Abstract
Objective
It has been reported that levels of soluble CD30 in serum and joint fluid are significantly elevated in patients with rheumatoid arthritis (RA). This study aimed to investigate whether CD30 could be a therapeutic target for RA.
Methods
The expression and localization of CD30 were examined by immunohistochemical and double immunofluorescence staining on synovial tissue samples obtained from patients with RA or osteoarthritis (OA) during surgery. Changes in CD30 expression of fibroblast-like synoviocytes (FLS) from RA patients with or without TNFα and IL-1β stimulation were examined by the polymerase chain reaction (PCR) and flow cytometry. Collagen antibody-induced arthritis (CAIA) was created in DBA/1 mice, and the therapeutic effect of brentuximab vedotin (BV) was examined by clinical score, histological findings and measurement of serum levels of SAA, IL-6, and TNFα.
Results
CD30 expression was significantly higher in samples from patients with RA than from those with OA. Double immunofluorescence showed a low rate of co-localization of CD30 with CD20 or CD90, but a high rate of co-localization of CD30 and CD138. CD30 mRNA expression was upregulated 11.7-fold in FLS following stimulation by inflammatory cytokines. The clinical scores of CAIA mice were significantly lower following both BV treatments, however, the histological scores of CAIA mice were significantly lower only following treatment with high dose BV (70 mg/kg).
Conclusions
CD30 was expressed on immunocompetent cells in synovial tissue from RA patients and in cytokine-stimulated FLS in vitro. High dose BV (70 mg/kg) showed significant therapeutic effects in ameliorating inflammation and joint destruction in CAIA mice, but low dose BV (30 mg/kg) was insufficient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease affecting approximately 1% of the population and characterized by chronic inflammation of synovial tissue and progressive bone and joint destruction [1]. There have been significant advances in therapeutic options for RA over the past 20 years, evolving to therapeutic regimens such as methotrexate (MTX), a biological and targeting disease-modifying antirheumatic drug (DMARD) that effectively suppresses disease activity [2]. However, approximately one-third of patients with RA among those who fail to respond to conventional DMARD treatment show an inadequate response to tumour necrosis factor (TNF) inhibitors [3]. Biological DMARDs other than TNF inhibitors include IL-6 receptor inhibitors, T cell co-stimulation blockers, and B cell depletion agents, but only 69% of patients achieve a good or moderate EULAR response to a second non-TNF-targeted biologic after an inadequate response to a first TNF inhibitor [4]. Thus, there is still a need to develop novel efficacious agents with different modes of action.
CD30, a type 1 transmembrane glycoprotein, is a member of the tumour necrosis factor receptor (TNFR) superfamily. CD30 is mainly expressed by a variety of lymphoid neoplasms [5,6,7], and known to activate molecules on T cells and B cells. In areas other than oncology, the serum level of sCD30 has been reported to be associated with viral infection and chronic inflammatory diseases including lupus erythematosus, asthma, atopic dermatitis, and RA [8,9,10,11,12]. However, the primary function of CD30 has remained obscure.
In late 1990’s, Gerli R et al. reported that sCD30 levels in serum and synovial fluid were higher in patients with early RA than controls and hypothesized that preactivated CD30-committed T cell subset might be recruited from the peripheral blood (PB) into the inflamed joint, to downmodulate inflammation possible through IL-4 and IL-10 production [12, 13]. Tinazzi et al. found that high levels of sCD30L in the serum and synovial fluid of RA patients. They also described that sCD30 levels seem to reflect the recruitment of CD30 + T cells into the inflamed joints and are predictive of a positive response to classical and biological immunosuppressive therapy [14]. More recently, Barbieri also confirmed that in synovial fluid samples, not in PB samples, RA patients showed much higher percentage of CD4 + T cells and regulatory T cells expressing the CD30 than in controls, suggesting the local anti-inflammatory role of these cells. Interestingly, they also found that the stimulation of CD30 ligand (CD30L) + T cells with the CD30/Fc chimera, a molecule that behaves as sCD30 can induce the polarization of T cells towards a Th17 phenotype with proinflammatory characteristics [15]. They also reported that CD30L + neutrophils activated by stimulation with CD30/Fc release IL-8 amplifying the inflammatory response. The fact the CD30L/CD30 plays a critical role in Th17 differentiation [16] stimulated the investigations for complex CD30L/CD30 signaling pathways using CD30L or CD30 knockout mice, leading to the of attempts to the therapeutic modulation targeting CD30L/CD30 interaction for inflammatory autoimmune diseases including multiple sclerosis [17], inflammatory bowel diseases [18], and immune-mediated glomerulonephritis [19].
Brentuximab vedotin (BV; ADCETRIS®) is a novel antibody–drug conjugate (ADC) consisting of a chimeric anti-CD30 monoclonal antibody bound to monomethyl-auristatin E (MMAE), a potent microtubule inhibitor, via a valine-citrulline linker. When bound to CD30, a receptor on the cell membrane, BV is internalised and MMAE is released by the action of lysosomal enzymes on the linker. MMAE binds to microtubules to prevent mitosis and induce apoptosis [20]. In a clinical trial, BV was proven to be highly effective for relapsed and refractory classic Hodgkin lymphoma (CHL) and anaplastic large cell lymphomas (ALCL) that express CD30 on the surface of tumour cells [21].
