Abstract
The heterotrimeric carboxy-terminal domain kinase I (CTDK-I) in yeast is a cyclin-dependent kinase complex that is evolutionally conserved throughout eukaryotes and phosphorylates the C-terminal domain of the largest subunit of RNA polymerase II (RNApII) on the second-position serine (Ser2) residue of YSPTSPS heptapeptide repeats. CTDK-I plays indispensable roles in transcription elongation and transcription-coupled processing, such as the 3′-end processing of nascent mRNA transcripts. However, recent studies have revealed additional roles of CTDK-I beyond its primary effect on transcription by RNApII. Here, we describe recent advances in the regulation of genomic stability and rDNA integrity by CTDK-I and highlight the previously underappreciated cellular roles of CTDK-I in rRNA synthesis by RNA polymerase I and translational initiation and elongation. These multiple roles of CTDK-I throughout the cell expand our understanding of how this complex functions to coordinate diverse cellular processes through gene expression and how the human orthologue exerts its roles in diseased states such as tumorigenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In eukaryotes, gene expression is a tightly controlled process that involves many processes throughout the cell, which include transcription of mRNA, its processing in the nucleus, and translation of the exported mRNA in the cytoplasm. In past decades, researchers have made extensive efforts to unveil how the complicated steps of gene expression are coordinated and which steps are associated with which cellular processes [1]. In this regard, one of the most significant coordinators is RNApII, which primarily transcribes the protein-coding regions of genes. It also facilitates mRNA processing during transcription and further connects transcription with a variety of proteins that are associated with modifying histones or implicated in repairing DNA damage [2,3,4,5].
Rpb1 is the largest subunit of RNApII. It contains an unusual repetitive C-terminal domain (CTD) that consists of tandem heptapeptide repeats with the consensus sequence Y1-S2-P3-T4-S5-P6-S7, varying in number from 52 in humans to 26 in yeast [3]. Significantly, each CTD residue can be chemically modified by several CTD modifiers, allowing the repeats to serve as binding platforms to recruit and assemble proteins for the different stages of transcription [2, 5,6,7].
In Saccharomyces cerevisiae, four different cyclin-dependent kinases targeting the RNApII CTD for phosphorylation have been identified, namely, Kin28, Srb10, Bur1, and Ctk1 [3, 7]. Both Kin28 and Srb10 catalyze the phosphorylation of the fifth-position serine (Ser5) of the RNApII CTD during transcription initiation, but they have opposite effects; Kin28 phosphorylates the CTD after the formation of the RNApII initiation complex on the promoter and then promotes transcription, while Srb10 phosphorylates the CTD prior to initiation complex formation to inhibit transcription [8]. Srb10 also targets the subunits of the transcription factor IID complex in the transcriptional machinery [9] or Gal4 for transcription activation [10, 11]. The other two kinases, Bur1 and Ctk1, phosphorylate CTD Ser2 and belong to distinct complexes, Bur1/Bur2 and CTDK-I, respectively [12, 13]. Although both Bur1 and Ctk1 target the CTD Ser2, they regulate transcription elongation in different ways [14, 15]. Bur1 preferentially phosphorylates Ser2 at a low level near the 5′ end of the gene during early elongation, thereby enhancing the rest of the Ser2 phosphorylation, most of which is performed by Ctk1. Ser2 increases towards the 3′ ends of genes, then decreases beyond the poly(A) sites. This phosphorylation plays an essential role in recruiting 3′-end processing factors [3, 14].
Recent structural studies showed that the formation of the elongation complex is promoted by Bur1/Bur2 [16] or its mammalian homolog, the positive transcription elongation factor b (P-TEFb/Cdk9) [17]. In this context, Bur1/P-TEFb phosphorylates the RNApII linker to the CTD, which is bound by the elongation factor SPT6. Additionally, besides targeting the CTD of the RNApII, yeast Bur1 phosphorylates the Spt5, a subunit of the elongation factor Spt4/5 complex (also termed DRB sensitivity-inducing factor, DSIF), both in vivo and in vitro to stimulate recruitment of the PAF complex elongation factor [18].
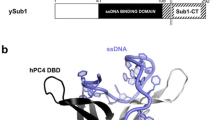
In general, CTDK-I is considered the major kinase complex targeting CTD Ser2 during transcription elongation by RNApII in budding yeast [14]. It consists of three subunits: the cyclin-dependent kinase Ctk1 (homologous to human CDK12), the cyclin Ctk2 (homologous to human cyclin K) and the yeast-specific subunit Ctk3, a co-cyclin factor (Fig. 1a) [13, 19, 20]. The subunits of Ctk1, Ctk2, and Ctk3 in budding yeast are homologous to those of Lsk1, Lsc1, and Lsg1 in fission yeast [21, 22]. In addition, sequence alignments throughout eukaryotes, together with a previous report, show that Ctk1 and Ctk2 are highly conserved from yeast to human [23] (Fig. 1b, c). The Ctk3 subunit in budding yeast has a low level of homology with the Lsg1 subunit in fission yeast and lacks homologs with other higher eukaryotes (Fig. 1d). A previous report identifies Lsg1 as a functional homolog of Ctk3 [22].
