Abstract
Background
Previous studies showed that CD4+ T cells play a critical role in Con A-induced hepatitis in wild-type mice. However, the role of CD8+ T cells in the setting of Con A-induced hepatitis is enigmatic. The aim of study is to investigate the function of CD8+ T cells in the context of Con-A-induced hepatitis.
Materials and subjects
Two different mouse models of Con A-induced hepatitis, T cell-transferred Rag2−/− mice and wild-type C57BL/6 mice, were used in the present study. IL-33 gene knockout mice were used to confirm the role of alarmin in Con A-induced hepatitis.
Results
Opposing to the previous results obtained in wild-type mice, transferred CD4+ T cells alone into Rag2-knockout mice cannot cause hepatitis upon Con A challenge. In stark contrast, transferred CD8+ T cells play an important role in the pathogenesis of Con A-induced liver injury in T cell-transferred Rag2-deficient mice. Furthermore, we found that hepatocytes injured by perforin-based CD8+ T cell cytotoxicity release the alarmin IL-33. This cytokine promotes ST2+ ILC2 development and the secretion of cytokines IL-5 and IL-13 to mediate liver inflammation triggered by Con A challenge. In addition, these type 2 cytokines, including those originated from CD4+ T cells, result in eosinophils accumulation in liver to exacerbate the liver injury after Con A administration.
Conclusion
Our data for the first time revealed that CD8+ T cells play an indispensable role in the pathogenesis of Con A-induced liver injury in T cell-transferred Rag2-deficient mice. Therefore, the CD8+ T cell/IL-33/ILC2 axis is a potential therapeutic target for acute immune-mediated liver injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Concanavalin A (Con A)-induced murine hepatitis has enabled the development of a novel hepatitis model induced by T cell activation. CD4+ T lymphocytes have been identified as being responsible for organ damage in the murine model of experimental liver injury induced by intravenous injection of Con A. However, the cellular and molecular mechanisms underlying Con A-induced hepatitis are not fully understood [1, 2]. Upon Con A administration, hepatic natural killer T (NKT) cells rapidly up-regulate cell surface Fas ligand (FasL) expression and FasL-mediated cytotoxicity. In addition, NKT cells undergo apoptosis, leading to their rapid disappearance from the liver. These results have implicated FasL expression on liver NKT cells in the pathogenesis of Con A-induced hepatitis [3, 4]. CD8+ T cells were not observed to play an important role in Con A-induced hepatitis in this mouse model, as antibody-dependent depletion of CD4+ T cells fully protected against Con A-induced hepatitis, whereas depletion of CD8+ T cells failed to prevent liver injury [1, 5]. Nevertheless, Con A-induced hepatitis is thought to be predominantly mediated by a perforin-dependent pathway. The cytotoxicity appears to be caused by CD8+ T cells [6].
It has been reported that activated T cells alone are not sufficient for the development of chronic liver inflammation. Thus, cell populations involved in the innate immune response also play important roles in the pathogenesis of autoimmune liver diseases [7, 8]. Innate lymphoid cells (ILCs) are cells that lack rearranged antigen-specific receptors, expressing many of the transcription factors and effector molecules expressed by CD4+ T helper (Th) cell populations [9, 10]. The type 1 ILC (ILC1) population consists of natural killer (NK) cells, producing interferon-γ (IFN-γ), similar to Th1 cells. Type 2 ILCs (ILC2s) are analogous to GATA-3-expressing Th2 cells that produce interleukin (IL)-5 and IL-13. Type 3 ILCs (ILC3s) are described as cells analogous to Th17 cells that produce IL-17A, IL-17F and IL-22 [10, 11]. ILC2s populate lymphoid organs at epithelial barrier surfaces and in other tissues, including skin, intestine, lung, adipose and liver tissue [12,13,14,15]. IL-25 and IL-33 have been reported to be crucial for inducing KLRG1+ ILC2 or ST2+ ILC2 populations both in humans and mice [16]. ILC2s express the cytokines IL-4, IL-5, IL-9 and IL-13; participate in the immune response against helminths; promote airway allergies, lung inflammation, and skewed gastric disease; and also function in hepatic fibrosis [17,18,19,20,21,22].
IL-33 is a prominent member of the IL-1 family, and produced by endothelial and epithelial cells and by some hematopoietic cell types, including dendritic cells (DCs), mast cells, and macrophages. High expression of IL-33 has also been found in endothelial cells of various tissues, including liver, skin, lung, and stomach tissue [23,24,25]. IL-33 is thought to be released during cellular death as an alarmin cytokine during the acute phase of disease, and hepatocytes are a major and novel cellular source of IL-33 in vivo during Con A-induced acute hepatitis [25]. The production of IL-33 is regulated by NKT cells, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and perforin [20, 25]. IL-33 is also described as a regulator of inflammation and tissue homeostasis. The receptors of IL-33 are ST2 and IL-1RAcP, which are expressed on Th1 and Th2 cells, regulatory T (Treg) cells, macrophages, CD8+ T cells, eosinophils, ILC2s, NK cells and NKT cells, and these receptors regulate type 1 and type 2 immunity, organismal metabolism, thermogenesis, and tissue repair [21, 22, 26]. We previously demonstrated that the endogenous alarm molecules high-mobility group box 1 (HMGB1) and IL-33 exacerbate Con A-induced hepatic injury in mice [27, 28]. Therefore, IL-33 released by injured hepatocytes could be the key cytokine promoting the ILC2 response to liver damage upon Con A challenge.
The aim of this study was to determine the role and mechanisms by which CD8+ T and type 2 innate lymphoid cells participate in a murine model of Con A-induced hepatitis in T cell-transferred Rag2-deficient mice. Our data show that CD8+ T cells injure hepatocytes, which results in IL-33 release. This mechanism promotes hepatic ST2+ ILC2 development and responses triggered by Con A, and induce severe damage to the liver.