According to the English literature to date, BV has been administered in six patients with RA with methotrexate (MTX)-associated lymphoproliferative disorder (MTX-LPD) with (n = 3) or without AVD (doxorubicin, vinblastine, dacarbazine) (n = 3), and achieved complete remission of HL [22]. Interestingly, in five of six RA cases (one case died) clinical remission or good disease control was maintained. None of the six cases developed any serious adverse events. The mechanism of action of BV in RA patients remains unknown, and it was uncertain whether BV independently acted on RA pathology, or disease control was achieved only by a manifestation of lymphoma in these case reports. These results stimulated us to examine the localization and expression of CD30 in RA synovial tissue. Next, we investigated whether BV has an effect on RA using an anti-type II collagen-induced arthritis model (CAIA), in which is an animal model of RA. To date, there are no treatment of RA complicated by MTX-HL, and if BV has a therapeutic effect on RA, it might be a promising treatment. The results of the current study may also provide an important clue to improve our understanding of the pathogenesis of RA, as well as for the development of a new CD30-targeted therapy against RA.
Materials and methods
Reagents
Brentuximab vedotin (BV) was obtained from Takeda Pharmaceutical Company Limited (Osaka, Japan). CD30 polyclonal antibody (PA5-86,095; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) was used in all histological analyses.
Patients and cells
Fresh synovial tissue was obtained during open joint replacement surgery from a total of 12 patients with a clinical diagnosis of RA as well as seven patients with a clinical diagnosis of osteoarthritis (OA). All RA patients fulfilled the American Rheumatism Association criteria for RA [23]. All OA patients had primary OA diagnosed by the criteria for OA knee by the American Rheumatism Association [24]. The background characteristics of patients are summarised in Supplemental Table 1. All OA patients had no clinical symptoms in joints other than the contralateral knee and did not have systemic inflammatory disease. They did not have a history of intra-articular injections of corticosteroids or hyaluronic acid at least 3 months before the surgery and not treated by oral corticosteroids. Fibroblast-like synoviocytes (FLS) were isolated from the RA synovial tissues as described previously [25]. Tissues were minced and digested in phosphate-buffered saline (PBS) containing TrypLE Express (Thermo Fisher Scientific), and digested with collagenase (Wako Pure Chemical Industries Ltd., Osaka, Japan) in Dulbecco’s Modified Eagle Medium (DMEM) for 1 h at 37 °C. Tissue debris was removed with a cell strainer, then cells were centrifuged twice at 1500 rpm for 5 min. The supernatant was removed, and the pelleted cells were dispensed into a 100 mm dish. Synovial tissue cell cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2 until the primary cultures reached confluence. FLS were used for experiments after three to four passages.
Immunohistochemistry of human synovial tissue
Synovial tissue was harvested from 14 patients with RA or OA at the time of joint surgery at our hospital (n = 7 each). All samples used in this study were immediately fixed in 4% paraformaldehyde (PFA) and embedded in paraffin, from which serial 3-μm-thick sections were cut and stained with haematoxylin and eosin. Other sections were stained immunohistochemically using an automated BOND-III stainer (Leica Biosystems, Wetzlar, Germany). The CD30 primary antibody PA5-86095 (1:100) was used and colour was developed using DAB Refine (Leica Biosystems). Sections were examined under an Olympus BX50 upright research microscope. Five different randomly selected high power fields (eyepiece, 10×; lens, 40×) were examined to calculate the mean number of CD30-positive cells per field in sections of OA and RA synovial tissues.
Double immunofluorescence assessment of CD30-positive cells
Double immunofluorescence staining was performed on paraffin-embedded RA synovial tissue using an automated BOND-III stainer (Leica Biosystems) (n = 7 each). Sections were incubated with the first primary antibody (CD30; 1:100) for 30 min, followed by the second primary antibody CD3 (ab16669, polyclonal antibody, 1:200; Abcam, Cambridge, UK), CD20 (ab64088, monoclonal antibody, 1:100; Abcam), CD138 (M7228, monoclonal antibody, 1:200; DAKO, Glostrup, Denmark), CD68 (M0814, monoclonal antibody, 1:400; DAKO) and CD90 (ab181469, monoclonal antibody, 1:500; Abcam). Sections were treated with goat anti-mouse IgG H&L Alexa Fluor 555 (ab150114; Abcam) and goat anti-rabbit IgG H&L Alexa Fluor 488 (ab150077; Abcam) as secondary antibodies. They were observed and analysed using a confocal laser scanning microscope (LSM780; Carl Zeiss, Jena, Germany) (Central Research Laboratory, Okayama University Medical School).
Real-time polymerase chain reaction (PCR) for the quantitative detection of CD30 (TNFRSF8) messenger RNA (mRNA)
FLS were seeded at a density of 3 × 104/well into 24-well dishes and stimulated with TNFα (10 ng/mL), IL-1β (5 ng/mL) or both TNFα and IL-1β for 24 h (n = 5). Total RNA was isolated from cultured cells using an RNeasy mini kit (Qiagen, Valencia, CA, USA). The RNA was reverse-transcribed into DNA using PrimeScript RT master mix (TaKaRa Bio Inc., Shiga, Japan). For CD30 (TNFRSF8) detection, the Hs0174277_m1 Taqman probe (Thermo Fisher Scientific) was used. PCR was performed using an AriaMX real-time PCR system (Agilent Technologies, Santa Clara, CA, USA). GAPDH was used as an endogenous control. The expression level of CD30 was calculated using the 2−ΔΔCt method.