The cyclin-dependent kinase complex is evolutionarily conserved. a The cyclin-dependent kinase complexes that are responsible for the phosphorylation of the RNApII CTD Ser2 are shown in several eukaryotes. Colors indicate homology among species; light red, gray, and light brown colors indicate the kinase, the cyclin, and the co-cyclin factors in each species, respectively. b–d Multiple sequence alignments of the CTDK-I homologs. Sequence alignments were generated using Clustal Omega from the protein sequences of kinase, cyclin, and co-cyclin factor from S. cerevisiae, S. pombe, C. elegans, Drosophila and H. sapiens. The numbers in the sequences indicate the positions of amino acid residues. Amino acids are colored according to the degree of conservation and physicochemical properties; blue, red, magenta, green, pink, orange, yellow, cyan and white indicate hydrophobic, positively charged, negatively charged, polar, cysteine, glycine, proline, aromatic and unconserved residues, respectively [77]. The symbols at the top are as follows: (*) indicates identity between residues, (:) conservation and (.) less conservation. b and c show only the most conserved sequences of Ctk1/Ctk2 and their homologs, whereas d shows the whole sequence of Ctk3 and its homologs. Note that the level of homology between Ctk3 in S. cerevisiae and Lsg1 in S. pombe is not very high, but they are functionally conserved
The genes encoding yeast CTDK-I subunits are all not essential for viability, but each disruption causes a cold-sensitive phenotype and abolishment of the RNApII CTD Ser2 phosphorylation [13, 20, 24]. Notably, the CTD kinase activity of Ctk1 is dependent on its association with the stable heterodimer of Ctk2 and Ctk3, showing that CTD Ser2 phosphorylation requires an intact CTDK-I complex in cells [24]. Similarly, the three subunits of the CTDK-I complex in fission yeast interact with each other to perform their activity [25]. Consistent with those associations, in higher eukaryotes, the CTD Ser2 kinases always have a stable association with their cyclin partners [19, 26, 27] (Fig. 1a).
By phosphorylating the RNApII CTD at Ser2, the CTDK-I complex plays a pivotal role in coupling transcription with the 3′-end processing of nascent mRNA transcripts by recruiting 3′-end processing factors or in regulating different steps in transcription by recruiting different kinds of histone-modifying enzymes [14, 28,29,30,31,32]. However, recent studies on the CTDK-I complex have revealed a few additional pathways that take advantage of its localizations in both the nucleus and cytoplasm [33] (see Fig. 2). Here, we highlight these multiple aspects of the CTDK-I complex in addition to its conventional role in RNApII transcription to emphasize its widespread roles across the cell. Recent findings concerning the role of the mammalian CTDK-I in ovarian and prostate cancers will be briefly discussed.
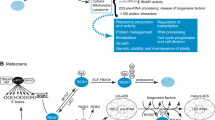
CTDK-I functions throughout the cell. The CTDK-I kinase contributes to various cellular processes throughout the cells via its widespread localization. (1) Yeast CTDK-I associates with RNApI in the nucleolus, where the kinase is necessary for efficient 35S rDNA transcription. (2) In the nucleoplasm, CTDK-I functions as a primary kinase for Ser2 phosphorylation of the RNApII CTD to facilitate the transcription elongation of protein-coding genes, including genes for DNA damage repair response. In human ovarian and prostate cancers, inactivation of CTDK-I is associated with a particular genome instability pattern consisting of a high number of TDs. (4) In the cytoplasm, yeast CTDK-I is important for the formation of the intact 80S ribosomal complex during translation initiation and plays a vital role in enhancing accurate translational decoding fidelity during translational elongation by phosphorylating the ribosomal protein Rps2. Human CTDK-I phosphorylates 4E-BP1 to regulate mTORC1-dependent translation and mitotic genome stability
The function of CTDK-I in genome stability
The repair process of damaged DNA often occurs in DNA regions adjacent to the places where transcription proceeds. Indeed, RNApII contributes extensively to DNA repair and stress responses by playing a significant role in identifying and signaling the occurrence of DNA damage to other cellular machinery [4, 5]. Importantly, the forms of RNApII that are correctly modified at the CTD are regarded as crucial during the DNA damage repair response; the evidence shows that deletion of yeast CTK1 leads to high sensitivity to certain DNA-damaging agents, such as doxorubicin (DOX), hydroxyurea (HU), methyl methanesulfonate (MMS) and 4-nitroquinoline oxide, or to UV irradiation [34,35,36,37]. In CycK-depleted human HeLa cells, sensitivity to a variety of DNA-damaging agents such as mitomycin C, etoposide, or camptothecin is reported [27, 38].
