Abstract
Aims and objective
Wogonin has multiple pharmacological effects, including anti-inflammatory effects. Here, we hypothesize that wogonin can protect intestinal barrier function in lipopolysaccharide (LPS)-induced Caco-2 cells, which is an in vitro model of intestinal inflammation.
Methods
We measured intestinal barrier function in LPS-induced Caco-2 cells by using transepithelial electrical resistance (TEER) and transport of fluorescent markers. A quantitative (q) RT-PCR and immunofluorescent staining analysis was used to detect the expression of tight junction proteins (claudin-1 and ZO-1) in LPS-induced Caco-2 cells. We measured inflammatory molecules in LPS-induced Caco-2 cells using ELISA and qRT-PCR. In addition, the expression of TLR4, MyD88 and TAK1 and their interaction, and NF-κB activity in LPS-induced Caco-2 cells were investigated by western blot analysis and immune-precipitation.
Results
We found that exposing Caco-2 cells to wogonin (10 and 50 μM for 24 h) attenuated the LPS-induced changes in TEER and transport of fluorescent markers. In addition, wogonin suppressed LPS-induced down-regulation of tight junction proteins (claudin-1 and ZO-1). Furthermore, LPS-induced up-regulation of inflammatory mediators, including interleukin (IL)-1β, IL-6 and IL-8, cyclooxygenase-2 (COX-2), inducible nitric oxide synthases (iNOS) were reduced after being pre-treated with wogonin. Moreover, wogonin not only inhibited the expression of TLR4, MyD88 and TAK1 and the interaction between these molecules, but also reduced NF-κB translocation to nucleus and its DNA-binding activity in LPS-induced Caco-2 cells.
Conclusion
Our results suggested that pre-treatment with wogonin could attenuate the TLR4-mediated inflammatory response and maintain intestinal barrier function in LPS-induced Caco-2 cells, thus might be a potential therapy for treating IBD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease and ulcerative colitis are two serious chronic inflammatory disorders of the gastrointestinal tract afflicting millions of patients worldwide [1]. These two disorders are grouped together as inflammatory bowel disease (IBD) and are often characterized by intestinal inflammation and mucosal tissue damage [1]. Patients with long-standing IBD are at great risk of developing colitis-associated colon cancer [2]. Although updated therapeutic strategies such as biological agents have been developed and have been shown to be effective for the treatment of IBD, patients with IBD frequently experience flare-ups and occasionally need surgical intervention.
Intestinal barrier dysfunction is considered to be the initiating event in the pathogenesis of IBD [3]. The intestinal mucosa barrier in IBD is characterized by highly permeably paracellular space [4], which is restricted primarily by the tight junction. The proteins claudin-1 and zona occludens-1 (ZO-1) maintain tight junction integrity and intestinal barrier function. The abnormal expression of tight junction proteins in epithelial cells may be involved in IBD pathogenesis [3]. An increased permeability of the intestinal mucosa barrier allows toxic substances and microorganisms to cross the intestinal wall, thereby inducing the activation of TLR4 [5]. Activation of TLR4 results in the activation of NF-κB via multiple downstream intracellular signals, and then stimulates synthesis of pro-inflammatory cytokines, including interleukin (IL)-1β [6], IL-6 [7] and IL-8 [5], cyclooxygenase-2 (COX-2) [8], inducible nitric oxide synthases (iNOS) [9], which promotes inflammatory cell recruitment and activation, therefore, it has potential to stimulate the acute phase inflammatory reaction in IBD. Hence, inhibiting the intestinal inflammation and improving intestinal barrier function is the research focus of IBD treatment.
Wogonin, a flavonoid isolated from Scutellaria baicalensis Georgi, has been shown to exert potent anti-inflammatory effects both in vitro and in vivo studies [10]. For example, wogonin could alleviate inflammatory responses in skin inflammation and carrageenan-induced hindpaw edema in animal studies [11, 12]. In addition, treatment by wogonin could suppress LPS-induced production of pro-inflammatory cytokines in macrophages and microglial cells via inhibiting NF-κB activity [13–16]. Moreover, wogonin treatment attenuated the TLR4/NF-κB-mediated inflammatory response and improved long-term functional and histological outcomes in mouse traumatic brain injury [17]. Despite evidence indicating the benefits of wogonin treatment in inflammatory diseases, there is a lack of data describing the function of wogonin on the intestinal inflammation and intestinal barrier function. The present study was the first to investigate the protective effects of wogonin on the intestinal barrier function and the controlling mechanisms in the LPS-induced Caco-2 cells.