Flow cytometric analysis of FLS
FLS were analysed by flow cytometry (FCM) to characterise the expression of surface markers and changes in CD30 expression following cytokine stimulation. FLS were incubated for 24 h with or without stimulation with TNFα and IL-1β. The cells were harvested and washed with PBS. Surface staining was performed in fluorescence-activated cell sorting (FACS) staining buffer [PBS plus 2% foetal bovine serum (FBS)] on ice for 20 min. The following fluorophore-labelled anti-human antibodies were used: CD90-PE (Biolegend, San Diego, CA, USA, 328109), CD30-APC (Biolegend, 333910), CD68-FITC (Biolegend, 333805), and CD3-PB (Biolegend, 300418). FCM data were acquired on a BD FACS Aria III (BD Biosciences, Franklin Lakes, NJ, USA), and analysed using FlowJo software (BD Biosciences). All procedures were performed according to the manufacturer’s instructions.
Apoptosis assay of FLS
To investigate apoptosis of FLS with BV treatment, caspase activation was measured using a Caspase-Glo 3/7 assay kit (Promega, Madison, WA, USA) according to the manufacturer’s instructions. FLS incubated with or without TNFα and IL-1β for 24 h were seeded into 96-well plates and treated with BV (50 μg/mL). After 24 h, 100 µL/well Caspase-Glo reagent was added. Following a 1 h incubation at room temperature, samples were read on a FlexStation 3 (Molecular Devices, Sunnyvale, CA, USA).
Induction of CAIA and in vivo treatment of mice with BV
CAIA mice were established as a model of RA, as they have a stable incidence rate of arthritis [26, 27]. Eighteen 7-week-old male DBA/1 mice (Japan SLC, Shizuoka, Japan) were used to evaluate the effect of BV in vivo. Mice were injected intraperitoneally with 2 mg of an arthritogenic cocktail of 5-clone monoclonal antibodies (mAb) to type II collagen (Chondrex, Redmond, WA, USA) on day 0 and 50 μg of lipopolysaccharide on day 3. After the onset of clinically distinct arthritis, the treatment group received intraperitoneal injections of BV on days 4, 7, 10, and 13. We set the dose to BV 70 mg/kg as the high dose group (n = 6) and BV 30 mg/kg as the low dose group (n = 6). The no treatment group (n = 6) received saline injections of the same volume. Mice were monitored for body weight and the development of arthritis every day. Clinical scores for each paw were graded individually on a scale of 0–4 (maximum cumulative clinical arthritis score 16 per mouse) (0, normal paw; 1, mild but definite redness and swelling in any one joint of the digit or ankle/ wrist; 2, moderate to severe redness and swelling of the ankle and wrist; 3, redness and swelling of the entire foot including digits; 4, maximally inflamed limb with involvement of multiple joints) [26].
Serum collection and biomarker measurements
On day 15, mice were euthanized by terminal blood collection via cardiac puncture under general anaesthesia. Whole blood was collected in a serum separator tube (CAPIJECT Micro Collection Tubes®, Terumo Medical, Tokyo, Japan) and spun at 1450 × g for 15 min after standing for 7 h. This supernatant was collected as serum and stored at − 80 °C until assayed. Levels of mouse serum amyloid A (SAA) (Life Diagnostics, Inc., West Chester, PA, USA), IL-6 and TNFα (Protein Tech Group, Chicago, IL, USA) were measured by ELISAs according to the manufacturer’s protocol.
Histological analysis of hind paws
On day 15, mice were euthanized by terminal blood collection via cardiac puncture under general anaesthesia. Hind paws were dissected and fixed in 4% PFA for 24 h. Samples were then decalcified in 0.3 M EDTA (pH 7.5) for 10 days, divided into two blocks along the sagittal plane, dehydrated through a graded series of ethanol, and embedded in paraffin. Standard sagittal sections measuring 3.5 μm were prepared and stained with haematoxylin–eosin and safranin O-fast green. We performed histological assessment according to a semi-quantitative scoring system. Histological examinations for synovial inflammation and bone and cartilage destruction were performed independently by two of the authors (MM and YN). We used the scoring system described by Sancho et al. [28], where 0 indicated no inflammation; 1 meant slight thickening of the synovial cell layer and/or some inflammatory cells in the sublining; 2 represented thickening of the synovial lining, infiltration of the sublining, and localized cartilage erosions; and 3, infiltration in the synovial space, pannus formation, cartilage destruction, and bone erosion.
Immunohistological analysis of hind paws
We performed immunohistochemical staining of CD30 in the hind paws. All sections were subjected to heat-induced antigen retrieval with citrate buffer (pH 6.0) for 10 min at 95 °C prior to further treatment. Endogenous peroxidase was blocked by immersing the specimens in 0.3% H2O2 in PBS, and then treating with 10% normal goat serum. An antibody against CD30 (PA5-86,095, 1:150; Invitrogen) was used as the primary antibody and incubated overnight at 4 °C. The next day, sections were incubated with N-Histofine® Simple Stain MAX PO® (Nichirei Biosciences Inc., Tokyo, Japan) at room temperature for 30 min. Staining was visualized using DAB (Nichirei Biosciences Inc.), and sections were counterstained with haematoxylin.
Statistical analysis
Data are expressed as mean ± SEM. Where appropriate, the different groups were tested for statistical significance using Student’s t-test (two-tailed) and two-way ANOVA with Dunnett’s post-hoc analysis. A P-value of less than 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism software (version 9.0; GraphPad Software Inc., La Jolla, CA, USA).
Ethical approval and consent to participate
All procedures involving human tissue samples used in this research were approved by the Ethics of Human Experiments Committee at Okayama University Graduate School of Medicine (Approval No. 1712-026) and carried out in accordance with relevant guidelines and regulations. All patients gave informed consent to take part in the study. All the animal experiments were approved by the Animal Care and Use Committee, Okayama University (Approval No. OKU-2018446) and carried out in accordance with relevant guidelines and regulations.
