Abstract
Introduction
Urinary trypsin inhibitor (UTI) decreases inflammatory cytokine levels and mortality in experimental animal models of inflammation. Here, we observed the effect of UTI on lipopolysaccharide (LPS)-induced hyperpermeability in human umbilical vein endothelial cells (HUVECs) and explored the role of vascular endothelial-cadherin (VE-cadherin) in its effect.
Methods
The effect of UTI on endothelial barrier hyperpermeability was detected by an electrical cell–substrate impedance sensing (ECIS) system and a transwell chamber system. The expression of VE-cadherin in HUVECs was examined by real-time PCR and western blot.
Results
We demonstrated that the alleviation of LPS-induced barrier dysfunction could be achieved by pretreatment with 3000 U/mL of UTI. VE-cadherin monoclonal antibody (mAb) could inhibit the protective effects. UTI maintained VE-cadherin expression by increasing protein stability at both the transcriptional and post-transcriptional levels. Meanwhile, VE-cadherin expression on the cell surface increased when the cells were pretreated with UTI. Furthermore, pretreatment with UTI decreased the phosphorylation of VE-cadherin at Tyr658 but not Tyr731.
Conclusions
Our data show that prophylactic UTI maintains the endothelial barrier function, increases VE-cadherin expression, and inhibits the phosphorylation of VE-cadherin at Tyr658 under inflammatory conditions. It suggests a scientific and potential clinical therapeutic importance of UTI in treatment of inflammatory disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Permeability of vasculature plays a pivotal role in regulating many physiological and pathological processes, including angiogenesis, immunity, and inflammation. Endothelial cells (ECs) of the microvasculature represent a critical site of barrier regulation, selectively permitting passage of fluid, macromolecules, and cells into the extravascular tissue [1]. Lipopolysaccharide (LPS) or endotoxin, the bacterial cell envelope component responsible, activates macrophages, neutrophils, and other immune cells to produce multiple inflammatory mediators and free radicals that ultimately cause EC damage and vascular barrier dysfunction [2–4].
Interendothelial junctions (IEJs), important regulators of the permeability characteristics of the endothelium, are modulated to allow selective and specific passage of blood cells and macromolecules. Dysregulation of cell junctions can lead to pathologic situations, including many chronic inflammatory diseases and edema, which might subsequently lead to an exaggerated systemic inflammatory response known as systemic inflammatory response syndrome (SIRS) [5, 6].
IEJs comprise the tight junctions (TJs) and adherens junctions (AJs) [5, 7]. Inflammatory cytokines, thrombin and histamine, increase endothelial permeability and dismantle TJs and AJs. Vascular endothelial (VE)-cadherin, the major component of AJs, is a calcium-dependent cell–cell adhesion molecule exclusively located at endothelial intercellular junctions [7, 8]. Although the barrier function of the endothelium is supported by multiple intercellular adhesion systems, disruption of VE-cadherin is sufficient to disrupt other intercellular junctions, even including TJs [9]. It regulates the protein complexes that join adjacent endothelial cells and prevents leukocyte migration and vascular leakage [10]. After LPS treatment, the contiguous structure in the ECs is destructed accompanied by the downregulation of VE-cadherin expression, which contributes to the occurrence and development of sepsis [11, 12]. Blocking VE-cadherin expression with the specific antibodies is shown to increase vascular permeability [13, 14]. Therefore, VE-cadherin indeed chiefly organizes the opening and closing of the endothelial barrier, and is central in regulating EC permeability changes [15].
Urinary trypsin inhibitor (UTI; ulinastatin) was firstly identified in and purified from human urine in 1982 [16]. It is well known that UTI is a glycoprotein liberated into blood and urine from its liver-derived precursor that is inter-α-trypsin inhibitor, upon degradation by neutrophil elastase during inflammation [17]. As a serine protease inhibitor, UTI has anti-inflammatory activity that suppresses the infiltration of neutrophils and the release of elastase and chemical mediators from neutrophils [18]. Previous animal studies reported that UTI inhibited systemic inflammatory response and subsequent organ injury induced by LPS [19–21]. UTI also protects pulmonary vascular endothelial cells by reducing paraquat-induced serum vascular endothelial growth factor level and vascular permeability amplitude [22]. Clinically, UTI has been already used in Japan and China as a drug for acute inflammatory disorders such as disseminated intravascular coagulation (DIC), shock, and pancreatitis [23, 24], and a series of randomized controlled trials were reported on the validity of UTI in septic patients [25–27]. However, current understanding regarding its accurate mechanisms remains obscure and the effect of UTI on endothelial barrier permeability and junction proteins during inflammation remains undiscovered. We therefore conducted this study to investigate the underlying mechanisms of UTI for anti-inflammation therapy.
Based on these above-mentioned findings, we hypothesized that UTI had a protective effect on LPS-induced EC hyperpermeability and VE-cadherin expression may play a key role in this effect. To test the hypothesis, we firstly observed the effect of pretreatment with UTI on LPS-induced human umbilical vein endothelial cells (HUVECs)’ barrier hyperpermeability as a model for LPS-challenged barrier dysfunction, by an electrical cell–substrate impedance sensing system (ECIS). Subsequently, we investigated the effect of prophylactic UTI on junction proteins under inflammatory condition, and further explored whether VE-cadherin expression is essential for the effect of UTI, especially using the prophylactic specific VE-cadherin monoclonal antibody (mAb). At last, we determined the level of VE-cadherin on the cell surface, expression of two types of VE-cadherin phosphorylation, as well as its synthesis and metabolism process under prophylactic UTI treatment, which would help us further understand its molecular mechanism.
