Abstract
Atherosclerosis is a chronic and progressive disease of the arterial walls and a leading cause of non-cardioembolic ischemic stroke. P2Y12 is a well-recognized receptor that is expressed on platelets and is a target of thienopyridine-type antiplatelet drugs. In the last few decades, P2Y12 receptor inhibitors, such as clopidogrel, have been applied for the secondary prevention of non-cardioembolic ischemic stroke. Recent clinical studies have suggested that these P2Y12 receptor inhibitors may be more effective than other antiplatelet drugs in patients with ischemic stroke/transient ischemic attack of atherosclerotic origin. Moreover, animal studies have also shown that the P2Y12 receptor may participate in atherogenesis by promoting the proliferation and migration of vascular smooth muscle cells (VSMCs) and endothelial dysfunction, and affecting inflammatory cell activities in addition to amplifying and maintaining ADP-induced platelet activation and platelet aggregation. P2Y12 receptor inhibitors may also exert neuroprotective effects after ischemic stroke. Thus, P2Y12 receptor inhibitors may be a better choice for secondary prevention in patients with atherosclerotic ischemic stroke subtypes because of their triple functions (i.e., their anti-atherosclerotic, anti-platelet aggregation, and neuroprotective activities), and the P2Y12 receptor may also serve as a noval therapeutic target for atherosclerosis. In this review, we summarize the current knowledge on the P2Y12 receptor and its key roles in atherosclerosis and ischemic stroke of atherosclerotic origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past several decades, stroke has become a global burden because of its long course and high morbidity and mortality. Antiplatelet treatment is recommended for secondary prevention of non-cardioembolic ischemic stroke. However, unlike cardiovascular disease, which is caused by coronary atherosclerosis, the etiology of ischemic stroke is very complicated and affects its prognosis, outcomes, and management. According to the TOAST classification system, non-cardioembolic ischemic stroke includes four subtypes: large-artery atherosclerosis, small-artery occlusion, stroke of other determined etiology, and stroke of undetermined etiology. Few antiplatelet drug trials have been performed according to ischemic stroke subtype [1]. Therefore, it is uncertain whether there is significant heterogeneity in the effects of antiplatelet therapy on secondary prevention among the different non-cardioembolic ischemic stroke subtypes. A recent prespecified exploratory subgroup analysis of the SOCRATES clinical trial demonstrated the superiority of ticagrelor, a P2Y12 antagonist, over aspirin in stroke/TIA patients with ipsilateral atherosclerotic stenosis but not in patients without ipsilateral atherosclerotic stenosis or those with potential causal small-vessel disease [2]. Atherosclerosis, a chronic disease of the medium and large arteries, is one of the main causes of stroke. Its pathological process is complicated and includes damage to vessel endothelial cells (ECs), the adhesion and activation of platelets, the recruitment of monocytes, the proliferation and migration of smooth muscle cells (SMCs), and the release of inflammatory mediators. Other clinical and basic studies have also indicated that the P2Y12 receptor plays a role in atherosclerosis. Thus, P2Y12 receptor inhibitors may be a better choice than other antiplatelet drugs in patients with atherosclerotic ischemic stroke subtypes because of their potential anti-atherogenic effect besides the antiplatelet effects. In this review, we summarize what is currently known about the P2Y12 receptor and the key role it plays in atherosclerosis and atherosclerotic ischemic stroke.
Introduction to the P2Y12 receptor
Discovery of the P2Y12 receptor
In 1978, Bennett et al. reported that a polypeptide involved in adenosine diphosphate (ADP)-induced platelet aggregation may represent an ADP receptor. This effect was inhibited by 5′-p-fluorosulfonyl-benzoyladenosine (FBSA), an adenine nucleotide analog that inhibits platelet aggregation. The receptor was then found to be sensitive to thienopyridines and to couple to inhibitory trimeric GTP-binding regulatory protein (Gi), which was called the P2TAC receptor [3]. Finally, this receptor was identified in humans and rats and designated P2Y12 by Hollopeter et al. [4], and it was shortly thereafter identified in humans [5, 6] and mice [7] by other groups. Patients with congenital P2Y12 receptor deficiency were first described in 1992 [8] and 1995. P2Y12 receptor deficiency is an autosomal recessive disorder characterized by a severe selective impairment of platelet reactivity to ADP and low concentrations of collagen. In Hollopeter’s study, evidence was provided indicating that affected patients have a defect in the p2y12-gene [4].
Structure of the P2Y12 receptor
The P2Y12 gene resides on chromosome 3q24-25 [4, 9]. The P2Y12 receptor contains 342 amino acids and is a member of the P2Y purinergic receptor family, which is composed of two main groups: ligand-gated intrinsic ion channel P2X receptor subtypes (P2X1–P2X7) [10, 11] and G-protein-coupled P2Y-receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14) [4, 12, 13]. Similar to other classes of G-protein-coupled receptors (GPCRs), the crystal structure of the P2Y12 receptor contains seven α-transmembrane helices that interact with each other to stabilize overlapping regions. In all of these transmembrane domains, helix III is located in the center of the structure. In the P2Y12 receptor, helix V is straight and long, which is a distinct difference between this receptor and other GPCRs [14]. In addition, four extracellular cysteines located at positions 17, 97, 175, and 270 form two disulfide bonds that link the amino terminus (C17) to helix VII (C270) and helix III (C97) to the extracellular loop (EL2, C175) [14,15,16] (Fig. 1). P2Y12 receptors localize on the surfaces of mammalian cells and freshly isolated platelets as monomers, dimers, and oligomers, each of which may be associated with different physiological characteristics and functions [16, 17]. ADP is the endogenous agonist of P2Y12 receptors [18], and adenosine triphosphate (ATP) and its triphosphate analog act as its antagonists [19,20,21]. The P2Y12 receptor triggers different intracellular signaling cascades in different cells, resulting in different functions.

Adapted from Ref. [16])
Predicted secondary structure of the P2Y12 receptor. The P2Y12 receptor is a member of GPCRs, with seven α-transmembrane helices, three extracellular loops (EL1–EL3) and three intracellular loops (IL1–IL3), and consists of 342 amino acids in total. There are four extracellular cysteines at positions 17, 97, 175 and 270, five cysteine within transmembrane domain at positions 194, 208, 248, 292, and 302, and one intracellular cysteine at position 315. In addition, there are two disulfide bonds bridging Cys97 with Cys 175, and Cys17 with Cys 270, respectively. Cys cysteine; EL extracellular loop; IL intracellular loop; TM transmembrane domain (
Distribution of the P2Y12 receptor
In the past, the P2Y12 receptor was thought to be expressed on platelets and in subregions of the brain including the amygdala, caudate nucleus, corpus callosum, hippocampus, substantia nigra, and thalamus [4]. In recent years, P2Y12 receptors have also been found on M2 microglia cells [22, 23], dendritic cells [24], oligodendrocytes [25], oligodendrocyte precursor cells [26], astrocytes [27], endothelial cells [28,29,30], vascular smooth muscle cells (VSMCs) [31, 32], osteoclasts [33], macrophages [34, 35], and subpopulations of leukocytes [36].
Blocking the P2Y12 receptor ameliorates atherosclerosis and atherosclerotic ischemic stroke
Genetic abnormalities and P2Y12 receptor inhibitors induce structural and functional deficiencies, respectively, in this receptor. Here, we summarize the anti-atherosclerotic effects of P2Y12 receptor blockade.