Results
Localization of CD30-expressing cells in human synovium
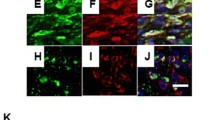
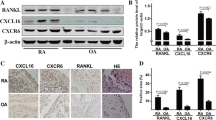
Immunohistochemical staining for CD30 showed a few CD30-positive cells in the synovial tissue of OA samples. On the other hand, in the synovial tissue of RA, large numbers of CD30-positive cells were observed around lymphoid follicles, and weak CD30 staining was observed in the synovial lining cells and stromal cells (Fig. 1a, b). The average number of CD30-positive cells in RA synovial tissue was significantly higher than that in OA synovial tissue (P < 0.01) (Fig. 1c). Double immunofluorescence staining showed expression of CD30 in 58–98% of CD138-positive cells. In addition, it was observed in an average of 4.1% (1–9%) of CD20-positive cells among three of seven cases, and in an average of 3.7% (1–10%) of CD90-positive cells among four of seven cases. No expression of CD30 was observed in CD3- or CD68-positive cells (Fig. 2). These results indicated that CD30 was predominantly expressed on plasma cells in RA synovial tissue specimens, and in some B cells and synovial fibroblasts.
Analysis of CD30-positive cells in synovial tissue of patients with osteoarthritis (OA) and rheumatoid arthritis (RA). a, b Immunohistochemical staining (diaminobenzidine) of CD30 in synovial tissue of patients with OA (a) and RA (b). There is the accumulation of CD30-positive cells, which are thought to be plasma cells, around the area of lymphoid follicles (circles). Original magnifications: 200×. c Number of CD30-positive cells in synovial tissue of OA or RA patients (n = 7 each). Asterisks indicate statistical significance (*P < 0.05: Student’s t- test)
Double-fluorescent staining images of CD3, CD20, CD138, CD68, CD90 and CD30 in RA synovial tissues. a–e CD3 (a), CD20 (b), CD138 (c), CD68 (d) or CD90 (e) expression is shown in red; CD30 expression is shown in green. Cell nuclei were counter-stained blue with DAPI. The cells marked with an arrow show double-positivity for CD30 and CD20, CD90 or CD138 expression
Overexpression of CD30 and effectiveness of BV in FLS of RA patients with cytokine stimulation
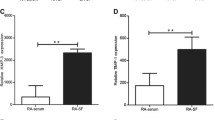
To investigate changes in CD30 expression in cultured FLS of RA patients, we evaluated CD30 mRNA with and without cytokine stimulation by PCR. The mRNA expression level of CD30 was elevated in cells stimulated with TNFα and IL-1β, and it was highest in cells stimulated with both cytokines (Fig. 3a). To assess the protein expression levels of CD30, we performed FCM analysis of FLS with or without cytokine stimulation (both TNFα and IL-1β). The analysis revealed that most cells were CD90-positive, moreover, stimulation with the cytokines increased CD30 expression in CD 90-positive cells (Fig. 3b–d). PCR and FCM experiments showed that stimulation of FLS with cytokines increased expression of CD30 in FLS of RA. A caspase-Glo kit was used to analyse the caspase 3/7 activity to determine whether the effect of BV on CD30 caused apoptosis in FLS. In FLS without cytokine stimulation, caspase activity did not change by BV treatment; however, in cytokine stimulated FLS, caspase activity was significantly increased by BV treatment (Fig. 4). It was assumed that BV did not work well probably due to low expression of CD30 in FLS without cytokine stimulation. On the other hand, since CD30 expression was increased by cytokine stimulation, BV could act on CD30-positive FLS to induce apoptosis, resulting in increased caspase activity.
Changes of CD30 expression induced by stimulation with TNFα and IL-1β in fibroblast-like synoviocytes (FLS) of RA patients. a Real-time polymerase chain reaction analysis of early changes in the expression of CD30 mRNA, with and without cytokine stimulation for 24 h. Relative gene expression of CD30 differed significantly between unstimulated (n = 6) samples and IL-1β-treated (n = 6) samples or those treated with both IL-1β and TNFα (n = 6). Error bars represent mean relative values normalized to GAPDH expression ± SEM. Asterisks indicate statistical significance versus no stimulation (*P < 0.05: Two-way ANOVA with Dunnett’s post-hoc analysis). b-d Flow cytometry (FCM) analysis of CD30 expression in FLS with and without cytokine stimulation for 24 h. b Plots showing representative data of CD30-expressing cells among live CD90 cells. The percentage of double-positive cells for CD90 and CD30 was 2.39% in non-stimulated FLS (left), whereas it increased to 28.3% in cytokine-stimulated FLS (right). c Histogram showing the right shift of CD30 positive signal in cytokine stimulated FLS compared to non-stimulated FLS. d The mean fluorescence intensity (MFI) of CD30 in gated CD90 cells. Asterisks indicate statistical significance versus no stimulation (n = 7) (*P < 0.05: Student’s t- test)
In vivo efficacy of BV for CAIA
We used 18 mice with CAIA to investigate the efficacy of BV in vivo (Fig. 5a). Clinically apparent arthritis developed on day 4 with acute body weight loss, and all control mice (no treatment group) developed severe arthritis with swelling of all limb joints. In the no treatment group, clinical symptoms of active arthritis such as joint redness and swelling peaked on day 10 (average clinical score: 13.5). BV treatment suppressed the clinical symptoms of arthritis in BV treatment groups. Clinical scores were significantly lower in both the BV 30 mg/kg and the BV 70 mg/kg groups compared to CAIA mice without treatment. In the BV 70 mg/kg group, joint inflammation was almost completely suppressed after day 12 (Fig. 5b, d). There was a significant difference in body weight change only between the no treatment group and the BV 70 mg/kg group (Fig. 5c). The weight in the BV 70 mg/kg group tended to increase, suggesting that BV suppressed inflammation and had no systemic side effects. In addition, mouse serum was collected at day 15. In one of the 18 mice, we were unable to measure cytokine levels because it was not possible to collect a sufficient amount of blood (n = 1: control group). The CAIA mice without treatment showed higher SAA and IL-6 levels in serum compared to BV treatment groups. The level of TNFα showed no significant difference between the three groups (Fig. 5e). Histological analysis of the hind paw joints of mice on day 15 revealed that mice treated with BV 70 mg/kg showed amelioration of bone and cartilage destruction and synovial inflammation (Fig. 6a–i). However, histological analysis showed no improvement in inflammation in mice treated with BV 30 mg/kg. Histological scores were significantly lower in the BV 70 mg/kg groups compared to CAIA mice without treatment. There was no statistically significant difference in score between the BV 30 mg/kg group and CAIA without treatment (Fig. 6j). Immunohistochemical staining of CD30 in the hind paw showed that CD30-positive cells were present in the expanded synovium of no treatment mice and mice treated with BV 30 mg/kg (Fig. 6k). In mice treated with BV 70 mg/kg, there was no hyperplastic synovium and almost no CD30-positive cells were observed. Because these mouse specimens easily peeled off the slides, it was not possible to evaluate the sections by multiple immuno-staining.