Among many different types of DNA damage, DNA double-strand breaks (DSBs) are regarded as the type of lesion most dangerous to cells. The deleterious DSB is preferentially repaired by homologous recombination (HR), which requires an intact homolog or a sister chromatid to serve as a template for the DNA repair [39]. By employing a gene deletion study of diploid cells, a subset of conserved genes, including CTK1, were identified as needed for resistance to the DNA-damaging agent DOX [37]. The ctk1Δ/ctk1Δ diploid cells cause a severely impaired G1/S transition and an enhanced gene conversion frequency when exposed to DOX, suggesting a role of Ctk1 in HR-mediated DNA damage repair. In parallel, spontaneous mitotic recombination is believed to be strictly dependent on Ctk1 because, in ctk1Δ/ctk1Δ diploid cells, the rate of loss of heterozygosity is dramatically reduced at several auxotrophic loci, such as CAN1, LEU1, TRP5 and URA3 loci [34]. The intimate connection between the CTDK-I complex and HR-dependent DNA repair is further supported by a global genetic analysis; several single-deletion mutations in the Rad52 epistasis group, which are essential for DSB repair by mitotic HR, are synthetically lethal when combined with ctk1Δ, ctk2Δ or ctk3Δ [34, 40, 41]. In addition, each deletion of any CTDK-I subunit is synthetically lethal when combined with the deletion of many genes involved in DNA integrity [34, 40]. Thus, these reports support an indispensable role of the CTDK-I complex in maintaining genome integrity by facilitating HR in response to DSB.
CDK12, the metazoan orthologue of yeast Ctk1 in C. elegans [26], Drosophila [19] and human [27] (see Fig. 1), is critical in maintaining the genomic stability by regulating the expression of the functional DNA damage response genes, especially in the HR-induced DNA lesion repair [27, 42]. Genome-wide expression studies show that CDK12 globally suppresses intronic polyadenylation events in mouse embryonic stem cells, enabling the production of full-length HR gene products [43]. In CDK12-deficient cells, only a small set of the assayed human genes are altered (2.67%), of which the downregulated group includes genes encoding critical regulators of genomic stability, including BRCA1, ATR, FANCI, and FANCD2. Consequently, the knockdown of CDK12 or cyclin K sensitizes cells to a variety of DNA damage agents and increased the spontaneous DNA damage [27]. Sharp et al. further show that CDK12 loss enriches the intronic polyadenylation sites events at the expense of distal sites, which leads to a cumulative defect by downregulating multiple gene products and thus causes the enhanced sensitivity of HR gene expression to CDK12 loss [43].
It is particularly notable that inactivation of the CDK12 gene in ovarian and prostate cancers has been associated with a particular genome instability pattern consisting of a high number of tandem duplications (TDs) up to 10 Mb in size [44, 45]. TDs were quasi-randomly distributed along the genomes and affected more than 10% of the genomic content. The genomic instability caused by the inactivation of CDK12 gene, which was referred to as ‘the CDK12 TD-plus phenotype’, was observed in approximately 4% of serous ovarian carcinomas and 1–2% of prostate adenocarcinomas. Significantly, analysis of the TDs in the CDK12-mutated context gave no preference for TD localization and no presence of homologous fragments around the breakpoints, excluding any possibility of a recombination mechanism, such as misalignment of the sister chromatids or unbalanced translocations. To address the connection of CDK12 with HR, they examined the number of large-scale state transitions (LSTs), which are defined as chromosomal breaks between adjacent regions of at least 10 Mb and are considered a genomic HR signature in basal-like breast carcinomas [46]. They found that CDK12-inactivated tumors resemble the group of non-BRCA1/2 tumors and mostly display fewer LSTs than the minimal level of BRCA1/2-inactivated ovarian cancers, suggesting that genomic instability associated with CDK12 inactivation does not display substantial genomic HR deficiency [44]. Similarly, another recent study using integrative genomic analysis of metastatic castration-resistant prostate cancers found that CDK12 loss was associated with genomic instability and focal TDs, leading to highly recurrent gains of genes involved in the cell cycle and DNA replication [45]. Importantly, they did not observe any DNA mutational signatures typically associated with defects in HR, nor was the expression of BRCA1 or BRCA2 affected by CDK12 mutation. So far, it is unclear whether the CDK12 TD-plus phenotype results from an indirect effect of CDK12 mutation disrupting RNApII transcriptional regulation or is instead a direct result of aberrant cellular processes, e.g., a defect in DNA re-replication during S-phase.
