Abstract
Tristetraprolin (TTP) is an anti-inflammatory molecule known to post-transcriptionally regulate cytokine production and is, therefore, an attractive drug target for chronic respiratory diseases driven by inflammation, such as asthma and chronic obstructive pulmonary disease. Our recent in vitro studies in primary human airway smooth (ASM) cells have confirmed the essential anti-inflammatory role played by TTP as a critical partner in a cytokine regulatory network. However, several unanswered questions remain. While prior in vitro studies have suggested that TTP is regulated in a cAMP-mediated manner, raising the possibility that this may be one of the ways in which β2-agonists achieve beneficial effects beyond bronchodilation, the impact of β2-agonists on ASM cells is unknown. Furthermore, the effect of prostaglandin E2 (PGE2) on TTP expression in ASM cells has not been reported. We address this herein and reveal, for the first time, that TTP is not regulated by cAMP-activating agents nor following treatment with long-acting β2-agonists. However, PGE2 does induce TTP mRNA expression and protein upregulation in ASM cells. Although the underlying mechanism of action remains undefined, we can confirm that PGE2-induced TTP upregulation is not mediated via cAMP, or EP2/EP4 receptor activation, and occurred in a manner independent of the p38 MAPK-mediated pathway. Taken together, these data confirm that β2-agonists do not upregulate TTP in human ASM cells and indicate that another way in which PGE2 may achieve beneficial effects in asthma and COPD may be via upregulation of the master controller of inflammation—TTP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tristetraprolin [TTP; zinc-finger protein 36 (Zfp36)] is an anti-inflammatory protein that functions to induce mRNA decay of a number of critical molecules, including cytokines that drive pathogenesis of respiratory disorders [1]. Our recent studies have demonstrated that TTP plays an essential anti-inflammatory and pro-resolving role in airway disease by serving as a critical partner in a cytokine regulatory network [with p38 MAPK and mitogen-activated protein kinase (MKP-1) phosphatase] [2,3,4]. We have utilized a key airway structural cell responsible for both bronchomotor tone and inflammation—human primary airway smooth muscle (ASM) cells—to show that TTP operates as a negative feedback effector, controlling the extent and duration of inflammation [2, 4]. Moreover, we have revealed that one of the important mechanisms by which the first-line anti-inflammatory therapies used in chronic respiratory diseases—corticosteroids—exert their anti-inflammatory impact is via a MKP-1/TTP-dependent pathway [4]. To date, however, whether β2-agonists also act on TTP in ASM is unknown.
β2-agonists are widely used medicines in respiratory disease. They potently and efficaciously increase cAMP in airway smooth muscle to reverse bronchoconstriction in asthma and alleviate breathlessness in chronic obstructive pulmonary disease (COPD). It has been known for some time that β2-agonists have beneficial effects beyond bronchodilation and that they exert anti-inflammatory actions in a cAMP-dependent manner [5]. We have shown that this beneficial impact is due in part to the upregulation of MKP-1; that is, in ASM cells, cAMP-elevating agents (including β2-agonists) can also increase the expression of anti-inflammatory molecules (MKP-1) to repress inflammation [6,7,8,9]. But what is the effect of cAMP-elevating agents on TTP in ASM cells? This is currently unknown. cAMP has been shown to increase expression of TTP in macrophages, and through this pathway, the β2-agonist salbutamol was proposed to have beneficial effects in airway inflammatory disease [10]. In other disease contexts, including obesity [11], cAMP was shown to increase TTP expression in adipocytes. Thus, it was of interest to examine the effect of β2-agonist on TTP expression in ASM cells and whether cAMP was an inducing stimulus.
