Abstract
Background
Fecal calprotectin has been extensively investigated as a screening marker for the detection of necrotizing enterocolitis (NEC). However, there is a complete lack of consensus regarding its efficacy as a diagnostic test.
Objective
The purpose of the present systematic review is to evaluate the effectiveness of fecal calprotectin as a screening marker for the detection of NEC.
Materials and methods
We conducted a systematic review of studies published in the Medline (1966–2016), Scopus (2004–2016), ClinicalTrials.gov (2008–2016), Cochrane Central Register of Controlled Trials CENTRAL (1999–2016), and Google Scholar (2004–2016) databases, combined with studies found in the reference lists of the included studies. All prospective and retrospective observational cohort studies were included.
Results
Thirteen studies that included 601 neonates were identified in the international literature. The presence and severity of NEC was evaluated with the modified Bell’s criteria. Ten studies found significantly elevated fecal calprotectin levels among infants with NEC (p < 0.05). One study found that this effect was observed irrespective of the stage of the disease. Five studies evaluated the efficacy of fecal calprotectin as a diagnostic test. The reported sensitivity ranged between 76 and 100 %, and the specificity varied from 39 to 96.4 %. However, the proposed cut-off values were not similar.
Conclusion
Current evidence suggests that fecal calprotectin is elevated in newborns suffering from NEC. However, its significance as an early screening marker remains unknown. Future studies are needed and should focus on the identification of specific cut-off values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Necrotizing enterocolitis (NEC) is characterized by variable damage to the intestinal tract that ranges from mild mucosal defects to full-thickness necrosis of the bowel wall. The incidence of NEC ranges from 10 to 15 % and the estimated mortality rate fluctuates between 10 and 30 % in very low birth weight (VLBW) infants [1]. Its exact pathogenetic mechanisms remain, to date, unknown. However, gastrointestinal ischemia, alimentation, and microbiota in combination with immature gastrointestinal functions and host defense mechanisms are believed to play a key role in the progression of NEC [2]. The pattern described by Bell et al. in 1978 was the first systematic description of NEC [3]. Current treatment strategies for the treatment of advanced NEC with intestinal perforation include laparotomy and primary peritoneal drainage [4].
NEC prognosis can be improved by early detection and accurate evaluation of disease severity and activity over time. To date, no specific markers of inflammation of the gastrointestinal tract exist in neonates. Recently, calprotectin has been investigated extensively as a potential biomarker. However, there is still no consensus regarding its use in current clinical practice [5, 6]. Calprotectin is a 36-kDa calcium- and zinc-binding cytosolic protein and a member of the S100 family of proteins, which are released from neutrophils or monocytes and followed by the pro-inflammatory activation of pattern recognition receptors [7]. It is secreted into the intestinal lumen during the early phases of intestinal mucosal damage and thus could be useful for the early detection of NEC and possibly for tracking the course of the disease. Fetal calprotectin is easily found in stool and is measured by ELISA [8–10].
The purpose of our systematic review is to synthesize the current knowledge in the field and to reach firm conclusions regarding the use of calprotectin as a potential screening marker of NEC in preterm neonates.
Methods
Study design
The present study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. Eligibility criteria were predetermined by the authors. No language or date restrictions were applied during the literature search. All observational studies (both prospective and retrospective) were included in the systematic review. Case reports and review articles were excluded from tabulation and analysis. Two researchers tabulated the selected indices using structured forms. Any discrepancies in the methodology, article retrieval, and statistical analysis were resolved by reaching consensus among all authors.
Literature search and data collection
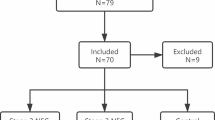
We used the Medline (1966–2016), Scopus (2004–2016), Popline (1974–2016), ClinicalTrials.gov (2008–2016), and Cochrane Central Register of Controlled Trials CENTRAL (1999–2016) databases in our primary search, along with the reference lists of electronically retrieved full text papers. Our search was restricted to a minimum number of keywords to assess an eligible number that could be hand searched, minimizing the potential loss of articles. All the articles that met or were presumed to meet the inclusion criteria were retrieved in full text. Search strategies and results are shown in Fig. 1.
Our search strategy included the terms calprotectin, enterocolitis, S100A8, S100A9. We specifically searched Pubmed using the MeSH terms “(“leukocyte l1 antigen complex”[MeSH Terms] OR (“leukocyte”[All Fields] AND “l1”[All Fields] AND “antigen”[All Fields] AND “complex”[All Fields]) OR “leukocyte l1 antigen complex”[All Fields] OR “calprotectin”[All Fields]) AND (“enterocolitis”[MeSH Terms] OR “enterocolitis”[All Fields])”. The PRISMA flow diagram depicts the method of article retrieval (Fig. 1).