The function of CTDK-I in the synthesis of rRNA and implications in maintaining rDNA integrity
In budding yeast, the rDNA region encoding the ribosomal RNAs (rRNAs) is organized as tandem arrays of a 9.1-kb-long unit that repeats 100–200 times on chromosome XII [47]. This rDNA unit consists of a 5S rRNA gene that is transcribed by RNA polymerase III (RNApIII) and a long precursor 35S rRNA gene that is transcribed by RNA polymerase I (RNApI). These two genes are separated by two nontranscribed spacers, namely, NTS1 and NTS2 [48]. Intriguingly, CTDK-I physically interacts with RNApI in vivo; its kinase subunit Ctk1 can physically interact with RNApI in vitro as well [49]. The copurification of Ctk1 with the RNApI–Rrn3 complex, which is the competent form of transcription initiation after assembling onto the 35S rDNA promoter, further supports the necessary association of CTDK-I with rRNA-transcribing RNApI [50, 51]. Loss of either the Ctk1 kinase or the Ctk2 cyclin subunit leads to reduced recruitment of RNApI to the 35S promoter region [49]. Indeed, the synthesis of 35S pre-rRNA, as well as the production of each 25S, 18S or 5.8S rRNA, is severely impaired by ctk1Δ or ctk3Δ and by a kinase-dead mutation of Ctk1 [49, 52]. Phosphorylated RNApI is a prerequisite to forming a stable RNApI–Rrn3 complex for efficient transcription initiation [53]. Although the kinase that is responsible for RNApI phosphorylation is not known, the previous findings, together with the fact that Ctk1 colocalizes in the nucleoplasm and nucleolus [49, 54], strongly suggest the importance of Ctk1 kinase for the synthesis of functional rRNAs.
The rDNA region is very unstable and prone to inducing HR primarily due to its tandemly repeated structure. Especially in the event of a DSB, the instability of the rDNA array may cause unequal sister chromatid exchange and lead to contraction or expansion of the rDNA repeats [48, 55, 56]. It is notable that the loss of Ctk1 results in contraction of the rDNA tandem array, a phenotype that is reversed by the reintroduction of CTK1 [52]. However, little is known about the molecular mechanism by which Ctk1 contributes to rDNA integrity. At the rDNA loci, Fob1, a protein that binds to the replication fork barrier within the NTS1 region of the rDNA repeat and induces HR in response to DSB, is required for rDNA expansion [52, 57, 58]. However, it appears unlikely that Ctk1 shares the pathway with Fob1 to regulate rDNA integrity because the rDNA contraction caused by ctk1Δ is not concomitant with the formation of Fob1-dependent extrachromosomal rDNA circles (ERC). Additionally, the loss of Ctk1 impairs RNApI transcription without affecting Sir2-dependent silencing at rDNA loci [52]. Thus, considering that Sir2 is critical in inhibiting ERC formation [59], it appears that Ctk1 affects rDNA integrity in a distinct manner from Fob1 and Sir2.
The function of CTDK-I in translation
Translation is one of the essential cellular processes during gene expression, where ribosomes produce polypeptides with the assistance of multiple factors in the cytoplasm. Several lines of evidence suggest that, in addition to the primary location and role of CTDK-I in the nucleus [20], the kinase translocates to the cytoplasm and even affects translation by interacting with ribosomal subunits, monosomes and polysomes [33, 60, 61].
In eukaryotes, translation initiation is an intricate process that involves the formation of the translation-competent 80S ribosome by the assembly of the 40S and 60S ribosomal subunits on the start codons of mRNAs with the help of ribosome-bound eukaryotic initiation factors (eIFs) [62]. Briefly, before joining with 60S ribosomal subunits, the 43S preinitiation complex (PIC) is formed from the 40S ribosomal subunits associated with the eIF2-GTP-Met-tRNAMet ternary complex (eIF2 TC) and a subset of initiation factors, such as eIF1, eIF1A, and eIF3. Subsequently, the 43S PIC attaches to the 5′-end of mRNA, associating with the eIF4F complex composed of eIF4E, eIF4G, and eIF4A to generate the 48S PIC. The 48S complex scans mRNA from 5′ to 3′ to recognize the AUG start codon, which triggers the hydrolysis of eIF2-bound GTP and phosphate release. Finally, a 60S ribosomal subunit joins the 48S initiation complex to form an elongation-competent 80S initiation complex (see reviews [62, 63]).
Notably, the phosphorylation of the α subunit of eIF2 (eIF2α) at Ser51 by protein kinase Gcn2 reduces eIF2 TC formation and blocks protein synthesis under various stressful circumstances, such as amino acid deprivation or treatment with chlorpromazine or peroxide [64,65,66]. Recently, Sträßer et al. reported that the yeast Ctk1 kinase is necessary for full translation initiation activity [60]. They found that the deletion of CTK1 increases the phosphorylated form of eIF2α, leading to impaired translation initiation and defective 80S complex formation. Nevertheless, it remains obscure how Ctk1 affects translation initiation activity. The approach of stable isotope labeling with amino acids in cell culture (SILAC) showed that the depletion of endogenous Ctk1 decreased the phosphorylation levels of several proteins that are involved in ribosome biogenesis and/or translation in vivo, but none of the proteins were found to be associated with Ctk1 in vitro [60].