Coupled to this is the need to explore the impact of the prostanoid PGE2 on TTP expression and the potential involvement of cAMP in mediating the response. PGE2 is a potent prostanoid that has both positive and negative effects in airway diseases (see recent review [12]). PGE2 acts via a family of prostanoid E (EP) receptors (EP1- EP4). EP2 and EP4 receptors exert their effects via cAMP-mediated signaling pathways [12] and we have shown that the prostanoid PGE2 dose-dependently increases cAMP production in ASM cells [13]. We recently published [14] that PGE2 upregulates mRNA expression and protein production of the anti-inflammatory molecule – MKP-1, in a dose-dependent manner. However, the effect of PGE2 on TTP in ASM cells is unknown, and potentially, this could occur via cAMP-dependent EP2/EP4 receptor pathways.
To determine the role that cAMP plays in regulating TTP expression in ASM cells, we first examined the direct and indirect activation of cAMP to increase TTP mRNA expression. Second, we investigated receptor-mediated pathways and induced production of cAMP as a secondary messenger by activation of Gs-coupled protein receptors (β2-adrenergic receptors and EP2/EP4 prostanoid receptors) with cognate ligands β2-agonists and prostaglandin E2 (PGE2), respectively. In all cases, MKP-1 was used as a positive control as it was well established as being upregulated by cAMP [6,7,8,9, 15]. Finally, we investigated the underlying signaling pathways involved. Collectively, our data clearly showed that cAMP-elevating agents, including β2-agonists, were unable to upregulate TTP mRNA expression in human ASM cells, but that PGE2-induced TTP mRNA expression and protein upregulation occur in a cAMP-, EP2/EP4 receptor-, and p38 MAPK-independent pathway.
Materials and methods
Chemicals
PGE2, PF-04418948, and GW 627368X were purchased from the Cayman Chemical Company (Ann Arbor, MI, USA). Unless otherwise specified, all chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Human bronchi were obtained from patients undergoing surgical resection for carcinoma or lung transplant donors in accordance with procedures approved by the Sydney South West Area Health Service and the Human Research Ethics Committee of the University of Sydney. ASM cells were dissected and purified as previously described by Johnson et al. [16]. A minimum of three different ASM primary cell lines was used for each experiment.
Real-time RT-PCR
Total RNA was extracted using the RNeasy Mini Kit (Qiagen Australia, Doncaster, VIC, Australia) and reverse transcription performed using the RevertAid First strand cDNA Synthesis kit (Fermentas Life Sciences, Hanover, MD, USA) according to the manufacturer’s protocol. Real-time RT-PCR was performed on an ABI Prism 7500 with TTP (Zfp36, Hs00185658_m1) and MKP-1 (DUSP1: Hs00610256_g1) TaqMan gene expression assays and the eukaryotic 18S rRNA endogenous control probe (Applied Biosystems, Foster City, CA) subjected to the following cycle parameters: 50 °C for 2 min, 1 cycle; 95 °C for 10 min, 1 cycle; 95 °C for 15 s, 60 °C for 1 min, 40 cycles and mRNA expression quantified by delta–delta Ct calculations.
Western blotting
TTP was measured by Western blotting using rabbit antisera against TTP (Sak21). Detection of α-tubulin was used as the loading control (mouse monoclonal IgG1, DM1A: Santa Cruz Biotechnology, Santa Cruz, CA, USA). p38 MAPK was detected using rabbit monoclonal or polyclonal antibodies against phosphorylated (Thr180/Tyr182) and total p38 MAPK (Cell Signaling Technology, Danvers, MA, USA). Primary antibodies were detected with goat anti-rabbit and anti-mouse HRP-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA, USA) and visualized by enhanced chemiluminescence (PerkinElmer, Wellesley, MA, USA).
Statistical analysis
Statistical analysis was performed using the Student’s unpaired t test or one-way ANOVA and then Fisher’s PLSD post-test. P values < 0.05 were sufficient to reject the null hypothesis for all analyses.