Effect of intraperitoneal administration of BV (30 mg/kg or 70 mg/kg body weight) on the development of clinical arthritis in mice with collagen antibody-induced arthritis (CAIA). a Induction of arthritis and treatment protocol. MAbs. monoclonal antibodies; i. p. intraperitoneally, LPS lipopolysaccharide, BV brentuximab vedotin. b The clinical score for every day of the experiment is given as mean ± SEM (see “Materials and Methods” for scoring system). c Body weight for every day of the experiment is given as mean ± SEM. Asterisks indicate statistical significance (b, c *P < 0.01: No treatment (CAIA) vs. BV 30 mg/kg or BV 70 mg/kg body weight, analysed by two-way ANOVA with Dunnett’s post-hoc analysis.) d Photographs of hind paws of no treatment CAIA mouse (left) and BV 70 mg/kg-treated mouse (right) at day 9. e Inflammatory biomarkers in the serum of CAIA mice. We analysed SAA (left), IL-6 (middle), and TNFα (right) by ELISA. Asterisks indicate statistical significance (*P < 0.05: No treatment (CAIA) vs. BV 30 mg/kg or BV 70 mg/kg body weight, analysed by two-way ANOVA with Dunnett’s post-hoc analysis)
Histological examination of the ankle joints of no treatment mice (a–c), mice treated with BV 30 mg/kg (d–f), or treated with BV 70 mg/kg (g–i). a, b, d, e, g, h Haematoxylin and eosin (H&E) staining. c, f, i Safranin O staining. Original magnification: 40 × (a, d, g), 100 × (b, e, h), and 200 × (c, f, i). j Quantification of histopathological changes in the ankle joint. Data are given as mean histopathological score ± SEM. Asterisks indicate statistical significance (*P < 0.05: Control (CAIA) vs. BV 70 mg/kg of histological score by two-way ANOVA with Dunnett’s post-hoc analysis). Fibroblast proliferation of BV 70 mg/kg was not more noticeable than no treatment and BV 30 mg/kg mice. k Immunohistochemical staining (diaminobenzidine) for CD30 in the synovial tissue of CAIA mice. Original magnifications: 100 × (upper panel) and 400 × (lower panel)
Discussion
Previous studies demonstrated the presence of high levels of soluble CD30 (sCD30) in serum and synovial fluid of RA patients compared with normal controls, and that levels of serum sCD30 were positively correlated with disease activity, as well as better response to immunosuppressive therapy for RA [12, 29, 30]. Increased level of sCD30 has been thought to be reflect the activation of CD30 + T cells through production of IL-4 and IL-10, which could play a role of counter-regulatory effects in RA pathology [31]. Thus, CD30L/CD30 signalling pathway has been thought to be preferentially involved in Th2 cell responses. However, after the demonstration of CD30L/CD30 signaling executed by the T-T cell interaction plays a critical role in Th17 cell differentiation [16], the role of CD30L/CD30 signaling pathway in the pathogenesis of several disorders needed to be re-considered in Th1/Th2/Th17 axis. In a murine model of anti-CD3-induced enteritis, intestinal damage was significantly reduced in CD30L knockout mice with reduced levels of serum IFN-g and IL-17 [18]. Blocking of CD30L/CD30 signals by CD30-immunoglobulin (CD30-Ig) fusion protein showed reduced inflammation of enteritis in vivo. Similar suppressive effects on Th17 differentiation or IL-17 production by CD30-Ig have been reported in experimental autoimmune encephalomyelitis [17] and dextran sulfate sodium-induced colitis [32], suggesting the blocking agent of CD30L/CD30 signaling, such as CD30-IG or antagonistic anti-CD30 antibodies could be a new therapeutic tool for these human disorders. More recently, Artinger et al. demonstrated combined blockade of the CD30 and OX40 (CD134) costimulatory pathways reduced pathogenic hyperproliferation in secondary lymphoid organs and the migration of disease-promoting Th17 cells to the kidney in nephrotoxic serum nephritis [19]. These results cumulatively suggested the complex CD30L/CD30 signaling pathway and functional activity of the soluble molecules might be involved in the pathogenesis of RA.