P2Y12 antagonists may be more effective than aspirin in patients with ischemic stroke/TIA of atherosclerotic Origin
Two main types of P2Y12 receptor inhibitors are used in a clinical setting: prodrug thienopyridines such as ticlopidine, clopidogrel, and prasugrel, and direct-acting antagonists such as ticagrelor and cangrelor [37,38,39]. In recent years, among these drugs, clopidogrel and ticagrelor have been the two most widely used clinically as antiplatelet agents, along with aspirin, for secondary prevention in patients who have experienced non-cardioembolic ischemic stroke. However, among trials of antiplatelet therapies intended for secondary prevention, few analyses have characterized patients according to ischemic stroke subtypes. In the recent SOCRATES Study, ticagrelor was found to produce effects that were no better than those of aspirin in preventing stroke, myocardial infarction (MI) or death at 90 days in patients with non-cardioembolic, non-severe ischemic stroke (IS), or high-risk transient ischemic attack (TIA) [40]. Surprisingly, a prespecified exploratory subgroup analysis of the SOCRATES data showed that ticagrelor was superior to aspirin as a secondary preventive agent in patients with ipsilateral large-artery atherosclerotic stenosis, but not in patients without ipsilateral large-artery atherosclerotic stenosis or in patients with potentially causal small-vessel disease [2]. The subgroup analysis focused on the presence or absence of symptomatic disease with a potential causal relationship with the index stroke or TIA, and provided evidence that a treatment effect was modified by atherosclerotic stenosis.
Coincidentally, some previous studies have produced similar results (Table 1). As early as 1996, the CAPRIE study suggested that clopidogrel exerts a better effect than that was achieved by aspirin in decreasing the occurrence of stroke, MI or vascular death in patients with atherosclerotic vascular disease consisting of recent IS or MI or symptomatic peripheral arterial disease [41]. Specifically, in the ischemic stroke subgroup, the risk of a reduced primary outcomes was relatively higher (7.3%) for clopidogrel treatment than for aspirin. The later CAPRIE-subgroup analysis showed that the benefit of clopidogrel was amplified in patients with documented prior symptomatic atherosclerotic disease [42]. In the CHARISMA-subgroup study, patients with prior ischemic events who were at high risk of atherothrombotic events received more benefit from a dual therapy consisting of clopidogrel and aspirin than from aspirin therapy alone. It is a pity that the atherosclerotic disease origin was not assessed in patients with ischemic stroke or TIA as this may have decreased the power of the test [43]. The results of the CAPRIE and CHARISMA studies indicated that clopidogrel may benefit atherosclerotic patients when used for secondary prevention of cardiocerebrovascular events and that the additive effect of clopidogrel may derive from the fact that it likely exerts an anti-atherosclerotic effect in addition to its antiplatelet effect.
However, in the COMPRESS study, there was no significant difference between combination medication consisting of clopidogrel and aspirin and aspirin alone for preventing the recurrence of IS in patients with acute IS of the large-artery atherosclerosis type according to the TOAST classification [44]. The primary outcome of the COMPRESS study was new ischemic lesion on MRI within 30 days. First, this primary outcome is a surrogate marker, the detection of which was approximately 15-fold greater than the recurrent clinical ischemic stroke incidence. Second, the follow-up duration was 30 days, which might be slightly short to detect the anti-atherogenic effect of drug treatments for new ischemic lesions and particularly for symptomatic clinical events. The follow-up durations of the SOCRATES, CAPRIE, and CHARISMA studies were all more than 90 days. Therefore, the findings need to be confirmed by more randomized clinical trials that use an adequate clinical end point as a primary end point and follow-up for a longer duration.
An analogous result was shown in the CHANCE-subgroup study in which the addition of clopidogrel to aspirin did not significantly alter the risk of recurrent stroke in patients with minor stroke or high-risk TIA, regardless of the presence of intracranial arterial stenosis [45]. Clopidogrel requires conversion to an active metabolite by hepatic cytochrome p450 (CYP) isoenzymes to target the P2Y12 receptor, and polymorphisms of the CYP2C19 gene have been identified as strong predictors of clopidogrel non-responsiveness. The CHANCE study focused on Asian populations, and the percentage of carriers of CYP2C19 loss-of-function alleles is higher in Asians (52.5%) than in western populations (27.9%) [46, 47], which may have played an important role in reducing the efficacy of clopidogrel and narrowing the differences observed between groups [48].
In conclusion, P2Y12 receptor inhibitors may exert an anti-atherosclerotic effect in addition to its antiplatelet effect in non-cardioembolic ischemic stroke of presumed atherosclerotic disease origin. However, most of these studies were subgroup analyses or even post hoc subgroup analyses, and these designs dramatically diminish the reliability of their results. Additional multicenter, randomized, and controlled trials are needed to confirm these results.
Effects of P2Y12 receptor blockade in animal atherosclerotic models
Recently, pharmacologic and genetic studies have implicated the P2Y12 receptor in atherosclerosis in different animal models of atherosclerosis (Table 2).
p2y12-gene deletion improves atherosclerosis lesion development in mice
The effect of P2Y12 on the progression of atherosclerosis was tested in ApoE−/− mice. These mice develop atherosclerosis after being provided a high-fat diet. Li et al. reported that when the p2y12-gene was deleted in ApoE−/− mice, the size of the lesion area was reduced to different degrees in aortic arches (~ 60%), abdominal aortas (~ 60%), and the aortic root after 20 weeks on a high-fat diet [49]. Following this study, West et al. reported similar results in the aortic arch, aortic sinus and brachiocephalic artery in p2y12/ApoE double-knockout mice that were fed a high-fat diet for 4 weeks [50]. However, there were no significant decreases in lesion area in the carotid artery and descending aortae when p2y12 was knocked out, potentially due to differences in hemorheology. Compared with the aortic sinus and aortic arch, the carotid artery and descending aortae are exposed to lower levels of shear stress and faster blood flow velocities, which provide natural protection against atherosclerotic plaques. Furthermore, p2y12/ApoE double-knockout mice had a higher plaque fibrous content and exhibited preserved fiber integrity [49, 50], which may have increased the stability of atherosclerotic plaques by reducing the risk of rupture [51].
Some other studies have used the ldlr−/− mouse model. Similar to ApoE−/− mice, ldlr−/− mice with p2y12 deficiency had smaller plaque areas in the aortic sinus and aortic root than were found in ldlr−/− mice in addition to fewer macrophages and neutrophils within the plaques after they were fed a high-fat diet for 12 weeks; both of these effects indicate that atherosclerosis development was improved [52].
A transplanted-arteriosclerosis model was also established in p2y12 knockout mice and wild-type mice into which a carotid artery with atherosclerotic plaques was transplanted. The results showed that there was less luminal occlusion, a lower intima–media ratio, and fewer host-derived smooth muscle-like cells in the carotid allografts transplanted into the p2y12 knockout mice than in those transplanted into the wild-type mice [53, 54].
P2Y12 receptor inhibitors diminish the size of plaques in animal atherosclerosis models
P2Y12 receptor inhibitors play a role similar to that of p2y12 deficiency in ApoE−/− mice fed a high-cholesterol or normal diet. Quantitative analyses have shown that clopidogrel inhibits atherogenesis in the aortic sinus, aortic arch, carotid artery, brachiocephalic artery, and whole aortae [55,56,57,58]. The effect of P2Y12 antagonists on stabilizing plaques has also been explored in the past several years. Clopidogrel was found to decrease the area of low mass density, which is composed of lipids and macrophages, and to increase the area of high mass density, which is composed of SMCs and collagen [55, 57], thus leading to more stable plaques.
