Abstract
Objective and design
Postoperative cognitive dysfunction (POCD) is a common complication following surgery among elderly patients. Emerging evidence demonstrates that neuroinflammation plays a pivotal role in the pathogenesis of POCD. This study tested the hypothesis that fluoxetine can protect against POCD by suppressing hippocampal neuroinflammation through attenuating TLR4/MyD88/NF-κB signaling pathway activation.
Subjects
Aged C57BL/6 J male mice (18 months old) were studied.
Treatment
Aged mice were intraperitoneally injected with fluoxetine (10 mg/kg) or saline for seven days before splenectomy. In addition, aged mice received an intracerebroventricular injection of a TLR4 agonist or saline seven days before splenectomy in the rescue experiment.
Methods
On postoperative days 1, 3, and 7, we assessed hippocampus-dependent memory, microglial activation status, proinflammatory cytokine levels, protein levels related to the TLR4/MyD88/NF-κB signaling pathway, and hippocampal neural apoptosis in our aged mouse model.
Results
Splenectomy induced a decline in spatial cognition, paralleled by parameters indicating exacerbation of hippocampal neuroinflammation. Fluoxetine pretreatment partially restored the deteriorated cognitive function, downregulated proinflammatory cytokine levels, restrained microglial activation, alleviated neural apoptosis, and suppressed the increase in TLR4, MyD88, and p-NF-κB p65 in microglia. Intracerebroventricular injection of LPS (1 μg, 0.5 μg/μL) before surgery weakened the effect of fluoxetine.
Conclusion
Fluoxetine pretreatment suppressed hippocampal neuroinflammation and mitigated POCD by inhibiting microglial TLR4/MyD88/NF-κB pathway activation in aged mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative cognitive dysfunction (POCD) is increasingly recognized as a complication following intensive surgical procedures, particularly affecting elderly individuals [1]. POCD is defined as a decline in cognitive function at varying postoperative intervals, up to 3 months–7.5 years after surgery, manifested by memory impairment, deficiency in daily function, psychomotor retardation, and so on [2, 3]. As life expectancy prolongs and patients aged 65 years or older account for a tremendously expanding proportion of major operation consumers, growth in the number of patients susceptible to postoperative neurocognitive disorders is inevitable. Unfortunately, no medication has been approved for the prevention of POCD.
Although the mechanism for POCD remains elusive, mounting evidence has suggested the critical role of neuroinflammation, mainly labeled by microglial activation, in the pathological process of POCD [4]. Neuroinflammation increases neuronal apoptosis, reduces neurogenesis, and alters synaptic plasticity [5, 6]. These events may damage neural function, eventually resulted in POCD. The TLR4/MyD88/NF-κB signaling pathway was shown to facilitate neuroinflammation in the pathophysiology of neurodegenerative diseases, including POCD [7,8,9]. Thus, targeting the TLR4/MyD88/NF-κB signaling pathway to alleviate neuroinflammation may be instructive in exploring a prophylactic medication for POCD.
Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), is prescribed widely as an antidepressant [10]. Preclinical experiments have indicated that fluoxetine exhibits neuroprotective profiles due to its anti-neuroinflammatory properties [11]. Evidence that fluoxetine exerts an anti-neuroinflammatory effect through TLR4-mediated signaling pathways has been elaborated in several studies [12, 13]. Moreover, fluoxetine has been reported to protect cognitive function in neurodegenerative conditions, such as Alzheimer’s and Parkinson’s diseases [14, 15]. However, the effect of fluoxetine pretreatment on POCD has not been explored. Therefore, we tested the hypothesis that fluoxetine pretreatment might benefit POCD protection by suppressing TLR4/MyD88/NF-κB signaling pathway activation and attenuating neuroinflammation.
Materials and methods
Animals
C57BL/6 J male mice (18 months old, weighing 28–36 g) were obtained from the Model Animal Research Center of Fujian Medical University. We conducted the experiments according to the Care and Use of Laboratory Animals guidelines published by the US National Institutes of Health. All protocols were approved by the Animal Care and Use Committee of Fujian Medical University (No. FJMUIACUC2021-0430).