Materials and methods
Cell culture and treatments
The Caco-2 cells (passage 20) were purchased from ATCC (Rockville, MD) and maintained in a culture medium composed of DMEM with 4.5 mg/ml glucose, 50 U/ml penicillin, 50 U/ml streptomycin, 4 mM glutamine, 25 mM HEPES, and 10 % heat-inactivated fetal bovine serum (FBS, Invitrogen, Grand Island, NY, USA). The cells were cultured in 75 cm2 flasks at 37 °C in a humidified atmosphere of 5 % CO2. Culture medium changed every 2 days. Caco-2 cells were used between passages 22 and 28 in this study. An additional 2 μg/ml LPS (Sigma, St. Louis, MO) for 24 h was added to the medium for the LPS groups as previously described [8]. In pharmacologic studies, the Caco-2 cells were randomly divided into five groups: control group, LPS group, and LPS plus 1 μM wogonin, 10 μM wogonin and 50 μM wogonin. Caco-2 cells (5 × 104 cells/well) were cultured in 96-well plates at 37 °C for 24 h then pre-treated with different concentrations of wogonin (0–50 μM) for 24 h, followed by LPS (2 μg/ml) for 24 h. The choice of wogonin concentrations was based on previous studies [18].
Transepithelial electrical resistance (TEER) measurement
TEER Measurement is used as an index of monolayer confluence and integrity in cell culture experiments [19]. TEER also has been used to measure paracellular permeability of cell mono-layers. Caco-2 cells were cultured in Transwell plates until the confluent monolayer achieved a transepithelial electrical resistance (TEER) >300 Ω/cm2 (about 15–18 days), demonstrating a tight monolayer. TEER was measured using a voltmeter (Millicell-ERS; Millipore, USA): TEER (Ω/cm2) = (Total resistance-Blank resistance) (Ω) × Area (cm2).
Transport studies
Caco-2 cells were seeded on a BD BioCoat Transwell system with 6.5-mm diameter, 0.4-μm polycarbonate pore size inserts (BD Biosciences, Oxford, UK) at a density of 20,000 cells/insert and incubated in 24-well culture plates with a medium change every other day. Confluent mono-layers were washed three times with PBS, left for 30 min at 37 °C to equilibrate, and then baseline TEER was measured. PBS containing increasing molecular weight fluorescent dextrans (FDs; 25 mg/ml) were then added to the apical side: 4 (FD4), 10 (FD10), and 20 kDa (FD20). After 1 h at 37 °C, aliquot samples were withdrawn from the basolateral sites. The amounts of different florescence in the samples were determined using a Fluoroskan Ascent FL2.5 fluorometer (Thermo Fisher Scientific, Waltham, MA), with excitation and emission wavelengths of 485 and 520 nm, respectively. The apparent permeability coefficients (Papp) of different fluorescent agents used were measured using the following equation: P app = (dq/dt) × (A × C°)−1, where dq is the amount of fluorescence in the basolateral side (milligrams per milliliter), dt is a function of time per second, A is the surface area of the inserts (0.64 cm2), and C° is the initial concentration of fluorescent applied in apical compartment (milligrams per milliliter).
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Caco-2 cells was isolated using RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Briefly, each reaction contained 2 μl of cDNA (0.1 μl of RNA equivalent), 0.8 μl primer, 2.2 μl of H2O, and 5 μl of Sofast EvaGreen supermix. qRT-PCR was performed (BioRad) in a three-step program (95 °C for 15 s, 60 °C for 30 s and 72 °C for 45 s for 50 cycles). qRT-PCR data were analyzed by CFX manager software (BioRad, Hercules, CA, USA). Gene expression fold change was obtained by dividing treated group signal by that of base expression level signal of corresponding genes in untreated cells. Results were normalized using qRT-PCR signal from β-actin of respective samples. The primers of target genes were showed in supplementary material Table 1.
Immunoprecipitation (IP)
To examine protein–protein interactions, Caco-2 cells were pretreated with wogonin for 24 h and then exposed to LPS for 90 min. The cells were lysed in 1 mL buffer consisting of 50 mM Trise HCl (pH 7.4), 150 mM NaCl, 1 % Triton X-100, 0.5 % sodium deoxycholate, 10 mM NaF, 1 mM Na3VO4, 10 g/mL leupeptin, 10 g/mL aprotinin and 20 mM PMSF after harvesting. Aliquots of the cellular lysates (containing 500 μg proteins) were incubated with proper primary anti-TLR4 and anti-MyD88 antibodies with rocking overnight at 4 °C. The immune complexes were allowed to bind to 40 μl of Recombinant Protein G Agarose beads (Invitrogen, USA) at 4 °C for 2 h, and the beads were washed three times with lysis buffer. The washed beads were re-suspended in electrophoresis sample buffer and boiled for 10 min. After centrifugation, the supernatants were obtained as immunoprecipitates for western blot analysis.