Results
Lack of effect of cell-permeable cAMP or adenylate cyclase activation on TTP mRNA expression, compared to MKP-1
Our early studies in ASM cells have shown that we can directly increase cAMP in ASM cells through treatment of the cell-permeable analogue, dibutyryl cAMP, or indirectly via activation of adenylate cyclase, with forskolin [13]. To examine the effect of these cAMP-elevating agents on TTP mRNA expression, growth-arrested cells were treated for 1 h with 1 mM dibutyryl cAMP or 10 µM forskolin and RNA extracted. TTP mRNA was measured and compared to MKP-1 mRNA as a positive control [6, 8]. As shown in Fig. 1, both dibutyryl cAMP and forskolin induced MKP-1 mRNA expression in accordance with our previous publications [6, 8]. However, the cAMP-elevating agents did not increase TTP mRNA expression in ASM cells (Fig. 1).
Lack of effect of cell-permeable cAMP or adenylate cyclase activation on TTP mRNA expression, compared to MKP-1. Growth-arrested ASM cells were treated with vehicle, dibutyryl cAMP (1 mM), or forskolin (10 µM) for 1 h. TTP and MKP-1 mRNA expression was quantified by real-time RT-PCR (results expressed as fold increase compared to vehicle-treated cells). Statistical analysis was performed using the Student’s unpaired t test [where * denotes a significant increase (P < 0.05)]. Data are mean + SEM values from n = 8 primary ASM cell cultures
Long-acting β2-agonists salmeterol and formoterol do not upregulate TTP mRNA expression, while MKP-1 is induced
In ASM cells, cAMP can also be elevated by the activation of Gs-coupled proteins of the β2-adrenergic receptors. We then examined the impact of two β2-agonists, salmeterol (100 nM) and formoterol (10 nM), on TTP mRNA expression in ASM cells. These studies were performed in parallel with the measurement of MKP-1 mRNA expression. In confirmation of our prior studies [8, 9], cAMP-mediated MKP-1 was significantly upregulated with salmeterol (P < 0.05: Fig. 2a), while TTP mRNA expression was not induced. Similarly, formoterol significantly increased MKP-1 mRNA expression by 11.5 ± 4.8-fold (P < 0.05: Fig. 2b), yet TTP was unaffected.
Long-acting β2-agonists salmeterol and formoterol do not upregulate TTP mRNA expression, while MKP-1 is induced. Growth-arrested ASM cells were treated with a salmeterol (100 nM) or b formoterol (10 nM) for 1 h, compared to vehicle. TTP and MKP-1 mRNA expression was quantified by real-time RT-PCR (results expressed as fold increase compared to vehicle-treated cells). Statistical analysis was performed using the Student’s unpaired t test [where * denotes a significant increase (P < 0.05)]. Data are mean + SEM values from an = 5 and bn = 7 primary ASM cell cultures
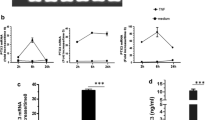
PGE2 induces MKP-1 and TTP mRNA expression in a temporally specific manner
To address the impact of PGE2 on TTP mRNA expression, we performed a temporal analysis of the effect of PGE2 (100 nM) on TTP mRNA expression in ASM cells. This was compared to a complete time-course for MKP-1 mRNA expression over a 24 h period, as our previous publication [14] solely focussed on MKP-1 mRNA expression at one time point only [i.e., 1 h, where 100 nM PGE2 increased MKP-1 mRNA by 11.9 ± 3.3-fold (P < 0.05)]. As shown in Fig. 3a, 100 nM PGE2 induced a rapid and significant peak of MKP-1 mRNA expression at 1 h (14.8 ± 4.1-fold: P < 0.05) and remained significantly upregulated at 2 h, but then subsided at 4 h and remained at basal levels at 8 h and 24 h. Interestingly, in Fig. 3b, we show, for the first time, in ASM cells, that PGE2 induced a small, but significant, increase in the expression of TTP mRNA at 1 h (P < 0.05).