Experimental design
A schematic diagram of the experimental procedure is depicted in Fig. 1. In part A, mice were randomly allocated to four different groups: saline-pretreated control group (C + S), fluoxetine-pretreated control group (C + F), saline-pretreated surgery group (S + S), and fluoxetine-pretreated surgery group (S + F). Fluoxetine hydrochloride (F132, Sigma, USA) was dissolved in 0.9% saline and intraperitoneally injected (10 mg/kg) once daily for seven consecutive days in the C + F and S + F groups [16]. Meanwhile, mice in the C + S and S + S groups were administered saline at an equivalent volume. In the S + S and S + F groups, splenectomy was performed to establish the POCD model after fluoxetine or saline pretreatment. The hippocampus was harvested for further analyses after completing the behavioral experiment at 1, 3, and 7 days postoperatively. In part B, mice were randomly divided as follows: saline-pretreated control group (C + S), saline-pretreated surgery group (S + S), fluoxetine-pretreated surgery group (S + F), fluoxetine-pretreated surgery group with intracerebroventricular lipopolysaccharide (LPS) injection (S + F + LPS), and fluoxetine-pretreated surgery group with an intracerebroventricular injection of vehicle (S + F + VEH). LPS (055: B5, Sigma, USA) was dissolved in sterile 0.9% saline and infused into the right lateral ventricle (1 μg, 0.5 μg/μL) seven days before surgery in the S + F + LPS group. An equivalent volume of vehicle (0.9% saline) was intracerebroventricularly injected seven days before surgery in the S + F + VEH group. The other interventions were identical to those described in Part A.
Schematic diagram of the experimental program. Part A: The C + F and S + F groups were administered 10 mg/kg intraperitoneal fluoxetine every day for 7 days before surgery. Splenectomy was carried out on day 0 for the S + S and S + F groups. The Morris water maze trial was performed for 5 consecutive days before surgery for training and on postoperative days 1, 3, and 7 for testing. Hippocampal tissues were harvested for further analyses after the behavioral tests. Part B: The TLR4 agonist LPS was microinjected into the lateral ventricle 7 days before surgery in the S + F + LPS group
Splenectomy
Mice were anesthetized with 1% pentobarbital sodium (40 mg/kg, i.p.) and fixed on the operating platform in the right lateral decubitus position. The vital signs of mice, including heart rate, blood oxygen saturation, and respiratory rate, were continuously monitored throughout the surgery. Heat management was conducted with a rectal temperature probe and maintained at 36.0−37.0 °C with a thermal blanket (WB-1, Shanghai Yuyan Instruments Co., Ltd. China). After an adequate depth of anesthesia was achieved, a 1 cm incision was performed in the abdominal wall. Then, the spleen was gently exposed, and the blood vessels were ligated, followed by removal of the spleen. After the surgical procedures, the wound was infiltrated with 0.15% ropivacaine for analgesia. All mice were allowed to resume normal activities, diet, and water after recovery.
Stereotaxic injection
Mice were anesthetized using intraperitoneal injection with 1% pentobarbital sodium (40 mg/kg) and transferred to a brain stereotaxic apparatus (71000; RWD Life Science Co., Ltd., China). The TLR4 agonist LPS (1 μg, 0.5 μg/μL) was administered into the right lateral ventricle (from bregma, AP − 0.3 mm, ML 1.0 mm, DV − 2.5 mm) by a Hamilton syringe (80630, Hamilton Co., USA) with a microinjection pump (KDS LEGATO 130, RWD Life Science Co., Ltd., China) [17]. The infusion rate was 0.2 μL/min, and the needle was removed after 10 min of additional residence time to prevent reflux. All infusions of LPS were conducted 30 min before fluoxetine or saline injection.
Cognitive function assessment
We used the Morris water maze (MWM) to assess hippocampus-related spatial learning and memory abilities on postoperative days 1, 3, and 7. In the initial training phase, mice underwent training to use visual cues to swim toward a hidden platform that was submerged 1 cm under the water's surface. At the end of each trial, mice remained on the platform (if they could not find the platform within 60 s, we guided them toward it) for 10 s. After five days of training, splenectomy was performed, and a single 60-s probe trial was scheduled 1, 3, and 7 days after the operation, with the platform removed. The swimming track, escape latency, swimming speed, time spent in the target quadrant, and crossing times were acquired and analyzed by the MWM video analysis system (Zhenghua Biological Instrument Equipment Co., Ltd, China).
