Abstract
Objective and design
Arthritic gout is caused by joint inflammation triggered by the damaging effects of monosodium uric acid (MSU) crystal accumulation in the synovial space. Neutrophils play a major role in mediating joint inflammation in gout. Along with neutrophils, other immune cells, such as macrophages, are present in inflamed joints and contribute to gout pathogenesis. Neutrophils form neutrophil extracellular traps (NETs) in response to MSU crystals. In the presence of MSU crystals, macrophages release IL-1β, a cytokine crucial to initiate gout pathogenesis and neutrophil recruitment. Our research investigated interactions between human macrophages and neutrophils in an in vitro model system and asked how macrophages affect NET formation stimulated by MSU crystals.
Materials or subjects
Human neutrophils and PBMCs were isolated from peripheral blood of healthy volunteers. PBMCs were differentiated into macrophages in vitro using human M-CSF.
Treatment
Human neutrophils were pretreated with macrophage-conditioned media, neutrophil-conditioned media, recombinant human IL-1β or anakinra prior to stimulation by MSU crystals.
Method
Interaction of neutrophils with MSU crystals was evaluated by live imaging using confocal microscopy. The presence of myeloperoxidase (MPO) and neutrophil elastase (NE) was measured by ELISA. NET formation was quantitated by Sytox Orange-based extracellular DNA release assay and NE-DNA ELISA. AggNET formation was assessed by macroscopic evaluation.
Results
We found that crystal- and cell-free supernatants of macrophages stimulated with MSU crystals promote MSU crystal-stimulated NET formation in human neutrophils. This observation was confirmed by additional assays measuring the release of MPO, NE, and the enzymatic activity of NE. MSU crystal-induced NET formation remained unchanged when neutrophil supernatants were tested. IL-1β is a crucial cytokine orchestrating the onset of inflammation in gout and is known to be released in large amounts from macrophages following MSU crystal stimulation. We found that recombinant IL-1β strongly promoted MSU crystal-induced NET formation in human neutrophils. Interestingly, IL-1β alone did not induce any NET release. We also found that clinical grade anakinra, an IL-1 receptor blocker, strongly reduced the NETosis-enhancing effect of macrophage supernatants indicating that IL-1β is mainly responsible for this effect.
Conclusions
Macrophage-derived IL-1β enhances MSU crystal-induced NET release in neutrophils. We identified a new mechanism by which macrophages and IL-1β affect neutrophil functions, and could contribute to the inflammatory conditions present in gout. Our results also revealed a new anti-inflammatory mechanism of anakinra.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gout is a sterile inflammation of the joints mediated mainly by infiltrated macrophages and polymorphonuclear neutrophilic granulocytes (PMN) driven by monosodium urate (MSU) crystal deposition [1]. These innate immune cells mistakenly recognize MSU crystals as dangers and undergo unnecessary activation resulting in painful, tissue-damaging inflammation [2]. While macrophages are the initiators of the inflammatory cascade, PMN infiltration and activation play a major role in bringing inflammation to its full expansion in gout [3–6]. IL-1β is a crucial and potent proinflammatory cytokine which also orchestrates gouty inflammation [7–9]. IL-1β is abundant in the synovial fluid of gout patients [3]. Its central role in gout inflammation is shown by successful IL-1β-inhibitory therapies [9–11]. Anakinra, a recombinant IL-1Ra protein, rilonacept, an IL-1 receptor fusion protein, and canakinumab, an anti-IL-1β antibody have all been shown to successfully treat acute gout attacks [9]. The primary role of IL-1β in mediating the inflammatory cascade in gout is thought to be its potent effect to recruit PMNs to the synovial space [9, 12]. MSU crystals activate the NLRP3 inflammasome and induce release of IL-1β in macrophages [7]. We and others have shown that MSU crystal-induced NLRP3 inflammasome activation in macrophages requires calcium and NADPH oxidase-independent production of reactive oxygen species (ROS) [13–15]. Caspase-1 activated by the inflammasome cleaves pro-IL-18 and pro-IL-1β forming bioactive cytokines [16, 17].
Polymorphonuclear neutrophilic granulocytes are the most abundant leukocytes during gout inflammation reaching very high concentrations in the synovial fluid [5, 18]. PMNs are known to engulf MSU crystals triggering ROS production, cytokine release, and neutrophil extracellular trap (NET) formation [5, 6, 18–20]. In a previous study, we described robust NET release of human PMNs in response to calcium pyrophosphate dihydrate (CPPD) microcrystals, the causative agents of pseudogout, a condition similar to gout [21]. NETs are composed of extracellular DNA network associated with histones and PMN granule proteins, including myeloperoxidase (MPO) and neutrophil elastase (NE) [22]. NET formation in gout is mistakenly initiated and leads to the release of dangerous PMN cargo into the synovium. In gout, the primary antimicrobial function of NETs (trapping extracellular microbes) manifests in MSU crystal immobilization [5, 6]. MSU crystal-induced NET release requires autophagy, NADPH oxidase-dependent ROS production, and RIP3K-MLKL signaling pathway [5, 18, 23]. A recent study shed new light on a potential anti-inflammatory role of NETs in gout [5]. At high PMN densities, NETosed PMNs and MSU crystals form ‘aggregated NETs’ (aggNETs), the proposed basis of gouty tophi, a characteristic white material that appears at the beginning of the resolution phase of acute gout flares [5, 24]. Although our view on the role of NETs in gout pathogenesis is improving, less is known how the two most important cell types initiating and mediating inflammation in gout, macrophages, and PMNs interact.