PGE2 induces MKP-1 and TTP mRNA expression in a temporally specific manner. Growth-arrested ASM cells were treated with PGE2 (100 nM) for 0, 1, 2, 4, 8, and 24 h. a MKP-1 and b TTP mRNA expression was quantified by real-time RT-PCR (results expressed as fold increase compared to vehicle-treated cells at 0 h). Statistical analysis was performed using one-way ANOVA and then Fisher’s PLSD post-test [where * denotes a significant increase compared to 0 h (P < 0.05)]. Data are mean + SEM values from n = 6 primary ASM cell cultures
PGE2 increases TTP protein upregulation
We then performed an additional experimentation to examine the temporal kinetics of PGE2-induced TTP protein upregulation. Growth-arrested ASM cells were treated with PGE2 for 0, 0.25, 0.5, 1, 2, 4, 8, and 24 h and Western blotting for TTP performed. As shown in Fig. 4a, PGE2 induced an increase in immunoreactive bands detected with the rabbit antisera Sak21. Our previous studies (with tumour necrosis factor as the stimulus [17]) revealed that this antisera detects TTP and that the change in electrophoretic mobility is indicative of phosphorylation. Although an oversimplification, we have indicated on Fig. 4a that there are two bands of immunoreactivity for TTP: bands at higher molecular weight are stable phosphorylated TTP, while lower bands are unphosphorylated but unstable TTP. Our results in Fig. 4a show that there is temporally specific increase in TTP phospho-forms in ASM cells after stimulation with PGE2. These data were analyzed with densitometry (where all TTP phospho-forms were normalized to α-tubulin and compared to 0 h) to show that PGE2 significantly induced TTP protein upregulation at 2, 4, and 8 h (Fig. 4b: P < 0.05).
PGE2 increases TTP protein upregulation. Growth-arrested ASM cells were treated with PGE2 (100 nM) for 0, 0.25, 0.5, 1, 2, 4, 8, and 24 h. Western blotting for TTP was performed (compared to α-tubulin as loading control). Please note two bands of immunoreactivity for TTP: bands at higher molecular weight indicate stable phosphorylated TTP (inactive), while lower bands are unphosphorylated (active) but unstable. Results are a representative Western blots or b densitometric analysis [normalized to α-tubulin and expressed as PGE2-induced TTP protein upregulation (results expressed as fold increase compared to vehicle-treated cells at 0 h)]. Statistical analysis was performed using one-way ANOVA and then Fisher’s PLSD post-test [where * denotes a significant increase compared to 0 h (P < 0.05)]. Data are mean + SEM values from n = 4 primary ASM cell cultures
Effect of EP2/EP4 receptor antagonists on PGE2-induced TTP and MKP-1 mRNA expression
We were then interested to explore the molecular mechanisms responsible for PGE2-induced TTP expression. As mentioned, PGE2 can induce cAMP in ASM cells [13], and it is known that EP2 and EP4 receptors exert their effects via cAMP-mediated signaling pathways [12]. Next step, therefore, was to examine the potential involvement of these receptor-mediated pathways, although earlier studies with dibutyryl cAMP and β2-agonists predict that PGE2-induced TTP mRNA expression will not be induced via this pathway. We utilized the specific EP2 and EP4 receptor antagonists PF-04418948 and GW 627368X, respectively. ASM cells were pretreated with either 1 µM PF-04418948 or GW 627368X prior to PGE2 (100 nM) stimulation. RNA was extracted at indicated time points over a 24 h period and TTP (Fig. 5a) and positive control MKP-1 mRNA expression (Fig. 5b) measured. PF-04418948 or GW 627368X had no effect on TTP mRNA expression at any time point following PGE2 stimulation (Fig. 5a). We observed a significant repression of PGE2-induced MKP-1 mRNA expression with 1 µM PF-04418948, but not GW 627368X, at 4 h and 8 h (Fig. 5b, P < 0.05), suggesting that MKP-1 mRNA is upregulated via downstream signaling of the EP2 receptor (in confirmation of our earlier study [14]).