In the current study, number of CD30 positive cells was significantly higher in RA synovial tissues than in OA synovial tissues. This might have been related that OA sample was from end stage disease obtained at total joint replacement surgery, however, it is difficult to obtain the early stage of the OA due to ethical problem. In the future study, it would be interesting to see the change of CD30 expression relevant to the disease progression in the same OA and RA patient.
Which cells in the synovial membrane are responsible for the expression of CD30 under inflammatory conditions in RA has not been well understood. As CD30 expression is generally restricted to activated T and B lymphocytes and some activated macrophages [33,34,35,36], we performed double immunofluorescence staining for CD30 with CD3, CD20, CD68, CD90 and CD138 in the synovial tissue of RA patients. In contrast to previous reports, CD30 expression was confirmed in the synovial tissue of RA by immuno-histological assay [13]. Interestingly, CD30 was expressed mainly on plasma cells and on a very small population of B cells and synovial fibroblasts, while T cells and macrophages in RA synovial tissue showed no positive reaction for CD30.
Shanebeck et al. confirmed that activated mouse B cells expressed significant levels of CD30. They demonstrated that stimulation of mouse B cells by CV-1 cells transfected with CD30L in the presence of cytokines (IL-2, IL-4 and/or IL-5) induced the B cells to proliferate, differentiate and produce antibodies [37]. It has been described that only a few CD20+ B cells might be activated to contribute to the germinal centre reaction, and this small population of activated B cells would be selected to differentiate into plasma cells [38]. In addition, the turnover of the B cell population infiltrated into the synovium is rapid. These B cell behaviours might be one of the reasons why immunohistochemical analysis only indicated the presence of a very small population of CD30+ B cells in RA synovial tissue.
The expression of CD30 by plasma cells has not received much attention in the literature. It has been reported that 5–15% of CD38 reactive mucosal plasma cells also react with CD30 in frozen sections of primary gastric plasmacytoma [39]. In the current study, our results showed that most of the plasma cells in the synovial tissue were positive for both CD30 and CD138. The plasma cells may survive for a long time in the synovial tissue, thus they are thought to be exposed to the BV therapy as long as they express CD30 on the cell surface. However, plasma cells are thought to be less involved in the CAIA model because in this case arthritis is induced without involvement of the adaptive or innate immune response. Therefore, it was not clear whether the effect of BV on the CAIA model was caused by plasma cell depletion.
FLS, which are non-immunological cells and drive inflammation, have recently regained attention as a therapeutic target for RA [40]. A previous study showed that Cadherin-11 is a FLS-specific surface receptor, and Cadherin-11-deficient mice have a hypoplastic synovial lining, a disordered synovial response to inflammation, and resistance to inflammatory arthritis [41]. In the current study on synovial tissues of RA patients, CD30-positive cells were observed in 3.7% (1–10%) of the CD90-positive synovial fibroblasts. Surprisingly, FLS expressed CD30 in response to stimulation by inflammatory cytokines in vitro. In the FCM study, the positive rate of CD30 expression before cytokine stimulation was only approximately 3%; however, it increased about 11.8-fold after stimulation with TNFα and IL-1β. Because a soluble form of the extracellular domain of the CD30 molecule (sCD30) is cleaved from the cell membrane of CD30-positive cells by the activity of a disintegrin and metalloproteinase (ADAM) 10 [42], shedding of CD30 from the cell surface might lead to the apparently lower numbers of CD30-positive cells by immunohistochemistry. Our in vitro experiments also demonstrated that BV, a microtubule inhibitor-binding anti-CD30 monoclonal antibody, did not affect FLS without stimulation by TNFα and IL-1β, whereas BV induced apoptosis of cytokine-stimulated FLS.
In the current study, we examined for the first time that the effects of BV, an anti-human CD30-directed antibody–drug conjugate, on an experimental model of RA. The results showed that BV successfully suppressed synovial inflammation and development of bone erosion of in CAIA mice treated with high dose BV (70 mg/kg). The beneficial effects of BV seen in CAIA model mice might be partially due to its pro-apoptotic effects on CD30 + FLS and plasma cells. Another possible mechanism would be the suppression of Th17 differentiation of T cells through the blockade of CD30L/CD30 signaling pathway. CD30 expressed by plasma cells or fibroblast-like synoviocytes could directly interact with CD30L on CD4 + T cells, activate CD30L/CD30 signalling pathway, and promote Th17 cell differentiation. Binding of BV might indirectly inhibit the T cell differentiation to proinflammatory Th17 cells. Barbieri et al. demonstrated the stimulation of CD30L with the CD30/Fc chimera, a molecule that behaves as sCD30, is able to favor the polarization of T cells towards a Th17 phenotype with proinflammatory features in RA [15]. They also found that the percentage of CD4 + CD30L + T cell is higher in synovial fluid than in PB samples in both RA patients suggesting CD30L is preferentially expressed by cells present at sites of inflammation. BV also might have a role in binding with sCD30 to block the CD30L/CD30 signaling pathway, thereby contributed to the amelioration of joint inflammation.