Western blot analysis
Caco-2 cells were harvested, washed once in ice-cold phosphate-buffered saline, gently lysed in ice-cold lysis buffer (250 mM sucrose, 1 mM EDTA, 0.05 % digitonin, 25 mM Tris, pH 6.8, 1 mM dithiothreitol, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml aprotinin, 1 mM benzamidine and 0.1 mM phenylmethylsulphonyl fluoride) for 30 min, and centrifuged at 12,000 bpm at 4 °C. Protein concentration was measured using BioRad Bradford protein assay reagent, and subjected to SDS-PAGE. Proteins were transferred to polyvinylidene fluoride membranes and incubated successively in 5 % bovine serum albumin in TBST (25 mmol/L Tris, pH 7.5, 150 mmol/L NaCl and 0.1 % Tween 20) for 1 h, then incubated overnight at 4 °C with inhibitory kappa B (IκB), phosphor (p)-IκB, p-TAK1, TAK1, MyD88, TLR4, or GAPDH antibody(Cell Signaling Technology, Beverly, MA, USA), followed by reaction with horseradish peroxidase-labeled secondary antibody (Santa Cruz Biotechnology) for 1 h. After each incubation, membranes were washed extensively in TBST and the immunoreactive band was detected using ECL-detecting reagents.
Measurement of IL-1β, IL-6 and IL-8
For the cytokine assay, Caco-2 cells were collected and centrifuged 24 h after treatment with LPS in the presence or absence of wogonin. The concentrations of the pro-inflammatory-associated cytokines, IL-1β, IL-6 and IL-8, were measured using ELISA, according to the manufacturer’s instructions (BioLegend ELISA MAX™ Deluxe kit; BioLegend, San Diego, CA, USA).
NF-κB assay
Nuclear extracts from treated Caco-2 cells were prepared using the Nuclear Extract Kit (Active Motif, Carlsbad, CA). NF-κB activity was measured by a NF-κB p65 assay kit (Active Motif) according to the manufacturer’s protocol.
Immunofluorescence staining analysis
Caco-2 cells were harvested, washed with PBS and fixed in 4 % formaldehyde in PBS for 15 min, permeabilized with 0.3 % Triton-X100 in PBS for 1 h with blocking of non-specific binding sites using 2 % BSA. After Caco-2 cells were fixed, Caco-2 cells then incubated with ZO-1 antibody (1:100, Cell Signaling Technology, Danvers, MA, USA) or claudin-1 antibody (1:100, Cell Signaling Technology, Danvers, MA, USA) overnight and then exposed to the secondary antibody (FITC-conjugated goat anti-mouse IgG at 1:100 dilution), followed by DNA staining with DAPI. Photomicrographs were obtained using a Leica TCS SP2 Confocal Spectral Microscope.
Statistical analysis
The data were analyzed using Statistical Analysis System software (PRISM). All the data are expressed as mean ± S.E.M. Statistical comparisons between the different treatments were performed using one-way ANOVA with Tukey’s multiple comparison post test. p values of <0.05 were considered to be statistically significant.
Results
Effect of wogonin on LPS-induced TEER changes in Caco-2 cells
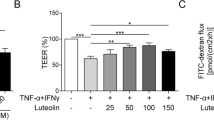
We tested various concentrations of wogonin and found that 10 and 50 μM produced the strongest effects on TEER (Fig. 1a). Compared with UT group, TEER was reduced in Caco-2 cells treated with LPS. Exposure to 10 μM wogonin for various durations significantly increased TEER in LPS-induced Caco-2 cells, with the most substantial increase observed at 24 h (Fig. 1b).