Effect of EP2/EP4 receptor antagonists on PGE2-induced TTP and MKP-1 mRNA expression. Growth-arrested ASM cells were pretreated for 30 min with vehicle, 1 µM PF-04418948 (EP2 receptor antagonist), or GW 627368X (EP4 receptor antagonist), and then treated with PGE2 (100 nM) for 0, 1, 2, 4, 8, and 24 h. a TTP and b MKP-1 mRNA expression was quantified by real-time RT-PCR (results expressed as fold difference to vehicle-treated cells at 0 h). Statistical analysis was performed using the Student’s unpaired t test [where * denotes significant repression by the EP2 receptor antagonist PF-04418948 at the same time point (P < 0.05)]. Data are mean + SEM values from n = 6 primary ASM cell cultures
PGE2 increases TTP mRNA expression and protein upregulation in a p38 MAPK-independent manner
Taken together, our data thus far show that PGE2-induced TTP mRNA expression and protein upregulation occur in a cAMP-independent manner. When then turned to examining the role of the p38 MAPK pathway as this MAPK phosphoprotein family member is known to play a critical role in TTP expression, upregulation, and activity (reviewed in [1]). First, to confirm that PGE2 induces MAPK phosphorylation in ASM, cells were treated with PGE2 for 30 min and p38 MAPK phosphorylation detected by Western blotting (Fig. 6a). As shown by densitometric analysis (Fig. 6b), PGE2 significantly increased p38 MAPK phosphorylation by 4.8 ± 1.5-fold (P < 0.05). Then, to examine whether PGE2-induced TTP mRNA expression (at 1 h) and subsequent TTP protein upregulation (at 2 h) were induced via a p38 MAPK-mediated pathway, ASM cells were pretreated with the p38 MAPK inhibitor SB203580 (1 µM) for 30 min prior to real-time RT-PCR for TTP (Fig. 5c) and Western blotting for TTP (Fig. 5d, e). There was no effect of SB203580, and thus, our studies revealed that PGE2 increases TTP mRNA expression and protein upregulation in a p38 MAPK-independent manner.
PGE2 increases TTP mRNA expression and protein upregulation in a p38 MAPK-independent manner. a, b Growth-arrested ASM cells were treated with PGE2 (100 nM) and p38 MAPK phosphorylation detected by Western blotting (compared to total p38 MAPK as loading control) at 0 min and 30 min. Results are a representative Western blots or b densitometric analysis (normalized to p38 MAPK and expressed as PGE2-induced p38 MAPK phosphorylation (expressed as fold increase compared to 0 min)). c–e Growth-arrested ASM cells were pretreated with the p38 MAPK inhibitor SB203580 (1 µM) for 30 min then treated with PGE2 (100 nM). c After 1 h, TTP mRNA expression was quantified by real-time RT-PCR (results expressed as percentage of PGE2-induced TTP mRNA expression). d, e After 2 h, Western blotting for TTP was performed (compared to α-tubulin as loading control). Results are d representative Western blots or e densitometric analysis (normalized to α-tubulin and expressed as percentage of PGE2-induced TTP protein upregulation). Statistical analysis was performed using one-way ANOVA and then Fisher’s PLSD post-test [where * denotes a significant increase (P < 0.05)]. Data are mean + SEM values from n = 4 primary ASM cell cultures
Discussion
There are two notable findings from this study. First, that TTP is not upregulated by cAMP in ASM cells. Elevating cAMP in ASM cells via cell-permeable analogies of adenylate cyclase inhibitors did not induce TTP mRNA expression, nor did receptor-mediated induction of cAMP via β2-adrenergic receptor ligand engagement with salmeterol and formoterol. These studies are the first in ASM cells and demonstrate that the impact of cAMP on TTP is cell-type specific. Second, that PGE2 induced TTP mRNA expression and protein upregulation in ASM cells in a time-dependent manner. Given that it is known that PGE2 induces cAMP and p38 MAPK phosphorylation in ASM cells, two signaling molecules known to regulate TTP, we then examined the involvement of these pathways in the impact of PGE2 in ASM cells. We explored this using pharmacological antagonists of the EP2 and EP4 prostanoid receptors (Gs-coupled E prostanoid receptors that mediate via cAMP), where PF-04418948 (EP2 receptor antagonist) or GW 627368X (EP4 receptor antagonist) had no effect on PGE2-induced TTP mRNA expression. Moreover, a pharmacological inhibitor of the p38 MAPK pathway was also without impact on PGE2-induced TTP mRNA expression and protein upregulation. These data support the notion that upregulation of TTP after PGE2 stimulation in ASM cells occurs in a manner independent of cAMP and p38 MAPK.