Delaying treatment with clopidogrel after atherogenesis has been reported to inhibit the infiltration of macrophages and CD4+T cells into the plaques [58]. Delayed treatment with ticagrelor was also investigated and was found to reduce the sizes of necrotic areas and to thicken the fibrous caps of plaques [59]. However, neither treatment reversed lesion remodeling [58], [59]. In agreement with this finding, Li et al. reported that clopidogrel induced significant decreases in the intimal thickness, the intimal area, and the intima–media ratio by reducing both the area and the thickness to lower than was achieved by treatment with aspirin or atorvastatin. In addition, clopidogrel additively decreased the media thickness and area to levels lower than were observed in the placebo group in rabbit vascular-injury models [60].
As shown in Table 2, the majority of animal experiments have verified the anti-atherogenic effect of P2Y12 receptor inhibitors, with the exception of West’s Study. In West’s study, both clopidogrel and ticagrelor were administered for only 4 weeks, which may be too short to affect plaque formation and development in a high-fat/cholesterol-fed ApoE−/− mouse model. The authors found that there was no significant difference in the sizes of the lesion areas among the clopidogrel, ticagrelor and control groups.
In summary, experimental evidence from animal models, especially p2y12-gene knockout animal models, generally supports the idea that inducing a blockade against the P2Y12 receptor via either gene deficiency or an antagonist exerts inhibitory effects on atherosclerosis initiation and progression. The effects may at least partially explain the additive effect of P2Y12 receptor inhibitors observed in patients with non-cardioembolic ischemic stroke of presumed atherosclerotic disease origin. However, these conclusions remain under investigation in humans.
Mechanisms of P2Y12 receptor involvement in atherosclerosis progression
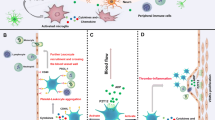
As previously mentioned, inducing a blockage against P2Y12 receptors ameliorates atherosclerosis. P2Y12 receptors are expressed on the surface of different types of cells, and each of these cell types can potentially contribute to the progression of atherosclerosis [61]. Some of the relevant mechanisms have been confirmed, while others remain unclear (Fig. 2).

Adapted from Ref. [61])
Mechanisms of P2Y12 receptor involvement in pathological process of atherosclerosis. The P2Y12 receptor on platelet, upregulated under nicotine or high glucose condition, activates platelets, leading to platelet aggregation to damaged ECs and increased expression and release of inflammatory molecules, which attracting recruitment and infiltration of inflammatory cells, such as monocytes and neutrophils. The platelets, inflammatory cells and damaged ECs interact with each other and induce inflammatory cascades. The SMC P2Y12 receptor plays a key role in the proliferation, IL-6 secretion and migration of SMCs into the intima and plaque. The expression of P2Y12 receptor on SMC can be upregulated by oxLDL or thrombin via NF-κB pathway. Levels of P2Y12 receptor on SMCs and ECs can be increased by nicotine via nAChR. EC endothelial cell; IL-6 interleukin-6; LDL low density lipoprotein; nAChR nicotinic acetylcholine receptor; NF-κB nucleus factor-κB; oxLDL oxidized low density lipoprotein; PAR-1 protease activated receptors-1; PDGF platelet-derived growth factor; PF4 platelet factor 4; PSGL-1 P-selectin glycoprotein ligand-1; SMC smooth muscle cell (
The role of P2Y12 receptors expressed on platelets in atherosclerosis
Activated platelets, represent an important resource of inflammatory mediators, and play a critical role in atherogenesis. The P2Y12 receptor is expressed on platelets, in which its expression is upregulated by nicotine and high glucose levels [30, 62] to mediate the activation of different downstream effectors. To date, three main signaling pathways have been shown to act downstream of P2Y12 in platelets (Fig. 3).
Mechanisms of platelet P2Y12 receptor involvement in progression of atherosclerosis. ADP binds to the P2Y12-receptor and activates the PI3K via coupled Gβγ protein. PI3K isoform p110β and (or) p110γ and regulates activation of PKB/Akt and RAP1 as well as inhibition of RASA3, which balances the circle of Rap1-GDP and Rap1-GTP with CDGI. The phosphorylated Akt mediates an Akt-p38-ERK pathway and TXA2 generation. Both Akt and RAP1 pathways stimulate the inside-out activation of integrin αIIbβ3, which allows for amplification and stabilization of platelet aggregation. The P2Y12-promoted release of dense granule amplifies the platelet aggregation. On the other hand, the activated Gαi protein mediates the inhibition of AC and decrease of cAMP and PKA levels, resulting in α-granules release containing PDGF and PF4, and the exposure of P-selectin and CD40L, which leads to inflammation and coagulation. AC adenylyl cyclase; ADP adenosine diphosphate; ATP adenosine triphosphate; cAMP cyclic adenosine monophosphate; CDGI Ca2+ and diacylglycerol regulated guanine nucleotide exchange factor I; RASA Ras activator; CD40L CD40 ligand; ERK extracellular signal-regulated kinase; GTP guanosine triphosphate; PDGF platelet-derived growth factor; PF4 platelet factor 4; PI3K phosphoinositide 3-kinase; PKA protein kinase A; PKB protein kinase B; PKC protein kinase C; PLC phospholipase C; RAP Ras-related protein; TXA2 thromboxaneA2; VASP vasodilator-stimulated phosphoprotein
The Gαi2–AC–cAMP–PKA pathway
Once ADP binds to a P2Y12 receptor, the coupled Gi2 protein dissociates into Gαi2 and Gβγ. The dissociated Gαi2 inhibits the activation of adenylyl cyclase (AC), the production of cyclic adenosine monophosphate (cAMP), and the phosphorylation of protein kinase A (PKA), which causes a change in the level of vasodilator-stimulated phosphoprotein (VASP), an upstream effector of platelet shape [63, 64] and the release of α-granules [49]. However, the relationship between VASP and α-granules is currently uncertain. As a result of granule release, plasma levels of pro-inflammatory factors, such as platelet-derived growth factor (PDGF) and platelet factor 4 (PF4) increase, and the exposure levels of P-selectin and CD40 ligand (CD40L) are upregulated [65]. These factors trigger inflammatory cascades, and may promote the development of atherosclerosis.
The Gβγ-PI3K–Rap1 pathway
Rap1, a member of the small GTPase family, plays a key role in platelet aggregation. After ADP binds to the P2Y12 receptor, the dissociated Gβγ activates phosphoinositide 3-kinase (PI3K) [66]. Then, RAP1 is activated via GTP-loading from the inactive GDP-bound form and the subsequent inside-out activation of integrin αIIbβ3 is synergized by CalDAG-GEFI (CDGI) [67, 68]. Integrin αIIbβ3 is an important signaling molecule known to contribute to the amplification and stabilization of platelet aggregation [69,70,71]. Deficiency in one of two PI3K isoforms (p110β or p110γ) inhibits ADP-induced RAP-1b and platelet aggregation to some degree, but does not completely block either [72, 73]. However, Jackson’s team proposed an opposite view, whereby p110γ may mediate non-catalytic signaling to activate αIIbβ3 [74].
In addition, the P2Y12 receptor and PI3K also play key roles in downregulating the activity of RASA3, one of the most highly expressed GTPase activating proteins (GAPs) to hydrolyze GTP in platelets. They thereby exert a synergistic effect that results in the upregulation of PI3K-Rap1 and CDGI-Rap1 activity to sustain the integrin activation [75, 76]. As a result, CDGI induces the rapid but reversible activation of αIIbβ3 and platelet aggregation, while PKC and P2Y12–PI3K complementarily induce the delayed but sustained activation of αIIbβ3 and platelet aggregation.
The Gβγ-PI3K–PKB/Akt–ERK pathway
PI3K also induces the sequential phosphorylation of protein kinase B(PKB)/Akt and activation of mitogen-activated protein kinase (MAPK) p38 and extracellular regulated protein kinases (ERK). Briefly, the P2Y12-receptor mediates the PI3K–PKB/Akt–p38-ERK pathway, leading to the generation of thromboxane A2 and platelet aggregation [77, 78]. In addition, because it shares this signaling pathway in common with integrin activation, the p38-ERK pathway may also act as a stimulator of the inside-out activation of αIIbβ3 [79].