Materials and methods
Mice
Male 6–8-week-old C57BL/6 mice weighting 20–25 g were purchased from Beijing HFK Bioscience (China). Male 6–8-week-old Rag2−/− mice weighting 20–25 g with a C57BL/6 background were purchased from Jackson Laboratory (Bar Harbor, ME, USA). IL-33-deficient mice with C57BL/6 background were obtained from the laboratory of Dr. Fang Zheng in Department of Immunology, Tongji Medical College, HUST (China). Mice were maintained under specific-pathogen-free (SPF) conditions and studied in compliance with the animal care and use committee guidelines of Tongji Medical College, HUST (China), and the study protocols were specifically reviewed and approved by this ethics committee.
Treatment of mice
Con A (Sigma-Aldrich, USA) was dissolved in pyrogen-free phosphate-buffered saline (PBS), intravenously injected at 15 mg/kg body weight. For some experiments, mice were challenged with Con A at a lower dose (10 mg/kg body weight), together with rmIL-33 prepared as described previously [29]. Sera and tissues were collected at various time points after Con A challenge. Sera were collected for measuring alanine aminotransferase (ALT) and cytokine levels. The livers were excised for RNA isolation, and portions of the livers were used for tissue sectioning, hematoxylin/eosin (HE) staining and immunofluorescence (IF) analysis. For the cell reconstitution experiments, cells were transferred intravenously 1 h before injection of Con A.
Assessment of liver injury
Serum ALT was analyzed (Backman LX20 autoanalyzer, CA, USA). Liver tissue was fixed in 4% paraformaldehyde and cut into 4-µm-thick sections for HE staining, TUNEL staining or IF. Serum IFN-γ, IL-6, and IL-10 were examined using ELISA kits (BioLegend).
Cell isolation and culture
Hepatocyte isolation was performed as described previously [29, 30]. Briefly, additional 8–10-week-old C57BL/6 mice (> 25 g body weight) were used as a source of hepatocytes. Hepatocytes were obtained via a two-step collagenase perfusion method. Anesthetized C57BL/6 mice were perfused through the inferior vena cava with D-Hank’s buffer and then perfused with type IV collagenase (Sigma-Aldrich, USA) to initiate in vivo hepatocyte dissociation. The liver was then transferred to a Petri dish with pre-cooled Dulbecco’s modified Eagle’s medium (DMEM) and gently shaken to dissociate single hepatocytes. Hepatocytes were purified using 30% Percoll (Sigma-Aldrich, USA). Approximately 4 million cells were obtained with a viability between 90% and 95% (evaluated by Trypan Blue exclusion) and cultured for 3 days. The hepatocyte culture medium was prepared with high-glucose DMEM medium plus hydrocortisone, 500 U/L insulin, and 1% penicillin–streptomycin. Twelve-well plates were pre-coated with type I rat tail collagen. Hepatic mononuclear cells (HMCs) were prepared by Percoll density centrifugation. The livers were pressed through a 200-gauge stainless-steel mesh. After washing with pre-cooled PBS, cells were resuspended in 40% Percoll, overlaid onto 70% Percoll and then centrifuged at 500 g for 30 min. HMCs were then collected in the interphase and washed with cold PBS.
CD4+ T cells and CD8+ T cells were obtained from the spleen and mesenteric lymph nodes (mLN) and negative selection was performed using a mouse MicroBead Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). To isolate hepatic ST2+ ILC2s from HMCs, lineage-negative (Lin−) cells with FITC-conjugated antibodies for CD3, CD5, CD8, CD19, B220, CD11b, CD11c, Ter119, F4/80, Gr-1, TCRβ, TCRγt, FcR1α, and CD49b (eBioscience, Germany) were enriched by magnetic cell sorting. Lin− cell-enriched HMCs were then stained with fluorescence-labeled antibodies for CD127-APC/cy7, Sac-1-PercyPCy5.5, and ST2-APC (eBioscience, Germany), and Lin−CD127+Sac-1+ST2+ ILC2s were sorted with a BD FACSAria III sorter (BD Biosciences). In some experiments, the concentration of Con A was 0.1 mg/ml, and the concentration of IL-33 was 20 ng/ml in the cell culture.
Adoptive cell transfer
Sorting of T cells and ILC2s for the adoptive transfer study was conducted as described above. Before transfer, isolated cell populations were washed twice with pyrogen-free PBS. Cell suspensions (150 µl), containing the indicated cell numbers, were injected i.v. into the tail veins of recipient mice.
Real-time PCR
Total RNA of the liver tissues was extracted using TRIzol reagent (TaKaRa, Japan) and then reverse-transcribed to cDNA, which was synthesized from 5 µg RNA using a Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA USA). Primers of GAPDH, IL-33, perforin and FasL for real-time PCR were from Sangon Biotech, China. The amount of the target gene messenger RNA (mRNA) was calculated from the standard curve and normalized to actin mRNA.
Flow cytometry analysis
HMCs were stimulated for 4 h in the presence of 50 ng/ml phorbol myristate acetate (PMA) (Sigma-Aldrich, USA), 1 µg/ml ionomycin (Sigma-Aldrich, USA) and 0.67 µg/ml GolgiStop (BD biosystems, San Jose, CA, USA). After stimulation, cells were incubated with surface markers for 30 min at 4 °C. Subsequently, cells were fixed and permeabilized using a Cytofix/Cytoperm kit (BD) per the instructions of the manufacture’s manual and then incubated with intracellular antibodies. Antibodies (CD3-FITC, CD4-FITC, CD5-FITC, CD8-FITC, CD19-FITC, CD11c-FITC, Ter119-FITC, F4/80-FITC,Gr-1-FITC,FcR1a-FITC, NK1.1-FITC, CD127-APC-cy7, Sca-1-Percyp/cy5.5, ST2-APC, IL-4-PE, IL-5-PE, IL-13-PE) were purchased from BioLegend (San Diego, CA). Data were acquired using a BD LSR Fortessa cytometer (BD Biosciences) and analyzed by FlowJo software (Tree star, Ashland, OR).