Our study addressed the question how macrophages influence NET formation stimulated by gout-causing MSU crystals. Our data show that proinflammatory macrophages stimulated with MSU crystals release mediators that cause neutrophils to have over-exaggerated release of NETs induced by the crystals. We also show that IL-1β is a potent enhancer of NET formation and it is mainly responsible for the NETosis-promoting effect of macrophages. We propose that macrophage- and IL-1β-enhanced NET formation can be a novel contributor to gout inflammation.
Materials and methods
Human subjects
De-identified healthy human volunteers were recruited at the University of Georgia to donate blood. The studies were performed according to the guidelines of the World Medical Association’s Declaration of Helsinki. Enrolled blood donors signed consent forms as described previously [25, 26]. The human blood protocol (UGA# 2012-10769) and the associated consent form were reviewed and approved by the Institutional Review Board (IRB) of the University of Georgia.
Human neutrophils and autologous serum
Human neutrophils were isolated as described previously [21, 26]. Briefly, coagulation was prevented with heparin and red blood cells were removed by Dextran sedimentation (GE Healthcare). Neutrophils were isolated using five-step Percoll gradient centrifugation. Cell viability was >98% (Trypan Blue, Sigma-Aldrich, St. Louis, MO, USA) and neutrophil purity was >95% (cytospin and flow cytometry). Autologous serum was prepared by centrifugation and sterile filtration. Calcium- and magnesium-containing HBSS (Mediatech, Manassas, VA, USA) supplemented with 1% autologous serum, 5 mM glucose, and 10 mM HEPES was used as assay buffer.
PBMC isolation and in vitro macrophage differentiation
Peripheral blood mononuclear cells were isolated from 65% Percoll gradient interphase, washed twice with PBS, and seeded at 2–3 million cells/well concentration in RPMI medium supplemented with Glutamax (Gibco Thermo Fischer, Waltham, MA, USA). The next day, media were supplemented with 50 ng/ml M-CSF and 1% autologous serum or FBS, and macrophages were differentiated for 5–7 days into M1 phenotype as described previously [13].
Macrophage supernatant collection (macrophage-conditioned medium)
Differentiated macrophage cultures were primed with 10 μg/ml LPS for 30 min to mimic proinflammatory conditions found in the gout synovium. Macrophages were then washed 4× with HBSS to remove LPS and were subsequently incubated with or without 250 μg/ml MSU crystals for 5 h. Supernatants were collected and then spun at 10,000 g for 5 min. The pellet was discarded; cell-, crystal- and debris-free supernatants were frozen at −80 °C and called the ‘macrophage-conditioned medium’.
Extracellular DNA release assay
Neutrophil extracellular traps were quantified essentially as described previously [21, 27]. Briefly, PMNs were allowed to adhere to poly d-lysine coated 96-well black transparent bottom plates (Thermo Scientific, Rochester, NY, USA) in assay buffer with 0.2% Sytox Orange (Life Technologies, Grand Island, NY, USA). Fluorescence (excitation: 530 nm, emission: 590 nm) was measured in a fluorescence microplate reader (Varioskan Ascent, Thermo Scientific) for 6 h at 37 °C. Increase in fluorescence normalized on maximal value (saponin-treated PMNs) was referred to as “extracellular DNA release” and expressed as “% of max”. The obtained relative fluorescence units (RFU) were sometimes also normalized against PMA.
Confocal microscopy of NETs
As previously detailed [21, 26], 2 × 106 human neutrophils in assay medium were incubated for 4 h at 37 °C with or without 100 nM PMA and 100 μg/ml MSU crystals in 35 mm glass bottom dishes (MatTek, Ashland, MA, USA) pre-coated for 1 h with 1% human serum albumin (Sigma Aldrich, St Louis, MO, USA). 2.5 μM Sytox Orange (Invitrogen, Grand Island, NY, USA) was added prior to fluorescence imaging (exc/em: 547/570 nm). Images were collected using a Nikon A1R confocal microscope system equipped with a Nikon Eclipse Ti-E inverted microscope, built in Perfect Focus, high-speed motorization, and NIS Elements software (Nikon Instruments, Melville NY, USA). Live cell imaging was carried out using a Tokai Hit INY-G2A-TIZ incubator (Tokai Hit CO, Ltd., Shizuoka-kenwith Japan) for temperature, humidity, and CO2 control. The Coherent Sapphire 561 nm 20 mW laser was used to excite Sytox Orange using a CFI Plan APO VC 60X Oil NA 1.4 WD 0.13 mm objective. Optical sections of neutrophils were analyzed, Z-stacks spanning 1 μm were acquired, and 3-D image reconstructions were processed using the Nikon NIS Element Version 4 software. Final image preparation was carried out using Adobe Photoshop.