In summary, the activation of P2Y12 receptors located on platelets mainly results in three effects. First, the activated platelets release a collection of cytokines, adhesion molecules and chemokines to attract leukocytes such as neutrophils, lymphocytes and monocytes, to the area around the damaged endothelium. The leukocytes release more inflammatory molecules, adhere to the endothelium and migrate through the endothelial layer, where they become involved in atherogenesis. Second, membrane proteins and receptors, such as glycoprotein (GP) IIb/IIIa, are activated to recruit many more platelets, resulting in the induction of platelet aggregation, which plays a key role in plaque formation after endothelial damage and thrombogenesis after plaque rupture. Third, the ADP-induced activation promotes the release of the dense granules induced by other agonists [80,81,82] and then activates the recruitment of more platelets and initiates a positive feedback mechanism that contributes to an inflammatory storm and amplifies and stabilizes platelet aggregation.
The role of P2Y12 receptors expressed on VSMCs in atherosclerosis
Fewer studies have explored the functions of the P2Y12 receptor in VSMCs. The first report, by Wihlborg et al. in 2004, showed that P2Y12 receptors are expressed on human internal mammary artery SMCs [32]. Sane et al. then reconfirmed that P2Y12 is expressed on human aortic and saphenous vein SMCs and that its expression is enhanced by nicotine via nicotinic acetylcholine receptors (nAChR) [30] and by thrombin via the NF-κB pathway [83]. Subsequently, the P2Y12 receptor was also found to be expressed in human carotid atherosclerotic plaques, especially at plaque ruptures, and that it colocalizes with SMCs [83]. The level of P2Y12 expression observed in culprit coronary plaques, in which it mostly colocalizes with ECs and SMCs but not macrophages, was higher in acute MI patients than in stable angina pectoris patients [84], implicating the P2Y12 receptor in plaque destabilization. It has been reported that in mice, the level of expression of the P2Y12 receptor on VSMCs is only approximately 7% of that on platelets, and that its expression on VSMCs may have less effect than its expression on platelets with regard to the size of plaques during atherogenesis [49]. However, Storey’s team performed an interesting experiment, in which they obtained platelet p2y12-deficient and vessel wall p2y12-deficient ApoE−/− mice from ApoE−/− and ApoE−/−p2y − /− 12 male mice undergoing bone marrow transplantation. After the mice were fed a high-fat diet for 4 weeks, the authors surprisingly found that in the vessel wall p2y12-deficient mice compared with ApoE−/− controls, plaque sizes were significantly lower in the aortic sinus, aortic arch, and brachiocephalic artery, while in the platelet p2y12-deficient mice, there were no significant differences, indicating that the expression of P2Y12 receptors in vessel walls, but not platelets, promotes early atherogenesis [50].
As was observed in platelets, P2Y12 receptors reduced the cAMP levels via Gi proteins in human VSMCs [32, 83], but the influence of this function remains unclear. In vitro, the P2Y12 receptors present on SMCs were also found to enhance the expression of IL-6 [83], thereby leading to an increase in the production of chemokines, an increase in intercellular adhesion molecule-1 (ICAM-1) levels via the endothelial cell response, and the subsequent recruitment and transmigration of leukocytes [85, 86]. Moreover, in vitro, P2Y12 receptors enhanced the proliferation of human SMCs [83], which is an important step in the development of atherosclerosis. Furthermore, our group found that in a high-fat-diet-fed ApoE−/− mouse model, the number of P2Y12-positive SMCs in plaques was linearly correlated with the plaque area in the aortic arch. In an ApoE−/− mouse model, in the clopidogrel-treated group compared with a placebo group, the abundance of SMCs was lower in the lesions of mice fed a western diet for 12 weeks, potentially because SMC migration was suppressed by the P2Y12 receptor blockade-mediated inhibition of PKA and cofilin dephosphorylation [56]. In addition, the P2Y12 receptors on VSMCs mediate the 2-MesADP-induced contraction of human blood vessels, especially small veins [32], an effect that may be associated with vasospasm and could further contribute to atherosclerosis. However, it is a pity that these effects were not inhibited by clopidogrel in humans [32].
Thus, the P2Y12 receptor-mediated proliferation and migration of VSMCs play important roles during atherogenesis. It has been reported that up to 50% of the macrophage-like cells in plaques are derived from SMCs [87] and VSMC phenotypic switching are also critical in both atherogenesis and advanced plaques. However, to indentify whether the P2Y12 receptors expressed on VSMCs involve in phenotypic switching and SMC-derived foam cells formation in atherosclerotic lesions and further effect plaque vulnerability, more evidence need to be provided.
The role of P2Y12 receptors expressed on inflammatory cells in atherosclerosis
Although there is almost no P2Y12 receptor expression in white blood cells in mice [49], P2Y12 receptors could affect inflammatory cell activities, such as rolling and recruitment, via the cytokines, adhesion molecules and chemokines that are released from the abovementioned cells to promote atherosclerotic processes.
Reports have shown that in ApoE−/− mice, genetically knocking out the p2y12 gene decreases monocyte/macrophage infiltration within vascular walls [49]. A recent study reported that prasugrel inhibited the platelet-induced release of interferon-γ (IFN-γ) and the differentiation of CD4+ T cells into pro-inflammatory CD4+ phenotypes; that report investigated the role of P2Y12 receptor inhibitors in indirect immune responses to platelet activation [88]. Clopidogrel induced a similar effect in addition to reducing the levels of P-selectin, E-selectin, PDGF-β, ICAM-1, vascular adhesion molecule-1 (VCAM-1) and monocyte chemotactic protein-1 (MCP-1) and decreasing the numbers of CD4+ and CD8+ T cells in the vascular walls of animal models [58, 60]. Its effects on cellular infiltration may represent an outcome of the P2Y12 blockade-mediated down-regulation of cytokines, adhesion molecules and chemokines [89], as no report to date has shown that P2Y12 receptors exert a direct effect on monocytes, macrophages, or T cells.
Surprisingly, clopidogrel was found to upregulate CD4+CD25+ regulatory T cells (Tregs) in the spleen in addition to splenic but not bone marrow-derived endothelial progenitor cells in a mouse model, and these increases may have contributed to decreases in the volumes and increases in the stability of plaques [55]. Clopidogrel inhibited the proliferation of splenocytes in vitro but had no influence on the oxLDL-mediated immunoproliferative response of splenocytes [55]. The effects of clopidogrel on the spleen suggest that P2Y12 receptors play a role in systemic inflammation, for which inflammatory factor levels in the blood are much more commonly used indicators. For example, P2Y12 antagonists decrease serum levels of C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), IL-6 and IL-8, chemokine (C–C motif) ligand (CCL)-2, and growth colony-stimulating factor (G-CSF) and platelet–leukocyte aggregation in humans [83, 90,91,92,93]; the levels of ICAM-1, VACM-1, MCP-1, PF4, PDGF-β and platelet aggregations in experimental animal models [49, 58, 60]; and P-selectin and soluble CD40L levels in both humans and experimental animal models [58, 83]. All of these inflammatory mediators interact with each other and give rise to a cascade of inflammatory events, including inflammatory cell recruitment and infiltration and the release of reactive oxygen species and matrix degrading enzymes, which are partially responsible for the pathological processes that lead to atherosclerosis.