In contrast to the effect of high dose BV (70 mg/kg), our results showed that CAIA treated with low dose BV (30 mg/kg) significantly improved clinical scores of mice but failed to suppress the pathology of synovial inflammation and cartilage destruction. Even when the human clinical trial volume of BV of 0.1 to 3.6 mg/kg is converted into the mouse dose by body surface area, the maximum BV is about 45 mg/kg, and BV 70 mg/kg was an overdose. Previous reports have demonstrated that there were no side effects up to 30 mg/kg, but weight loss was reported above 40 mg/kg of BV in mice [43]. The possible reasons why low dose BV failed to show complete suppression of joint inflammation of CAIA mice might include the difference of pattern and amount of CD30 expression between human and mice, difference in sensitivity to BV toxicity, and route of BV administration. As the previous studies have shown high structural homology between human and mouse CD30 [44], we had expected that BV might work well in this murine model. The reason for the inadequate effect of the human dose equivalent of BV is that BV might not be able to completely neutralize CD30 in mice. In addition, CAIA can cause arthritis similar to CIA without the priming phase of the immune response when the anti-collagen antibody cocktail is given alone or combination with LPS. Therefore, in the early phase of CAIA model, T cell response, B cell response and plasma cell function might not largely related [45]. Experiment using other RA mice model such as CIA mice in which plasma cells are more involved in the onset or SCID mice engrafted with human RA tissue might add further information.
To summarize, CD30 was mainly expressed on plasma cells in human RA synovial tissue, and CD30 expression was increased in inflammatory cytokine-stimulated FLS. CD30 expressed on these cells and sCD30 might be the targets of anti-CD30 therapy. In vitro study showed that higher dose BV (70 mg/kg) significantly attenuated the severity of arthritis and improved histological findings in CAIA mice, however the effects of low dose BV (30 mg/kg) comparable to human clinical use were not sufficient. Further study would be needed to explore the efficacy of CDL/CD30 targeting therapy in experimental model of RA.
References
Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62:1576–82.
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001.
Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129–43.
Gottenberg JE, Brocq O, Perdriger A, Lassoued S, Berthelot JM, Wendling D, et al. Non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. JAMA. 2016;316:1172–80.
Smith CA, Gruss HJ, Davis T, Anderson D, Farrah T, Baker E, et al. CD30 antigen, a marker for Hodgkin’s lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349–60.
Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–58.
Kim WY, Nam SJ, Kim S, Kim TM, Heo DS, Kim C-W, et al. Prognostic implications of CD30 expression in extranodal natural killer/T-cell lymphoma according to treatment modalities. Leuk Lymphoma. 2015;56:1778–86.
Caligaris-Cappio F, Bertero MT, Converso M, Stacchini A, Vinante F, Romagnani S, et al. Circulating levels of soluble CD30, a marker of cells producing Th2-type cytokines, are increased in patients with systemic lupus erythematosus and correlate with disease activity. Clin Exp Rheumatol. 1995;13:339–43.
Bengtsson A, Holm L, Back O, Fransson J, Scheynius A. Elevated serum levels of soluble CD30 in patients with atopic dermatitis (AD). Clin Exp Immunol. 1997;109:533–7.
Heshmat NM, El-Hadidi ES. Soluble CD30 serum levels in atopic dermatitis and bronchial asthma and its relationship with disease severity in pediatric age. Pediatr Allergy Immunol. 2006;17:297–303.
Pizzolo G, Vinante F, Nadali G, Krampera M, Morosato L, Chilosi M, et al. High serum level of soluble CD30 in acute primary HIV-1 infection. Clin Exp Immunol. 1997;108:251–3.
Gerli R, Muscat C, Bistoni O, Falini B, Tomassini C, Agea E, et al. High levels of the soluble form of CD30 molecule in rheumatoid arthritis (RA) are expression of CD30+ T cell involvement in the inflamed joints. Clin Exp Immunol. 1995;102:547–50.
Gerli R, Pitzalis C, Bistoni O, Falini B, Costantini V, Russano A, et al. CD30+ T cells in rheumatoid synovitis: mechanisms of recruitment and functional role. J Immunol. 2000;164:4399–407.
Tinazzi E, Barbieri A, Rigo A, Patuzzo G, Beri R, Gerli R, et al. In rheumatoid arthritis soluble CD30 ligand is present at high levels and induces apoptosis of CD30+ T cells. Immunol Lett. 2014;161:236–40.
Barbieri A, Dolcino M, Tinazzi E, Rigo A, Argentino G, Patuzzo G, et al. Characterization of CD30/CD30L+ cells in peripheral blood and synovial fluid of patients with rheumatoid arthritis. J Immunol Res. 2015; 2015:729654.
Sun X, Yamada H, Shibata K, Muta H, Tani K, Podack ER, et al. CD30 ligand/CD30 plays a critical role in Th17 differentiation in mice. J Immunol. 2010;185:2222–30.
Shinoda K, Sun X, Oyamada A, Yamada H, Muta H, Podack ER, et al. CD30 ligand is a new therapeutic target for central nervous system autoimmunity. J Autoimmun. 2015;57:14–23.
Somada S, Muta H, Nakamura K, Sun X, Honda K, Ihara E, et al. CD30 ligand/CD30 interaction is involved in pathogenesis of inflammatory bowel disease. Dig Dis Sci. 2012;57:2031–7.
Artinger K, Kirsch AH, Mooslechner AA, Cooper DJ, Aringer I, Schuller M, et al. Blockade of tumor necrosis factor superfamily members CD30 and OX40 abrogates disease activity in murine immune-mediated glomerulonephritis. Kidney Int. 2021;100(2):336–48.
Katz J, Janik JE, Younes A. Brentuximab vedotin (SGN-35). Clin Cancer Res. 2011;17:6428–36.
Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–21.