Several studies further imply a role of Ctk1 in the elongation step of translation to maintain high decoding fidelity during protein synthesis. Loss of Ctk1 causes hypersensitivity against drugs such as paromomycin, hygromycin B and geneticin, which prevent aminoacyl-tRNA dissociation from the ribosomal A site [67,68,69]. Moreover, Röther and Sträßer previously demonstrated that Ctk1 is necessary for a high rate of decoding fidelity in translation elongation; depletion of Ctk1 impairs the explicit incorporation of amino acids and increases miscoding frequency [61]. In this maintenance step of high decoding fidelity, Ctk1 phosphorylates the Ser238 residue of Rps2, a protein component of the small 40S subunit, and tightly controls translational accuracy to enhance translational decoding fidelity [61, 70,71,72].
In higher eukaryotes, the kinase subunit of the mammalian P-TEFb, CDK9, shuttles between the nucleus and cytoplasm, similarly to yeast Ctk1 [73], and likewise associates with translating polysomes [61]. Moreover, a recent study in the human osteosarcoma U2OS cells reported that CDK12 is involved in the translation of a specific subset of mRNAs by collaborating with the mechanistic target of rapamycin (mTORC1) kinase [74]. CDK12 directly phosphorylates the mRNA 5′ cap-binding repressor, 4E-BP1, at Ser65 and Thr70, to promote the translation of mTORC1-dependent mRNAs. This phosphorylation is facilitated by the phosphorylation of 4E-BP1 by mTORC1 at Thr37 and Thr46 and followed by the exchange of 4E-BP1 with eIF4G at the 5′ cap of many mTORC1 target mRNAs. Additionally, by genome-wide ribosome profiling (Ribo-seq), they showed that CDK12 mediates a specialized translation network that coordinates the expression of crucial factors in the centrosome, centromere, and kinetochore proteins to direct mitotic progression. Parallel with this, cells deprived of CDK12 display profound mitotic chromosomal instability, including chromosome misalignment, bridging and segregation defects, raising the possibility that the CDK12-involved translation network correlates with the genomic stability, a defect hallmark of CDK12 mutant cancers [44, 45, 75, 76]. Unlike the global effect of mRNA translation initiation and elongation by yeast CTDK-I, CDK12 controls only a specific subset of mRNAs, including mTORC1 target mRNAs. As they mentioned, this may occur due to the absence of a 4E-BP1 homolog in yeast and reflects the greater diversity and regulation of mRNA cap complex assemblies in metazoans [74].
Conclusions and perspectives
The highly conserved CTDK-I complex plays a central role in RNApII transcription by phosphorylating its CTD at Ser2 and thus recruiting 3′-end processing factors or various histone-modifying enzymes to the chromatin. In this review, we focus on the previously underappreciated roles of CTDK-I, such as its involvement in the regulation of the genomic stability and the rDNA integrity, rRNA synthesis by RNApI, and translational initiation and elongation and mTORC1-dependent translation. Our review shows that CTDK-I is multifunctional and significantly influences many steps in gene expression across the cell (see Figs. 2, 3 for details). The widespread distribution of Ctk1 and its multiple functions within the cell reflect that the CTDK-I complex coordinates diverse cellular processes required for gene expression, from transcription to translation, and these functions are important to maintain genome stability and prevent deleterious mutations. Therefore, continued effort to identify novel substrates for CTDK-I kinase is important for comprehending the underlying mechanisms by which it contributes to the coordination of many cellular processes. Moreover, it appears necessary to define the pathways by which CTDK-I shuttles between the nucleus and cytoplasm; the pre-40S subunit that includes Rps2 would be one plausible partner because it is synthesized in the nucleoplasm but functions in the cytoplasm, where it interacts with and is phosphorylated by yeast CTDK-I. It will also be promising to test whether the multifunctional nature of CTDK-I contributes to the regulation of lifespan, as cellular aging is generally correlated with attenuated gene expression, including both transcription and translation, and with increased genome instability. Thus, a comprehensive understanding of the role of the evolutionarily conserved CTDK-I in many cellular processes would provide insight into how multiple steps in gene expression are coordinated and utilized under physiological conditions.