The role of P2Y12 receptors expressed on ECs in atherosclerosis
ECs, as important components of the vessel wall, act as a natural barrier and maintain vascular integrity. The P2Y12-receptor is likely to be expressed on ECs in culprit coronary plaques in patients with acute MI, indicating a potential association between EC-P2Y12 and atherosclerosis [84]. Similar to platelets and SMCs, the expression of the P2Y12 receptor is upregulated by nicotine on ECs via nAChR [30]. A recent study suggested that ADP-P2Y12 signaling mediated JNK activation, induced increases in the levels of ICAM-1, VACM-1, and MCP-1, and impaired endothelium-dependent vascular vasodilation, all of which form the basis for atherogenesis and the recruitment of inflammatory cells, including monocytes [94]. The ameliorated inflammation and endothelial dysfunction induced by ticagrelor may partially contribute to its protective effect against the formation or rupture of plaques.
The neuroprotective effects of P2Y12 receptor inhibitors in animal model of ischemic stroke
In addition to its antiplatelet and anti-atherogenic effects, some animal studies have also suggested that P2Y12 receptor inhibitors may have neuroprotective effects. The P2Y12 receptor is expressed on resting microglia but not activated microglia, and it at least partly mediates microglia activation and migration towards damage sites following brain injury [23, 95]. However, ticagrelor reduced infarct volumes and ameliorated the neurological deficits induced by cerebral focal ischemia in a rat permanent middle cerebral artery occlusion (MCAO) model, potentially by inhibiting the activation and chemotaxis of microglia [23]. Yamauchi et al. reported that ticagrelor exerted a similar protective effect, including improved cerebral blood flow, to the ischemia reperfusion injury area in a mouse transient MCAO model by modulating endothelial nitric oxide synthase (eNOS) and ERK1/2 signaling in ECs during the early phase after reperfusion [96]. This neuroprotective effect was also confirmed in prasugrel and non-human primate models. The significantly increased MCA patency, reduced infarct volume and reduced neurological deficits observed in these models support the notion that these agents may act by inhibiting P2Y12 to limit the development of ischemic stroke [97, 98]. These findings indicate that neurological function scores may also be considered an end point in clinical trials.
Conclusion
P2Y12 receptor inhibitors are considered antiplatelet agents and are widely used in clinical settings, especially to benefit patients with acute coronary syndromes or non-cardioembolic ischemic stroke. However, a growing amount of both clinical and experimental evidences suggests that in addition to their well-known antiplatelet effects, they also have anti-atherosclerotic effects that could be achieved through targeting platelets or VSMCs or other cells, such as inflammatory cells and ECs, and neuroprotective effects. In this review, we summarized the roles of the P2Y12 receptor and its inhibitors in atherosclerotic ischemic stroke and atherosclerosis and considered the mechanisms potentially underlying these effects, thereby providing a fundamental basis for the multitarget effects of P2Y12 receptors.
In future studies, some points should be taken into consideration, such as the limitation of data for ischemic stroke of atherosclerotic origin, the choice of target study population (i.e., gene polymorphisms associated with drug metabolism), the size of the study population, the length of follow-up and the precise definition of the end point. More evidence is needed to increase our understanding of the role of the P2Y12 receptor in atherosclerotic ischemic stroke and atherosclerosis.
References
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24(1):35–41
Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Hill MD, Jonasson J, Kasner SE, Ladenvall P, Minematsu K, Molina CA, Wang Y, Wong KSL, Johnston SC, Committee SS, Investigators (2017) Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol 16(4):301–310. https://doi.org/10.1016/S1474-4422(17)30038-8
Gachet C, Hechler B, Léon C, Vial C, Ohlmann P, Cazenave JP (1996) Purinergic receptors on blood platelets. Platelets 7(5–6):261–267. https://doi.org/10.3109/09537109609023587
Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409(6817):202–207. https://doi.org/10.1038/35051599
Herbert J-M, Savi P (2003) P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med 3(2):113–122. https://doi.org/10.1055/s-2003-40669
Takasaki J, Kamohara M, Saito T, Matsumoto M, Matsumoto S, Ohishi T, Soga T, Matsushime H, Furuichi K (2001) Molecular cloning of the platelet P2T(AC) ADP receptor: pharmacological comparison with another ADP receptor, the P2Y(1) receptor. Mol Pharmacol 60(3):432–439
Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS (2001) Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest 107(12):1591–1598. https://doi.org/10.1172/JCI12242
Cattaneo M, Lecchi A, Randi AM, McGregor JL, Mannucci PM (1992) Identification of a new congenital defect of platelet function characterized by severe impairment of platelet responses to adenosine diphosphate. Blood 80(11):2787–2796
Gachet C (2012) P2Y(12) receptors in platelets and other hematopoietic and non-hematopoietic cells. Purinergic Signal 8(3):609–619. https://doi.org/10.1007/s11302-012-9303-x
Norenberg W, Illes P (2000) Neuronal P2X receptors: localisation and functional properties. Naunyn Schmiedebergs Arch Pharmacol 362(4–5):324–339
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58(3):281–341. https://doi.org/10.1124/pr.58.3.3
von Kugelgen I, Wetter A (2000) Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol 362(4–5):310–323
Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G (2003) Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci 24(2):52–55. https://doi.org/10.1016/S0165-6147(02)00038-X
Zhang K, Zhang J, Gao ZG, Zhang D, Zhu L, Han GW, Moss SM, Paoletta S, Kiselev E, Lu W, Fenalti G, Zhang W, Muller CE, Yang H, Jiang H, Cherezov V, Katritch V, Jacobson KA, Stevens RC, Wu B, Zhao Q (2014) Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature 509(7498):115–118. https://doi.org/10.1038/nature13083
Zhang J, Zhang K, Gao ZG, Paoletta S, Zhang D, Han GW, Li T, Ma L, Zhang W, Muller CE, Yang H, Jiang H, Cherezov V, Katritch V, Jacobson KA, Stevens RC, Wu B, Zhao Q (2014) Agonist-bound structure of the human P2Y12 receptor. Nature 509(7498):119–122. https://doi.org/10.1038/nature13288
Savi P, Zachayus JL, Delesque-Touchard N, Labouret C, Herve C, Uzabiaga MF, Pereillo JM, Culouscou JM, Bono F, Ferrara P, Herbert JM (2006) The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci USA 103(29):11069–11074. https://doi.org/10.1073/pnas.0510446103