Definitions
The severity of NEC was assessed using the modified Bell’s criteria, which stratifies cases into the following categories: suspected (Stage I), mild (Stage IIa), moderate (Stage IIb), severe without bowel perforation (Stage IIIa), and severe with bowel perforation (Stage IIIb). Evaluation is based on the presence of systemic findings (i.e., metabolic or respiratory acidosis, acidemia, neutropenia, etc.), intestinal findings (i.e., abdominal distention, blood stool, signs of peritonitis, abdominal cellulitis, etc.), radiological findings (i.e., pneumatosis intestinalis, ileus, pneumoperitoneum), and according to treatment needs (i.e., antibiotics, intropes, surgery, etc.).
Quality assessment
The methodological quality of the included studies was assessed with the Ottawa-Newcastle scale, which uses a star-based system to evaluate the strength of each paper’s study group selection, the comparability of the groups, and the ascertainment of the outcome of interest [12].
Results
Heterogeneity between studies and risk of bias
Our systematic review included 13 studies that involved 601 neonates. In Table 1, we present the methodological characteristics of the included studies. The majority of the included studies used the modified Bells criteria to assess patients for NEC. However, we observed heterogeneity in the NEC population included in these studies.
Most studies used a similar methodology for conducting the calprotectin assays. The majority of the included studies used the commercially available Calprotectin ELISA Kits and conducted the assay according to the manufacturer’s instructions [13–21]. The reference value given by all commercially available ELISA is 50 μg/g feces for healthy adults and children aged 4–17 years, regardless of sex [22]. Two studies used the Bühlmann Quantum Blue rapid test [23, 24]. One study did not report the exact methods used for calprotectin measurement [25].
Calprotectin values among necrotizing enterocolitis and healthy neonates
Table 2 presents the neonatal characteristics (i.e., gestational age, birth weight, age at diagnosis, and gender) and calprotectin values for the NEC and healthy neonates. We observed that ten studies found significantly elevated fecal calprotectin (FC) levels among infants with NEC (p < 0.05) [13, 15–17, 19, 21, 23, 26, 27]. One study noted that this effect was observed at all stages of the stage of the disease (Wei-Hong et al.) (p = 0.01) [27]. The remaining three studies did not find significant differences between NEC and healthy neonates [20, 28, 29].
Some studies investigated the correlation of FC with gestational age, birth weight, age at diagnosis, and gender. They did not find significant differences. Yoon et al. were the sole exception, who showed a possible relation between FC and gestational age in healthy neonates that were born prior to 26 weeks (p = 0.001). Interestingly, however, the same researchers reported a clear negative linear relationship for neonates born between 26 and 30 weeks of gestational age (p = 0.03) [21]. Furthermore, Carroll et al. mentioned that there was a wide variation in the concentration of calprotectin in controls and many values were outside the normal reference range for adults [13].
Accuracy of the test
Five studies evaluated the efficacy of FC as a diagnostic test. Aydemir et al. observed that a cut-off value of 792 μg/g had a 76 % sensitivity, 92 % specificity, and that the area under the curve (AUC) was 0.89 (p < 0.001, 95 % CI 0.81–0.98) [16]. Reisenger et al. used a lower cutoff value (286.3 μg/g) and reported a sensitivity of 81 %, a specificity of 79 %, and an AUC of 0.82 (95 % CI 0.68–0.96) [17]. However, these findings were not reproduced by Shenoy et al., who found that a cut-off value of 280 μg/g had a sensitivity of 93.3 %, specificity of 39 %, and that the AUC was 0.652 (95 % CI 0.516–0.789) [26]. Bin-Nun et al. used a cut-off value of 480 μg/g, which resulted in 100 % sensitivity and 84.6 % specificity [23]. Finally, Terrin et al. used a cutoff value of 3.0 mg/ml and reported that the sensitivity was 100 % (79.4–100 %) and the specificity was 96.4 % (87.6–99.6 %) [18].
Discussion
Necrotizing enterocolitis is a life-threatening disease that presents in preterm neonates. Early identification reduces morbidity and mortality. However, to date no specific markers to screen for the disease have been identified. The association of FC with intestinal inflammatory diseases has been thoroughly investigated during the last decade [30]. It is believed that the presence of calprotectin in stool indicates increased neutrophil migration towards the intestinal mucosa, which is triggered by bowel inflammation [31]. Although the actual pathophysiologic pathways remain vague, it seems that the activated neutrophils gradually degranulate and secrete calprotectin, which inhibits matrix metalloproteinases and triggers apoptosis [32].