Multiple cellular roles of CTDK-I beyond mRNA transcription. A schematic diagram shows multiple roles of the CTDK-I complex. The primary roles of the CTDK-I complex are during transcription elongation and mRNA 3′-end processing. However, recent studies have found that the CTDK-I complex also has significant direct or indirect roles in other cellular processes in various locations across the cell, including the regulation of genome stability in both the nucleoplasm and the nucleus, rRNA synthesis in the nucleolus, and translation initiation and elongation in the cytoplasm. The CTDK-I complex is shown in the center, and its multiple cellular roles are indicated in the gray box. Detailed descriptions of the function of each pathway are listed below the box
References
Komili S, Silver PA (2008) Coupling and coordination in gene expression processes: a systems biology view. Nat Rev Genet 9:38
Hsin JP, Manley JL (2012) The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev 26:2119–2137
Srivastava R, Ahn SH (2015) Modifications of RNA polymerase II CTD: connections to the histone code and cellular function. Biotechnol Adv 33:856–872
Lindsey-Boltz LA, Sancar A (2007) RNA polymerase: the most specific damage recognition protein in cellular responses to DNA damage? Proc Natl Acad Sci USA 104:13213–13214
Hanawalt PC, Spivak G (2008) Transcription-coupled DNA repair: two decades of progress and surprises. Nat Rev Mol Cell Biol 9:958–970
Cramer P, Bushnell DA, Kornberg RD (2001) Structural basis of transcription: RNA polymerase II at 2.8 Ångstrom resolution. Science 292:1863–1876
Zaborowska J, Egloff S, Murphy S (2016) The pol II CTD: new twists in the tail. Nat Struct Mol Biol 23:771
Hengartner CJ, Myer VE, Liao SM, Wilson CJ, Koh SS, Young RA (1998) Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol Cell 2:43–53
Liu Y, Kung C, Fishburn J, Ansari AZ, Shokat KM, Hahn S (2004) Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Mol Cell Biol 24:1721–1735
Ansari AZ, Ogirala A, Ptashne M (2005) Transcriptional activating regions target attached substrates to a cyclin-dependent kinase. Proc Natl Acad Sci 102:2346–2349
Allen BL, Taatjes DJ (2015) The mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol 16:155
Yao S, Neiman A, Prelich G (2000) BUR1 and BUR2 encode a divergent cyclin-dependent kinase–cyclin complex important for transcription in vivo. Mol Cell Biol 20:7080–7087
Sterner DE, Lee JM, Hardin SE, Greenleaf AL (1995) The yeast carboxyl-terminal repeat domain kinase CTDK-I is a divergent cyclin-cyclin-dependent kinase complex. Mol Cell Biol 15:5716–5724
Qiu H, Hu C, Hinnebusch AG (2009) Phosphorylation of the Pol II CTD by KIN28 enhances BUR1/BUR2 recruitment and Ser2 CTD phosphorylation near promoters. Mol Cell 33:752–762
Cho E-J, Kobor MS, Kim M, Greenblatt J, Buratowski S (2001) Opposing effects of Ctk1 kinase and Fcp1 phosphatase at Ser 2 of the RNA polymerase II C-terminal domain. Genes Dev 15:3319–3329
Sdano MA, Fulcher JM, Palani S, Chandrasekharan MB, Parnell TJ, Whitby FG, Formosa T, Hill CP (2017) A novel SH2 recognition mechanism recruits Spt6 to the doubly phosphorylated RNA polymerase II linker at sites of transcription. Elife 6:e28723
Vos SM, Farnung L, Boehning M, Wigge C, Linden A, Urlaub H, Cramer P (2018) Structure of activated transcription complex Pol II-DSIF-PAF-SPT6. Nature 560:607–612
Liu Y et al (2009) Phosphorylation of the transcription elongation factor Spt5 by yeast Bur1 kinase stimulates recruitment of the PAF complex. Mol Cell Biol 29:4852–4863
Bartkowiak B et al (2010) CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev 24:2303–2316
Lee JM, Greenleaf AL (1991) CTD kinase large subunit is encoded by CTK1, a gene required for normal growth of Saccharomyces cerevisiae. Gene Expr J Liver Res 1:149–167
Karagiannis J, Balasubramanian MK (2007) A cyclin-dependent kinase that promotes cytokinesis through modulating phosphorylation of the carboxy terminal domain of the RNA Pol II Rpb1p sub-unit. PLoS One 2:e433
Sukegawa Y, Yamashita A, Yamamoto M (2011) The fission yeast stress-responsive MAPK pathway promotes meiosis via the phosphorylation of Pol II CTD in response to environmental and feedback cues. PLoS Genet 7:e1002387
Bowman EA, Kelly WG (2014) RNA polymerase II transcription elongation and Pol II CTD Ser2 phosphorylation: a tail of two kinases. Nucleus 5:224–236