Materials and methods
Reagents
Bacterial LPS (Escherichia coli O55:B5) and the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). UTI was purchased from Acris (San Diego, CA, USA) and dissolved in endothelial growth medium (EGM-2, Walkerville, MD, USA). The mouse macrophage cell line RAW264.7 was purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China). Primary antibodies against VE-cadherin (1:750) and β-actin were purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse mAb against the extracellular domain of human VE-cadherin (50 µg/mL) was obtained from Transduction Laboratories Inc (Lexington, KY, USA). The anti-phospho-VE-cadherin (Tyr658: 1:1000, Tyr731: 1:1000) and FITC-dextran (average MW ~40,000) were purchased from Millipore (Billerica, MA, USA). All other chemicals were of the highest purity commercially available.
Endothelial cell culture
HUVECs were obtained from ATCC (Manassas, VA, USA). The purity of the HUVECs was >98 %. They were grown in EGM-2 with 2 % FBS, incubated with 5 % CO2 at 37 °C, and cultured to confluence for 72 h. Once confluent, the cells were used in barrier function assays. HUVECs were only used within the first five passages.
MTT assay
HUVECs were seeded in 96-well plates (1 × 105 cells/well in 100 μL of medium; Corning; Tewksbury, MA, USA), and were treated with various concentrations of UTI (500, 1000, 2000, 3000, 5000, and 10,000 U/mL) for 24 h at 37 °C. Cell viability was determined by the MTT assay as described previously [28]. The absorbance was measured at 570 nm in a microplate reader.
Transendothelial monolayer electrical resistance (TER)
The method is based on an established process to monitor cell behavior by an ECIS Model 1600R and electrode arrays obtained from Applied BioPhysics (Troy, NY, USA). HUVECs were seeded in polycarbonate wells containing evaporated gold microelectrodes in EBM-2 with 2 % FBS. After 72 h, the cells were grown to confluence and TER measurements were performed using an ECIS as previously described [29]. Only the wells with a resistance of 800–1800 Ohm were used in the experiments. TER value from each microelectrode was pooled at discrete time points and plotted against time as the mean ± SEM. Baseline. Initial experiments were conducted by treating the cells in the presence or absence of 3000 U/mL UTI for a period of 1 h prior to the addition of 1 μg/mL of LPS [30], in order to evaluate the effect of UTI on LPS-induced changes in barrier function. EGM-2 medium served as a negative control. Especially, the VE-cadherin mAb was added in the upper compartment 6 h ahead of UTI treatment [13]. All experiments were carried out with 5 % CO2 at 37 °C.
Macrophage transmigration assay
The transwell migration assay was performed as previously described [31]. Fibronectin-coated transwell filters (diameter, 6.5 mm; pore size, 8 mm; Corning; Tewksbury, MA, USA) were used for the migration assays. HUVECs were seeded at a density of 1.0 × 105 cells/well and cultured to confluence for 72 h. HUVEC monolayer was treated with or without UTI (3000 U/mL) for 1 h and then stimulated with LPS (1 μg/mL) for additional 4 h.
The VE-cadherin mAb was also added in the upper compartment 6 h ahead of UTI treatment. Subsequently, macrophages (5 × 105 cells in 100 μL of medium) were added to each upper chamber and incubated for 3 h to allow migration. Macrophages that migrated across the filter were fixed and stained with 0.05 % crystal violet solution, and the absorbance of eluted crystal violet was measured in a microplate reader (TECAN; Mannedorf, ZRH, CH).
FITC-dextran permeability assay
HUVECs (1 × 105 cells/well) were grown to confluence on transwell permeable supports for 72 h. HUVEC monolayers were treated with or without UTI (3000 U/mL) for 1 h and then stimulated with LPS (1 μg/mL) for additional 4 h. The VE-cadherin mAb was also added in the upper compartment 6 h ahead of UTI treatment. Subsequently, all media were removed and 0.6 mL of phosphate-buffered saline was added to the lower chamber. Then, 0.2 μL of FITC-dextran (0.5 mg/mL) was added to the upper chamber for 1-h incubation. The amount of FITC-dextran that diffused into the bottom chamber was determined by a fluorescence microplate reader and expressed as arbitrary units.
Quantitative real-time polymerase chain reaction
For the comparison of gene expression levels, total mRNA was isolated from HUVECs according to the manufacturer’s protocol. Reverse transcription into cDNA was performed with 1 μg of total RNA using the SuperScript II polymerase (Invitrogen; Carlsbad, CA, USA). The resulting cDNA was used for PCR with the SYBR-Green Master PCR Mix (Bio-Rad; Richmond, CA, USA). mRNA expression levels of the VE-cadherin, ZO-1, claudin-3, claudin-4, claudin-5, JAM-1, and JAM-2 were detected by real-time PCR. Primers for VE-cadherin, ZO-1, JAM-1, JAM-2, claudin-3, claudin-4, claudin-5, and GAPDH are listed in Table 1. The expression levels of samples were determined with the standard curve method.