In 2010 Thuigls et al. suggested that FC might be a useful diagnostic marker for NEC [8]. Since then, several studies have investigated its efficacy. In our systematic review we sought to gather all available evidence from the international literature to evaluate the effectiveness of using FC levels in the diagnosis of NEC. According to our findings, FC is significantly elevated in neonates that suffer from NEC. To date, we do not know whether this effect takes place early during this process. However, at least one study points towards early elevation of FC levels. Although several studies have studied the differences in FC levels between NEC and healthy neonates, there seems to be a complete lack of consensus regarding the utility of specific cut-off values. This might be partially influenced by the fact that FC values show high inter-individual variation during the neonatal period. This observation is encountered both in term and preterm infants [33–36]. Therefore, the efficacy of this protein in detecting NEC remains unknown.
In this context, we strongly believe that future prospective studies that use similar techniques of protein detection are needed and that the results should be reported in specific units of measurement. Furthermore, future studies should first evaluate the cut-off values that have previously been described before proposing new ones, so as to assess the reproducibility of the test and to specifically direct future research.
Strengths and weaknesses of our study
The findings of our study are based on a systematic review of the literature. No language or date restriction was applied; therefore, there is only a small possibility that articles were omitted.
However, the risk of bias in our findings remains high due to the presence of several factors. First, all included studies were retrospective; therefore, selection bias might be an issue. Furthermore, their methodological heterogeneity in terms of patient recruitment (stage of the disease), units of measurement, and implemented assays precludes safe interpretation of results.
Last, despite the fact that the majority of included studies reported high sensitivity and the specificity of the test, the suggested cut-off points vary widely. Therefore, we could not perform a meta-analysis of these results to provide a summary receiver operating characteristic curve.
Conclusion
The current evidence suggests that FC is elevated in newborns suffering from NEC. Its significance as an early screening marker remains unknown; however, at least one study found elevated calprotectin levels during the early stages of the disease. The reported sensitivity and specificity of the test remain unknown, as there is a lack of consensus regarding the implementation of specific cut-off values. Given the absence of specific early markers for the detection of NEC, further research is needed to reach firm conclusions.
References
Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44:1072–5 (discussion 1075–6).
Thompson AM, Bizzarro MJ. Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs. 2008;68:1227–38.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
Moss RL, Dimmitt RA, Barnhart DC, Sylvester KG, Brown RL, Powell DM, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. 2006;354:2225–34.
Young C, Sharma R, Handfield M, Mai V, Neu J. Biomarkers for infants at risk for necrotizing enterocolitis: clues to prevention? Pediatr Res. 2009;65:91R–7R.
Oh S, Young C, Gravenstein N, Islam S, Neu J. Monitoring technologies in the neonatal intensive care unit: implications for the detection of necrotizing enterocolitis. J Perinatol Off J Calif Perinat Assoc. 2010;30:701–8.
Striz I, Trebichavsky I. Calprotectin—a pleiotropic molecule in acute and chronic inflammation. Physiol Res Acad Scientiarum Bohemoslov. 2004;53:245–53.
Thuijls G, Derikx JP, van Wijck K, Zimmermann LJ, Degraeuwe PL, Mulder TL, et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg. 2010;251:1174–80.
Burri E, Beglinger C. Faecal calprotectin—a useful tool in the management of inflammatory bowel disease. Swiss Med Wkly. 2012;142:w13557.
Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:524–34.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
Wells GA, Shea B, O`Connel D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 27 Nov 2015.
Carroll D, Corfield A, Spicer R, Cairns P. Faecal calprotectin concentrations and diagnosis of necrotising enterocolitis. Lancet. 2003;361:310–1.
Altamimi EM, Murthy P, Urbaniak C, Grey VL, Denou E, Issenman R. Prospective non-randomized diagnostic pilot study using fecal calprotectin for diagnosing necrotizing enterocolitis (NEC) in very low birth weight infants. Gastroenterology. 2011;140:S-685–6.
Aydemir G, Cekmez F, Tanju IA, Canpolat FE, Genc FA, Yildirim S, et al. Increased fecal calprotectin in preterm infants with necrotizing enterocolitis. Clin Lab. 2012;58:841–4.
Aydemir O, Aydemir C, Sarikabadayi YU, Emre Canpolat F, Erdeve O, Biyikli Z, et al. Fecal calprotectin levels are increased in infants with necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2012;25:2237–41.
Reisinger KW, Van der Zee DC, Brouwers HA, Kramer BW, van Heurn LW, Buurman WA, et al. Noninvasive measurement of fecal calprotectin and serum amyloid A combined with intestinal fatty acid-binding protein in necrotizing enterocolitis. J Pediatr Surg. 2012;47:1640–5.
Terrin G, Passariello A, De Curtis M, Paludetto R, Berni Canani v. S100 A8/A9 protein as a marker for early diagnosis of necrotising enterocolitis in neonates. Arch Dis Child. 2012;97:1102.