Hautbergue G, Goguel V (2001) Activation of the cyclin-dependent kinase CTDK-I requires the heterodimerization of two unstable subunits. J Biol Chem 276:8005–8013
Saberianfar R, Cunningham-Dunlop S, Karagiannis J (2011) Global gene expression analysis of fission yeast mutants impaired in Ser-2 phosphorylation of the RNA pol II carboxy terminal domain. PLoS One 6:e24694
Bowman EA, Bowman CR, Ahn JH, Kelly WG (2013) Phosphorylation of RNA polymerase II is independent of P-TEFb in the C. elegans germline. Development 140:3703–3713
Blazek D et al (2011) The cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes Dev 25:2158–2172
Ahn SH, Keogh MC, Buratowski S (2009) Ctk1 promotes dissociation of basal transcription factors from elongating RNA polymerase II. EMBO J 28:205–212
Ahn SH, Kim M, Buratowski S (2004) Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol Cell 13:67–76
Govind CK et al (2010) Phosphorylated Pol II CTD recruits multiple HDACs, including Rpd3C(S), for methylation-dependent deacetylation of ORF nucleosomes. Mol Cell 39:234–246
Ni Z, Schwartz BE, Werner J, Suarez JR, Lis JT (2004) Coordination of transcription, RNA processing, and surveillance by P-TEFb kinase on heat shock genes. Mol Cell 13:55–65
Wyce A et al (2007) H2B ubiquitylation acts as a barrier to Ctk1 nucleosomal recruitment prior to removal by Ubp8 within a SAGA-related complex. Mol Cell 27:275–288
Hampsey M, Kinzy TG (2007) Synchronicity: policing multiple aspects of gene expression by Ctk1. Genes Dev 21:1288–1291
Winsor TS, Bartkowiak B, Bennett CB, Greenleaf AL (2013) A DNA damage response system associated with the phosphoCTD of elongating RNA polymerase II. PLoS One 8:e60909
Jeong SJ, Kim HJ, Yang YJ, Seol JH, Jung BY, Han JW, Lee HW, Cho EJ (2005) Role of RNA polymerase II carboxy terminal domain phosphorylation in DNA damage response. J Microbiol 43:516–522
Ostapenko D, Solomon MJ (2003) Budding yeast CTDK-I is required for DNA damage-induced transcription. Eukaryot Cell 2:274–283
Westmoreland TJ et al (2009) Comparative genome-wide screening identifies a conserved doxorubicin repair network that is diploid specific in Saccharomyces cerevisiae. PLoS One 4:e5830
O’Connell BC et al (2010) A genome-wide camptothecin sensitivity screen identifies a mammalian MMS22L-NFKBIL2 complex required for genomic stability. Mol Cell 40:645–657
Jackson SP (2002) Sensing and repairing DNA double-strand breaks. Carcinogenesis 23:687–696
Pan X, Ye P, Yuan DS, Wang X, Bader JS, Boeke JD (2006) A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell 124:1069–1081
Symington LS (2002) Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol Mol Biol Rev 66:630–670
Ekumi KM et al (2015) Ovarian carcinoma CDK12 mutations misregulate expression of DNA repair genes via deficient formation and function of the Cdk12/CycK complex. Nucleic Acids Res 43:2575–2589
Dubbury SJ, Boutz PL, Sharp PA (2018) CDK12 regulates DNA repair genes by suppressing intronic polyadenylation. Nature 564:141–145
Popova T et al (2016) Ovarian cancers harboring inactivating mutations in CDK12 display a distinct genomic instability pattern characterized by large tandem duplications. Cancer Res 76:1882–1891
Wu YM et al (2018) Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell 173:1770–1782
Popova T et al (2012) Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res 72:5454–5462
Petes TD (1979) Yeast ribosomal DNA genes are located on chromosome XII. Proc Natl Acad Sci 76:410–414
Srivastava R, Srivastava R, Ahn SH (2016) The epigenetic pathways to ribosomal DNA silencing. Microbiol Mol Biol Rev 80:545–563
Bouchoux C, Hautbergue G, Grenetier S, Carles C, Riva M, Goguel V (2004) CTD kinase I is involved in RNA polymerase I transcription. Nucleic Acids Res 32:5851–5860
Yamamoto RT, Nogi Y, Dodd JA, Nomura M (1996) RRN3 gene of Saccharomyces cerevisiae encodes an essential RNA polymerase I transcription factor which interacts with the polymerase independently of DNA template. EMBO J 15:3964–3973
Peyroche G, Milkereit P, Bischler N, Tschochner H, Schultz P, Sentenac A, Carles C, Riva M (2000) The recruitment of RNA polymerase I on rDNA is mediated by the interaction of the A43 subunit with Rrn3. EMBO J 19:5473–5482
Grenetier S, Bouchoux C, Goguel V (2006) CTD kinase I is required for the integrity of the rDNA tandem array. Nucleic Acids Res 34:4996–5006