Western blot analysis
Cell surface proteins of HUVECs were isolated using a cell surface protein extraction kit, according to the manufacturer’s protocol (Thermo Fisher; Pittsburgh, PA, USA). Total protein of HUVECs was extracted and western blot analysis was performed as previously described [32]. Immunoreactive protein was visualized by the LumiGLO chemiluminescence reagent and peroxide.
Analysis of mRNA stability by actinomycin D chase
For detecting RNA stability, HUVECs were grown in complete medium until reaching confluence. Firstly, HUVECs were pretreated with UTI at 3000 U/mL for 1 h. Then actinomycin D (10 μg/mL, Sigma) [33] was added to stop RNA synthesis. After the addition of actinomycin D, the total RNA was harvested at 0, 2, 4, 6, and 8 h, and the remaining level of VE-cadherin mRNA was measured by real-time PCR. Blots were reprobed with a GAPDH cDNA probe to ensure equal loading and VE-cadherin mRNA transcript levels were normalized for GAPDH mRNA.
Protein synthesis inhibition with cycloheximide
After grown in complete medium until reaching confluence, HUVECs were treated with UTI (3000 U/mL) for 1 h, then subsequently treated with cycloheximide (100 μg/mL) [34] for 8 h. Protein was extracted from the cells using Trizol reagent.
Statistical analysis
All data were expressed as mean ± SEM. Statistical analysis was performed by SAS 9.3 for windows (SAS Institute Inc., Cary, NC, USA). One-way analysis of variance (ANOVA) was used to compare different concentrations of UTI on HUVEC viability and analyze dose–response and time-course of UTI treatment on VE-cadherin expression. For evaluating the effect of UTI on barrier function and comparing mRNA or protein expression of VE-cadherin, a 2 × 2 factorial design ANOVA or two-way ANOVA was performed. The effect of VE-cadherin mAb was analyzed by three-way ANOVA. Student–Newman–Keuls tests were used for post hoc multiple treatment comparisons. Statistical significance was defined as P < 0.05. All graphs were generated using GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA).
Results
Effect of different concentrations of UTI on HUVEC viability
Treatment of HUVECs with 500, 1000, 2000, 3000, 5000, and 10,000 U/mL of UTI for 24 h did not significantly affect the cell viability (one-way ANOVA; F = 0.81, P = 0.56; Fig. 1).
Prophylactic UTI attenuated LPS-induced HUVEC hyperpermeability
Using the ECIS system, we detected the TER value to monitor changes in monolayer permeability. As shown in Fig. 2, the wells containing media, which served as the negative control, did not demonstrate significant changes in resistance over time. When compared with that in the control group, treatment with 3000 U/mL of UTI alone for 9 h did not affect the TER in HUVECs. After treatment with 1 μg/mL of LPS, the TER value began to decrease after 2 h (P = 0.02) and decreased by 49.7 % after 4 h (P < 0.01) [2 × 2 Factorial design ANOVA; 2 h: (LPS: F = 6.74, P = 0.03; UTI: F = 0.25, P = 0.63; LPS*UTI: F = 0.07, P = 0.80); 4 h: (LPS: F = 27.22, P < 0.01; UTI: F = 5.27, P = 0.05; LPS*UTI: F = 2.25, P = 0.17)]. However, prophylactic UTI led to the increase in the TER value 4 h after the LPS treatment compared with the LPS group (P = 0.03). Its protective effect maintained until 9 h after the LPS treatment (P < 0.01) [2 × 2 Factorial design ANOVA; 9 h: (LPS: F = 47.21, P < 0.01; UTI: F = 8.22, P = 0.02; LPS*UTI: F = 4.95, P = 0.06)].
Prophylactic UTI attenuated LPS-induced endothelial barrier hyperpermeability. HUVECs were incubated with 1 μg/mL of LPS alone, 3000 U/mL of UTI alone, or prophylactic UTI treatment 1 h ahead of LPS treatment. Then, the transendothelial monolayer electrical resistance values were measured 0–9 h after the incubation. Data represent mean ± SEM (n = 3). *P < 0.05, **P < 0.01, compared with the control group; # P < 0.05, ## P < 0.01, compared with the LPS group. CON control
Pretreatment with UTI increased mRNA expression of VE-cadherin in HUVECs exposed to LPS
As indicated in Fig. 3, the mRNA levels of seven candidate junctions (VE-cadherin, JAM-1, JAM-2, claudin-3, claudin-4, claudin-5, and ZO-1) in HUVECs, treated with or without UTI (3000 U/mL) for 1 h and then stimulated with LPS (1 µg/mL) for additional 1 h, were validated by real-time PCR. As to the mRNA expression of VE-cadherin in HUVECs, prophylactic UTI treatment considerably attenuated its decreased level induced by the LPS treatment (P < 0.01) (2 × 2 Factorial design ANOVA; LPS: F = 42.00, P < 0.01; UTI: F = 170.84, P < 0.01; LPS*UTI: F = 0.85, P = 0.37; Fig. 3a). Meanwhile, when compared with those in their corresponding control groups, neither the LPS treatment alone nor treatments combined with prophylactic UTI ahead of LPS treatment changed the mRNA expression of JAM-1, JAM-2, and Claudin-5 in HUVECs [2 × 2 Factorial design ANOVA; JAM-1: (LPS: F = 21.16, P < 0.01; UTI: F = 27.35, P < 0.01; LPS*UTI: F = 3.85, P = 0.07); JAM-2: (LPS: F = 4.74, P = 0.04; UTI: F = 2.09, P = 0.17; LPS*UTI: F = 3.01, P = 0.10); Claudin-5: (LPS: F = 6.91, P = 0.02; UTI: F = 5.99, P = 0.03; LPS*UTI: F = 6.94, P = 0.02); Fig. 3b, c, f]. Furthermore, although LPS treatment decreased the mRNA levels of Claudin-3, Claudin-4, and ZO-1 in the HUVECs (Claudin-3: P < 0.01; Claudin-4: P < 0.01; ZO-1: P = 0.02), prophylactic UTI treatment for 1 h failed to attenuate its inhibitory effect on these three TJs’ mRNA expression [2 × 2 Factorial design ANOVA; Claudin-3: (LPS: F = 60.33, P < 0.01; UTI: F = 2.60, P = 0.13; LPS*UTI: F = 3.39, P = 0.08); Claudin-4: (LPS: F = 37.27, P < 0.01; UTI: F = 2.78, P = 0.12; LPS*UTI: F = 0.63, P = 0.44); ZO-1: (LPS: F = 14.58, P < 0.01; UTI: F = 0.97, P = 0.34; LPS*UTI: F = 0.01, P = 0.91); Fig. 3d, e, g].