High-throughput live imaging of NET formation
A microplate-based NET imaging assay was established using a motorized stage support of a confocal microscope. PMNs were seeded at a concentration of 250,000 cells/well in a 96-well plate Optical Btm Pit Polymer Base black plate (Thermoscientific, Rochester, NY, USA). PMNs were preincubated with 10 ng/ml IL-1β prior to imaging. PMNs were then stimulated with 200 μg/ml MSU crystals for 16 h. Nikon A1R confocal microscope system with a 10× lens was used to capture transmitted light and fluorescence images. A field of interest was chosen to perform imaging and their position was defined using the NIS elements software. The chosen field was a true representation of the entire well. Images were taken every 15 min for 16 h using automated capture component of the NIS elements software. Mean Sytox Orange intensities of the fluorescent images were quantified using the measure region-of-interest (ROI) feature of the Nikon A1 Elements software. The entire image which represented the field was selected as the ROI. These measurements were used to calculate changes in Sytox Orange intensities over time (∆Mean Sytox Orange intensity). Samples were always run as three biological replicates.
Measurement of AggNET formation
Visualization of aggregated NET formation was modified after adaptation [5]. 1,000,000 PMNs in assay medium were added in a 48-well plate with or without 1 mg/ml MSU crystals at 37 °C with or without 10 ng/ml IL-1β. Images of aggNETs were taken after 5 h incubation using an inverted light microscope [5].
Elisa
Myeloperoxidase (MPO), IL-1β, or IL-8 levels were quantitated using commercial ELISA kits from R&D systems (Minneapolis, MN, USA), Millipore (Billerica, MA, USA), and BioLegends (San Diego, CA, USA), respectively. Manufacturer’s instructions were followed for sample dilutions and processing. 250,000 PMNs/well were seeded in 24-well plates and stimulated with 250 μg/ml MSU crystals in HBSS for up to 4 h at 37 °C. Cell supernatants collected were either immediately processed or stored (−80 °C) for later analysis. Undiluted supernatants were used to measure IL-8 and IL-1β, whereas supernatants were diluted 1:100 to determine MPO and HNE concentrations. HNE release was assessed as described [25, 26] by ELISA using anti-HNE rabbit polyclonal antibody (Merck Millipore, Billerica, MA). Supernatant samples diluted with coating buffer (25 mM carbonate, 25 mM bicarbonate, pH 9.6) were incubated overnight at 4 °C in 96-well high-binding microlon ELISA plates (Greiner bio-one, Fricken-hausen, Germany). After blocking with 1% bovine serum albumin (BSA) for 1 h, anti-rabbit mouse polyclonal Ab (1:500 in PBS [Calbiochem], 481001 [Merck Millipore]) was added for 2 h at room temperature (RT). After repeated washes, samples were incubated with horseradish peroxidase-linked donkey anti-rabbit Ab (1:2000 in PBS, NA934V; GE Healthcare) for 1 h. Yellow coloration of the TMB substrate (Thermo Scientific, Rockford, IL, USA) after the addition of 1 N hydrochloric acid (Sigma-Aldrich, St. Louis, MO, USA) was read at 450-nm wavelength with Eon microplate photometer (BioTek, Winooski, VT, USA). Commercially available HNE (stock: 1 mg/ml; Cell Sciences, Canton, MA, USA) was used as standard.
HNE-DNA ELISA
NETs were quantitated as described previously [25, 27]. Briefly, PMN supernatants were exposed to DNAse1 digestion for 15 min that was stopped by adding 2.5 mM EGTA. Diluted supernatants and “NET standard samples” were added to 96-well high-binding microlon ELISA plates (Greiner bio-one, Fricken-hausen, Germany) coated with anti-HNE rabbit antibody. Horse radish peroxidase-labeled anti-DNA secondary antibody (1:500 in PBS) was added for 2 h followed by repeated washes. Absorbance of the TMB substrate was read after addition of 1 N hydrochloric acid at 450 nm with Eon microplate photometer (BioTek, Winooski, VT, USA). NET’s were quantitated as percentage of the NET standard in the undiluted supernatants.
Quantification of enzymatic activity
To measure HNE activity, the Neutrophil Elastase Activity Assay Kit (Cayman Chemical Ann Arbor, MI, USA) was used following manufacturer’s protocol. Briefly, supernatants (50 μl) were placed into 96-well black plates. Substrate (Z-Ala-Ala-Ala-Ala 2Rh110, cat no. 600613) solution was added to assess elastase activity by measuring production of the highly fluorescent product (compound R110) using 485 nm excitation and 525 nm emission wavelengths in microplate fluorimeter (Varioskan Flash, Thermo Scientific). Data are expressed either as kinetics (RFU) or endpoint values normalized on maximal (PMA-stimulated) data.
Statistical analysis
Data are represented as mean ± SEM. p value was calculated with ANOVA and Dunnett’s or Tukey’s post hoc tests, and was marked as * when p < 0.05, ** when p < 0.01 and *** when p < 0.001.
Results
Secreted neutrophil products do not affect MSU crystal-induced NET release
PMNs release NETs in response to gout-causing MSU crystals [5, 6, 18]. First, we exposed human PMNs to different doses of MSU crystals and measured NET formation using the extracellular DNA-binding dye, Sytox Orange. MSU crystals induced NET release in a dose-dependent manner (Fig. 1a). An MSU crystal concentration of 100 μg/ml was required to trigger a minimal NET release that is higher than the signal without crystals (Fig. 1a). Throughout the manuscript, 100 or 250 μg/ml MSU crystal doses were used that are significant and detectable but still small enough to leave room to record any NETosis-enhancing effects.