Enzyme-linked immunosorbent assay (ELISA)
Hippocampal IL-1β, IL-6, and TNF-α were assayed using ELISA kits (Biosource, Invitrogen, BMS6002, BMS603-2, BMS607-3, USA) on postoperative days 1, 3, and 7. All experimental protocols were conducted according to the manufacturer’s instructions. The concentrations of IL-1β, IL-6, and TNF-α were calculated following the standard curve and are presented as pg/mg protein.
Western blot
The western blot protocol was conducted as previously described [18]. Briefly, the protein concentration of hippocampal samples on postoperative day 1 was determined using a BCA protein assay kit (P0010, Beyotime, China). After blocking with 5% nonfat dry milk in TBS-Tween 20 (0.05%) (TBST), the membranes were incubated overnight at 4 ℃ with the appropriate primary antibodies and then incubated for 60 min at 25 ℃ with secondary antibodies after three washes with TBST. The primary and secondary antibodies are listed in Supplementary Table 1. Blots were developed with an enhanced chemiluminescence reagent (AS1059, ASPEN, China) and detected using X-ray film (XBT-1, Kodak, USA). The band intensity was quantified by densitometric analysis using ImageJ for Windows (V 1.8.0, NIH, USA). Relative protein expression levels were obtained by normalization to β-actin.
Nissl staining
On postoperative day 1, brain tissues were harvested and processed with paraformaldehyde (4%) for fixation, embedded in paraffin, and sectioned into 4 μm slices using a sliding microtome (RM2235, Leica, Germany). The sections were stained with toluidine blue (89640, Sigma, USA) and observed using an automated acquisition system (TissueFAXS Confocal Plus SL, Austria). Six high-power domains were randomly selected from the same part of the hippocampus, and the number of normal neurons per domain was determined.
Immunofluorescence and TUNEL staining
Mice were perfusion-fixed, and their brains were removed and sectioned (thickness of 4 μm) on postoperative days 1 and 7. Immunofluorescence staining was performed following routine immunostaining protocols [19]. The primary and secondary antibodies used are listed in Supplementary Table 2. TUNEL staining on postoperative day 7 was performed using a TUNEL assay kit (11684795910, Roche, USA). Finally, the slices were covered with Fluoroshield mounting medium with DAPI (P-36931, Invitrogen, USA) and observed using an automated acquisition system (TissueFAXS Confocal Plus SL, Austria). We randomly selected six high-power areas from different mice to quantify positive cells. The numbers from these fields were averaged and expressed as positive cells per area for each mouse. Tissue sections were analyzed by an observer blinded to the experimental cohorts.
Statistical analysis
We performed statistical analysis using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., USA). Data are presented as the mean ± standard deviation (SD) and were compared using the independent-samples t test or one-way analysis of variance followed by the Bonferroni post hoc test. Differences were considered significant if the two-tailed P value was less than 0.05.
Results
Fluoxetine partially restored neurocognitive function after splenectomy in aged mice
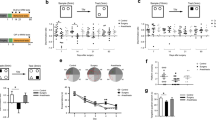
The MWM test was conducted to assess spatial learning and memory capacity before and after splenectomy. During the five consecutive preoperative days of the MWM training phase, the four groups displayed similar spatial learning abilities, as demonstrated by the variation tendency of the escape latency. (Fig. 2A, B). A diminished percentage of time in the target quadrant (Fig. 2C) and platform crossing number (Fig. 2D) were observed in the S + S group compared to the C + S group during the MWM testing phase. However, compared to the S + S group, the S + F group mice displayed a higher percentage of time spent in the target quadrant (Fig. 2C) and an increased number of platform crossings (Fig. 2D). Given that splenectomy alone and fluoxetine treatment did not change swimming speed (Fig. 2E), the above results indicate that fluoxetine pretreatment can preserve learning and memory and potentially mitigate POCD.