ELISA
Blood samples were harvested and kept at room temperature for 30 min before centrifuging. Plasma was collected and stored at − 80 °C until analysis. For in vitro experiments, the supernatants were collected after mixed cell co-culture for 10 h. The levels of IFN-γ, IL-6, and IL-10 and IL-33 were determined by ELISA kits (eBioscience).
HE staining and immunofluorescence (IF)
Details of the procedures used for HE staining and IF were described in a previous study [28]. IL-33 and perforin were detected using anti-perforin antibody (Abcam) and anti-IL-33 antibody (R&D System), respectively. Propidium iodide (PI)/Annexin V was determined using an Annexin V-FITC Apoptosis Detection Kit I (BD).
Statistical analysis
Data were analyzed using GraphPad Prism software. Statistical differences were identified by Student’s t test or one-way ANOVA. The data are presented at means ± standard deviation (SD). Values of *p < 0.05 and **p < 0.01 were considered significant.
Results
CD8+ T cells play a necessary complementary role in T cell-transferred Con A-induced hepatitis in Rag2-deficient mice
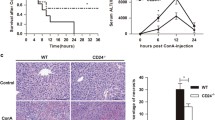
We first sought to determine the roles of CD4+ T and CD8+ T cells in Con A-induced hepatitis. For this purpose, a murine T cell-transferred Con A-induced hepatitis Rag2 deficiency model was developed. As expected, we observed liver tissue damage in the wild-type mice challenged with Con A compared with PBS control mice (Fig. 1a, lower panel). Interestingly, in the T cell-transferred Con A-induced hepatitis Rag2-deficient mice, liver tissue damage was more severe in Rag2−/− mice co-reconstituted with CD4+ T cells and CD8+ T cells than in those transferred with CD4+ T cells or CD8+ T cells alone after Con A injection (Fig. 1a, upper panel). Consistently, after administration of Con A, serum ALT levels were markedly higher in the Rag2−/− mice co-reconstituted with both CD4+ T cells and CD8+ T cells than in those transferred with CD4+ T cells or CD8+ T cells alone (Fig. 1b). In line with the above observations and consistent with the Con A-induced hepatitis in wild-type mice, serum IFN-γ, IL-6, and IL-10 levels in Rag2−/− mice co-reconstituted with CD4+ T cells and CD8+ T cells were significantly up-regulated compared with PBS control group. (Fig. 1c). The results above indicate that CD8+ T cells play a necessary complementary pathogenic role in hepatitis induced by Con A in T cell-transferred Rag2-deficient mice.
CD8+ T cells play an important role in Con A-induced hepatitis in T cells-transferred Rag2−/− mice. C57BL/6 or Rag2−/− mice were intravenously (i.v.) injected with 2 × 106 CD4+ T cells and/or 1 × 106 CD8+ T cells, and Con A was administered at a dose of 15 mg/kg body weight 1 h later. Samples were harvested 10 h after Con A injection. a HE staining and TUNEL staining of tissue samples from C57BL/6 and reconstituted Rag2−/− mice (original magnification × 200). b Serum ALT was determined to evaluate hepatitis in the various groups. c Serum levels of IFN-γ, IL-6 and IL-10 were detected by ELISA in wild-type C57/B6 mice and T cell-transferred Rag2−/− mice after Con A challenge versus PBS control treatment. Data are representative of an average of n = 5–7 individual mice from n = 5 independent experiments. Values are the means ± SD; **p ≤ 0.01, ***p ≤ 0.001. The experiments were replicated at least twice
CD8+ T cells enhance the production of IL-33 by hepatocytes via cytotoxicity in a perforin-dependent manner
To further address the involvement of CD8+ T cells in Con A-induced hepatitis, cytometry analysis revealed that CD69+ CD8+ T cells in HMCs, the activated CD8+ T cell population, increased with time after Con A challenge (Fig. 2a). Even in the early phase of Con A-induced hepatitis (at the 3rd hour after Con A administration), CD69 was dramatically up-regulated in CD8+ T cells, which led to a significantly increased number of CD69+ CD8+ T cells for 10 h after Con A injection compared with PBS control (Fig. 2b). To elucidate the underlying mechanism of CD8+ T cell function, we detected the expression of FasL and perforin in liver tissue. FasL was not significantly up-regulated (data not shown). In contrast, similar to wild-type C57BL/6 mice after Con A administration, perforin mRNA was markedly up-regulated in liver tissue in Rag2−/− mice co-reconstituted with both CD4+ T cells and CD8+ T cells compared with in liver tissue in mice transferred with CD4+ T cells alone plus Con A challenge or PBS control (Fig. 2c lower panel). Interestingly, following the up-regulation of perforin mRNA in liver tissue, IL-33 mRNA in liver tissue and IL-33 protein in plasma were also significantly increased (Fig. 2c upper panel). To further confirm the role of CD8+ T cells, we cultured hepatocytes with or without CD8+ T cells from the livers of mice with Con A present in the medium for 10 h. PI staining revealed that hepatocytes were clearly detected in the co-culture of hepatocytes and CD8+ T cells. In contrast, there was very little PI fluorescence (red) among the hepatocytes cultured without CD8+ T cells (Fig. 2d). In parallel, IF revealed increased expression of perforin and IL-33 in the liver tissue after Con A challenge compared with the PBS control (Fig. 2e). Together, these data indicated that CD8+ T cells attacked hepatocytes through perforin, the latter producing alarmin IL-33.