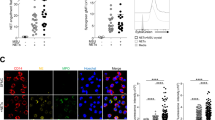
Supernatants of macrophages, but not neutrophils, enhance extracellular DNA release from MSU crystal-stimulated human neutrophils. a Dose-dependence of MSU crystal-stimulated NET release in human PMNs measured in the presence of the fluorescent, extracellular DNA-binding dye, Sytox Orange. Mean ± SEM, n = 3. b–d Human macrophages differentiated with M-CSF for 5–7 days or neutrophils were exposed to 250 μg/ml MSU crystals for 5 h. Collected supernatants were centrifuged to remove cells, crystals and debris and referred to as “PMN-conditioned medium” or “macrophage-conditioned medium”, respectively. The effects of conditioned media on NET formation were subsequently tested on human neutrophils stimulated in the absence (UT, untreated) or presence (MSU) of 250 μg/ml MSU crystals by Sytox Orange-based fluorescence. Fluorescence data were normalized on the 100 nM PMA-triggered signal as the positive control for NET formation. b “PMN-conditioned media” of neither MSU crystal-stimulated (MSU PMN) nor untreated neutrophils (UT PMN) had any significant effect on NET release (No Sup). Mean ± SEM, n = 4, Dunnett’s test. c “Macrophage-conditioned media” of MSU crystal-stimulated macrophages (MSU MAC) significantly increased NET formation in comparison to that seen in the absence of conditioned media (No Sup). Conditioned media of macrophages without crystal stimulation (UT MAC, untreated) had no significant effect. Mean ± SEM, n = 11, Dunnett’s test. d Representative confocal microscopy images of MSU crystal-stimulated human neutrophils show enhanced DNA release (Sytox Orange) by the MSU crystal-stimulated macrophage-conditioned media compared (right panels) to that without addition of conditioned media (left panels) (n = 3). (1) Merged images of transmitted light and Sytox Orange fluorescence and (2) three-dimensional Z-stack fluorescent images are shown for both experimental conditions. Black bars indicate 10 μm distance. RFU relative fluorescence unit, PMA phorbol myristate acetate, MAC macrophage, PMN neutrophil, MSU monosodium urate, n.s. not significant; ****p < 0.0001
To assess whether PMNs themselves promote NET formation, we first stimulated human PMNs with MSU crystals and collected supernatants after 5 h. Cells, debris, and crystals were removed by centrifugation resulting in a clean supernatant referred to as “PMN-conditioned medium” that was kept frozen. This conditioned medium was then added to unchallenged PMNs isolated from another donor in the presence or absence of MSU crystals and DNA release was followed. As shown in Fig. 1b, the PMN-conditioned medium did not affect NET formation indicating that PMNs do not intrinsically accelerate MSU crystal-induced NETs in a positive feedback fashion.
Macrophages release NETosis-enhancing mediators upon MSU crystal stimulation
Since macrophages are present in the gout synovium in large numbers and are responsible for initiating inflammation [2], we repeated the same experiment as before using macrophages, not PMNs, to collect the conditioned medium. When this “macrophage-conditioned medium” was added to human PMNs, MSU crystal-induced DNA release was significantly enhanced (Fig. 1c). Spontaneous NET formation remained unaffected (Fig. 1c). These data were also confirmed by confocal microscopy showing the larger amount of three-dimensional extracellular DNA release in the presence of macrophage-conditioned medium than in its absence (Fig. 1d). These results indicate that MSU crystal-exposed macrophages release secretory products that strongly accelerate NET formation of PMNs stimulated by MSU crystals.
Macrophage-conditioned medium increases the release of primary granule markers and NETs from MSU crystal-stimulated PMNs
Neutrophil extracellular traps are composed of an extracellular DNA meshwork associated with histones and PMN granule components, such as myeloperoxidase (MPO) and neutrophil elastase (NE) [22]. The definition of NETs requires the detection of DNA–protein complexes characteristic for NETs. First, we confirmed that we can detect MPO (p = 0.0469) and HNE (p = 0.0694) by ELISA in the supernatants of human PMNs after MSU crystal stimulation (Fig. 2a, b). Second, we observed that addition of macrophage-conditioned medium significantly enhanced MSU crystal-induced MPO (p < 0.0001) and HNE (p = 0.0003) release (Fig. 2a, b). To prove that released MPO and DNA are present in a complex defining NETs, we utilized an ELISA developed in our laboratory capable of quantitating NET-specific MPO-DNA complexes [25, 27]. MSU crystals initiated NET release that was further enhanced by mediators secreted by macrophages (Fig. 2c). Overall, our results presented so far describe a novel mechanism by which NET formation is enhanced in PMNs by macrophages.