Ding Z, Bynagari YS, Mada SR, Jakubowski JA, Kunapuli SP (2009) Studies on the role of the extracellular cysteines and oligomeric structures of the P2Y12 receptor when interacting with antagonists. J Thromb Haemost 7(1):232–234. https://doi.org/10.1111/j.1538-7836.2008.03202.x
Zhang FL, Luo L, Gustafson E, Lachowicz J, Smith M, Qiao X, Liu YH, Chen G, Pramanik B, Laz TM, Palmer K, Bayne M, Monsma FJ Jr (2001) ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J Biol Chem 276(11):8608–8615. https://doi.org/10.1074/jbc.M009718200
Garcia AE, Mada SR, Rico MC, Dela Cadena RA, Kunapuli SP (2011) Clopidogrel, a P2Y12 receptor antagonist, potentiates the inflammatory response in a rat model of peptidoglycan polysaccharide-induced arthritis. PLoS One 6(10):e26035. https://doi.org/10.1371/journal.pone.0026035
Kauffenstein G, Hechler B, Cazenave JP, Gachet C (2004) Adenine triphosphate nucleotides are antagonists at the P2Y receptor. J Thromb Haemost 2(11):1980–1988. https://doi.org/10.1111/j.1538-7836.2004.00926.x
Park HS, Hourani SM (1999) Differential effects of adenine nucleotide analogues on shape change and aggregation induced by adnosine 5-diphosphate (ADP) in human platelets. Br J Pharmacol 127(6):1359–1366. https://doi.org/10.1038/sj.bjp.0702690
Moore CS, Ase AR, Kinsara A, Rao VT, Michell-Robinson M, Leong SY, Butovsky O, Ludwin SK, Seguela P, Bar-Or A, Antel JP (2015) P2Y12 expression and function in alternatively activated human microglia. Neurol Neuroimmunol Neuroinflamm 2(2):e80. https://doi.org/10.1212/NXI.0000000000000080
Gelosa P, Lecca D, Fumagalli M, Wypych D, Pignieri A, Cimino M, Verderio C, Enerback M, Nikookhesal E, Tremoli E, Abbracchio MP, Sironi L (2014) Microglia is a key player in the reduction of stroke damage promoted by the new antithrombotic agent ticagrelor. J Cereb Blood Flow Metab 34(6):979–988. https://doi.org/10.1038/jcbfm.2014.45
Ben Addi A, Cammarata D, Conley PB, Boeynaems JM, Robaye B (2010) Role of the P2Y12 receptor in the modulation of murine dendritic cell function by ADP. J Immunol 185(10):5900–5906. https://doi.org/10.4049/jimmunol.0901799
Amadio S, Montilli C, Magliozzi R, Bernardi G, Reynolds R, Volonte C (2010) P2Y12 receptor protein in cortical gray matter lesions in multiple sclerosis. Cereb Cortex 20(6):1263–1273. https://doi.org/10.1093/cercor/bhp193 (New York, NY: 1991)
Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, Franchini L, Volonte C, Aloisi F, Visentin S (2005) Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia 50(2):132–144. https://doi.org/10.1002/glia.20160
Franke H, Krugel U, Schmidt R, Grosche J, Reichenbach A, Illes P (2001) P2 receptor-types involved in astrogliosis in vivo. Br J Pharmacol 134(6):1180–1189. https://doi.org/10.1038/sj.bjp.0704353
Simon J, Filippov AK, Goransson S, Wong YH, Frelin C, Michel AD, Brown DA, Barnard EA (2002) Characterization and channel coupling of the P2Y(12) nucleotide receptor of brain capillary endothelial cells. J Biol Chem 277(35):31390–31400. https://doi.org/10.1074/jbc.M110714200
Cavelier C, Ohnsorg PM, Rohrer L, von Eckardstein A (2012) The beta-chain of cell surface F(0)F(1) ATPase modulates apoA-I and HDL transcytosis through aortic endothelial cells. Arterioscler Thromb Vasc Biol 32(1):131–139. https://doi.org/10.1161/ATVBAHA.111.238063
Shanker G, Kontos JL, Eckman DM, Wesley-Farrington D, Sane DC (2006) Nicotine upregulates the expression of P2Y12 on vascular cells and megakaryoblasts. J Thromb Thrombolysis 22(3):213–220. https://doi.org/10.1007/s11239-006-9033-4
Hogberg C, Svensson H, Gustafsson R, Eyjolfsson A, Erlinge D (2010) The reversible oral P2Y12 antagonist AZD6140 inhibits ADP-induced contractions in murine and human vasculature. Int J Cardiol 142(2):187–192. https://doi.org/10.1016/j.ijcard.2008.12.091
Wihlborg A-K, Wang L, Braun OO, Eyjolfsson A, Gustafsson R, Gudbjartsson T, Erlinge D (2004) ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol 24(10):1810–1815. https://doi.org/10.1161/01.ATV.0000142376.30582.ed
Su X, Floyd DH, Hughes A, Xiang J, Schneider JG, Uluckan O, Heller E, Deng H, Zou W, Craft CS, Wu K, Hirbe AC, Grabowska D, Eagleton MC, Townsley S, Collins L, Piwnica-Worms D, Steinberg TH, Novack DV, Conley PB, Hurchla MA, Rogers M, Weilbaecher KN (2012) The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest 122(10):3579–3592. https://doi.org/10.1172/JCI38576
Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, Schon P, Schwab A, Hanley PJ (2010) Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal 3(132):ra55. https://doi.org/10.1126/scisignal.2000588
Isfort K, Ebert F, Bornhorst J, Sargin S, Kardakaris R, Pasparakis M, Bahler M, Schwerdtle T, Schwab A, Hanley PJ (2011) Real-time imaging reveals that P2Y2 and P2Y12 receptor agonists are not chemoattractants and macrophage chemotaxis to complement C5a is phosphatidylinositol 3-kinase (PI3K)- and p38 mitogen-activated protein kinase (MAPK)-independent. J Biol Chem 286(52):44776–44787. https://doi.org/10.1074/jbc.M111.289793
Diehl P, Olivier C, Halscheid C, Helbing T, Bode C, Moser M (2010) Clopidogrel affects leukocyte dependent platelet aggregation by P2Y12 expressing leukocytes. Basic Res Cardiol 105(3):379–387. https://doi.org/10.1007/s00395-009-0073-8
Cattaneo M (2011) The platelet P2Y(1)(2) receptor for adenosine diphosphate: congenital and drug-induced defects. Blood 117(7):2102–2112. https://doi.org/10.1182/blood-2010-08-263111
Adamski P, Kozinski M, Ostrowska M, Fabiszak T, Navarese EP, Paciorek P, Grzesk G, Kubica J (2014) Overview of pleiotropic effects of platelet P2Y12 receptor inhibitors. Thromb Haemost 112(2):224–242. https://doi.org/10.1160/TH13-11-0915
Savi P, Herbert JM (2005) Clopidogrel and ticlopidine: p2Y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis. Semin Thromb Hemost 31(2):174–183. https://doi.org/10.1055/s-2005-869523
Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Jonasson J, Minematsu K, Molina CA, Wang Y, Wong KS, Committee SS, Investigators (2016) Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med 375(1):35–43. https://doi.org/10.1056/NEJMoa1603060
Committee CS (1996) A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 348(9038):1329–1339
Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W (2004) Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 35(2):528–532. https://doi.org/10.1161/01.str.0000110221.54366.49
Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Fabry-Ribaudo L, Hu T, Topol EJ, Fox KA (2007) Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 49(19):1982–1988. https://doi.org/10.1016/j.jacc.2007.03.025
Hong KS, Lee SH, Kim EG, Cho KH, Chang DI, Rha JH, Bae HJ, Lee KB, Kim DE, Park JM, Kim HY, Cha JK, Yu KH, Lee YS, Lee SJ, Choi JC, Cho YJ, Kwon SU, Kim GM, Sohn SI, Park KY, Kang DW, Sohn CH, Lee J, Yoon BW, Investigators C (2016) Recurrent ischemic lesions after acute atherothrombotic stroke: clopidogrel plus aspirin versus aspirin alone. Stroke 47(9):2323–2330. https://doi.org/10.1161/STROKEAHA.115.012293