Con A-activated CD8+ T cells destroyed hepatocytes to release IL-33 by perforin in T cell-transferred Rag2−/− mice after Con A challenge. C57BL/6 and reconstituted Rag2−/− mice were challenged with Con A for 10 h, and then, HMCs were isolated and subjected to flow cytometry. a The population of CD69+ CD8+ T cells was examined by flow cytometry. b The absolute number of CD8+ T cells in HMCs was calculated for the Con A challenge group and the PBS control group. c IL-33 and perforin mRNA expression levels and sera IL-33 levels were determined using real-time PCR and ELISA and normalized to the levels in PBS-treated mice. d Hepatocytes co-cultured with/without CD8+ T cells and treated with Con A for 10 h. PI/Annexin V staining was performed to evaluate the apoptosis of injured hepatocytes attacked by CD8+ T cells. e Perforin and IL-33 protein levels were measured in liver samples of C57BL/6 mice by IF. Values are the means ± SD; *p ≤ 0.05, **p ≤ 0.01. Experiments were repeated at least 3 times, n = 5 per group
IL-33 promotes the pathology of hepatitis induced by Con A and the generation of hepatic ST2 + ILC2s
To directly examine the link between the cytotoxicity of CD8+ T cells and IL-33 derived from injured hepatocytes, C57BL/6 mice were transferred with CD8+ T cells or pretreated with rmIL-33 before Con A challenge. As shown by the tissue samples, the damage after Con A treatment was exacerbated by CD8+ T cell transfer or rmIL-33 administration compared with treatment with Con A alone, rmIL-33, or PBS, respectively, and the level of ALT in the serum showed the same trend (Fig. 3a). ST2 is the receptor of IL-33, and ST2+ ILC2s were markedly expanded in multiple organs of C57BL/6 mice treated with rmIL-33 for 48 h (Fig. 3b). Furthermore, hepatic ST2+ ILC2s were expanded after Con A injection, particularly in the mice transferred with CD8+ T cells or pretreated with rmIL-33 (Fig. 3c). To further examine whether IL-33 augmented Con A-induced liver injury by ST2+ ILC2s, we performed a cell transfer experiment and analyzed disease pathogenesis. For this purpose, we first transferred CD4+ T cells into Rag2−/− mice and then treated the mice with rmIL-33 and Con A 1 h after cell transfer. ALT was dramatically increased by rmIL-33 treatment (Fig. 3d), and ST2+ ILC2s were also expanded (Fig. 3e). In addition, we co-cultured hepatocytes with CD8+ T cells (from mLN) and lymphocytes (from mLN with CD8+ T cells removed) in the presence or absence of rmIL-33/Con A. Both rmIL-33 and Con A promoted the proliferation of ST2+ ILC2s, especially in the group treated with Con A (Fig. 3f). The results above suggest that IL-33 expands ST2+ ILC2s and promotes pathological hepatic immune responses.
IL-33 released from hepatocytes enhanced by CD8+ T cells stimulates ST2+ ILC2 proliferation. C57BL/6 mice (n = 5) were injected (i.v.) with CD8+ T cells (1 × 106) or rmIL-33 (200 ng/mouse) and administered Con A 1 h later. Samples were taken 10 h after Con A challenge. a Liver samples and serum ALT levels in each group are displayed. b Rag2−/− mice were treated with rmIL-33 (200 ng/mouse) for 48 h, and ST2+ ILC2s in multiple organs were determined by flow cytometry. c Rag2−/− mice were first adoptively transferred with CD4+ T cells (2 × 106) and then treated with rmIL-33 or rmIL-33 plus CD8+ T cells (1 × 106); Con A was administered 1 h later. Mice treated with rmIL-33 alone or PBS were used as controls. After the above treatments, hepatic ST2+ ILC2s in various groups were examined by flow cytometry. d Bar graphs showing the serum levels of ALT in various groups. e Bar graphs showing ST2+ ILC2s of HMCs in various groups. f Bar graphs showing ST2+ ILC2s of lymphocytes in cell culture with CD8+ T cells and/or IL-33 and/or Con A, respectively. Values are the means ± SD; *p ≤ 0.05, **p ≤ 0.01. All data shown here are representative of independent experiments with similar results
CD8+ T cells promote the expression of IL-33 in hepatocytes and expand ST2+ ILC2s in vitro
To further confirm the results presented above, we cultured hepatocytes with CD4+ T cells and/or CD8+ T cells and challenged them with Con A for 10 h. IF staining for perforin and IL-33 revealed that the expression levels of perforin and IL-33 were significantly increased in hepatocytes treated with Con A combined with CD4+ T cells plus CD8+ T cells compared with controls treated with Con A combined with CD4+ T cells or CD8+ cells alone, PBS with CD4+ T cells, or CD8+ T cells (Fig. 4a). Hepatocytes produced more IL-33 when cultured with CD8+ T cells and Con A as compared to other groups (Fig. 4b). Furthermore, the PI/Annexin V staining revealed that the number of PI and/or Annexin V-stained hepatocytes was markedly increased in the group cultured in the presence of both CD8+ T cells and CD4+ T cells than that of in the presence of CD8+ T cells or CD4+ T cells alone (Fig. 4c). We also co-cultured hepatocytes with CD8+ T cells (mLN) and/or lymphocytes (mLN, with CD8+ T cells removed) to verify the differences with ST2+ ILC2s. ST2+ ILC2 proliferation was increased when the cells were cultured with CD8+ T cells and challenging by Con A for 10 h. To remove the interference of Con A, we administered Con A to CD8+ T cells for 6 h, and the supernatant was then discarded after centrifugation. Finally, the cells were cultured with lymphocytes (without CD8+ T cells) for 10 h. ST2+ ILC2 proliferation was dramatically increased compared with the proliferation of normal treated cells (Fig. 4d). To further prove the role of endogenous IL-33 in promoting the generation of ST2+ ILC2, hepatocytes that originated from IL-33 gene-deficient mice were used in the in vitro culture system. We found that IL-33 deficiency of hepatocytes resulted in a significant decrease in ST2+ ILC2 generation compared with wild-type control hepatocytes (Fig. 4e). This evidence suggests that activated CD8+ T cells destroy hepatocytes by perforin, thus increasing the expression of IL-33 and expanding ST2+ ILC2s in Con A-induced hepatitis.