Prophylactic UTI increased mRNA expression of VE-cadherin inhibited by LPS treatment in HUVECs. HUVECs were treated with or without 3000 U/mL of UTI for 1 h and then stimulated with or without 1 µg/mL of LPS for additional 1 h. Then, the mRNA expression of seven candidate junctions (VE-cadherin, JAM-1, JAM-2, claudin-3, claudin-4, claudin-5, and ZO-1) in HUVECs was detected by real-time PCR. Data represent mean ± SEM (n = 5). *P < 0.05, **P < 0.01, compared with the control group; # P < 0.05, ## P < 0.01, compared with the LPS group. CON control
Dose–response and time-course curves of UTI on VE-cadherin protein and mRNA expression in HUVECs
To examine the dose–response effect of UTI on LPS-induced changes in VE-cadherin protein expression, HUVECs were pretreated for 1 h in a cell medium containing different concentrations of UTI (1000, 2000, 3000, 4000, and 5000 U/mL), and then stimulated with LPS (1 μg/mL) for additional 4 h (Fig. 4a). Compared with the control group, VE-cadherin protein expression decreased by 43.8 % (P = 0.01) in the cells treated with LPS alone, but increased by 1.64-fold (P < 0.01) in the cells pretreated with 3000 U/mL of UTI (two-way ANOVA; UTI: F = 21.42, P < 0.01; LPS: F = 30.13, P < 0.01). Furthermore, pretreatment with 3000 U/mL of UTI considerably increased the protein expression of VE-cadherin when compared with that in the LPS group (P < 0.01). So, we selected 3000 U/mL of UTI as pretreatment in our experiment.
Dose–response and time-course of effects of UTI on VE-cadherin protein and mRNA expression induced by LPS in HUVECs. a VE-cadherin protein expression was detected after HUVECs were incubated with different concentrations of UTI for 1 h, then followed by stimulation with 1 μg/mL of LPS for 4 h. b HUVECs were pretreated with 3000 U/mL of UTI for 1 h, followed by stimulation with LPS (1 μg/mL) for 0, 0.5, 1, 2, and 3 h. Then, VE-cadherin mRNA expression was detected by real-time PCR. c HUVECs were pretreated with 3000 U/mL of UTI for 1 h, followed by stimulation with LPS (1 μg/mL) for 0, 1, 2, 4, 8, and 24 h. Then, VE-cadherin protein expression was detected by western blot. The bands were quantified and analyzed (a, c). Data represent mean ± SEM (n = 5). *P < 0.05, **P < 0.01, compared with the control group in a and 0 time point in b and c, respectively; # P < 0.05, ## P < 0.01, compared with the LPS group. CON control
Next, we investigated time-dependent effect of UTI on LPS-induced VE-cadherin mRNA level by real-time PCR (Fig. 4b). The cells were pretreated for 1 h in a cell medium containing 3000 U/mL of UTI, and then stimulated with LPS. The results revealed that, at 1 and 2 h later (1 h, P < 0.01; 2 h, P = 0.03), VE-cadherin mRNA level increased when compared with that at 0 h time point (one-way ANOVA; F = 8.57, P < 0.01). Meanwhile, we also detected a time-dependent effect of UTI on LPS-induced VE-cadherin protein expression by western blot analysis (Fig. 4c). The results demonstrated that, when compared with that at 0 h time point, VE-cadherin protein expression increased after 4 h (P < 0.01), but maintained at the decreased level after 1 and 2 h (1 h, P < 0.01; 2 h, P < 0.01) (one-way ANOVA; F = 38.74, P < 0.01). From the above-mentioned data, we chose 1 h time for detecting VE-cadherin mRNA level and 4 h for detection of its protein expression.