Macrophage supernatants increase MPO, HNE, and NET release from human neutrophils following MSU crystal stimulation. Differentiated human macrophages were exposed to 250 μg/ml MSU crystals for 5 h to collect and centrifuge their supernatants. This “macrophage-conditioned medium” (MSU MAC) was added to MSU crystal-stimulated human neutrophils and releases of a MPO (n = 9), b HNE (n = 9) and c NETs (MPO-DNA complexes, n = 6) were quantitated by ELISA assays (mean ± SEM, Dunnett’s test). As comparisons, PMNs were also treated with mock only (No MAC) or supernatants of macrophages without crystal stimulation (UT MAC, untreated). MPO myeloperoxidase, HNE human neutrophil elastase, MAC macrophage. *p < 0.05; **p < 0.01; ***p < 0.001
IL-1β potentiates MSU crystal-induced DNA release from PMNs
As the next step, we aimed at identifying which macrophage-secreted component is responsible for the NETosis-enhancing effect. Macrophages have been described to release several cytokines and other inflammatory mediators upon MSU crystal exposure [2]. IL-1β was our primary suspect potentially responsible for the NET-promoting effect of macrophages, because IL-1β is a central cytokine orchestrating gout inflammation and it is released in copious amounts from MSU crystal-stimulated macrophages [2, 7, 9]. We confirmed that differentiated macrophages produced IL-1β upon MSU crystal activation in our hands, as well (Fig. 3a). Human PMNs failed to release any detectable IL-1β (Fig. 3a). We stimulated human PMNs with MSU crystals in the presence of recombinant human IL-1β and measured extracellular DNA release. While IL-1β had only a minimal NETosis-enhancing effect when 100 μg/ml MSU crystals were used, it strongly amplified NET formation in a dose-dependent manner when 250 μg/ml MSU crystal concentration was applied (Fig. 3b, c). The highest dose of IL-1β enhanced NET formation to the extent observed with the positive control PMA (Fig. 3b, c). Interestingly, IL-1β alone (without MSU crystals) did not induce NET release (Fig. 3b, c). Lower doses of MSU crystals were chosen to be used in these experiments that allow detection of any potential, NETosis-enhancing effect of IL-1β. These results indicate a novel effect of IL-1β, a central cytokine in gout inflammation, on PMN activation by MSU crystals.
IL-1β promotes DNA release from human neutrophils stimulated with MSU crystals. a MSU crystal stimulation resulted in IL-1β release in human macrophages (MF) but not in human PMNs as measured by ELISA. Mean ± SEM, n = 4. Student’s t test. b Following treatment with increasing doses of recombinant human IL-1β, human neutrophils were left unstimulated or were stimulated with 100 or 250 μg/ml MSU crystals and DNA release was measured in the presence of the membrane-impermeable DNA-binding dye, Sytox Orange. DNA release over 4 h was presented as endpoint values (mean ± SEM, n = 3). c Kinetics of the previous experiment (b) (one representative, n = 4). Fluorescence data were normalized on the maximal signal obtained by stimulation with 100 nM PMA. RFU relative fluorescence unit, MSU monosodium urate, UT untreated, PMA phorbol myristate acetate, n.s. not significant; *p < 0.05; ***p < 0.001; ****p < 0.0001
IL-1β enhances MPO, HNE, and NET release following MSU crystal stimulation
To confirm that the DNA release-promoting effect of IL-1β is also accompanied by enhanced levels of released granule markers and NETs, we quantitated MPO, HNE, HNE-DNA, and HNE enzymatic activity levels in supernatants of MSU crystal-induced PMN in the absence or presence of IL-1β. We found that IL-1β significantly promoted the release of MPO and HNE from human PMNs after MSU crystal stimulation (Fig. 4a, b, p < 0.05 in each combination between untreated and MSU crystal-treated samples). In addition, HNE remained enzymatically active and bound to DNA in PMN supernatants after MSU crystal activation (Fig. 4c, d). These data are in accordance with those presented in Fig. 3, indicating that IL-1β alone does not induce NET formation, but it significantly promotes NET release triggered by MSU microcrystals.
IL-1β enhances MSU crystal-stimulated releases of MPO, HNE, and NETs from human neutrophils. Human neutrophils were treated with recombinant human IL-1β (0–5 ng/ml) prior to addition of 250 μg/ml MSU crystals. Neutrophils were also left unstimulated (UT, no crystals). Five hours later, the following parameters were quantitated in the supernatants: a MPO by commercial ELISA (n = 5), b HNE by ELISA (n = 9), c NETs (HNE-DNA ELISA, n = 7), and d HNE activity by commercial kit (n = 7). NET release values were normalized on PMA (100%). Data are shown as mean ± SEM Significance was calculated using Dunnett’s test. *p < 0.05; **p < 0.01. MPO myeloperoxidase, HNE human neutrophil elastase, PMA phorbol myristate acetate
Imaging the NET-promoting effect of IL-1β by high-throughput confocal live microscopy
Next, we performed high-throughput imaging to visualize NET formation real-time using confocal microscopy. This method developed in our laboratory enables simultaneous imaging of NET-forming live PMNs on a 96-well-based format in presence of Sytox Orange. Recorded videos allow spatiotemporal resolution of the NET-forming process, while kinetic data enable concomitant quantitative comparisons.
The recorded videos confirmed the previous data. IL-1β alone did not induce DNA release, while MSU crystals triggered NET formation (Fig. 5a, b). Addition of IL-1β to MSU crystals resulted in more robust NET release than MSU crystals alone (Fig. 5a, b). In addition, video recordings show that PMNs start to aggregate soon after MSU crystal exposure (Fig. 5a). This microaggregate formation is unique to MSU crystals among NET-inducing stimuli and forms the microscopic basis of later, macroscopic aggNET formation.