IL-33 released from injured hepatocytes promoted ST2+ ILC2 generation in vitro. Murine hepatocytes cultured with lymphocytes (1 × 106) and/or CD8+ T cells (1 × 106) and treated with Con A for 10 h. a IF (×200) of perforin (green), IL-33 (red) and merged images are shown. b Bar graph displaying IL-33 levels in the supernatant determined by ELISA in various cell culture conditions. c PI (red)/Annexin V (green) staining (×200) was examined to evaluate the damage to hepatocytes cultured in various conditions. d Bar graph showing the population of ST2+ ILC2s of lymphocytes after Con A or PBS treatment detected by flow cytometry. In the group, CD8+ T cells were pretreated with Con A for 6 h, then centrifuged to remove the supernatant, and subsequently cultured with hepatocytes and lymphocytes for 10 h. Bar graph displaying the population of ST2+ ILC2s determined by flow cytometry. e Hepatocytes from male IL-33 gene knockout mice were added to the culture system and incubated for 3 days, as indicated in the lower part of the figure. Lymphocytes from male wild-type C57BL/6 mice lymph glands were isolated and co-cultured with hepatocytes for 1 h, after which Con A and/or IL-33 were added. Bar graph displaying the population of ST2+ ILC2s determined by flow cytometry. Values are the means ± SD; *p < 0.05, **p ≤ 0.01. The experiments were repeated at least 3 times. (Color figure online)
Con A-induced hepatic ST2+ ILC2s exhibit increased production of IL-5 and IL-13 cytokines
ST2+ ILC2s have been shown to act as activated resident ILC2s to express IL-5 and IL-13 necessary for the accumulation of eosinophils [31]. Therefore, we hypothesized that hepatic ST2+ ILC2s may augment hepatitis by causing the accumulation of eosinophils via enhancing type 2 cytokine production. To test this hypothesis, we performed flow cytometry and intracellular cytokine staining to examine the ST2+ ILC2s and eosinophils, as well as IL-4, IL-5 and IL-13 cytokine production by ST2+ ILC2s. Cytometry analysis revealed that hepatic ST2+ ILC2s were increased in the Con A-induced hepatitis group compared with the PBS control group (Fig. 5a). We then evaluated the functional role of these cells by intracellular cytometric analysis of cytokine secretion (Fig. 5b). Notably, ILC2s are capable of producing IL-4, IL-5 and IL-13. In particular, IL-5 and IL-13 production by hepatic ST2+ ILC2s was significantly increased in the Con A treatment group compared with the PBS control group (Fig. 5c). In addition, the number of eosinophils (CD45+CD11b+-Siglec-F+) was dramatically increased in the liver after Con A challenge compared with PBS control administration (Fig. 5d). These results indicated that ST2+ ILC2s play a proinflammatory role in Con A-induced hepatitis.
IL-5 and IL-13 cytokines produced by ST2+ ILC2s augmented eosinophil accumulation after Con A challenge in the mouse livers. C57BL/6 mice treated with Con A (15 mg/kg body weight) for 10 h. HMCs were prepared and activated with PMA (50 ng/ml) and ionomycin (1 µg/ml) in the presence of GolgiStop (0.67 µg/ml), followed by intracellular cytokine staining flow cytometric analysis for IL-4, IL-5 and IL-13. a ST2+ ILC2s were increased in the livers of mice treated with Con A compared with control mice treated with PBS. b The gating strategy for intracellular cytokine staining flow cytometric analysis on lineage CD127+sac-1+ST2+IL-5+ILC2s as an example. c Bar graphs showing the frequency of IL-4+ ILC2s, IL-5+ ILC2s and IL-13+ ILC2s after Con A challenge versus PBS control. d The frequency of eosinophils (CD45+CD11b+Siglec-F+) evaluated by flow cytometry in the livers of mice challenged with Con A compared with control mice treated with PBS. Values are the means ± SD; **p ≤ 0.01. Experiments were independently carried out at least 3 times, n = 3–5
ST2+ ILC2s in Rag2-deficient mice enhance the production of IL-5 and IL-13 in vitro
To further examine the implication of the production of IL-5 and IL-13 by hepatic ST2+ ILC2s in Con A-induced hepatitis, we reconstituted Rag2−/− mice with CD4+ T cells and/or CD8+ T cells and then treated the mice with Con A for 10 h, as evidenced by serum ALT levels (Fig. 6a). Flow cytometry analysis revealed that the number of hepatic ST2+ ILC2s was significantly increased. Moreover, the production of IL-5 and IL-13 by these ST2+ ILC2s was dramatically up-regulated in Rag2−/− mice transferred with CD4+ T cells combined with CD8+ T cells, similar to the levels in C57BL/6 mice, but not in mice transferred with CD4+ T cells or CD8+ T cells alone (Fig. 6b). We also confirmed the results above in vitro. For this purpose, hepatocytes were cultured with lymphocytes (without CD8+ T cells) and/or CD8+ T cells and then treated with Con A for 10 h. As expected, the addition of CD8+ T cells significantly promoted the production of IL-5 and IL-13 by ST2+ ILC2s (Fig. 6c). Collectively, these results revealed that IL-5 and IL-13 secretion by ST2+ ILC2s was up-regulated in the liver upon Con A challenge and contributed to Con A-induced hepatitis.