Liu L, Wong KS, Leng X, Pu Y, Wang Y, Jing J, Zou X, Pan Y, Wang A, Meng X, Wang C, Zhao X, Soo Y, Johnston SC, Wang Y (2015) Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of CHANCE. Neurology 85(13):1154–1162. https://doi.org/10.1212/wnl.0000000000001972
Man M, Farmen M, Dumaual C, Teng CH, Moser B, Irie S, Noh GJ, Njau R, Close S, Wise S, Hockett R (2010) Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans. J Clin Pharmacol 50(8):929–940. https://doi.org/10.1177/0091270009355161
Niu X, Mao L, Huang Y, Baral S, Li JY, Gao Y, Xia YP, He QW, Wang MD, Li M, Zou L, Miao XP, Hu B (2015) CYP2C19 polymorphism and clinical outcomes among patients of different races treated with clopidogrel: a systematic review and meta-analysis. J Huazhong Univ Sci Technol Med Sci 35(2):147–156. https://doi.org/10.1007/s11596-015-1404-7
Wang Y, Zhao X, Lin J, Li H, Johnston SC, Lin Y, Pan Y, Liu L, Wang D, Wang C, Meng X, Xu J, Wang Y (2016) Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA 316(1):70–78. https://doi.org/10.1001/jama.2016.8662
Li D, Wang Y, Zhang L, Luo X, Li J, Chen X, Niu H, Wang K, Sun Y, Wang X, Yan Y, Chai W, Gartner TK, Liu J (2012) Roles of purinergic receptor P2Y, G protein-coupled 12 in the development of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 32(8):e81–e89. https://doi.org/10.1161/ATVBAHA.111.239095
West LE, Steiner T, Judge HM, Francis SE, Storey RF (2014) Vessel wall, not platelet, P2Y12 potentiates early atherogenesis. Cardiovasc Res 102(3):429–435. https://doi.org/10.1093/cvr/cvu028
van der Wal AC, Becker AE (1999) Atherosclerotic plaque rupture–pathologic basis of plaque stability and instability. Cardiovasc Res 41(2):334–344
Boulaftali Y, Owens AP 3rd, Beale A, Piatt R, Casari C, Lee RH, Conley PB, Paul DS, Mackman N, Bergmeier W (2016) CalDAG-GEFI deficiency reduces atherosclerotic lesion development in mice. Arterioscler Thromb Vasc Biol 36(5):792–799. https://doi.org/10.1161/atvbaha.115.306347
Yashiro K, Matsumoto Y, Ihara H, Suzuki Y, Kondo K, Urano E, Umemura K (2009) Involvement of platelet activation by P2Y receptor in the development of transplant arteriosclerosis in mice. Transplantation 87(5):660–667. https://doi.org/10.1097/TP.0b013e318196305a
Harada K, Matsumoto Y, Umemura K (2011) Adenosine diphosphate receptor P2Y12-mediated migration of host smooth muscle-like cells and leukocytes in the development of transplant arteriosclerosis. Transplantation 92(2):148–154. https://doi.org/10.1097/TP.0b013e318221d407
Afek AKE, Maysel-Auslender S, Mor A, Regev E, Rubinstein A, Keren G, George J (2009) Clopidogrel attenuates atheroma formation and induces a stable plaque phenotype in apolipoprotein E knockout mice. Microvasc Res 77(3):364–369
Niu X, Pi SL, Baral S, Xia YP, He QW, Li YN, Jin HJ, Li M, Wang MD, Mao L, Hu B (2017) P2Y12 promotes migration of vascular smooth muscle cells through cofilin dephosphorylation during atherogenesis. Arterioscler Thromb Vasc Biol 37(3):515–524. https://doi.org/10.1161/atvbaha.116.308725
Takeda M, Yamashita T, Shinohara M, Sasaki N, Tawa H, Nakajima K, Momose A, Hirata K (2012) Beneficial effect of anti-platelet therapies on atherosclerotic lesion formation assessed by phase-contrast X-ray CT imaging. Int J Cardiovasc Imaging 28(5):1181–1191. https://doi.org/10.1007/s10554-011-9910-6
Heim C, Gebhardt J, Ramsperger-Gleixner M, Jacobi J, Weyand M, Ensminger SM (2016) Clopidogrel significantly lowers the development of atherosclerosis in ApoE-deficient mice in vivo. Heart Vessels 31(5):783–794. https://doi.org/10.1007/s00380-015-0696-7
Preusch MR, Rusnak J, Staudacher K, Mogler C, Uhlmann L, Sievers P, Bea F, Katus HA, Blessing E, Staudacher I (2016) Ticagrelor promotes atherosclerotic plaque stability in a mouse model of advanced atherosclerosis. Drug Des Devel Ther 10:2691–2699. https://doi.org/10.2147/DDDT.S105718
Li M, Zhang YJ, Ren HS, Zhang YC, Zhu XL (2007) Effect of clopidogrel on the inflammatory progression of early atherosclerosis in rabbits model. Atherosclerosis 194(2):348–356. https://doi.org/10.1016/j.atherosclerosis.2006.11.006
Madamanchi NR, Vendrov A, Runge MS (2005) Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25(1):29–38. https://doi.org/10.1161/01.atv.0000150649.39934.13
Hu L, Chang L, Zhang Y, Zhai L, Zhang S, Qi Z, Yan H, Yan Y, Luo X, Zhang S, Wang Y, Kunapuli SP, Ye H, Ding Z (2017) Platelets express activated P2Y12 receptor in patients with diabetes mellitus. Circulation 136(9):817–833. https://doi.org/10.1161/circulationaha.116.026995
Daniel JL, Dangelmaier C, Jin J, Kim YB, Kunapuli SP (1999) Role of intracellular signaling events in ADP-induced platelet aggregation. Thromb Haemost 82(4):1322–1326
Weber AA, Hohlfeld T, Schror K (1999) cAMP is an important messenger for ADP-induced platelet aggregation. Platelets 10(4):238–241. https://doi.org/10.1080/09537109976086
Gawaz M, Langer H, May AE (2005) Platelets in inflammation and atherogenesis. J Clin Invest 115(12):3378–3384. https://doi.org/10.1172/JCI27196
Trumel C, Payrastre B, Plantavid M, Hechler B, Viala C, Presek P, Martinson EA, Cazenave JP, Chap H, Gachet C (1999) A key role of adenosine diphosphate in the irreversible platelet aggregation induced by the PAR1-activating peptide through the late activation of phosphoinositide 3-kinase. Blood 94(12):4156–4165
Cifuni SM, Wagner DD, Bergmeier W (2008) CalDAG-GEFI and protein kinase C represent alternative pathways leading to activation of integrin alphaIIbbeta3 in platelets. Blood 112(5):1696–1703. https://doi.org/10.1182/blood-2008-02-139733
Stefanini L, Bergmeier W (2010) CalDAG-GEFI and platelet activation. Platelets 21(4):239–243. https://doi.org/10.3109/09537101003639931
Lova P, Paganini S, Sinigaglia F, Balduini C, Torti M (2002) A Gi-dependent pathway is required for activation of the small GTPase Rap1B in human platelets. J Biol Chem 277(14):12009–12015. https://doi.org/10.1074/jbc.M111803200
Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF (2002) Activation of Rap1B by G(i) family members in platelets. J Biol Chem 277(26):23382–23390. https://doi.org/10.1074/jbc.M202212200
Cosemans JM, Iserbyt BF, Deckmyn H, Heemskerk JW (2008) Multiple ways to switch platelet integrins on and off. J Thromb Haemost 6(8):1253–1261. https://doi.org/10.1111/j.1538-7836.2008.03041.x
Hirsch E, Bosco O, Tropel P, Laffargue M, Calvez R, Altruda F, Wymann M, Montrucchio G (2001) Resistance to thromboembolism in PI3Kgamma-deficient mice. FASEB J 15(11):2019–2021. https://doi.org/10.1096/fj.00-0810fje
Canobbio I, Stefanini L, Cipolla L, Ciraolo E, Gruppi C, Balduini C, Hirsch E, Torti M (2009) Genetic evidence for a predominant role of PI3Kbeta catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood 114(10):2193–2196. https://doi.org/10.1182/blood-2009-03-208074