IL-1β increases MSU crystal-induced aggregated NET formation. 250,000 human PMNs/well were incubated in wells of a 96-well microplate with IL-1β (10 ng/ml) and stimulated with 100 μg/ml MSU crystals or 100 nM PMA for 15 h in the presence of the extracellular DNA-binding dye, Sytox Orange. Interactions of PMNs and crystals were followed using confocal microscopy every 15 min. a Representative images taken at t = 0, 2, 4, 6 h are shown (n = 3). Black bars indicate 100 μm distances. Insets indicated by white frames are enlarged on the right side. b Representative kinetics of Sytox Orange fluorescence curves followed for 8 h (n = 3, mean ± S.D. at each timepoint). c AggNET formation was evaluated by light microscopy after a 5-h incubation of human PMNs with 1 mg/ml MSU crystal in the presence or absence of 10 ng/ml human IL-1β (one representative image, n = 3). MSU monosodium urate, aggNET aggregated NETs, RFU relative fluorescence unit
IL-1β enhances aggregated NET formation
Monosodium urate crystals induce the formation of aggregated NETs (aggNET) at higher PMN densities that provide the structural basis of gout tophi [5]. Since aggNET formation depends on MSU crystal-induced NET release that is enhanced by IL-1β, we wanted to study how IL-1β affects aggNET formation. Human PMNs at higher cell density were exposed to enhance MSU crystal dose (1 mg/ml) modeling peak phases of gout inflammation [5]. As shown in Fig. 5c, MSU crystals initiated aggNET formation that was further enhanced by IL-1β, as assessed by light microscopy. Thus, acceleration of MSU crystal-triggered NET release by IL-1β also leads to faster and more robust aggregated NET formation in vitro.
Anakinra abolishes the NET-promoting effect of macrophages
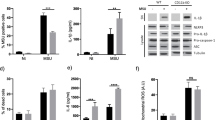
To prove that IL-1β is the main component found in macrophage supernatants promoting MSU crystal-induced NET release, we pretreated PMNs with clinical grade anakinra, an IL-1R antagonist, prior to addition of MSU crystals and macrophage supernatants. Supernatants were collected from macrophages obtained from nine independent volunteers (donors #4–12) and were tested in groups of three on PMNs isolated from three, additional blood donors (donors #1–3) (Fig. 6a). Although we experienced donor-to-donor variations (Fig. 6a), anakinra generally efficiently inhibited the NET-enhancing effect of the MSU crystal-stimulated macrophage supernatants (Fig. 6b). Anakinra treatment resulted in a 76.5 ± 6.3% (mean ± SEM, n = 12) inhibition of the signal (Fig. 6b). Thus, we described a novel effect of anakinra that could be a significant component of its in vivo mechanism of action.
Anakinra inhibits the NET-enhancing effect of macrophage supernatants. a Macrophages were isolated and differentiated as described from nine independent donors (donors #4–12) and macrophage-conditioned media were collected when cells were left untreated (UT, no crystals) or stimulated with 250 μg/ml MSU crystals for 5 h (MSU). Supernatants were cleaned, frozen, and used in groups of three on PMNs isolated from three independent blood donors as indicated. PMNs were pretreated with 30 mg/ml anakinra for 1 h before addition of 100 μg/ml MSU crystals and/or macrophage supernatants. EcDNA release was measured in PMNs for 6 h following stimulation by the crystals using Sytox Orange dye. Results are expressed as percentage of maximal DNA release and shown as mean ± S.D. of triplicates. b Data shown in a were further analyzed by calculating the MSU crystal-induced DNA release signal and its averages. Data are shown as mean ± SEM (n = 12) and were analyzed by one-way ANOVA and Tukey’s post hoc test. *p < 0.05; ***p < 0.001. MAC macrophage, PMN neutrophil, UT untreated
Discussion
In line with previous reports of other groups, we confirmed that gout-causing MSU crystals elicit robust NET formation in human PMNs in vitro [5, 18, 23, 28]. Concentrations of MSU crystals higher than 100 μg/ml induce a significant extracellular DNA release in PMNs. The 100–1000 μg/ml MSU crystal dose range used here represents pathophysiological concentrations found in the synovial fluid of gout patients [5]. Therefore, the in vitro findings described here likely have human in vivo clinical relevance.
PMN-conditioned media did not alter MSU crystal-induced NET release indicating that PMNs themselves do not secrete any inflammatory mediators that would promote NET formation in a positive feedback fashion. Macrophages, however, were found to significantly enhance NET release following MSU crystal exposure in PMNs. The interaction between PMNs and macrophages is an essential component of gout inflammation in joints [16, 29]. It is known that resident macrophages at the synovium represent the first cell type to come in contact with MSU microcrystals and release several cytokines that attract additional macrophages and PMNs to the site of inflammation [16, 29]. The previous reports described how macrophages react to already formed NETs [30–32], but their NETosis-enhancing effect described here is a new finding that adds to the complex interplay between macrophages and PMNs.