Frequency and cytokine production by ST2+ ILC2s were increased in reconstituted Rag2−/− mice administered with Con A. a Bar graph showing that plasma ALT levels in Rag2−/− mice reconstituted with CD4+ T cells and/or CD8+ T cells after Con A challenge compared with PBS control mice. b Hepatic ST2+ ILC2s from Con A-treated mice were analyzed for frequency and IL-5 and IL-13 cytokine production by flow cytometry. c The gating strategy of lineage−CD127+sac-1+ST2+IL-5+/IL-13+ ILC2s. Values are the means ± SD; **p ≤ 0.01. A representative experiment of at least three independent experiments with three or five mice per group is shown
Hepatic ST2+ ILC2s promote inflammation in liver and cooperate with CD4+ T cells
Finally, to further confirm the above observations, we performed adoptive transfer of ST2+ ILC2s in C57BL/6 mice followed by treatment with Con A. ST2+ ILC2s were isolated from the livers of naïve male C57BL/6 mice by cell sorting. HE staining showed more necrosis in the transferred mice than in the Con A-only-treated mice (Fig. 7a). Consistently, ST2+ ILC2 transfer followed by Con A challenge evoked increases in serum ALT and IL-6 and IL-10 cytokines compared with treatment with Con A alone or PBS control (Fig. 7b). To exclude the influence of CD4+ T cells, we also examined hepatic CD4+ T cells. The results showed that there was no significant difference between mice treated with Con A alone and mice transferred with ST2+ ILC2s followed by Con A challenge, while mice transferred with ST2+ ILC2s combined with Con A challenge and mice treated with Con A alone both exhibited increased numbers of CD4+ T cells in the livers (Fig. 7c). Furthermore, ST2+ ILC2-transferred mice displayed increases in the production of IL-4 and IL-5 type 2 cytokines by CD4+ T cells (Fig. 7d). Taken together, these data indicate that hepatic ST2+ ILC2s enhance the production of type 2 cytokines by CD4+ T cells to promote the progression of Con A-induced hepatitis.
ST2+ ILC2s promote inflammation by augmenting cytokine expression by CD4+ T cells in Con A-induced hepatitis in mice. ST2+ ILC2s were obtained by flow cytometric sorting from naïve C57BL/6 mice, and the isolated ST2+ ILC2s (2 × 105) were adoptively transferred to C57BL/6 mice; reconstituted mice were sequentially administered with Con A for 10 h. a HE staining used to evaluate hepatitis is shown (×200 and ×4) for the various groups. b Sera levels of ALT, IL-6 and IL-10 were examined in the various groups. c Bar graph showing that the numbers of CD4+ T cells in the liver were not significantly different between the Con A-treated groups with and without ST2+ ILC2 adoptive transfer. d The cytokine expression levels of hepatic CD4+ T cells were augmented after adoptive transfer of ST2+ T cells following Con A treatment. Values are the means ± SD; *p ≤ 0.05, **p ≤ 0.01. All data shown here are representative of independent experiments with similar results
Discussion
In the present study, we investigated the roles and mechanisms of CD8+ T cells and ILC2s on experimental hepatitis induced by Con A in mice. We demonstrated that IL-33 is an important cytokine that promotes injury of hepatocytes by CD8+ T cells and ILC2s.
Con A-induced liver injury is mainly mediated by T cells, and CD4+ T cells are more important than CD8+ T cells in sensitive mouse strains such as NMRI and BALB/c mice [1]. However, we found that CD8+ T cells play a critical role in liver injury in T cell-transferred Rag2-deficient mice challenged with Con A. This contradiction suggests that the mechanism of Con A-induced hepatitis remains elusive. Interestingly, CD4+ cells do not induce cytotoxicity to Con A-treated hepatocytes, while CD8+ cells are cytotoxic to these hepatocytes. This cytotoxicity is predominantly mediated by a perforin-dependent pathway [32]. In line with these observations, we found that perforin mRNA levels were dramatically increased in Rag2-deficient mice transferred with both CD4+ T cells and CD8+ T cells compared with mice transferred with only CD4+ T cells after Con A challenge. Furthermore, the mRNA and protein levels of IL-33 were significantly higher after the transfer of CD4+ T cells and CD8+ T cells to Rag2 knockout mice than after the transfer of only CD4+ T cells. Moreover, these phenomena were seen in C57/B6 wild-type mice with Con A-induced hepatitis. Together, these data strongly suggest that perforin expression by CD8+ T cells and IL-33 released by injured hepatocytes are important elements for Con A-induced hepatitis in T cell-transferred Rag2-deficient mice.
It has been shown that hepatic ILC2s are poised to respond to the release of IL-33 upon liver tissue damage through the expression of type 2 cytokines, thereby participating in the pathogenesis of immune-mediated hepatitis [6]. Consistently, we observed that ILC2s are an important innate cell subset that can injure the liver when triggered by Con A challenge. Importantly, we provide compelling evidence for the first time that CD8+ T cell-mediated injured hepatocytes are a major source of IL-33 release, which promotes the ILC2 development and response.
Regarding the cytokine profiles elicited by Con A administration, proinflammatory cytokines, including TNF-α, IFN-γ, and IL-1, have been shown to play a crucial role in the pathogenesis and development of Con A-induced liver injury [2, 33]. However, it has been proposed that type 2 cytokines such as IL-4, IL-5 and IL-10 also participate in the pathogenesis of Con A-induced hepatitis [34, 35]. In addition to cytokines that are triggered by Con A challenge, we have previously shown that alarm molecules such as HMGB1 and IL-33 participate in the pathogenesis of Con A-induced hepatitis [27, 28]. Here, we demonstrate that downstream IL-33 elicits high levels of IL-5 and IL-13 production by IL-33-stimulated ILC2s, and these type 2 cytokines also injure the liver. Future studies are warranted to investigate the balanced mechanisms by which IL-33 regulates innate or adaptive immunity in hepatitis.