This publication adds to the earlier research performed by the Moilanen group [10, 18] that explored the impact of cAMP on TTP expression in cell types apart from ASM. In 2007, Jalonen et al. [10] utilized J774 murine macrophages and THP-1 human macrophages to show that cAMP analogues, forskolin and the short-acting β2-agonist, salbutamol, increased TTP mRNA and protein expression. The authors then went on to demonstrate that compounds that mimic or elevate cAMP, when added with inflammatory stimuli such as lipopolysaccharide, can enhance TTP protein degradation in J774 murine macrophages [18]. The latter study [18] was amongst the first to underscore the complexities of TTP expression, protein activation, and control by the proteasome, and the precise temporal regulation exerted by TTP in concert with p38 MAPK and MKP-1 (reviewed in [1, 19]). In our current study, we demonstrate that, in contrast to Jalonen et al. [10], none of the cAMP-elevating agents tested (i.e., dibutyryl cAMP, forskolin, salmeterol, or formoterol) increase TTP mRNA expression in human ASM cells. Notably, other studies that show that cAMP increases TTP were performed in mouse 3T3 fibroblasts [20], rat pheochromocytoma cells [21], and 3T3-L1 adipocytes [11], suggesting that, perhaps, the cAMP dependence of TTP is cell type and/or species specific. It is also important to note that Jalonen et al. demonstrated that murine macrophages were more sensitive to the effects of cAMP than human macrophages [10]. Moreover, the mechanism responsible for TTP upregulation by cAMP warrants further investigation as Rajah et al. [22] recently commented that classical cAMP response element was not identified within the − 2000 base-pair upstream of the transcriptional start site of TTP.
Our current study demonstrated that TTP mRNA expression is induced by PGE2, via a mechanism independent of cAMP in ASM cells. TTP mRNA is rapidly and significantly upregulated (at 1 h), but is unaffected by the EP2 and EP4 receptors that signal via cAMP. In contrast, MKP-1 is cAMP-dependent and is upregulated (as expected [14]) at 1 h by PGE2 and is inhibited by the EP2 receptor antagonist PF-04418948, especially at later time points. We, therefore, examined whether TTP induction by PGE2 may occur in a p38 MAPK-dependent manner, as we have previously published that TTP mRNA expression in ASM cells is p38 MAPK-mediated [2]. Intriguingly, although PGE2 was shown to increase p38 MAPK phosphorylation, there was no repressive effect of a p38 MAPK inhibitor on PGE2-induced TTP mRNA expression and protein regulation. Unanswered by this study is the mechanism by which PGE2 induces TTP. Other signaling molecules may be responsible, for example phosphoinositide-3-kinase, both known to be activated by PGE2 [23] and involved in TTP regulation [24].
These data build our understanding of the regulation of anti-inflammatory proteins (TTP and MKP1) in ASM cells and the impact of the prostanoid PGE2 upon the dynamic regulatory network known to exert significant repression on cytokine secretion in a post-transcriptional manner [2, 4]. In support, a recent study from the Clark lab [25] showed that PGE2 negatively regulates cytokine expression post-transcriptionally via induction of MKP-1 and enhancement of TTP anti-inflammatory function. Further studies are warranted.
