co-aligned perijunctional bundles and as a consequence are unable to form an extrusion ring [52].
In summary, there are currently two different mechanisms that explain the communication needed to coordinate the apoptotic extruding cell with its neighbors: one based on S1P localized secretion and the other on E-cadherin-mediated mechanosensing. Although there are no specific studies investigating whether these two mechanisms can coexist, the complex coordination among the extruding and neighboring cells likely requires multiple and redundant signals, including others yet to be discovered.
Recently, using the Drosophila pupal notum, Moreno et al. showed that tissue compaction induces apoptotic cell extrusion through the downregulation of EGFR/ERK pathway and upregulation of the pro-apoptotic protein Hid [59]. Therefore, the mechanosensing through EGFR/ERK could also contribute to the process of apoptotic cell extrusion, although the mechanism modulating EGFR/ERK pathway by mechanical stress is not known [59].
Although apoptotic extrusion is proving to be a highly coordinated and efficient process, not all the apoptotic epithelial cells are efficiently extruded and followed by a neighbor-mediated gap closure. Interestingly, some of the apoptotic cells can continue apoptosis until they are fragmented into apoptotic bodies within the epithelium [34]. This phenomenon is probably explained by communication defects between the apoptotic and neighboring cells, faults in the organization of the actomyosin cytoskeleton, topological defects, or an excessive number of apoptotic events. The latter is likely the case with TNF treatment on intestinal epithelial cells leading to simultaneous and massive cell death and formation of large gaps in the epithelial layer. This extreme situation is probably due to the high number of apoptotic cells which goes over the minimum number of healthy neighbors needed to extrude the dying cells and to repair the gap [60].
Apoptotic contractility in development and tissue homeostasis
Apoptosis is fundamental in the maintenance of tissue homeostasis by eliminating old or damaged cells. There are also several examples of apoptosis playing a part in organ or organism development. One clear case is the cavitation of terminal end buds in mammary gland growth: during puberty or pregnancy, terminal end buds grow in the breast fat tissue as a compact mass of cells, which are then reduced to a bilayer by apoptosis [61]. Several massive apoptotic events take place in embryo development, such as removing cells from the inner cell mass in blastocysts, removing cells between the fingers and toes, removing chondrocytes during ossification, or removing neurons which are in excess or inappropriately connected [62]. However, in all these phenomena, apoptosis has only a passive role, by eliminating certain groups of cells and leaving space for other cells or leaving a cavity.
Apoptosis has recently been shown to have an active role in shaping tissues and organs by means of its intrinsic contractile ability [5]. Two prototypical examples are dorsal closure in the embryo [63] and fold formation in the developing leg [64]. Dorsal closure is a fundamental process in the final phases of Drosophila embryo morphogenesis. It is characterized by the gradual closure of an eye-shaped dorsal gap temporarily occupied by the amnioserosa, an extra-embryonic tissue, by means of the migration of two lateral epithelial cell sheets. The mechanical forces required for this closure are partly generated by a collective actomyosin ring at the migration edge and partly by the amnioserosa itself. During dorsal closure, a subset of amnioserosa cells undergoes apical constriction, followed by basal extrusion, while neighboring cells acquire a “rosette” morphology, which is a typical consequence of epithelial extrusion [63].
The sum of multiple extrusion events generates tension in the amnioserosa tissue which eventually stretches the edges of the two lateral epithelial sheets thus contributing in their reciprocal migration. The importance of apoptosis in dorsal closure has been experimentally proven by reducing apoptosis, which slows down the migration of epithelial cells, or by promoting apoptosis, which accelerates migration. Other experiments with laser ablation of amnioserosa cells demonstrated that at least one-third of the force necessary for dorsal closure derives from apoptosis [63].