IL-33 is a pivotal alarm cytokine in infection and allergy that directly stimulates not only ILCs but also macrophages, osteoclasts, Th2 cells, mast cells, DCs, and other subsets that express IL-33 receptors [36]. Just as one coin has two sides, IL-33 may play different roles in various diseases based its mechanisms of expression and delivery [37]. On one hand, in the setting of Con A-induced hepatitis, IL-33 is released from injured hepatocytes as a proinflammatory alarm cytokine by signaling liver tissue damage; on the other hand, IL-33 exhibits a protective role as a Th2 cytokine against Th1 proinflammatory cytokines, and these contrasting roles depend on how IL-33 is administered and what animal model is used [30, 38]. Supporting this idea, Oboki et al. showed that IL-33 acts as a crucial alarmin for innate-type, but not acquired-type, tissue injury-related inflammatory responses, such as lipopolysaccharide (LPS)-induced endotoxin shock [39].
Our unique T cell-transferred Rag2−/− Con A-induced hepatitis model has the advantage over wild-type models of being able to be used to define the cellular and molecular mechanisms underlying Con A-induced hepatitis. In this model, we can clearly evaluate the functional contributions of different immune cell subsets to Con A-induced hepatitis because Rag2-deficient mice are devoid of adaptive immune cells, such as T cells and B cells, unlike wild-type mice, while these Rag2 gene knockout mice do have innate lymph cells, such as ILCs. Our results in T cell-transferred Rag2-deficient mice point to a CD8+ T cell/injured hepatocyte IL-33 release/ILC2 axis in Con A-induced hepatitis.
In contrast to the traditional concept of CD4+ T cells playing an essential role in Con A-induced hepatitis, a novel finding of this study is that CD8+ T cells play an important role in the pathogenesis of Con A-induced liver injury through promoting the development and response of ILC2s via IL-33 released from injured hepatocytes. Indeed, acute hepatitis A (AHA) involves severe CD8+ T cell-mediated liver injury. The severity of liver injury in AHA patients correlates with activation of hepatitis A virus (HAV)-unrelated virus-specific CD8+ T cells and the innate-like cytolytic activity of CD8+ T cells, but not the activation of HAV-specific T cells. Thus, host injury in AHA is associated with innate-like cytotoxicity of bystander-activated CD8+ T cells, a result with implications for acute viral diseases [40]. In patients with hepatitis C virus (HCV) infection, for efficient control of HCV infection, CD8+ cytotoxic T cells (CTL) are pivotal, but the persistence of activated T cells may lead to liver toxicity [41]. A better understanding of how adaptive immunity mediates pathogen clearance and tumor elimination may lead to improved vaccinations and immunotherapy treatment strategies of infectious diseases and cancer [42].
Currently, it was recognized that eosinophils are recruited by IL-5 produced by ILC2s in many disease conditions [19, 32, 43]. The relationship of CD8+ T cells, ST2+ ILC2 and eosinophils is as follows: Con A-activated CD8+ T cells attack the hepatocytes, as a result, the injured hepatocytes released large amount of IL-33 which activate ST2+ ILC2 to produce IL-5 and IL-13. Consequently, IL-5 produced by ST2+ ILC2 and CD4+ Th2 cells recruit eosinophils to the sites of hepatitis to enhance inflammation (Fig. 8).
Schematic representation of the pathologic role of hepatic CD8+ T cells in Con A-induced hepatitis. Hepatic CD8+ T cells were activated by Con A challenge and destroyed hepatocytes, thus causing the release of IL-33. The latter stimulates ST2+ ILC2s to secrete IL-5 and IL-13. These cytokines promote inflammation by causing the accumulation of eosinophils and further enhancing IL-4 and IL-5 cytokine production by CD4+ T cells in the liver, which promotes Con A-induced liver damage
In conclusion, our data demonstrate for the first time that CD8+ T cells play an indispensable role in the pathogenesis of Con A-induced liver injury in T cell-transferred Rag2-deficient mice. Downstream of CD8+ T cell cytotoxicity, the IL-33-mediated innate ILC2 response is part of a vicious circle that drives the execution of hepatic necrosis and tissue damage. Our data highlight the CD8+ T cell/IL-33/ILC2 axis as a potential therapeutic target for treatment of acute immune-mediated liver injury.
References
Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203.
Mizuhara H, O’Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, et al. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994;179:1529–37.
Knolle PA, Gerken G, Loser E, Dienes HP, Gantner F, Tiegs G, et al. Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology. 1996;24:824–9.
Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498–503.
Tiegs G. Experimental hepatitis and role of cytokines. Acta Gastroenterol Belg. 1997;60:176–9.
Watanabe Y, Morita M, Akaike T. Concanavalin A induces perforin-mediated but not Fas-mediated hepatic injury. Hepatology. 1996;24:702–10.
Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47:729–36.
Bogdanos DP, Gao B, Gershwin ME. Liver immunology. Compr Physiol. 2013;3:567–98.
Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–75.
Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566.
Zhang Y, Tang J, Tian Z, van Velkinburgh JC, Song J, Wu Y, et al. Innate lymphoid cells: a promising new regulator in fibrotic diseases. Int Rev Immunol. 2016;35:399–414.
Bernink JH, Germar K, Spits H. The role of ILC2 in pathology of type 2 inflammatory diseases. Curr Opin Immunol. 2014;31:115–20.