Pretreatment of HUVEC monolayer with VE-cadherin monoclonal antibody abolished protective effect of UTI on endothelial barrier dysfunction induced by LPS
Previous work showed that VE-cadherin monoclonal antibody (mAb) may inhibit adhesive properties of VE-cadherin [10]. We used it against the extracellular domain of human VE-cadherin to inhibit VE-cadherin reorganization. Three types of permeability assays (the macrophage transmigration assay, FITC-dextran assay, and TER assay) were used in our present study. Figure 5a shows the representative images of transmigrated macrophages stained with crystal violet. Treatment with LPS increased the amount of transmigrated macrophages and FITC-dextran in the lower chambers (P < 0.01, P < 0.01), and decreased the TER values (P < 0.01), which were all ameliorated when HUVECs were pretreated with 3000 U/mL of UTI (P < 0.01, P = 0.01, P = 0.03 in Fig. 5b–d). However, pretreatment with 50 µg/mL of VE-cadherin mAb 6 h ahead of UTI treatment abolished the protective effect of prophylactic UTI treatment (P = 0.04, P = 0.05, P = 0.03 in Fig. 5b–d, respectively) [three-way ANOVA; Fig. 5b: (UTI: F = 8.59, P < 0.01; LPS: F = 86.88, P < 0.01; mAb: F = 7.64, P = 0.02); Fig. 5c: (UTI: F = 6.66, P = 0.02; LPS: F = 37.75, P < 0.01; mAb: F = 4.43, P = 0.05); Fig. 5d: (UTI: F = 9.54, P < 0.01; LPS: F = 58.73, P < 0.01; mAb: F = 8.13, P = 0.02)].
Pretreatment of HUVEC monolayer with VE-cadherin mAb suppressed the protective effect of UTI on endothelial barrier dysfunction induced by LPS. HUVECs were exposed to 50 μg/mL of VE-cadherin mAb for 6 h, then treated with or without 3000 U/mL of UTI for 1 h, and stimulated with 1 µg/mL of LPS for additional 4 h. a Representative photos of migrated macrophages stained with crystal violet in the transwell migration assay. b The crystal violet dye in the migrated macrophages was dissolved. Then its optical density was determined. c The amount of FITC-dextran that diffused to the bottom chamber was analyzed in the FITC-dextran permeability assay. d HUVEC monolayer was treated as indicated and its electrical resistance was measured 4 h after LPS treatment. Data represent mean ± SEM (n = 5). *P < 0.05, **P < 0.01 compared with the control group; # P < 0.05, ## P < 0.01, compared with the LPS group; $ P < 0.05, compared with the UTI + LPS group. CON control (color figure online)
Regulation of VE-cadherin mRNA and protein degradation by prophylactic UTI
To understand the mechanism by which prophylactic UTI enhanced VE-cadherin protein expression, we investigated the effect of the UTI on the steady-state levels of VE-cadherin mRNA in the presence or absence of actinomycin (Act) D, an RNA synthesis inhibitor (Fig. 6a). Following the addition of Act D, the VE-cadherin mRNA level rapidly decreased to approximately 49 % (P = 0.02) of its starting level within 2 h and to 11 % (P < 0.01) within 8 h [two-way ANOVA; time: F = 0.02, P = 0.96; Act D: F = 1.35, P = 0.25]. Compared with that in the absence of Act D at the corresponding time point, UTI had no significant effect on the degradation rate of VE-cadherin mRNA.
The stability of VE-cadherin in UTI-treated HUVECs. a In this experiment, HUVECs were pretreated with UTI at 3000 U/mL for 1 h, followed by incubation with 10 μg/mL of actinomycin D. The total RNA was harvested at indicated time points and the remaining level of VE-cadherin mRNA was measured by real-time PCR. b HUVECs were treated with UTI (3000 U/mL) for 1 h, then subsequently treated with cycloheximide (100 μg/mL) for 8 h. The expression of VE-cadherin protein was detected by western blotting. The β-actin was determined using the internal standard. The bands were quantified and analyzed (c). Data represent mean ± SEM (n = 3). *P < 0.05, compared with the control (CHX-) group; ## P < 0.01, compared with the control (CHX+) group
To address whether UTI could reduce the degradation of VE-cadherin protein, protein translation was inhibited by treating the cells with cycloheximide (CHX). After the cells were treated with UTI for 1 h, they were subsequently treated with or without CHX. As shown in Fig. 6b, UTI treatment alone increased the amount of VE-cadherin protein (P = 0.03), but CHX treatment alone decreased the amount of VE-cadherin protein by 77.1 % (P = 0.02) when new protein synthesis was inhibited. The combined use of UTI and CHX considerably increased VE-cadherin protein expression (P < 0.01). The results showed that UTI might retard the degradation of VE-cadherin in septic condition [2 × 2 Factorial design ANOVA; (CHX: F = 7.75, P = 0.02; UTI: F = 16.91, P < 0.01; CHX*UTI: F = 1.83, P = 0.21); Fig. 6b].
Pretreatment with UTI upregulated VE-cadherin expression on the cell surface induced by LPS
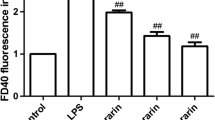
We used a cell surface protein extraction kit to isolate cell surface protein of HUVECs. As shown in Fig. 7, LPS treatment decreased VE-cadherin expression on the HUVEC surface (P < 0.01). The biotinylation assay showed that, compared with the LPS group, VE-cadherin expression on the cell surface increased by 2.75-fold when the cells were pretreated with UTI (P < 0.01) [2 × 2 Factorial design ANOVA; (LPS: F = 50.63, P < 0.01; UTI: F = 32.67, P < 0.01; LPS*UTI: F = 0.01, P = 0.91)].