Epithelium folding is observed in both vertebrates and invertebrates, and can generate three-dimensional structures from simple two-dimensional epithelial sheets [65]. In Drosophila developing leg, epithelium folding enables leg segmentation along with the definition of the site where the future leg-joint will develop. In this model, the apico-basal forces required for epithelium folding are generated through apoptosis of some of the epithelial cells. In fact, circumferential rings of cells in the site of the upcoming epithelial folding undergo apoptosis in a coordinated fashion. Apoptotic cells then activate a transient contraction process applied to apico-basal acto-myosin cables, which pulls the apical part of the cell toward the basal side. As a result of this potent apico-basal myosin contraction, apoptotic cells induce an increase in tissue tension in the neighboring cell, which eventually results in epithelium folding [64].
Conclusions
A prerequisite for pluricellular organisms is the ability to accurately remove elderly, overnumbered or critically damaged cells. Apoptosis is one of the various types of programmed cell death [48] that plays a key role in both embryo development and tissue homeostasis in adulthood. Although the pathways and mechanisms involved have been studied for around 50 years, only recently has its morphogenetic role been investigated.
Apoptosis directly controls several types of cytoskeletal proteins thereby promoting degradation in intermediate filaments; disassembly and reorganization in microtubules; and hyperactivation in the acto-myosin cytoskeleton. Since apoptotic pathways regulate the cytoskeleton, they can control cell shape by promoting plasma-membrane blebbing, nuclear fragmentation and apoptotic body formation. At the tissue scale, the mechanical force generated by the apoptotic cell can influence and modify surrounding cells or tissues, playing a major role in epithelial homeostasis or in shaping embryos.
However, there are several aspects regarding the morphological role of apoptosis that are still not understood. For example, the contribution of the actomyosin cytoskeleton or microtubules in apoptotic body fragmentation has never been clarified nor has how the gap is closed at the apical side during epithelial extrusion or how neighboring cells establish new adhesion. Finally, how epithelial communities are able to sense the extrusion events and compensate them with new cell division would contribute to understanding how epithelial homeostasis is maintained over time. Further studies are, thus, required to investigate the complexity and the dynamics of the morphological process observed in apoptotic cells and their impact on the tissue scale.
References
Gudipaty SA, Conner CM, Rosenblatt J, Montell DJ (2018) Unconventional ways to live and die: cell death and survival in development, homeostasis, and disease. Annu Rev Cell Dev Biol 34:311–332. https://doi.org/10.1146/annurev-cellbio-100616-060748
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239–257
Diamantis A, Magiorkinis E, Sakorafas GH, Androutsos G (2008) A brief history of apoptosis: from ancient to modern times. Onkologie 31(12):702–706. https://doi.org/10.1159/000165071
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Ambrosini A, Gracia M, Proag A, Rayer M, Monier B, Suzanne M (2017) Apoptotic forces in tissue morphogenesis. Mech Dev 144(Pt A):33–42. https://doi.org/10.1016/j.mod.2016.10.001
Saraste A, Pulkki K (2000) Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res 45(3):528–537
Model MA (2014) Possible causes of apoptotic volume decrease: an attempt at quantitative review. Am J Physiol Cell Physiol 306(5):C417–C424. https://doi.org/10.1152/ajpcell.00328.2013
Okada Y, Shimizu T, Maeno E, Tanabe S, Wang X, Takahashi N (2006) Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J Membr Biol 209(1):21–29. https://doi.org/10.1007/s00232-005-0836-6