Fath S, Milkereit P, Peyroche G, Riva M, Carles C, Tschochner H (2001) Differential roles of phosphorylation in the formation of transcriptional active RNA polymerase I. Proc Natl Acad Sci 98:14334–14339
Oakes M, Nogi Y, Clark MW, Nomura M (1993) Structural alterations of the nucleolus in mutants of Saccharomyces cerevisiae defective in RNA polymerase I. Mol Cell Biol 13:2441–2455
Kobayashi T, Heck DJ, Nomura M, Horiuchi T (1998) Expansion and contraction of ribosomal DNA repeats in Saccharomyces cerevisiae: requirement of replication fork blocking (Fob1) protein and the role of RNA polymerase I. Genes Dev 12:3821–3830
Kobayashi T (2014) Ribosomal RNA gene repeats, their stability and cellular senescence. Proc Jpn Acad Ser B Phys Biol Sci 90:119–129
Johzuka K, Horiuchi T (2002) Replication fork block protein, Fob1, acts as an rDNA region specific recombinator in S. cerevisiae. Genes Cells 7:99–113
Huang J, Moazed D (2003) Association of the RENT complex with nontranscribed and coding regions of rDNA and a regional requirement for the replication fork block protein Fob1 in rDNA silencing. Genes Dev 17:2162–2176
Kaeberlein M, McVey M, Guarente L (1999) The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13:2570–2580
Coordes B, Brunger KM, Burger K, Soufi B, Horenk J, Eick D, Olsen JV, Strasser K (2015) Ctk1 function is necessary for full translation initiation activity in Saccharomyces cerevisiae. Eukaryot Cell 14:86–95
Rother S, Strasser K (2007) The RNA polymerase II CTD kinase Ctk1 functions in translation elongation. Genes Dev 21:1409–1421
Jackson RJ, Hellen CU, Pestova TV (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 11:113–127
Sonenberg N, Hinnebusch AG (2009) Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136:731–745
Hinnebusch AG (2005) Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 59:407–450
Mascarenhas C, Edwards-Ingram LC, Zeef L, Shenton D, Ashe MP, Grant CM (2008) Gcn4 is required for the response to peroxide stress in the yeast Saccharomyces cerevisiae. Mol Biol Cell 19:2995–3007
Deloche O, de la Cruz J, Kressler D, Doere M, Linder P (2004) A membrane transport defect leads to a rapid attenuation of translation initiation in Saccharomyces cerevisiae. Mol Cell 13:357–366
Palmer E, Wilhelm JM, Sherman F (1979) Phenotypic suppression of nonsense mutants in yeast by aminoglycoside antibiotics. Nature 277:148
Singh A, Ursic D, Davies J (1979) Phenotypic suppression and misreading in Saccharomyces cerevisiae. Nature 277:146
Moazed D, Noller HF (1987) Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature 327:389
Stansfield I, Jones KM, Herbert P, Lewendon A, Shaw WV, Tuite MF (1998) Missense translation errors in Saccharomyces cerevisiae. J Mol Biol 282:13–24
Synetos D, Frantziou CP, Alksne LE (1996) Mutations in yeast ribosomal proteins S28 and S4 affect the accuracy of translation and alter the sensitivity of the ribosomes to paromomycin. Biochim Biophys Acta 1309:156–166
Eustice DC, Wakem LP, Wilhelm JM, Sherman F (1986) Altered 40 S ribosomal subunits in omnipotent suppressors of yeast. J Mol Biol 188:207–214
Napolitano G, Licciardo P, Carbone R, Majello B, Lania L (2002) CDK9 has the intrinsic property to shuttle between nucleus and cytoplasm, and enhanced expression of cyclin T1 promotes its nuclear localization. J Cell Physiol 192:209–215
Choi SH, Martinez TF, Kim S, Donaldson C, Shokhirev MN, Saghatelian A, Jones KA (2019) CDK12 phosphorylates 4E-BP1 to enable mTORC1-dependent translation and mitotic genome stability. Genes Dev 33:418
Menghi F et al (2018) The tandem duplicator phenotype is a prevalent genome-wide cancer configuration driven by distinct gene mutations. Cancer Cell 34:197–210
Chila R, Guffanti F, Damia G (2016) Role and therapeutic potential of CDK12 in human cancers. Cancer Treat Rev 33:418–435
Livingstone CD, Barton GJ (1993) Protein sequence alignments: a strategy for the hierarchical analysis of residue conservation. Comput Appl Biosci 9:745–756
Acknowledgements
We are grateful to Stephen Buratowski for suggestions and insightful comments on the manuscript. This work was supported by a National Research Foundation of Korea (NRF) Grant funded by the South Korean government (no. NRF-2016R1A2B2008217).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srivastava, R., Duan, R. & Ahn, S.H. Multiple roles of CTDK-I throughout the cell. Cell. Mol. Life Sci. 76, 2789–2797 (2019). https://doi.org/10.1007/s00018-019-03118-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03118-0