Fluoxetine relieved cognitive impairment after surgery. A Representative trajectories of mice during the MWM training and testing phases. B The line chart shows the escape latency represented by the time elapsed before the mice found the platform during the MWM training phase. C The bar charts represent the percentages of the time taken to swim in the target quadrant during the MWM testing stage at 1, 3, and 7 days postoperatively. D The bar charts represent the crossings over the target platform during the MWM testing stage at 1, 3, and 7 days postoperatively. E The line chart shows the swimming speed of the mice during the MWM training and testing phases. N = 10 for each group; data are presented as the mean ± SD, *P < 0.05
Fluoxetine downregulated neuroinflammation in the hippocampus of aged mice following surgery
We measured the levels of hippocampal TNF-α, IL-6, and IL-1β on postoperative days 1, 3, and 7. The proinflammatory cytokines in the S + S group within three days after surgery were above the levels of the control group, yet fluoxetine treatment depressed surgery-induced inflammation, as shown in Fig. 3. Intriguingly, following steep increases in cytokine levels on postoperative day 1, the surgical groups with or without fluoxetine treatment presented decreases in the protein levels of the three cytokines. This result implies that fluoxetine moderately curbs the upregulation of proinflammatory cytokines.
Fluoxetine attenuated the temporary increase in proinflammatory cytokine levels after surgery. ELISA kits were used to quantify the levels of TNF-α, IL-6, and IL-1β at 1, 3, and 7 days postoperatively. The bar charts show the proinflammatory cytokine levels on these three days. N = 6 for each group, data are presented as the mean ± SD, *P < 0.05
Fluoxetine suppressed hippocampal microglial activation in aged mice following surgery
Microglial activation promotes neuroinflammation [20]. Hence, we explored whether the anti-inflammatory effect of fluoxetine was associated with the inhibition of microglial activation. In the S + S group, the numbers of IBA-1-positive cells in the hippocampal CA1, CA3, DG, and HF regions increased at 24 h after splenectomy (Fig. 4). Notably, fluoxetine treatment remarkably restrained this increase, restoring the IBA-1-positive cell count to approximately normal levels in the hippocampal CA1, CA3, DG, and HF regions, as shown in Fig. 4. These results further reveal the possible anti-inflammatory mechanism of fluoxetine, inhibiting microglial activation behind its cognitive restorative effect.
Fluoxetine reduced the number of IBA-1-positive microglia in the hippocampus after surgery. A Representative fluorescence images show the immunoreactivity of IBA-1-positive microglia 24 h after surgery. B Regional magnification of the hippocampal CA1 field highlights fluoxetine’s effect on IBA-1-positive microglial increments. C The bar graphs represent IBA-1-positive microglial counts in the hippocampal CA1, CA3, DG, and HF regions. N = 6 for each group; data are presented as the mean ± SD, *P < 0.05
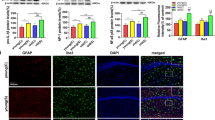
Fluoxetine attenuated hippocampal neuron apoptosis in aged mice following surgery
Neural apoptosis is associated with neuroinflammation and is a crucial pathological factor of cognitive dysfunction [21]. Therefore, we applied Nissl staining, western blotting, and confocal immunofluorescence analysis to examine the extent of hippocampal neuronal apoptosis. The number of surviving neurons on postoperative day 1 in the Nissl staining experiment decreased in the S + S group (Fig. 5A, B). In contrast, in the S + F group, this decrease was mitigated (Fig. 5A, B). The expression of the proapoptotic proteins Bax and Caspase 3 dramatically increased in the S + S group after surgery. In contrast, the apoptosis-inhibiting protein Bcl-2 decreased, which was reversed by fluoxetine treatment (Fig. 5C–F). As shown in the confocal immunofluorescence analysis on postoperative day 7, the percentage of apoptotic neurons labeled by TUNEL and NeuN was markedly increased in the S + S group, and the increase was alleviated in the S + F group (Fig. 5G, H). The above results illustrate that fluoxetine can mitigate neural apoptosis, possibly relieving POCD.
Fluoxetine inhibited neural apoptosis after surgery. A, B Nissl staining shows the extent of neural survival in the hippocampus on postoperative day 1. C–F Representative western blot bands for apoptosis-related proteins are presented in bar graphs. G, H Fluorescence images show NeuN (red) and TUNEL (green) coexpression in the hippocampus on postoperative day 7, and the statistics are represented in bar graphs. N = 5–6 for each group; data are presented as the mean ± SD, *P < 0.05
Fluoxetine inhibited TLR4/MyD88/NF-κB pathway activation in microglia of aged mice following surgery
To evaluate whether treatment with fluoxetine could alleviate surgery-induced activation of the TLR4/MyD88/NF-κB pathway in microglia, we performed colocalization of TLR4 and IBA-1 fluorescence immunostaining and detected protein expression. In the confocal immunofluorescence analysis, the number of TLR4+/IBA-1+ cells increased in the S + S group. In contrast, the increase in the S + F group was milder, preliminarily implicating the suppressive effect of fluoxetine on the TLR4/MyD88/NF-κB pathway of activated microglia (Fig. 6A, B). Then, we further quantified the levels of IBA-1, TLR4, MyD88, and phosphorylated NF-κB p65 (p-NF-κB p65). The levels of IBA-1, TLR4, MyD88, and p-NF-κB p65 were elevated in the S + S group, whereas this elevating trend was attenuated in the S + F group (Fig. 6C–E). These results indicated the suppressive effect of fluoxetine on the TLR4/MyD88/NF-κB pathway in microglia.