Beauvais F, Michel L, Dubertret L (1995) Human eosinophils in culture undergo a striking and rapid shrinkage during apoptosis. Role of K+ channels. J Leukoc Biol 57(6):851–855
Barry MA, Reynolds JE, Eastman A (1993) Etoposide-induced apoptosis in human HL-60 cells is associated with intracellular acidification. Cancer Res 53(10 Suppl):2349–2357
Moran J, Hernandez-Pech X, Merchant-Larios H, Pasantes-Morales H (2000) Release of taurine in apoptotic cerebellar granule neurons in culture. Pflugers Arch 439(3):271–277
Flamenco P, Galizia L, Rivarola V, Fernandez J, Ford P, Capurro C (2009) Role of AQP2 during apoptosis in cortical collecting duct cells. Biol Cell 101(4):237–250. https://doi.org/10.1042/BC20080050
Model MA, Schonbrun E (2013) Optical determination of intracellular water in apoptotic cells. J Physiol 591(23):5843–5849. https://doi.org/10.1113/jphysiol.2013.263228
Mills JC, Stone NL, Erhardt J, Pittman RN (1998) Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol 140(3):627–636
Tixeira R, Caruso S, Paone S, Baxter AA, Atkin-Smith GK, Hulett MD, Poon IK (2017) Defining the morphologic features and products of cell disassembly during apoptosis. Apoptosis 22(3):475–477. https://doi.org/10.1007/s10495-017-1345-7
Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, Rath N, Zander SA, Mleczak A, Sumpton D, Morrice N, Bienvenut WV, Olson MF (2013) Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ 20(10):1293–1305. https://doi.org/10.1038/cdd.2013.69
Atkin-Smith GK, Poon IKH (2017) Disassembly of the dying: mechanisms and functions. Trends Cell Biol 27(2):151–162. https://doi.org/10.1016/j.tcb.2016.08.011
Moss DK, Betin VM, Malesinski SD, Lane JD (2006) A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J Cell Sci 119(Pt 11):2362–2374. https://doi.org/10.1242/jcs.02959
Poon IK, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, Ravichandran KS (2014) Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 507(7492):329–334. https://doi.org/10.1038/nature13147
Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, Goodall KJ, Ravichandran KS, Hulett MD, Poon IK (2015) A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun 6:7439. https://doi.org/10.1038/ncomms8439
Atkin-Smith GK, Paone S, Zanker DJ, Duan M, Phan TK, Chen W, Hulett MD, Poon IK (2017) Isolation of cell type-specific apoptotic bodies by fluorescence-activated cell sorting. Sci Rep 7:39846. https://doi.org/10.1038/srep39846
Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ (2007) TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27(6):927–940. https://doi.org/10.1016/j.immuni.2007.11.011
Sachet M, Liang YY, Oehler R (2017) The immune response to secondary necrotic cells. Apoptosis 22(10):1189–1204. https://doi.org/10.1007/s10495-017-1413-z
Rosenblatt J, Raff MC, Cramer LP (2001) An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol 11(23):1847–1857
Schutte B, Henfling M, Kolgen W, Bouman M, Meex S, Leers MP, Nap M, Bjorklund V, Bjorklund P, Bjorklund B, Lane EB, Omary MB, Jornvall H, Ramaekers FC (2004) Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res 297(1):11–26. https://doi.org/10.1016/j.yexcr.2004.02.019
Byun Y, Chen F, Chang R, Trivedi M, Green KJ, Cryns VL (2001) Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ 8(5):443–450. https://doi.org/10.1038/sj.cdd.4400840
Rao L, Perez D, White E (1996) Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol 135(6 Pt 1):1441–1455
Seo MY, Rhee K (2018) Caspase-mediated cleavage of the centrosomal proteins during apoptosis. Cell Death Dis 9(5):571. https://doi.org/10.1038/s41419-018-0632-8
Sanchez-Alcazar JA, Rodriguez-Hernandez A, Cordero MD, Fernandez-Ayala DJ, Brea-Calvo G, Garcia K, Navas P (2007) The apoptotic microtubule network preserves plasma membrane integrity during the execution phase of apoptosis. Apoptosis 12(7):1195–1208. https://doi.org/10.1007/s10495-006-0044-6