In summary, our study shows that, in contrast to earlier studies (predominately performed in rodent cell types), cAMP does not directly induce TTP mRNA expression in primary cell cultures of human ASM. Thus, the mechanism by which β2-agonists regulate cytokine expression and display anti-inflammatory properties in airway inflammation may not be via TTP mRNA expression. In addition, we revealed a hitherto unrecognized role for PGE2 as a stimulus for TTP upregulation in ASM cells, highlighting a novel mechanism by which the prostanoid may exert anti-inflammatory actions in respiratory disease.
References
Prabhala P, Ammit A. Tristetraprolin and its role in regulation of airway inflammation. Mol Pharmacol. 2015;87:629–38.
Prabhala P, Bunge K, Rahman MM, Ge Q, Clark AR, Ammit AJ. Temporal regulation of cytokine mRNA expression by tristetraprolin: dynamic control by p38 MAPK and MKP-1. Am J Physiol Lung Cell Mol Physiol. 2015;308:L973-80.
Rahman MM, Rumzhum NN, Morris JC, Clark AR, Verrills NM, Ammit AJ. Basal protein phosphatase 2A activity restrains cytokine expression: role for MAPKs and tristetraprolin. Scientific reports. 2015;5:10063.
Prabhala P, Bunge K, Ge Q, Ammit AJ. Corticosteroid-Induced. MKP-1 represses pro-inflammatory cytokine secretion by enhancing activity of tristetraprolin (TTP) in ASM cells. J Cell Physiol. 2016;231:2153–8.
Giembycz MA, Kaur M, Leigh R, Newton R. A Holy Grail of asthma management: toward understanding how long-acting beta(2)-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. Br J Pharmacol 2007.
Che W, Manetsch M, Quante T, Rahman MM, Patel BS, Ge Q, et al. Sphingosine 1-phosphate induces MKP-1 expression via p38 MAPK- and CREB-mediated pathways in airway smooth muscle cells. Biochim Biophys Acta. 2012;1823:1658–65.
Manetsch M, Rahman MM, Patel BS, Ramsay EE, Rumzhum NN, Alkhouri H, et al. Long-acting beta2-agonists increase fluticasone propionate-induced mitogen-activated protein kinase phosphatase 1 (MKP-1) in airway smooth muscle cells. PLoS One. 2013;8:e59635.
Patel BS, Prabhala P, Oliver BG, Ammit AJ. Inhibitors of phosphodiesterase 4, but not phosphodiesterase 3, increase beta2-agonist-induced expression of antiinflammatory mitogen-activated protein kinase phosphatase 1 in airway smooth muscle cells. Am J Respir Cell Mol Biol. 2015;52:634–40.
Patel BS, Rahman MM, Baehring G, Xenaki D, Tang FS, Oliver BG, et al. Roflumilast N-oxide in combination with formoterol enhances the antiinflammatory effect of dexamethasone in airway smooth muscle cells. Am J Respir Cell Mol Biol. 2017;56:532–8.
Jalonen U, Leppanen T, Kankaanranta H, Moilanen E. Salbutamol increases tristetraprolin expression in macrophages. Life Sci. 2007;81:1651–8.
Brahma PK, Zhang H, Murray BS, Shu FJ, Sidell N, Seli E, et al. The mRNA-binding protein Zfp36 is upregulated by beta-adrenergic stimulation and represses IL-6 production in 3T3-L1 adipocytes. Obesity. 2012;20:40–7.
Lebender LF, Prunte L, Rumzhum NN, Ammit AJ. Selectively targeting prostanoid E (EP) receptor-mediated cell signalling pathways: Implications for lung health and disease. Pulm Pharmacol Ther. 2018;49:75–87.
Ammit AJ, Hoffman RK, Amrani Y, Lazaar AL, Hay DWP, Torphy TJ, et al. TNFa-induced secretion of RANTES and IL-6 from human airway smooth muscle cells: modulation by cAMP. Am J Respir Cell Mol Biol. 2000;23:794–802.