Fluoxetine repressed the hippocampal TLR4/MyD88/NF-κB signaling pathway. A, B Fluorescence images show IBA-1 (red) and TLR4 (green) coexpression, and the statistics are displayed in a bar graph. C–E Representative western blot bands for IBA-1, TLR4, MyD88, p-NF-κB p65, and total NF-κB p65, with statistics presented in bar graphs. N = 5–6 for each group, data are presented as the mean ± SD, and *P < 0.05
The protective potency of fluoxetine against POCD is related to suppressing TLR4/MyD88/NF-κB signaling pathway activation in microglia
To investigate the possible relationship between fluoxetine’s suppressive effect on the TLR4/MyD88/NF-κB signaling pathway and its alleviating effect on POCD in aged mice, we injected the TLR4 agonist LPS (1 μg, 0.5 μg/μL) into the lateral ventricle of aged mice to overexpress TLR4 before splenectomy (Fig. 7A–C). Compared with the S + F group, the S + F + LPS group displayed a shorter time in the target quadrant (Fig. 7D) and fewer target crossings (Fig. 7E), similar to the S + S group. Correspondingly, TUNEL experiments showed that the percentage of apoptotic neurons labeled by NeuN markedly increased in the S + S and S + F + LPS groups, whereas neuronal apoptosis was reduced in the S + F and S + F + VEH groups (Fig. 7F, G). Meanwhile, the ELISA results indicated that the expression of TNF-α, IL-6, and IL-1β on postoperative day 1 in the S + F + LPS group was far above that in the S + F group (Fig. 8A).
Activation of TLR4 abolished the effects of fluoxetine in restoring cognitive function and mitigating neural apoptosis. A–C Microinjection of the TLR4 agonist LPS into the right lateral ventricle. Western blot analysis confirmed the activation of TLR4 in the hippocampus in the S + LPS group. D, E The bar charts represent the percentages of the time taken to swim in the target quadrant and platform crossings during the MWM testing phase on postoperative days 1, 3, and 7. F, G Fluorescence images show NeuN (red) and TUNEL (green) hippocampal coexpression on postoperative day 7, and the statistics are represented in bar graphs. N = 5–6 for each group; data are presented as the mean ± SD, *P < 0.05
Activation of the TLR4/MyD88/NF-κB pathway reversed the anti-inflammatory effect of fluoxetine. A The bar charts show the expression of TNF-α, IL-6, and IL-1β at 1 day postoperatively. B, C Fluorescence images show IBA-1 (red) and TLR4 (green) coexpression, and the statistics are displayed in a bar graph. D, E Representative western blot bands for TLR4, MyD88, p-NF-κB p65, and total NF-κB p65, with statistics presented in bar graphs. N = 5–6 for each group; data are presented as the mean ± SD,*P < 0.05
Then, to elucidate the effect of fluoxetine on TLR4 on microglia, we performed immunofluorescence colocalization of IBA-1 and TLR4, where the expression of TLR4 on microglia in the S + F + LPS group was above that in the S + F group (Fig. 8B, C). Furthermore, we obtained consistent results for the expression levels of TLR4, MyD88, and p-NF-κB p65 (Fig. 8D, E). These results suggested that fluoxetine may prevent neuroinflammation, attenuate neural apoptosis, and alleviate POCD by inhibiting the activation of the TLR4/MyD88/NF-κB pathway in microglia.
Discussion
In the current study, we demonstrated that preoperative application of fluoxetine protected against cognitive decline in aged mice following surgery, which was associated with alleviating hippocampal neuroinflammation via the inhibition of the TLR4/MyD88/NF-κB pathway in microglia. Fluoxetine pretreatment is promising as a candidate intervention for POCD.