Schoenwaelder SM, Ono A, Sturgeon S, Chan SM, Mangin P, Maxwell MJ, Turnbull S, Mulchandani M, Anderson K, Kauffenstein G, Rewcastle GW, Kendall J, Gachet C, Salem HH, Jackson SP (2007) Identification of a unique co-operative phosphoinositide 3-kinase signaling mechanism regulating integrin alpha IIb beta 3 adhesive function in platelets. J Biol Chem 282(39):28648–28658. https://doi.org/10.1074/jbc.M704358200
Stefanini L, Paul DS, Robledo RF, Chan ER, Getz TM, Campbell RA, Kechele DO, Casari C, Piatt R, Caron KM, Mackman N, Weyrich AS, Parrott MC, Boulaftali Y, Adams MD, Peters LL, Bergmeier W (2015) RASA3 is a critical inhibitor of RAP1-dependent platelet activation. J Clin Invest 125(4):1419–1432. https://doi.org/10.1172/jci77993
Stefanini L, Bergmeier W (2016) RAP1-GTPase signaling and platelet function. J Mol Med 94(1):13–19. https://doi.org/10.1007/s00109-015-1346-3
Garcia A, Kim S, Bhavaraju K, Schoenwaelder SM, Kunapuli SP (2010) Role of phosphoinositide 3-kinase beta in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways. Biochem J 429(2):369–377. https://doi.org/10.1042/bj20100166
Shankar H, Garcia A, Prabhakar J, Kim S, Kunapuli SP (2006) P2Y12 receptor-mediated potentiation of thrombin-induced thromboxane A2 generation in platelets occurs through regulation of Erk1/2 activation. J Thromb Haemost 4(3):638–647. https://doi.org/10.1111/j.1538-7836.2006.01789.x
Begonja AJ, Geiger J, Rukoyatkina N, Rauchfuss S, Gambaryan S, Walter U (2007) Thrombin stimulation of p38 MAP kinase in human platelets is mediated by ADP and thromboxane A2 and inhibited by cGMP/cGMP-dependent protein kinase. Blood 109(2):616–618. https://doi.org/10.1182/blood-2006-07-038158
Tournoij E, Koekman CA, Du VX, Roest M, Ruijtenbeek R, Moll FL, Akkerman JW (2012) The platelet P2Y12 receptor contributes to granule secretion through Ephrin A4 receptor. Platelets 23(8):617–625. https://doi.org/10.3109/09537104.2011.645924
Cattaneo M, Lecchi A, Lombardi R, Gachet C, Zighetti ML (2000) Platelets from a patient heterozygous for the defect of P2CYC receptors for ADP have a secretion defect despite normal thromboxane A2 production and normal granule stores: further evidence that some cases of platelet ‘primary secretion defect’ are heterozygous for a defect of P2CYC receptors. Arterioscler Thromb Vasc Biol 20(11):E101–E106
Judge HM, Buckland RJ, Holgate CE, Storey RF (2005) Glycoprotein IIb/IIIa and P2Y12 receptor antagonists yield additive inhibition of platelet aggregation, granule secretion, soluble CD40L release and procoagulant responses. Platelets 16(7):398–407. https://doi.org/10.1080/09537100500163226
Rauch BH, Rosenkranz AC, Ermler S, Bohm A, Driessen J, Fischer JW, Sugidachi A, Jakubowski JA, Schror K (2010) Regulation of functionally active P2Y12 ADP receptors by thrombin in human smooth muscle cells and the presence of P2Y12 in carotid artery lesions. Arterioscler Thromb Vasc Biol 30(12):2434–2442. https://doi.org/10.1161/ATVBAHA.110.213702
Lee CW, Hwang I, Park CS, Lee H, Park DW, Kang SJ, Lee SW, Kim YH, Park SW, Park SJ (2011) Comparison of differential expression of P2Y(1)(2) receptor in culprit coronary plaques in patients with acute myocardial infarction versus stable angina pectoris. Am J Cardiol 108(6):799–803. https://doi.org/10.1016/j.amjcard.2011.05.008
Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A (1997) Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6(3):315–325
Ikonomidis I, Stamatelopoulos K, Lekakis J, Vamvakou GD, Kremastinos DT (2008) Inflammatory and non-invasive vascular markers: the multimarker approach for risk stratification in coronary artery disease. Atherosclerosis 199(1):3–11. https://doi.org/10.1016/j.atherosclerosis.2008.02.019
Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21(6):628–637. https://doi.org/10.1038/nm.3866
Johnston LR, La Flamme AC, Larsen PD, Harding SA (2015) Prasugrel inhibits platelet-enhanced pro-inflammatory CD4+ T cell responses in humans. Atherosclerosis 239(1):283–286. https://doi.org/10.1016/j.atherosclerosis.2015.01.006
Libby P (2002) Inflammation in atherosclerosis. Nature 420(6917):868–874. https://doi.org/10.1038/nature01323
Steinhubl SR, Badimon JJ, Bhatt DL, Herbert JM, Luscher TF (2007) Clinical evidence for anti-inflammatory effects of antiplatelet therapy in patients with atherothrombotic disease. Vasc Med 12(2):113–122. https://doi.org/10.1177/1358863X07077462
Chew DP, Bhatt DL, Robbins MA, Mukherjee D, Roffi M, Schneider JP, Topol EJ, Ellis SG (2001) Effect of clopidogrel added to aspirin before percutaneous coronary intervention on the risk associated with C-reactive protein. Am J Cardiol 88(6):672–674
Xiao Z, Théroux P (2004) Clopidogrel inhibits platelet-leukocyte interactions and thrombin receptor agonist peptide-induced platelet activation in patients with an acute coronary syndrome. J Am Coll Cardiol 43(11):1982–1988. https://doi.org/10.1016/j.jacc.2003.10.071
Cha J-K, Jeong M-H, Lee K-M, Bae H-R, Lim Y-J, Park KW, Cheon S-M (2002) Changes in platelet P-selectin and in plasma C-reactive protein in acute atherosclerotic ischemic stroke treated with a loading dose of clopidogrel. J Thromb Thrombolysis 14(2):145–150
Ganbaatar B, Fukuda D, Salim HM, Nishimoto S, Tanaka K, Higashikuni Y, Hirata Y, Yagi S, Soeki T, Sata M (2018) Ticagrelor, a P2Y12 antagonist, attenuates vascular dysfunction and inhibits atherogenesis in apolipoprotein-E-deficient mice. Atherosclerosis 275:124–132. https://doi.org/10.1016/j.atherosclerosis.2018.05.053
Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9(12):1512–1519. https://doi.org/10.1038/nn1805
Yamauchi K, Imai T, Shimazawa M, Iwama T, Hara H (2017) Effects of ticagrelor in a mouse model of ischemic stroke. Sci Rep 7(1):12088. https://doi.org/10.1038/s41598-017-12205-w
Tomizawa A, Ohno K, Jakubowski JA, Mizuno M, Sugidachi A (2015) Prasugrel reduces ischaemic infarct volume and ameliorates neurological deficits in a non-human primate model of middle cerebral artery thrombosis. Thromb Res 136(6):1224–1230. https://doi.org/10.1016/j.thromres.2015.09.013
Sugidachi A, Mizuno M, Ohno K, Jakubowski JA, Tomizawa A (2016) The active metabolite of prasugrel, R-138727, improves cerebral blood flow and reduces cerebral infarction and neurologic deficits in a non-human primate model of acute ischaemic stroke. Eur J Pharmacol 788:132–139. https://doi.org/10.1016/j.ejphar.2016.06.023
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81571139 to LM, No. 81571119 to BH) and National Key Research and Development Program of China (No. 2018YFC1312200).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
These experiments comply with the current laws of the country in which they were performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gao, Y., Yu, C., Pi, S. et al. The role of P2Y12 receptor in ischemic stroke of atherosclerotic origin. Cell. Mol. Life Sci. 76, 341–354 (2019). https://doi.org/10.1007/s00018-018-2937-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2937-2