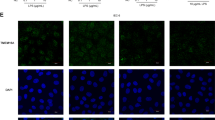
Effect of wogonin on LPS-induced intestinal barrier function changes in Caco-2 cells. a, b Effect of wogonin on LPS-induced concentration-course (a) and time-course (b) TEER changes induced by LPS in Caco-2 cells. c, e Effects of wogonin on the paracellular transport (permeability coefficients) of fluorescein isothiocyanate-dextrans of 4 (FD4), 10 (FD10), and 20 kDa (FD20). f, g mRNA levels of ZO-1 and claudin-1 were evaluated by qRT-PCR. h Immunofluorescent staining showing the effects of wogonin on the expressions of ZO-1 and claudin-1 exposed to LPS. The phenotype of nuclei was also investigated via DAPI staining. Scale Bar 25 μm. Data were shown as mean ± SEM (n = 3, # P < 0.01, compared to UT group; **P < 0.01, *P < 0.05 compared to LPS group). UT un-treatment
Effect of wogonin on LPS-induced transport of fluorescent markers changes in Caco-2 cells
Figure 1c–e showed the effects of various wogonin concentrations (1, 10 and 50 μM) on the permeability to increasing sizes of fluorescent markers FD4, FD10 and FD20 after LPS-induced. 1 μM wogonin did not alter the transport of any of the fluorescent markers. 10 and 50 μM wogonin significantly decreased transport of fluorescent markers: FD4 (Fig. 1c), FD10 (Fig. 1d) and FD20 (Fig. 1e) compared with LPS group.
Effect of wogonin on LPS-induced down-regulation of claudin-1 and ZO-1 in Caco-2 cells
qRT-PCR results demonstrated that claudin-1 and ZO-1 mRNA levels were reduced in Caco-2 cells treated with LPS. Treatment with 10 and 50 μM wogonin for 24 h attenuated the down-regulation of these tight junction proteins (Fig. 1f, g). Our immunofluorescent staining results (Fig. 1h) were consistent with the qRT-PCR results, and suggested significantly protective effect of wogonin on the tight junction proteins. Above all, the results of Fig. 1 showed the obviously protective effects of wogonin on the intestinal barrier function in the LPS-induced Caco-2 cells.
Effect of wogonin on LPS-induced expressions of key inflammatory mediators in Caco-2 cells
To confirm that LPS-induced inflammatory mediators release could be inhibited by wogonin in Caco-2 cells, we tested the COX-2 and iNOS expression levels. The qRT-PCR results showed that the mRNA expressions of COX-2 and iNOS were increased in LPS-induced Caco-2 cells. Conversely, these markers were decreased after wogonin treatment (Fig. 2d, e). Moreover, Our ELISA results indicated that the expression levels of IL-1β, IL-6 and IL-8 were significantly increased in LPS-induced Caco-2 cells compared to the UT group, which were reduced after wogonin treatment (Fig. 2a–c). This finding suggested that wogonin could inhibit the activation of the inflammatory cascade in LPS-induced Caco-2 cells.
Effect of wogonin on LPS-induced activation of inflammatory mediators in Caco-2 cells. a, c Levels of IL-1β, IL-6 and IL-8in Caco-2 cells were determined by ELISA analysis. d, e mRNA levels of iNOS and COX-2 were evaluated by qRT-PCR. Data were shown as mean ± SEM (n = 3, # P < 0.01, compared to UT group; **P < 0.01, *P < 0.05 compared to LPS group). UT un-treatment
Effect of wogonin on LPS-induced NF-κB activation in Caco-2 cells
As shown in Fig. 3a, LPS significantly enhanced the DNA binding activity of nuclear NF-κB p65 in Caco-2 cells. The increase in NF-κB activity was significantly decreased by pre-treating Caco-2 cells with 10 and 50 μM wogonin. NF-κB is inactivated in the cytosol by binding to IκB, and becomes active through translocation to the nucleus preceded by LPS-induced proteolytic degradation of IκB. As shown in Fig. 3b–d, IκB was phosphorylated and degraded 90 min after LPS treatment. Pretreatment of Caco-2 cells with 10 and 50 μM wogonin decreased phosphorylation and degradation of IκB in response to LPS, indicating that the subsequent NF-κB inactivation induced by wogonin in Caco-2 cells.
Effect of wogonin on LPS-induced activation of NF-κB in Caco-2 cells. a Nuclear extracts were prepared by using a nuclear extract kit. NF-κB activity was measured using an ELISA kit. b Protein levels of IκB and p-IκB were evaluated by western blot analysis. GAPDH was used to ensure equal loading. c, d Densitometric analysis of effects of wogonin on expression of p-IκB and IκB. Data were shown as mean ± SEM (n = 3, # P < 0.01, compared to UT group; **P < 0.01, *P < 0.05 compared to LPS group). UT un-treatment
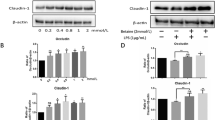
Effect of wogonin on LPS-induced TLR4 expression and its interactions with MyD88 or TAK1 in Caco-2 cells
As shown in Fig. 4a, b, western blot results showed LPS-induced significantly increased protein expression of TLR4 in Caco-2 cells, which was reduced after pre-treatment with 10 and 50 μM wogonin. In addition, qRT-PCR results were consistent with western blotting results (Fig. 4c), and suggested significant inhibitory effect of wogonin on LPS-induced TLR4 expression in Caco-2 cells.