Impairment of hippocampus-dependent spatial memory is the typical manifestation of POCD [22,23,24]. As a robust and reliable test sensitive to hippocampus-dependent cognitive dysfunction, the MWM test was chosen to evaluate the establishment of the POCD model in our study [25, 26]. The impaired water maze performance of mice following splenectomy indicated the successful establishment of the POCD model and was improved by fluoxetine pretreatment. Additionally, fluoxetine effectively prevented the acute aggravation of neuroinflammation induced by splenectomy in the hippocampus, which was correlated with the inhibition of microglial activation, in line with a previous study [27]. Intriguingly, following steep increases in cytokine levels on postoperative day 1, surgical mice presented decreases in the protein levels of the three cytokines. These results suggest that the rage of proinflammatory cytokines may be transient and that profound detrimental impacts on cognition may result from more extensive homeostatic or functional changes in the hippocampus triggered by proinflammatory cytokines, such as neuronal apoptosis or synaptic plasticity alteration [21, 28]. To test this hypothesis, we detected the apoptotic neuron rate. We found that neural apoptosis of surgical mice in the hippocampus was still observed along with cognitive decline on postoperative day 7, although proinflammatory cytokine levels had returned to normal. Although we discovered that neuronal apoptosis was curbed by fluoxetine, whether fluoxetine directly affects neurons is still unclear and worth exploring in vitro. At this point, the neuroprotective effect of fluoxetine was preliminarily clarified, which was associated with its anti-neuroinflammatory properties.
Of all TLR family members, TLR4 is the key receptor that activates microglia, which play a vital role in neuroinflammation [7]. Activated microglia cause an inflammatory cascade reaction, which may result in neurological dysfunction, including POCD. A preclinical study of cerebral ischemia–reperfusion injury indicated that upregulated TLR4, activated microglia, and elevated inflammatory cytokine levels might induce a neuroinflammatory response and reduce neuron viability [29]. Likewise, we found TLR4/MyD88/NF-κB signaling pathway activation in microglia of POCD mice, which was inhibited by fluoxetine pretreatment. More importantly, we used an intracerebroventricular injection of LPS, a TLR4 signaling pathway activator [17], to trigger overactivation of the TLR4/MyD88/NF-κB signaling pathway and found that the protective effect of fluoxetine was reversed. The above evidence elucidates TLR4/MyD88/NF-κB signaling pathway involved, at least in part, in POCD and the protective mechanisms of fluoxetine on POCD in aged mice, as shown in Fig. 9. Briefly, TLR4 and MyD88 are elevated in activated microglia induced by surgery, inducing NF-κB p65 phosphorylation and nuclear translocation, which upregulates the levels of inflammatory cytokines, promotes neuroinflammation, and induces neuronal apoptosis in the hippocampus. Fluoxetine ameliorates the neuroinflammatory response and mitigates apoptosis, which is closely related to inhibiting TLR4/MyD88/NF-κB pathway activation in microglia, thus presenting a potential protective effect on POCD.
Partial pathological mechanism of POCD and potential therapeutic effect of fluoxetine via inhibition of TLR4/MyD88/NF-κB pathway activation. Surgery triggers a peripheral inflammatory reaction extending to the central nervous system. Microglia are activated in the inflammatory setting, secrete proinflammatory factors, potentiate neural impairment, and eventually cause POCD. The magnified detailed graphic focuses on the anti-neuroinflammatory mechanism of fluoxetine explored in the current study
Our study indicated that the development of drugs targeting the TLR4/MyD88/NF-κB signaling pathway might be a candidate protective intervention for POCD. However, the development of new medications suffers from multiple challenges, including long development cycles, high costs, and undefined side effects, especially in elderly individuals [30, 31]. Searching for a drug commonly prescribed in clinical practice with a neuroprotective effect may be an alternative to prevent POCD. This study confirmed the effectiveness of fluoxetine pretreatment for POCD in aged mice. In addition, given the proven protective effects in treating neurological diseases [32,33,34] and the relatively high safety profile [35], fluoxetine may be a promising pharmaceutical intervention for POCD. We have registered and are conducting a randomized controlled trial [36] to verify the clinical efficacy of fluoxetine for POCD.