Oropesa-Avila M, Fernandez-Vega A, de la Mata M, Maraver JG, Cordero MD, Cotan D, de Miguel M, Calero CP, Paz MV, Pavon AD, Sanchez MA, Zaderenko AP, Ybot-Gonzalez P, Sanchez-Alcazar JA (2013) Apoptotic microtubules delimit an active caspase free area in the cellular cortex during the execution phase of apoptosis. Cell Death Dis 4:e527. https://doi.org/10.1038/cddis.2013.58
Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3(4):339–345. https://doi.org/10.1038/35070009
Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, Stewart CL, Olson MF (2005) Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol 168(2):245–255. https://doi.org/10.1083/jcb.200409049
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G, Nomenclature Committee on Cell Death (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16(1):3–11. https://doi.org/10.1038/cdd.2008.150
Gagliardi PA, Somale D, Puliafito A, Chiaverina G, di Blasio L, Oneto M, Bianchini P, Bussolino F, Primo L (2018) MRCKalpha is activated by caspase cleavage to assemble an apical actin ring for epithelial cell extrusion. J Cell Biol 217(1):231–249. https://doi.org/10.1083/jcb.201703044
Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J (2001) Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 3(4):346–352. https://doi.org/10.1038/35070019
Gagliardi PA, Primo L (2019) Irreversible activation of Rho-activated kinases resulted from evolution of proteolytic sites within disordered regions in coiled-coil domain. Mol Biol Evol 36(2):376–392. https://doi.org/10.1093/molbev/msy229
Iwasaki T, Katayama T, Kohama K, Endo Y, Sawasaki T (2013) Myosin phosphatase is inactivated by caspase-3 cleavage and phosphorylation of myosin phosphatase targeting subunit 1 during apoptosis. Mol Biol Cell 24(6):748–756. https://doi.org/10.1091/mbc.E11-08-0740
Nakazono-Kusaba A, Takahashi-Yanaga F, Morimoto S, Furue M, Sasaguri T (2002) Staurosporine-induced cleavage of alpha-smooth muscle actin during myofibroblast apoptosis. J Investig Dermatol 119(5):1008–1013. https://doi.org/10.1046/j.1523-1747.2002.19525.x
Brown SB, Bailey K, Savill J (1997) Actin is cleaved during constitutive apoptosis. Biochem J 323(Pt 1):233–237
Song Q, Wei T, Lees-Miller S, Alnemri E, Watters D, Lavin MF (1997) Resistance of actin to cleavage during apoptosis. Proc Natl Acad Sci USA 94(1):157–162
McKinley KL, Stuurman N, Royer LA, Schartner C, Castillo-Azofeifa D, Delling M, Klein OD, Vale RD (2018) Cellular aspect ratio and cell division mechanics underlie the patterning of cell progeny in diverse mammalian epithelia. eLife 7:e36739. https://doi.org/10.7554/elife.36739
Le Bras S, Le Borgne R (2014) Epithelial cell division—multiplying without losing touch. J Cell Sci 127(Pt 24):5127–5137. https://doi.org/10.1242/jcs.151472
Torres AY, Malartre M, Pret AM, Agnes F (2017) JAK/STAT signaling is necessary for cell monosis prior to epithelial cell apoptotic extrusion. Cell Death Dis 8(5):e2814. https://doi.org/10.1038/cddis.2017.166
Katoh H, Fujita Y (2012) Epithelial homeostasis: elimination by live cell extrusion. Curr Biol 22(11):R453–R455. https://doi.org/10.1016/j.cub.2012.04.036
Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J (2012) Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484(7395):546–549. https://doi.org/10.1038/nature10999
Kocgozlu L, Saw TB, Le AP, Yow I, Shagirov M, Wong E, Mege RM, Lim CT, Toyama Y, Ladoux B (2016) Epithelial cell packing induces distinct modes of cell extrusions. Curr Biol 26(21):2942–2950. https://doi.org/10.1016/j.cub.2016.08.057
Saw TB, Doostmohammadi A, Nier V, Kocgozlu L, Thampi S, Toyama Y, Marcq P, Lim CT, Yeomans JM, Ladoux B (2017) Topological defects in epithelia govern cell death and extrusion. Nature 544(7649):212–216. https://doi.org/10.1038/nature21718
Slattum G, Gu Y, Sabbadini R, Rosenblatt J (2014) Autophagy in oncogenic K-Ras promotes basal extrusion of epithelial cells by degrading S1P. Curr Biol 24(1):19–28. https://doi.org/10.1016/j.cub.2013.11.029