Effect of wogonin on LPS-induced expression of TLR4 and its interactions with MyD88 or TAK1 in Caco-2 cells. a Protein level of TLR4 was evaluated by western blot analysis. GAPDH was used to ensure equal loading. b Densitometric analysis of effects of wogonin on protein expression of TLR4. c mRNA levels of TLR4 was evaluated by qRT-PCR. d Protein levels of MyD88, p-TAK1 were evaluated by western blot analysis. e, f Densitometric analysis of effects of wogonin on expressions of MyD88 and p-TAK1. g The complexes of TLR4/MyD88 and TLR4/TAK1 were precipitated by antibody against TLR4 first and then analyzed by western blot analysis for MyD88 and TAK1. The protein levels of TLR4, MyD88 and TAK1 in whole cell lysate were analyzed by western blot analysis. h, i Densitometric analysis of effects of wogonin on interactions of TLR4 with MyD88 and TAK1. j The complexes of TLR4/MyD88 were precipitated by antibody against MyD88 first and then analyzed by western blot analysis for TLR4. The protein levels of TLR4 and MyD88 in whole cell lysate were analyzed by western blot analysis. k Densitometric analysis of effects of wogonin on interactions of MyD88 with TLR4. Data were shown as mean ± SEM (n = 3, # P < 0.01, compared to UT group; **P < 0.01, *P < 0.05 compared to LPS group). UT un-treatment
The interaction of TLR4 with adaptor molecule MyD88 and TAK1 is critical for TLR4 to activate downstream signaling pathways and induce inflammatory response. As shown in Fig. 4d, f, LPS stimulation of Caco-2 cells for 90 min caused an increase in MyD88 and phosphorylation of TAK1 protein expressions, which were inhibited by 10 and 50 μM wogonin pre-treatment.
As shown in Fig. 4g, i, LPS-stimulated Caco-2 cells pre-treated with wogonin showed a reduction in the intensity of the MyD88 band co-immunoprecipitated using anti-TLR4 antibody compared to LPS-induced group. Furthermore, the ligand association of TLR4 with TAK1 was detected after stimulation with LPS. 10 and 50 μM wogonin significantly attenuated the LPS-stimulated formation of TLR4/TAK1 complex. Likewise, the reverse co-immunoprecipitation of TLR4 using the MyD88 antibody was also diminished in Caco-2 cells pretreated with 10 and 50 μM wogonin compared to LPS group (Fig. 4j, k). These findings suggested that wogonin could inhibit the activation of the inflammatory cascade through the TLR4 and its relative signaling pathway, which might be involved the protective effects of wogonin on the intestinal barrier function in the LPS-induced Caco-2 cells.
Discussion
In IBD, gut barrier dysfunction and the increase of intestinal paracellular permeability to noxious luminal substances is closely associated with increases in pro-inflammatory cytokines [5–9]. Flavonoids are a group of polyphenolic compounds ubiquitously found in plants, they have been reported to exhibit a variety of biological effects including anti-viral, anti-bacterial, anti-inflammatory, anti-oxidant, and anti-cancer effects [10]. Recent study showed flavonoids might limit epithelial COX-2 expression under inflammatory conditions in an intestinal epithelmial cell line (IEC18) [20]. In this study, we revealed the biological functions underlying the protective effect of wogonin on LPS-induced intestinal inflammatory reaction and intestinal barrier dysfunction in Caco-2 cells. Wogonin not only decreased the secretion of IL-1β, IL-6, IL-8, COX-2 and iNOS, but also protect intestinal barrier function in LPS-induced Caco-2 cells.
Inflammation depends largely on gene expression and shares key regulators. The NF-κB signaling pathway, which comprises a family of transcription factors, functions as an essential intracellular messenger that enables cells to adapt and respond to intestinal environmental changes [21]. NF-κB transcription factors activate expression of genes encoding cytokines, chemokines, adhesion molecules, matrix metalloproteinases, COX-2, inducible nitric oxide synthase, and enzymes and molecules with microbicidal activity IBD models [21]. In our present study, LPS up-regulated the activation of NF-κB, but the pre-treatment of wogonin could significantly reduce the activating effect of LPS on NF-κB(10 and 50 μM), which might be involved in the inhibition of intestinal inflammatory reaction and protection of intestinal barrier function in LPS-induced Caco-2 cells.