Surface protein biotinylation following pretreatment with UTI. HUVECs were treated with or without UTI for 1 h and then stimulated with 1 μg/mL of LPS for 4 h. The expression of VE-cadherin protein on the HUVEC surface was detected by western blotting (a). The β-actin was determined using the internal standard. The bands were quantified and analyzed (b). Data represent mean ± SEM (n = 5). *P < 0.05 and **P < 0.01, compared with the control group; ## P < 0.01, compared with the LPS group. CON control
Prophylactic UTI downregulated the LPS-induced phosphorylation of VE-cadherin at Tyr658
As shown in Fig. 8, LPS treatment increased the phosphorylated tyrosine level of VE-cadherin at Tyr658 and Tyr731 by 4.17-fold (P < 0.01) and 1.86-fold (P = 0.02), respectively [2 × 2 Factorial design ANOVA; Tyr658: (LPS: F = 185.01, P < 0.01; UTI: F = 19.36, P < 0.01; LPS*UTI: F = 22.01, P < 0.01); Tyr731: (LPS: F = 24.49, P < 0.01; UTI: F = 2.21, P = 0.18; LPS*UTI: F = 0.98, P = 0.35)]. Pretreatment with UTI attenuated LPS-induced Tyr658 phosphorylation of VE-cadherin (P < 0.01), but did not change the level of its phosphorylation at Tyr731 induced by LPS (P = 0.74).
The effect of prophylactic UTI on the phosphorylation of VE-cadherin at Tyr658 and Tyr731. HUVECs were treated with or without UTI for 1 h and then stimulated with 1 μg/mL of LPS for 4 h. The expression of p-VE-cadherin (Tyr658), p-VE-cadherin (Tyr731), and VE-cadherin protein was detected by western blotting (a). The β-actin was determined using the internal standard. The bands were quantified and analyzed (b). Data represent mean ± SEM (n = 3). *P < 0.05 and **P < 0.01, compared with the control group; ## P < 0.01, compared with the LPS group. CON control
Discussion
Systemic capillary leak is an early feature of the inflammatory response to localized injury and is proportional to the severity of the inflammatory insult [6]. Clinically, an important goal in critical care medicine is to regulate vascular endothelial permeability that may impact favorably on septic patients’ outcome. Thus, preventing or ameliorating microvascular barrier dysfunction may be a widely applicable therapeutic strategy for inflammatory injury [35, 36]. While the application of molecular biology and recombinant techniques has produced major advances in our understanding of the mediation of the inflammatory response, there is no agent currently available which will reduce the incidence of capillary leak syndrome.
UTI treatment was reported to be associated with significantly decreased mortality, lower frequency of new organ dysfunction, and shorter durations of mechanical ventilation and hospital stay in patients with severe sepsis [27]. In clinical therapy, UTI is often applied to patients with sepsis and even in patients with multiple organ dysfunction syndrome at a dosage of 10,000 U/kg/day. So it is critical to investigate the preclinical mechanism of UTI and its underlined sites of action for anti-inflammation therapy. Our MTT data revealed that UTI had no effect on cell viability, even at the high dose of UTI (10,000 U/mL) tested. This result ruled out potential cytotoxicity effect of UTI on HUVECs. Therefore, the data indicated that UTI may have a high margin of safety.
In the present study, ECIS system and transwell system were used to determine the changes of permeability of endothelial barrier. Our findings showed that the treatment of HUVECs with LPS increased trans-HUVEC monolayer leak, and HUVEC hyperpermeability was obviously attenuated by UTI. The key pathophysiological mechanism of hyperpermeability associates with tight and adherens junctions [7]. Interestingly, TJs and AJs appear to have differential sensitivity to different inflammatory agents. For example, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) selectively disrupt TJs but does not affect AJs’ structure. However, some bacterial products such as endotoxin selectively disrupt AJs by cleaving endothelial-cadherin [37]. In comparison to the well-developed and spatially distinct TJs of epithelial cells, the TJs in ECs are less structured and more intermixed with the AJs [15, 38]. Increased VE-cadherin expression typically stabilizes AJs and promotes endothelial barrier function [39]. Our results revealed that UTI had no significant effect on TJs, but it could increase the expression of an AJ protein (VE-cadherin). Furthermore, pretreatment with VE-cadherin mAb ahead of UTI treatment abolished its protective effect on LPS-induced trans-HUVEC monolayer leak. The data suggest that the protective effect of UTI is dependent on the increased VE-cadherin expression.
At least three molecular pathways may be involved in regulating this process: (1) degradation of VE-cadherin at cell-to-cell contacts, (2) internalization of VE-cadherin at cell-to-cell contacts, (3) and tyrosine phosphorylation of VE-cadherin [40]. Treatment of HUVECs with LPS alone markedly reduced VE-cadherin expression at both the mRNA and protein levels. Pretreatment with UTI markedly attenuated this process. Regulation of gene expression might occur at multiple levels. So we applied Act D and CHX to study the accurate route of UTI enhancing VE-cadherin protein expression. In the absence or presence of Act D, the VE-cadherin mRNA levels appeared to decrease rapidly. The data confirmed that UTI did not interfere with the stability of VE-cadherin mRNA. Then, we speculated that the increased VE-cadherin mRNA level may be due to increased transcriptional activity. Inhibition of de novo protein synthesis with CHX did not alter the ability of UTI to upregulate VE-cadherin expression, suggesting that the rate of VE-cadherin degradation is decreased by pretreatment with UTI. Altogether, these data suggest that the levels of VE-cadherin expression regulated by UTI are caused by both transcriptional as well as post-transcriptional mechanisms and involve altered proteasomal degradation.