The following limitations of our study should be addressed. First, we only investigated hippocampus-dependent spatial learning function using the MWM test. However, POCD is a syndrome of multiple neurological disorders, including fear memory formation and mood disorders, possibly involving several brain regions in addition to the hippocampus [37]. The changes in neurological function in a single brain region may not comprehensively reflect the protective effect of fluoxetine on POCD. Second, the current study mainly focuses on the impact of fluoxetine in the hippocampus, yet there is a lack of experimental data to validate this hypothesis in peripheral blood. Third, we cannot exclude the influence of antidepressant effects on memory improvement in this study. Further studies are necessary to explore the correction between antidepressant and anti-neuroinflammation in the setting of fluoxetine for reducing POCD.
Conclusion
These results demonstrate the protective property of fluoxetine pretreatment on POCD through alleviating neuroinflammation, with putative involvement of repressing the TLR4/MyD88/NF-κB signaling pathway on activated microglia. Although the translatability of this study to humans and the safety of the novel fluoxetine application are unclear, the maturity of fluoxetine treatment in clinical practice provides reasonable enlightenment for further examination of its properties in preventing and alleviating POCD clinically.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Vizcaychipi MP, Watts HR, O’Dea KP, et al. The therapeutic potential of atorvastatin in a mouse model of postoperative cognitive decline. Ann Surg. 2014;259:1235–44.
Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anesthesia and surgery-2018. Br J Anaesth. 2018;121:1005–12.
Liu B, Huang D, Guo Y, et al. Recent advances and perspectives of postoperative neurological disorders in the elderly surgical patients. CNS Neurosci Ther. 2021. https://doi.org/10.1111/cns.13763.
Hovens IB, Schoemaker RG, van der Zee EA, Absalom AR, Heineman E, van Leeuwen BL. Postoperative cognitive dysfunction: involvement of neuroinflammation and neuronal functioning. Brain Behav Immun. 2014;38:202–10.
Mu RH, Tan YZ, Fu LL, et al. 1-Methylnicotinamide attenuates lipopolysaccharide-induced cognitive deficits by targeting neuroinflammation and neuronal apoptosis. Int Immunopharmacol. 2019;77: 105918.
Fakih D, Zhao Z, Nicolle P, et al. Chronic dry eye induced corneal hypersensitivity, neuroinflammatory responses, and synaptic plasticity in the mouse trigeminal brainstem. J Neuroinflammation. 2019;16:268.
Rahimifard M, Maqbool F, Moeini-Nodeh S, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Aging Res Rev. 2017;36:11–9.
El-Sahar AE, Shiha NA, El Sayed NS, Ahmed LA. Alogliptin attenuates lipopolysaccharide-induced neuroinflammation in mice through modulation of TLR4/MYD88/NF-kappaB and miRNA-155/SOCS-1 signaling pathways. Int J Neuropsychopharmacol. 2021;24:158–69.
Qiu LL, Pan W, Luo D, et al. Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca(2+)/calpain might contribute to postoperative cognitive dysfunction in aging mice. J Neuroinflammation. 2020;17:23.
Houwing DJ, Esquivel-Franco DC, Ramsteijn AS, et al. Perinatal fluoxetine treatment and dams’ early life stress history have opposite effects on aggressive behavior while having little impact on sexual behavior of male rat offspring. Psychopharmacology. 2020;237:2589–600.
Park BK, Kim NS, Kim YR, et al. Antidepressant and anti-neuroinflammatory effects of bangpungtongsung-san. Front Pharmacol. 2020;11:958.
Park J, Jang KM, Park KK. Apamin suppresses LPS-induced neuroinflammatory responses by regulating SK channels and TLR4-mediated signaling pathways. Int J Mol Sci. 2020;21:4319.
Cho KH, Kim DC, Yoon CS, et al. Anti-neuroinflammatory effects of citreohybridonol involving TLR4-MyD88-mediated inhibition of NF-small ka, CyrillicB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 cells. Neurochem Int. 2016;95:55–62.
Sierksma AS, de Nijs L, Hoogland G, et al. Fluoxetine treatment induces seizure behavior and premature death in APPswe/PS1dE9 mice. J Alzheimers Dis. 2016;51:677–82.
Boggio PS, Fregni F, Bermpohl F, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov Disord. 2005;20:1178–84.
Zheng ZH, Tu JL, Li XH, et al. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav Immun. 2021;91:505–18.