Results shown here suggest that IL-1β is the major macrophage-derived molecules promoting NET formation. These results complement previously published data showing that gout synovial fluid supernatants promote spontaneous NET release in human PMNs that are partially inhibited by anakinra [18]. MSU crystal stimulation of macrophages leads to NLRP3 inflammasome activation and production of the proinflammatory cytokine IL-1β [1, 7]. IL-1β initiates PMN chemotaxis that is thought to be the main mediator of PMN influx to the synovium in gout [7]. Accumulating data suggest more complex mechanisms by which macrophages interact with PMNs in gout indicating that macrophage-derived IL-1β not only attracts PMNs to the site of inflammation, but it also accelerates the release of PMN-derived inflammatory mediators, including NETs. Not only caspase-1 but neutrophil elastase can also cleave pro-IL-1β producing bioactive IL-1β [3, 33]. This mechanism would accelerate inflammation, since IL-1β would enhance its own activation by stimulating PMNs to release NET-related proteases. Data shown here also propose that targeting IL-1β in gout in vivo fulfills its beneficiary effects not only through diminished PMN recruitment but also via attenuated PMN activation by MSU crystals.
Interestingly, IL-1β alone does not induce NETs in our hands, but it enhances NET release induced by MSU crystals. Whether this observation is unique to MSU crystals or it is also true for other NET-triggering stimuli, such as bacteria, fungi, or other microcrystals, remains to be investigated. Several potential mechanisms could underlie the NETosis-enhancing effect of IL-1β. IL-1β could increase NET formation by its known beneficiary effect on the activation of the NADPH oxidase [34, 35]. The NADPH oxidase is required for MSU crystal-induced NET formation [5]. IL-1β could enhance the phosphorylation of the oxidase subunits or their expression. This mechanism is likely because human PMNs of IRAK4- or NEMO-deficient patients with impaired TLR/IL-1R signaling have reduced NADPH oxidase priming and activation [36]. IL-1β is a known chemoattractant for PMNs [37]. Although it remains to be studied, a migratory component of MSU crystal-induced NET/aggNET formation is likely that could be promoted by IL-1β.
At the same time, IL-1β also promotes aggNET formation, a novel mechanism proposed to shut down acute inflammation in gout [5]. The highly concentrated protease activity of aggNETs degrades several proinflammatory cytokines, including IL-1β, putting a break on the inflammatory process [5]. Therefore, our results implicate that enhancing the aggNET-promoting mechanism of IL-1β offers a novel anti-inflammatory strategy and could have therapeutic benefits.
Although IL-1β is the major proinflammatory molecule released by macrophages promoting gout inflammation, other secretory products could also promote NET formation. IL-8, C5a, G-CSF, IFN-γ, and TNF-α have all been reported to promote NET formation [5, 21, 38–40]. G-CSF is indispensable for neutrophil functions [41] and assists in the NETosis process [16, 28, 42]. TNF-α is released during the acute phase of gout and can trigger a NET response [16, 21, 28, 39, 42]. The potential contributions of these inflammatory mediators to the NETosis-enhancing effect of macrophages remain to be studied in the future.
In summary, our data presented here provide a new link between macrophages and PMNs, two important innate immune cell types mediating the general and pathological inflammatory pathways. IL-1β is a major player in gout, and NET formation promoted by IL-1β could contribute to gouty inflammation.
Abbreviations
- NETs:
-
Neutrophil extracellular traps
- AggNET:
-
Aggregated neutrophil extracellular traps
- PMN:
-
Polymorphonuclear neutrophils
- IL-1β:
-
Interleukin-1 beta
- PBMC:
-
Peripheral blood mononuclear cells
- NLRP3:
-
NACHT, LRR, and PYD domains-containing protein 3
- ROS:
-
Reactive oxygen species
- MSU:
-
Monosodium urate
- MPO:
-
Myeloperoxidase
- NE:
-
Neutrophil elastase
- HNE:
-
Human neutrophil elastase
- M-CSF:
-
Macrophage colony stimulating factor
References
Busso N, So A. Microcrystals as DAMPs and their role in joint inflammation. Rheumatology (Oxford). 2012;51:1154–60.
Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443–52.
Mitroulis I, Kambas K, Ritis K. Neutrophils, IL-1beta, and gout: is there a link? Semin Immunopathol. 2013;35:501–12.
Pope RM, Tschopp J. The role of interleukin-1 and the inflammasome in gout: implications for therapy. Arthritis Rheum. 2007;56:3183–8.
Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–7.
Schorn C, Janko C, Krenn V, Zhao Y, Munoz LE, Schett G, et al. Bonding the foe—NETting neutrophils immobilize the pro-inflammatory monosodium urate crystals. Front Immunol. 2012;3:376.
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41.
Oliveira SH, Canetti C, Ribeiro RA, Cunha FQ. Neutrophil migration induced by IL-1beta depends upon LTB4 released by macrophages and upon TNF-alpha and IL-1beta released by mast cells. Inflammation. 2008;31:36–46.
Schett G, Dayer JM, Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12:14–24.
Schlesinger N. Canakinumab in gout. Expert Opin Biol Ther. 2012;12:1265–75.
Schlesinger N. Treatment of chronic gouty arthritis: it is not just about urate-lowering therapy. Semin Arthritis Rheum. 2012;42:155–65.
Amaral FA, Costa VV, Tavares LD, Sachs D, Coelho FM, Fagundes CT, et al. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum. 2012;64:474–84.