Using a cell surface biotinylation assay, we found that UTI prevented LPS-induced loss of VE-cadherin in HUVECs by upregulating its expression on the cell surface. Tyrosine phosphorylation of VE-cadherin is sufficient to prevent the binding of p120 and β-catenin to its tail, leading to decreased intercellular adhesion and increased paracellular permeability [41]. An increase in tyrosine phosphorylation of VE-cadherin is associated with an increase in endothelial paracellular permeability [42–44]. Our data showed that UTI attenuated tyrosine phosphorylation of VE-cadherin at Tyr658 but not Tyr731. Therefore, we speculated that regulating phosphorylation of VE-cadherin at Tyr658 is likely to be involved in the protective effect of UTI on the endothelial barrier.
There are some limitations in our study. Although pretreatment with UTI might exert protective effect on LPS-induced hyperpermeability, post-treatment may be more practical than the former treatment in clinical situation. So further studies designed to investigate the overall mechanism of the UTI protective effect are warranted. The model of human umbilical vein endothelial cells (HUVECs) is often used for testing endothelial barrier function in vitro and has been shown to express large amounts of TJs and AJs [45]. The protective effect of UTI on HUVECs is confirmed; however, the effect on other endothelial cell lines is not clear. Moreover, while this study has shown protective effect of UTI on ECs, there is a great need for the studies in vivo.
Collectively, our findings demonstrate for the first time that UTI achieved significant retention of barrier integrity, which was paralleled by upregulation of VE-cadherin expression on the surface of the ECs and downregulation of phosphorylation of VE-cadherin. These results strongly suggested that VE-cadherin may play an important role in the protective effect of UTI by maintaining the integrity of AJs in ECs. UTI may be considered as a reasonable therapy for the treatment of vascular barrier dysfunction in inflammatory disorders.
References
Fox ED, Heffernan DS, Cioffi WG, Reichner JS. Neutrophils from critically ill septic patients mediate profound loss of endothelial barrier integrity. Crit Care. 2013;17:R226.
Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689–91.
Bogatcheva NV, Zemskova MA, Kovalenkov Y, Poirier C, Verin AD. Molecular mechanisms mediating protective effect of cAMP on lipopolysaccharide (LPS)-induced human lung microvascular endothelial cells (HLMVEC) hyperpermeability. J Cell Physiol. 2009;221:750–9.
Xing J, Wang Q, Coughlan K, Viollet B, Moriasi C, Zou MH. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am J Pathol. 2013;182:1021–30.
Aghajanian A, Wittchen ES, Allingham MJ, Garrett TA, Burridge K. Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J Thromb Haemost JTH. 2008;6:1453–60.
Gustot T. Multiple organ failure in sepsis: prognosis and role of systemic inflammatory response. Curr Opin Crit Care. 2011;17:153–9.
Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–70.
Herwig MC, Tsokos M, Hermanns MI, Kirkpatrick CJ, Muller AM. Vascular endothelial cadherin expression in lung specimens of patients with sepsis-induced acute respiratory distress syndrome and endothelial cell cultures. Pathobiol J Immunopathol Mol Cell Biol. 2013;80:245–51.
May C, Doody JF, Abdullah R, Balderes P, Xu X, Chen CP, et al. Identification of a transiently exposed VE-cadherin epitope that allows for specific targeting of an antibody to the tumor neovasculature. Blood. 2005;105:4337–44.
Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–34.
Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem. 1998;273:35371–80.
Angelini DJ, Hyun SW, Grigoryev DN, Garg P, Gong P, Singh IS, et al. TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1232–45.
Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, et al. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–84.
Gao X, Kouklis P, Xu N, Minshall RD, Sandoval R, Vogel SM, et al. Reversibility of increased microvessel permeability in response to VE-cadherin disassembly. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1218–25.
Gavard J. Endothelial permeability and VE-cadherin: a wacky comradeship. Cell Adhes Migr. 2013;7:455–61.
Sato H, Kajikawa S, Kuroda S, Horisawa Y, Nakamura N, Kaga N, et al. Impaired fertility in female mice lacking urinary trypsin inhibitor. Biochem Biophys Res Commun. 2001;281:1154–60.
Hirose J, Ozawa T, Miura T, Isaji M, Nagao Y, Yamashiro K, et al. Human neutrophil elastase degrades inter-alpha-trypsin inhibitor to liberate urinary trypsin inhibitor related proteins. Biol Pharm Bull. 1998;21:651–6.
Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003;98:465–73.
Inoue K, Takano H, Shimada A, Yanagisawa R, Sakurai M, Yoshino S, et al. Urinary trypsin inhibitor protects against systemic inflammation induced by lipopolysaccharide. Mol Pharmacol. 2005;67:673–80.
Bae HB, Jeong CW, Li M, Kim HS, Kwak SH. Effects of urinary trypsin inhibitor on lipopolysaccharide-induced acute lung injury in rabbits. Inflammation. 2012;35:176–82.
Molor-Erdene P, Okajima K, Isobe H, Uchiba M, Harada N, Okabe H. Urinary trypsin inhibitor reduces LPS-induced hypotension by suppressing tumor necrosis factor-alpha production through inhibition of Egr-1 expression. Am J Physiol Heart Circ Physiol. 2005;288:H1265–71.