Wang L, Yang JW, Lin LT, et al. Acupuncture attenuates inflammation in microglia of vascular dementia rats by inhibiting miR-93-mediated TLR4/MyD88/NF-kappaB signaling pathway. Oxid Med Cell Longev. 2020;2020:8253904.
Qian B, Yang Y, Yao Y, Liao Y, Lin Y. Upregulation of vascular endothelial growth factor receptor-1 contributes to sevoflurane preconditioning-mediated cardioprotection. Drug Des Devel Ther. 2018;12:769–76.
Liu Q, Sun YM, Huang H, et al. Sirtuin 3 protects against anesthesia/surgery-induced cognitive decline in aged mice by suppressing hippocampal neuroinflammation. J Neuroinflammation. 2021;18:41.
Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2: e91229.
Zhang X, Dong H, Li N, et al. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J Neuroinflammation. 2016;13:127.
Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61:1354–9.
Xiao JY, Xiong BR, Zhang W, et al. PGE2-EP3 signaling exacerbates hippocampus-dependent cognitive impairment after laparotomy by reducing expression levels of hippocampal synaptic plasticity-related proteins in aged mice. CNS Neurosci Ther. 2018;24:917–29.
Netto MB, de Oliveira Junior AN, Goldim M, et al. Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav Immun. 2018;73:661–9.
Meng B, Li X, Lu B, et al. The investigation of hippocampus-dependent cognitive decline induced by anesthesia/surgery in mice through integrated behavioral Z-scoring. Front Behav Neurosci. 2019;13:282.
Hovens IB, Schoemaker RG, van der Zee EA, Heineman E, Nyakas C, van Leeuwen BL. Surgery-induced behavioral changes in aged rats. Exp Gerontol. 2013;48:1204–11.
Tian M, Yang M, Li Z, et al. Fluoxetine suppresses inflammatory reaction in microglia under OGD/R challenge via modulation of NF-κB signaling. 2019. Biosci Rep. https://doi.org/10.1042/BSR20181584.
Rammes G, Starker LK, Haseneder R, et al. Isoflurane anesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology. 2009;56:626–36.
Gu J, Su S, Guo J, Zhu Y, Zhao M, Duan J. Anti-inflammatory and anti-apoptotic effects of the combination of ligusticum chuanxiong and radix paeoniae against focal cerebral ischemia via TLR4/MyD88/MAPK/NF-κB signaling pathway in MCAO rats. J Pharm Pharmacol. 2018;70:268–77.
Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58.
Orgeta V, Tabet N, Nilforooshan R, Howard R. Efficacy of antidepressants for depression in alzheimer’s disease: systematic review and meta-analysis. J Alzheimers Dis. 2017;58:725–33.
Chollet F, Tardy J, Albucher JF, et al. Fluoxetine for motor recovery after acute ischemic stroke (FLAME): a randomized placebo-controlled trial. Lancet Neurol. 2011;10:123–30.
Wang Y, Neumann M, Hansen K, et al. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma. 2011;28:259–68.
Liepert J. Update on pharmacotherapy for stroke and traumatic brain injury recovery during rehabilitation. Curr Opin Neurol. 2016;29:700–5.
Collaboration ET. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomized, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19:661–9.
Lin D, Yu L, Chen J, et al. Fluoxetine for reducing postoperative cognitive dysfunction in elderly patients after total knee replacement: study protocol for a single-center, double-blind, randomized, parallel-group, superiority, placebo-controlled trial. BMJ Open. 2022;12: e057000.
Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth. 2017;119:i115–25.
Acknowledgements
This study was supported by the Medical Innovation Project of Fujian Province (Grant No. 2022CXA007), Fujian Medical University Startup Fund for scientific research (Grant No. 2020QH1162), the Natural Science Foundation of Fujian Province (Grant No. 2021J01378), and the National Natural Science Foundation of China (Grant No. 821711186).
Author information
Authors and Affiliations
Contributions
YY, DL, and XZ: conceived and designed the experiments. DL, LL, and YW: performed the experiments. YC and YW: analyzed the data. DL and YC: wrote the draft. YY and XZ: revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, Y., Lin, D., Chen, Y. et al. Fluoxetine alleviates postoperative cognitive dysfunction by attenuating TLR4/MyD88/NF-κB signaling pathway activation in aged mice. Inflamm. Res. 72, 1161–1173 (2023). https://doi.org/10.1007/s00011-023-01738-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01738-8