Huang Y, Paul WE. Inflammatory group 2 innate lymphoid cells. Int Immunol. 2016;28:23–8.
Bjorkstrom NK, Kekalainen E, Mjosberg J. Tissue-specific effector functions of innate lymphoid cells. Immunology. 2013;139:416–27.
Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–9.
Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–7.
Liu M, Zhang C. The role of innate lymphoid cells in immune-mediated liver diseases. Front Immunol. 2017;8:695.
Gorski SA, Hahn YS, Braciale TJ. Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection. PLoS Pathog. 2013;9:e1003615.
Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–8.
Arshad MI, Piquet-Pellorce C, L’Helgoualc’h A, Rauch M, Patrat-Delon S, Ezan F, et al. TRAIL but not FasL and TNFalpha, regulates IL-33 expression in murine hepatocytes during acute hepatitis. Hepatology. 2012;56:2353–62.
Carriere V, Arshad MI, Le Seyec J, Lefevre B, Farooq M, Jan A, et al. Endogenous IL-33 deficiency exacerbates liver injury and increases hepatic influx of neutrophils in acute murine viral hepatitis. Mediators Inflamm 2017;2017:1359064.
McSorley HJ, Blair NF, Smith KA, McKenzie AN, Maizels RM. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014;7:1068–78.
Le Goffic R, Arshad MI, Rauch M, L’Helgoualc’h A, Delmas B, Piquet-Pellorce C, et al. Infection with influenza virus induces IL-33 in murine lungs. Am J Respir Cell Mol Biol. 2011;45:1125–32.
Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–103.
Arshad MI, Rauch M, L’Helgoualc’h A, Julia V, Leite-de-Moraes MC, Lucas-Clerc C, et al. NKT cells are required to induce high IL-33 expression in hepatocytes during ConA-induced acute hepatitis. Eur J Immunol. 2011;41:2341–8.
Vasanthakumar A, Kallies A. Interleukin (IL)-33 and the IL-1 family of cytokines-regulators of inflammation and tissue homeostasis. Cold Spring Harb Perspect Biol. 2017. https://doi.org/10.1101/cshperspect.a028506.
Gong Q, Zhang H, Li JH, Duan LH, Zhong S, Kong XL, et al. High-mobility group box 1 exacerbates concanavalin A-induced hepatic injury in mice. J Mol Med (Berl). 2010;88:1289–98.
Chen J, Duan L, Xiong A, Zhang H, Zheng F, Tan Z, et al. Blockade of IL-33 ameliorates Con A-induced hepatic injury by reducing NKT cell activation and IFN-gamma production in mice. J Mol Med (Berl). 2012;90:1505–15.
Cassim S, Raymond VA, Lapierre P, Bilodeau M. From in vivo to in vitro: Major metabolic alterations take place in hepatocytes during and following isolation. PLoS One. 2017;12:e0190366.
Moro K, Ealey KN, Kabata H, Koyasu S. Isolation and analysis of group 2 innate lymphoid cells in mice. Nat Protoc. 2015;10:792–806.
Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, et al. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells. Immunity. 2014;40:414–24.
Neumann K, Karimi K, Meiners J, Voetlause R, Steinmann S, Dammermann W, et al. A proinflammatory role of type 2 innate lymphoid cells in murine immune-mediated hepatitis. J Immunol. 2017;198:128–37.
Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–71.
Louis H, Le Moine A, Flamand V, Nagy N, Quertinmont E, Paulart F, et al. Critical role of interleukin 5 and eosinophils in concanavalin A-induced hepatitis in mice. Gastroenterology. 2002;122:2001–10.
Duran A, Rodriguez A, Martin P, Serrano M, Flores JM, Leitges M, et al. Crosstalk between PKCzeta and the IL4/Stat6 pathway during T-cell-mediated hepatitis. EMBO J. 2004;23:4595–605.
Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–10.
Villarreal DO, Weiner DB. Interleukin 33: a switch-hitting cytokine. Curr Opin Immunol. 2014;28:102–6.
Volarevic V, Mitrovic M, Milovanovic M, Zelen I, Nikolic I, Mitrovic S, et al. Protective role of IL-33/ST2 axis in Con A-induced hepatitis. J Hepatol. 2012;56:26–33.
Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA. 2010;107:18581–6.
Kim J, Chang DY, Lee HW, Lee H, Kim JH, Sung PS, et al. Innate-like cytotoxic function of bystander-activated CD8(+) T cells is associated with liver injury in acute hepatitis A. Immunity. 2018;48:161–73e5.
Balasiddaiah A, Davanian H, Aleman S, Pasetto A, Frelin L, Sallberg M, et al. Hepatitis C virus-specific T cell receptor mRNA-engineered human T cells: impact of antigen specificity on functional properties. J Virol. 2017;91:e00010–17.
Benechet AP, Iannacone M. Determinants of hepatic effector CD8(+) T cell dynamics. J Hepatol. 2017;66:228–33.
Doherty TA, Baum R, Newbury RO, Yang T, Dohil R, Aquino M, et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:792–4.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (91542110, 81373167 to M. Fang). We thank Mr. Yong Xu and Zhihui Liang for technical assistance with flow cytometry.
Author information
Authors and Affiliations
Contributions
YZ and CQ performed the experiments. LL and SH analyzed the data. FZ and FG provided the animal model and suggestions in experimental design. YZ and MF designed the experiments, analyzed data and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Responsible Editor: Mauro Teixeira.
Rights and permissions
About this article
Cite this article
Zhang, Y., Qi, C., Li, L. et al. CD8+ T cell/IL-33/ILC2 axis exacerbates the liver injury in Con A-induced hepatitis in T cell-transferred Rag2-deficient mice. Inflamm. Res. 68, 75–91 (2019). https://doi.org/10.1007/s00011-018-1197-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1197-9