Song Z, Chen G, Lin G, Jia C, Cao J, Ao G. The ultra-early protective effect of ulinastatin on rabbit acute lung injury induced by paraquat. BMC Emerg Med. 2013;13(Suppl 1):S7.
Takubo T. Human urinary trypsin inhibitor. Nihon rinsho Jpn J Clin Med. 2010;68(Suppl 7):810–3.
Linder A, Russell JA. An exciting candidate therapy for sepsis: ulinastatin, a urinary protease inhibitor. Intensive Care Med. 2014;40:1164–7.
Chen H, He MY, Li YM. Treatment of patients with severe sepsis using ulinastatin and thymosin alpha1: a prospective, randomized, controlled pilot study. Chin Med J. 2009;122:883–8.
Moon SW, Lee SW, Hong YS, Park DW, Jang IJ, Yoon YH, Lim SI. The effects of urinary trypsin inhibitor on the outcomes of severe sepsis and septic shock patients. J Korean Soc Emerg Med. 2009;20:80–5.
Karnad DR, Bhadade R, Verma PK, Moulick ND, Daga MK, Chafekar ND, et al. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med. 2014;40:830–8.
Winiarska M, Bil J, Wilczek E, Wilczynski GM, Lekka M, Engelberts PJ, et al. Statins impair antitumor effects of rituximab by inducing conformational changes of CD20. PLoS Med. 2008;5:e64.
Cowan CE, Kohler EE, Dugan TA, Mirza MK, Malik AB, Wary KK. Kruppel-like factor-4 transcriptionally regulates VE-cadherin expression and endothelial barrier function. Circ Res. 2010;107:959–66.
Wang L, Taneja R, Wang W, Yao LJ, Veldhuizen RA, Gill SE, et al. Human alveolar epithelial cells attenuate pulmonary microvascular endothelial cell permeability under septic conditions. PLoS One. 2013;8:e55311.
Redmond EM, Cahill PA, Hirsch M, Wang YN, Sitzmann JV, Okada SS. Effect of pulse pressure on vascular smooth muscle cell migration: the role of urokinase and matrix metalloproteinase. Thromb Haemost. 1999;81:293–300.
Prota LF, Cebotaru L, Cheng J, Wright J, Vij N, Morales MM, et al. Dexamethasone regulates CFTR expression in Calu-3 cells with the involvement of chaperones HSP70 and HSP90. PLoS One. 2012;7:e47405.
Payvandi F, Wu L, Haley M, Schafer PH, Zhang LH, Chen RS, et al. Immunomodulatory drugs inhibit expression of cyclooxygenase-2 from TNF-alpha, IL-1beta, and LPS-stimulated human PBMC in a partially IL-10-dependent manner. Cell Immunol. 2004;230:81–8.
Lee SH, Jee JG, Bae JS, Liu KH, Lee YM. A group of novel HIF-1alpha inhibitors, glyceollins, blocks HIF-1alpha synthesis and decreases its stability via inhibition of the PI3 K/AKT/mTOR pathway and Hsp90 binding. J Cell Physiol. 2015;230:853–62.
Ye X, Ding J, Zhou X, Chen G, Liu SF. Divergent roles of endothelial NF-kappaB in multiple organ injury and bacterial clearance in mouse models of sepsis. J Exp Med. 2008;205:1303–15.
Groger M, Pasteiner W, Ignatyev G, Matt U, Knapp S, Atrasheuskaya A, Bukin E, Friedl P, Zinkl D, Hofer-Warbinek R, Zacharowski K, Petzelbauer P, Reingruber S. Peptide Bbeta(15-42) preserves endothelial barrier function in shock. PLoS One. 2009;4:e5391.
Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–24.
Wolfson RK, Chiang ET, Garcia JG. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res. 2011;81:189–97.
Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367.
Li R, Ren M, Chen N, Luo M, Zhang Z, Wu J. Vitronectin increases vascular permeability by promoting VE-cadherin internalization at cell junctions. PLoS One. 2012;7:e37195.
Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–12.
Donners MM, Wolfs IM, Olieslagers S, Mohammadi-Motahhari Z, Tchaikovski V, Heeneman S, van Buul JD, Caolo V, Molin DG, Post MJ, Waltenberger J. A disintegrin and metalloprotease 10 is a novel mediator of vascular endothelial growth factor-induced endothelial cell function in angiogenesis and is associated with atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2188–95.
Shen W, Li S, Chung SH, Zhu L, Stayt J, Su T, Couraud PO, Romero IA, Weksler B, Gillies MC. Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-beta1-induced permeability of centrally derived vascular endothelium. Eur J Cell Biol. 2011;90:323–32.
Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, Nottebaum AF, Vestweber D. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol. 2014;15:223–30.
Schildberger A, Rossmanith E, Weber V, Falkenhagen D. Monitoring of endothelial cell activation in experimental sepsis with a two-step cell culture model. Innate Immun. 2010;16:278–87.
Acknowledgments
We thank Prof. Hang Xiao for his invaluable technical assistance throughout the study period. And this work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Natural Science Foundation of Jiangsu Province (BK20131444).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Artur Bauhofer.
J. Chen and J. Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, J., Wang, J., Su, C. et al. Urinary trypsin inhibitor attenuates LPS-induced endothelial barrier dysfunction by upregulation of vascular endothelial-cadherin expression. Inflamm. Res. 65, 213–224 (2016). https://doi.org/10.1007/s00011-015-0907-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0907-9