Sekiguchi Y, Iizuka H, Takizawa H, Sugimoto K, Sakajiri S, Inano T, et al. A case of methotrexate-associated Hodgkin lymphoma in a patient with rheumatoid arthritis successfully treated with brentuximab vedotin in combination with doxorubicin, vinblastine, and dacarbazine (BV+ AVD). Intern Med. 2020;59(17):2165-71.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis & Rheum. 1986;29:1039–49.
Nishida K, Komiyama T, Miyazawa S, Shen ZN, Furumatsu T, Doi H, et al. Histone deacetylase inhibitor suppression of autoantibody-mediated arthritis in mice via regulation of p16INK4a and p21(WAF1/Cip1) expression. Arthritis Rheum. 2004;50:3365–76.
Hutamekalin P, Saito T, Yamaki K, Mizutani N, Brand DD, Waritani T, et al. Collagen antibody-induced arthritis in mice: development of a new arthritogenic 5-clone cocktail of monoclonal anti-type II collagen antibodies. J Immunol Methods. 2009;343:49–55.
Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–8.
Sancho D, Gómez M, Viedma F, Esplugues E, Gordón-Alonso M, García-López MA, et al. CD69 downregulates autoimmune reactivity through active transforming growth factor-β production in collagen-induced arthritis. J Clin Investig. 2003;112:872–82.
Gerli R, Lunardi C, Bocci EB, Bobbio-Pallavicini F, Schillaci G, Caporali R, et al. Anti-tumor necrosis factor-alpha response in rheumatoid arthritis is associated with an increase in serum soluble CD30. J Rheumatol. 2008;35:14–9.
Ulusoy H, Kamanli A, Ilhan N, Kuru O, Arslan S, Alkan G, et al. Serum levels of soluble CD26 and CD30 and their clinical significance in patients with rheumatoid arthritis. Rheumatol Int. 2012;32:3857–62.
Gerli R, Lunardi C, Vinante F, Bistoni O, Pizzolo G, Pitzalis C. Role of CD30+ T cells in rheumatoid arthritis: a counter-regulatory paradigm for Th1-driven diseases. Trends Immunol. 2001;22:72–7.
Sun X, Yamada H, Shibata K, Muta H, Tani K, Podack ER, et al. CD30 ligand is a target for a novel biological therapy against colitis associated with Th17 responses. J Immunol. 2010;185:7671–80.
Durkop H, Foss HD, Eitelbach F, Anagnostopoulos I, Latza U, Pileri S, et al. Expression of the CD30 antigen in non-lymphoid tissues and cells. J Pathol. 2000;190:613–8.
Andreesen R, Brugger W, Löhr G, Bross K. Human macrophages can express the Hodgkin’s cell-associated antigen Ki-1 (CD30). Am J Pathol. 1989;134:187.
Agrawal B, Reddish M, Longenecker BM. CD30 expression on human CD8+ T cells isolated from peripheral blood lymphocytes of normal donors. J Immunol. 1996;157:3229–34.
Berro AI, Perry GA, Agrawal DK. Increased expression and activation of CD30 induce apoptosis in human blood eosinophils. J Immunol. 2004;173:2174–83.
Shanebeck KD, Maliszewski CR, Kennedy MK, Picha KS, Smith CA, Goodwin RG, et al. Regulation of murine B cell growth and differentiation by CD30 ligand. Eur J Immunol. 1995;25:2147–53.
Kim H-J, Krenn V, Steinhauser G, Berek C. Plasma cell development in synovial germinal centers in patients with rheumatoid and reactive arthritis. J Immunol. 1999;162:3053–62.
Moller P, Matthaei-Maurer DU, Moldenhauer G. CD30(Ki-1) antigen expression in a subset of gastric mucosal plasma cells and in a primary gastric plasmacytoma. Am J Clin Pathol. 1989;91:18–23.
Nygaard G, Firestein GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol. 2020;16:316–33.
Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–10.
Eichenauer DA, Simhadri VL, von Strandmann EP, Ludwig A, Matthews V, Reiners KS, et al. ADAM10 inhibition of human CD30 shedding increases specificity of targeted immunotherapy in vitro. Can Res. 2007;67:332–8.
Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–65.
Bowen MA, Lee RK, Miragliotta G, Nam SY, Podack ER. Structure and expression of murine CD30 and its role in cytokine production. J Immunol. 1996;156:442–9.
Nandakumar KS, Holmdahl R. Antibody-induced arthritis: disease mechanisms and genes involved at the effector phase of arthritis. Arthritis Res Ther. 2007;8:1–11.
Acknowledgements
Approval was obtained from the ethics committee of Okayama University Graduate School of Medicine (Approval No. 1712-026). The procedures used in this study adhered to the tenets of the Declaration of Helsinki. All patients gave informed consent to take part in the study. All the animal experiments were approved by the Animal Care and Use Committee, Okayama University (Approval No. OKU-2018446) and carried out in accordance with relevant guidelines and regulations.
Funding
This research was supported by JSPS KAKENHI under Grant Number JP20K09433. Partial financial support was received from Eisai Co., Ltd. (Grant number: HHCS20180824007), AbbVie GK (Grant number: 793), Mitsubishi Tanabe Pharma Corp. (Grant number: MTPS20190610039), and Chugai Pharmaceutical Co., Ltd (Grant number: AC-1-20200621233833-796001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsuhashi, M., Nishida, K., Sakamoto, M. et al. CD30-targeted therapy induces apoptosis of inflammatory cytokine-stimulated synovial fibroblasts and ameliorates collagen antibody-induced arthritis in mice. Inflamm. Res. 71, 215–226 (2022). https://doi.org/10.1007/s00011-021-01537-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01537-z