Rada B, Park JJ, Sil P, Geiszt M, Leto TL. NLRP3 inflammasome activation and interleukin-1beta release in macrophages require calcium but are independent of calcium-activated NADPH oxidases. Inflamm Res. 2014;63:821–30.
Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116:1570–3.
van Bruggen R, Koker MY, Jansen M, van Houdt M, Roos D, Kuijpers TW, et al. Human NLRP3 inflammasome activation is No 1–4 independent. Blood. 2010;115:5398–400.
Busso N, So A. Mechanisms of inflammation in gout. Arthritis Res Ther. 2010;12:206.
Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther. 2006;8(Suppl 1):S3.
Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, et al. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 2011;6:e29318.
Naccache PH, Grimard M, Roberge CJ, Gilbert C, Lussier A, de Medicis R, et al. Crystal-induced neutrophil activation. I. Initiation and modulation of calcium mobilization and superoxide production by microcrystals. Arthritis Rheum. 1991;34:333–42.
Popa-Nita O, Naccache PH. Crystal-induced neutrophil activation. Immunol Cell Biol. 2010;88:32–40.
Pang L, Hayes CP, Buac K, Yoo DG, Rada B. Pseudogout-associated inflammatory calcium pyrophosphate dihydrate microcrystals induce formation of neutrophil extracellular traps. J Immunol. 2013;190:6488–500.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5.
Desai J, Kumar SV, Mulay SR, Konrad L, Romoli S, Schauer C, et al. PMA and crystal-induced neutrophil extracellular trap formation involves RIPK1-RIPK3-MLKL signaling. Eur J Immunol. 2016;46:223–9.
Chhana A, Dalbeth N. The gouty tophus: a review. Curr Rheumatol Rep. 2015;17:19.
Yoo DG, Floyd M, Winn M, Moskowitz SM, Rada B. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes. Immunol Lett. 2014;160:186–94.
Yoo DG, Winn M, Pang L, Moskowitz SM, Malech HL, Leto TL, et al. Release of cystic fibrosis airway inflammatory markers from Pseudomonas aeruginosa-stimulated human neutrophils involves NADPH oxidase-dependent extracellular DNA trap formation. Journal of immunology. 2014;192:4728–38.
Sil P, Yoo DG, Floyd M, Gingerich A, Rada B. High throughput measurement of extracellular DNA release and quantitative NET formation in human neutrophils in vitro. J Vis Exp. 2016;(112). doi:10.3791/52779.
Schorn C, Janko C, Latzko M, Chaurio R, Schett G, Herrmann M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front Immunol. 2012;3:277.
Busso N, Ea HK. The mechanisms of inflammation in gout and pseudogout (CPP-induced arthritis). Reumatismo. 2011;63:230–7.
Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191:2647–56.
Nakazawa D, Shida H, Kusunoki Y, Miyoshi A, Nishio S, Tomaru U, et al. The responses of macrophages in interaction with neutrophils that undergo NETosis. J Autoimmun. 2016;67:19–28.
Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–20.
Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol. 2004;24:8055–68.
Chenevier-Gobeaux C, Lemarechal H, Bonnefont-Rousselot D, Poiraudeau S, Ekindjian OG, Borderie D. Superoxide production and NADPH oxidase expression in human rheumatoid synovial cells: regulation by interleukin-1beta and tumour necrosis factor-alpha. Inflamm Res. 2006;55:483–90.
Yan B, Han P, Pan L, Lu W, Xiong J, Zhang M, et al. IL-1beta and reactive oxygen species differentially regulate neutrophil directional migration and Basal random motility in a zebrafish injury-induced inflammation model. J Immunol. 2014;192:5998–6008.
Singh A, Zarember KA, Kuhns DB, Gallin JI. Impaired priming and activation of the neutrophil NADPH oxidase in patients with IRAK4 or NEMO deficiency. J Immunol. 2009;182:6410–7.
Perretti M, Appleton I, Parente L, Flower RJ. Pharmacology of interleukin-1-induced neutrophil migration. Agents Actions 1993;38 Spec No:C64–5.
Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–82.
Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–95.
Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218.
Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–23.
Torres R, Macdonald L, Croll SD, Reinhardt J, Dore A, Stevens S, et al. Hyperalgesia, synovitis and multiple biomarkers of inflammation are suppressed by interleukin 1 inhibition in a novel animal model of gouty arthritis. Ann Rheum Dis. 2009;68:1602–8.
Acknowledgements
We thank the personnel at the UGA University Health Center for blood collection and the College of Veterinary Medicine Imaging Core for technical assistance with confocal microscopy and flow cytometry. We are also grateful to Dr. Pramod Giri (UGA) for his help with optimizing the macrophage differentiation protocol. We are also thankful for Dr. Jeremy Sokolove (Stanford University) for providing clinical grade anakinra (Swedish Orphan Biovitrum; purchased via Stanford University Research Pharmacy) used in this study. This work was supported by the start-up fund of Dr. Rada provided by the Office of Vice President for Research, UGA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest to report.
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Sil, P., Wicklum, H., Surell, C. et al. Macrophage-derived IL-1β enhances monosodium urate crystal-triggered NET formation. Inflamm. Res. 66, 227–237 (2017). https://doi.org/10.1007/s00011-016-1008-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-1008-0