Wu SK, Lagendijk AK, Hogan BM, Gomez GA, Yap AS (2015) Active contractility at E-cadherin junctions and its implications for cell extrusion in cancer. Cell Cycle 14(3):315–322. https://doi.org/10.4161/15384101.2014.989127
Kuipers D, Mehonic A, Kajita M, Peter L, Fujita Y, Duke T, Charras G, Gale JE (2014) Epithelial repair is a two-stage process driven first by dying cells and then by their neighbors. J Cell Sci 127(Pt 6):1229–1241. https://doi.org/10.1242/jcs.138289
Lubkov V, Bar-Sagi D (2014) E-cadherin-mediated cell coupling is required for apoptotic cell extrusion. Curr Biol 24(8):868–874. https://doi.org/10.1016/j.cub.2014.02.057
Michael M, Meiring JCM, Acharya BR, Matthews DR, Verma S, Han SP, Hill MM, Parton RG, Gomez GA, Yap AS (2016) Coronin 1B reorganizes the architecture of F-actin networks for contractility at steady-state and apoptotic adherens junctions. Dev Cell 37(1):58–71. https://doi.org/10.1016/j.devcel.2016.03.008
Andrade D, Rosenblatt J (2011) Apoptotic regulation of epithelial cellular extrusion. Apoptosis 16(5):491–501. https://doi.org/10.1007/s10495-011-0587-z
Petrache I, Birukov K, Zaiman AL, Crow MT, Deng H, Wadgaonkar R, Romer LH, Garcia JG (2003) Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. FASEB J 17(3):407–416. https://doi.org/10.1096/fj.02-0672com
Gu Y, Forostyan T, Sabbadini R, Rosenblatt J (2011) Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J Cell Biol 193(4):667–676. https://doi.org/10.1083/jcb.201010075
Marshall TW, Lloyd IE, Delalande JM, Nathke I, Rosenblatt J (2011) The tumor suppressor adenomatous polyposis coli controls the direction in which a cell extrudes from an epithelium. Mol Biol Cell 22(21):3962–3970. https://doi.org/10.1091/mbc.E11-05-0469
Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22(1):50–60. https://doi.org/10.1016/j.tcb.2011.09.003
Slattum G, McGee KM, Rosenblatt J (2009) P115 RhoGEF and microtubules decide the direction apoptotic cells extrude from an epithelium. J Cell Biol 186(5):693–702. https://doi.org/10.1083/jcb.200903079
Moreno E, Valon L, Levillayer F, Levayer R (2019) Competition for space induces cell elimination through compaction-driven ERK downregulation. Curr Biol 29(1):23–34 e28. https://doi.org/10.1016/j.cub.2018.11.007
Kiesslich R, Goetz M, Angus EM, Hu Q, Guan Y, Potten C, Allen T, Neurath MF, Shroyer NF, Montrose MH, Watson AJ (2007) Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology 133(6):1769–1778. https://doi.org/10.1053/j.gastro.2007.09.011
Sternlicht MD (2006) Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 8(1):201. https://doi.org/10.1186/bcr1368
Brill A, Torchinsky A, Carp H, Toder V (1999) The role of apoptosis in normal and abnormal embryonic development. J Assist Reprod Genet 16(10):512–519
Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS (2008) Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science 321(5896):1683–1686. https://doi.org/10.1126/science.1157052
Monier B, Gettings M, Gay G, Mangeat T, Schott S, Guarner A, Suzanne M (2015) Apico-basal forces exerted by apoptotic cells drive epithelium folding. Nature 518(7538):245–248. https://doi.org/10.1038/nature14152
Pearl EJ, Li J, Green JB (2017) Cellular systems for epithelial invagination. Philos Trans R Soc Lond Ser B Biol Sci. https://doi.org/10.1098/rstb.2015.0526
Acknowledgements
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) investigator grants (IG 18675); Fondazione Piemontese per la Ricerca sul Cancro (5x1000 2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gagliardi, P.A., Primo, L. Death for life: a path from apoptotic signaling to tissue-scale effects of apoptotic epithelial extrusion. Cell. Mol. Life Sci. 76, 3571–3581 (2019). https://doi.org/10.1007/s00018-019-03153-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03153-x