Rumzhum NN, Ammit AJ. Prostaglandin E2 induces expression of MAPK phosphatase 1 (MKP-1) in airway smooth muscle cells. Eur J Pharmacol. 2016;782:1–5.
Kwak SP, Hakes DJ, Martell KJ, Dixon JE. Isolation and characterization of a human dual specificity protein-tyrosine phosphatase gene. J Biol Chem. 1994;269:3596–604.
Johnson PR, McKay KO, Armour CL, Black JL. The maintenance of functional activity in human isolated bronchus after cryopreservation. Pulm Pharmacol. 1995;8:43–7.
Rahman MM, Rumzhum NN, Hansbro PM, Morris JC, Clark AR, Verrills NM, et al. Activating protein phosphatase 2A (PP2A) enhances tristetraprolin (TTP) anti-inflammatory function in A549 lung epithelial cells. Cell Signal. 2016;28:325–34.
Jalonen U, Paukkeri EL, Moilanen E. Compounds that increase or mimic cyclic adenosine monophosphate enhance tristetraprolin degradation in lipopolysaccharide-treated murine j774 macrophages. J Pharmacol Exp Ther. 2008;326:514–22.
O’Neil JD, Ammit AJ, Clark AR. MAPK p38 regulates inflammatory gene expression via tristetraprolin: doing good by stealth. Int J Biochem Cell Biol. 2018;94:6–9.
DuBois RN, McLane MW, Ryder K, Lau LF, Nathans D. A growth factor-inducible nuclear protein with a novel cysteine/histidine repetitive sequence. J Biol Chem. 1990;265:19185–91.
Kaneda N, Oshima M, Chung SY, Guroff G. Sequence of a rat TIS11 cDNA, an immediate early gene induced by growth factors and phorbol esters. Gene. 1992;118:289–91.
Rataj F, Planel S, Desroches-Castan A, Le Douce J, Lamribet K, Denis J, et al. The cAMP pathway regulates mRNA decay through phosphorylation of the RNA-binding protein TIS11b/BRF1. Mol Biol Cell. 2016;27:3841–54.
Fujino H, Salvi S, Regan JW. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol Pharmacol. 2005;68:251–9.
Venigalla RKC, Turner M. RNA-binding proteins as a point of convergence of the PI3K and p38 MAPK pathways. Front Immunol. 2012;3:398–8.
Tang T, Scambler TE, Smallie T, Cunliffe HE, Ross EA, Rosner DR, et al. Macrophage responses to lipopolysaccharide are modulated by a feedback loop involving prostaglandin E2, dual specificity phosphatase 1 and tristetraprolin. Sci Rep. 2017;7:4350.
Acknowledgements
Funded by the: Woolcock Emphysema Centre; National Health and Medical Research Council of Australia; Centre for Health Technologies, Faculty of Science, University of Technology Sydney; and the Rebecca Cooper Medical Research Foundation. The authors wish to thank our colleagues at the Woolcock Institute of Medical Research (especially Dikaia Xenaki) and acknowledge the collaborative effort of the cardiopulmonary transplant team and the pathologists at St Vincent’s Hospital, Sydney, and the thoracic physicians and pathologists at Royal Prince Alfred Hospital, Concord Repatriation Hospital and Strathfield Private Hospital and Healthscope Pathology, Sydney.
Author information
Authors and Affiliations
Contributions
Conceived, designed, and performed the experiments: PB, BSP, AC, CPN, and AJA. Provision of ASM cells: BGO. Analysis and interpretation: PB and AJA. Wrote the paper: AJA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bradbury, P., Patel, B.S., Cidem, A. et al. Prostaglandin E2, but not cAMP nor β2-agonists, induce tristetraprolin (TTP) in human airway smooth muscle cells. Inflamm. Res. 68, 369–377 (2019). https://doi.org/10.1007/s00011-019-01224-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01224-0