Albanna EA, Ahmed HS, Awad HA. Stool calprotectin in necrotizing enterocolitis. Journal Clin Neonatol. 2014;3:16–9.
Selimoglu MA, Temel I, Yildirim C, Ozyaln F, Aktas M, Karabiber H. The role of fecal calprotectin and lactoferrin in the diagnosis of necrotizing enterocolitis. Pediatr Crit Care Med. 2012;13:452–4.
Yoon JM, Park JY, Ko KO, Lim JW, Cheon EJ, Kim HJ. Fecal calprotectin concentration in neonatal necrotizing enterocolitis. Korean J Pediatr. 2014;57:351–6.
Fagerberg UL, Loof L, Merzoug RD, Hansson LO, Finkel Y. Fecal calprotectin levels in healthy children studied with an improved assay. J Pediatr Gastroenterol Nutr. 2003;37:468–72.
Bin-Nun A, Booms C, Sabag N, Mevorach R, Algur N, Hammerman C. Rapid fecal calprotectin (FC) analysis: point of care testing for diagnosing early necrotizing enterocolitis. Am J Perinatol. 2015;32:337–42.
Shenoy M, Shenoy KT, Roseth A, Geir L, Keshavamurthy SR. Diagnostic utility of fecal calprotectin as a biomarker of gut inflammation in neonates to predict necrotizing enterocolitis: a prospective study. Indian J Child Health. 2014;1:99–104.
Avula S, Nantais-Smith L, Monga R, Lockwood L, Lansing E, Kadrofske M. Stool biomarkers to diagnose necrotizing enterocolitis in preterm infants: a pilot case-control study. AAP National Conference and Exhibition October 23–27, 2015.
Shenoy MT, Shenoy KT, Roseth A, Geir L, Keshavamurthy SR. Diagnostic utility of fecal calprotectin as a biomarker of gut inflammation in neonates to predict necrotizing enterocolitis: a prospective study. Indian J Child Health. 2014;1:99–104.
Tang WH, Guan MC, Jiang XJ, Teng CZ, Wang HT. The clinical significance of fecal calprotectin on premature infant with necrotizing enterocolitis. Chin J Health Lab Technol. 2013;4:23–8.
Altamimi EM, Murthy P, Urbaniak C, Grey VL, Denou E, Issenman R. Prospective non-randomized diagnostic pilot study using fecal calprotectin for diagnosing necrotizing enterocolitis (NEC) in very low birth weight infants. Gastroenterology. 2011;140:S-685–6.
Avula S, LN-S, Monga R, Lockwood L, Kadrofske M. Stool biomarkers to diagnose necrotizing enterocolitis in preterm infants: a pilot case-control study. American Academy of Pediatrics. Washington, DC; 24–27 Oct 2015.
Elgin TG, Kern SL, McElroy SJ. Development of the neonatal intestinal microbiome and its association with necrotizing enterocolitis. Clin Ther. 2016;38:706–15.
Erbayrak M, Turkay C, Eraslan E, Cetinkaya H, Kasapoglu B, Bektas M. The role of fecal calprotectin in investigating inflammatory bowel diseases. Clinics (Sao Paulo, Brazil). 2009;64:421–5.
Isaksen B, Fagerhol MK. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol MP. 2001;54:289–92.
Polycarpou E, Zachaki S, Papaevangelou V, Tsolia M, Kyriacou A, Kostalos C, et al. Oral l-arginine supplementation and faecal calprotectin levels in very low birth weight neonates. J Perinatol Off J Calif Perinat Assoc. 2013;33:141–6.
Laforgia N, Baldassarre ME, Pontrelli G, Indrio F, Altomare MA, Di Bitonto G, et al. Calprotectin levels in meconium. Acta Paediatr (Oslo, Norway 1992). 2003;92:463–6.
Campeotto F, Baldassarre M, Butel MJ, Viallon V, Nganzali F, Soulaines P, et al. Fecal calprotectin: cutoff values for identifying intestinal distress in preterm infants. J Pediatr Gastroenterol Nutr. 2009;48:507–10.
Yang Q, Smith PB, Goldberg RN, Cotten CM. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology. 2008;94:267–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to report.
Research involving human participants and/or animals
This is a systematic review of data already published in this field. In the present study we did not have direct involvement with humans or animals.
Informed consent
Not applicable because this study did not include individual patient data.
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Pergialiotis, V., Konstantopoulos, P., Karampetsou, N. et al. Calprotectin levels in necrotizing enterocolitis: a systematic review of the literature. Inflamm. Res. 65, 847–852 (2016). https://doi.org/10.1007/s00011-016-0963-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-0963-9