Multiple factors have been shown to alter intestinal barrier function, including LPS. Studies have demonstrated that LPS impairs intestinal barrier function in vitro and vivo models of IBD [22–24]. LPS binding to TLR4 results in the activation of NF-κB, causes the release of pro-inflammatory cytokines and significantly disrupted intestinal barrier function that contributes to the IBD [22, 23]. TLR4 was up-regulated in animals [22, 23] and humans [24] with IBD. Mice with a mutation in the TLR4 gene was observed to have reduced IBD severity [23]. Therefore, targeting TLR4 provides a promising intervention strategy to reduce the expression of inflammatory mediators induced by these pathologies. As mentioned above, we found that the mRNA and protein levels of TLR4 were up-regulated in LPS-induced Caco-2 cells. The pre-treatment of wogonin reduced the expression of TLR4, which might be a reason for anti-inflammatory effects in Caco-2 cells.
Activation of TLR4 signaling in the plasma membrane by LPS stimulates NF-κB pathway through the formation of MyD88-IRAK-TRAF6-TAK1 signaling complex. TLR4 was recruited to MyD88 and TAK1 after being stimulated by LPS, after that it dissociated from the receptor presumably to bifurcate the NF-κB-dependent cascade and initiates downstream inflammatory signaling [25, 26]. Therefore, MyD88 and TAK1 serve as a key TLR4 adaptor protein, linking the receptors to downstream kinases, suggesting that MyD88 and TAK1 act as specific targets for TLR4-induced inflammatory responses. The present results showed that wogonin significantly suppressed the LPS-induced expressions of MyD88 and p-TAK1 in Caco-2 cells. Moreover, wogonin also decreased the formation of the complexes of TLR4 with MyD88 and TAK1, which indicated that wogonin, could disturb the association of TLR4 with its adaptors, leading to inactivation of TLR4 in LPS-induced Caco-2 cells.
In conclusion, wogonin may inhibit TLR4-dependent inflammatory responses in LPS-induced Caco-2 cells, which may support the use of wogonin for the treatment of IBD. Further studies are required to explore potential efficacy of wogonin in the animal model of IBD against the inflammatory responses and impairment in intestinal barrier function.
References
McGilligan VE, Wallace JM, Heavey PM, Ridley DL, Rowland IR. Hypothesis about mechanisms through which nicotine might exert its effect on the interdependence of inflammation and gut barrier function in ulcerative colitis. Inflamm Bowel Dis. 2007;13:108–15.
Potack J, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease. Gut Liver. 2008;2:61–73.
Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34.
Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–63.
Bhattacharyya S, Gill R, Chen ML, Zhang F, Linhardt RJ, Dudeja PK, Tobacman JK. Toll-like receptor 4 mediates induction of the Bcl10-NFkappaB-interleukin-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J Biol Chem. 2008;283:10550–8.
Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–9.
Chiu YH, Lu YC, Ou CC, Lin SL, Tsai CC, Huang CT, Lin MY. Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol. 2013;13:190.
McElroy SJ, Hobbs S, Kallen M, Tejera N, Rosen MJ, Grishin A, Matta P, Schneider C, Upperman J, Ford H, Polk DB, Weitkamp JH. Transactivation of EGFR by LPS induces COX-2 expression in enterocytes. PLoS One. 2012;7:e38373.
Dou W, Zhang J, Li H, Kortagere S, Sun K, Ding L, Ren G, Wang Z, Mani S. Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J Nutr Biochem. 2014;25:923–33.
Tai MC, Tsang SY, Chang LY, Xue H. Therapeutic potential of wogonin: a naturally occurring flavonoid. CNS Drug Rev. 2005;11:141–50.
Chi YS, Lim H, Park H, Kim HP. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: in vivo regulation of inflammation-associated gene expression. Biochem Pharmacol. 2003;66:1271–8.
Huang WH, Lee AR, Yang CH. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci Biotechnol Biochem. 2006;70:2371–80.
Wakabayashi I, Yasui K. Wogonin inhibits inducible prostaglandin E(2) production in macrophages. Eur J Pharmacol. 2000;406:477–81.
Lee H, Kim YO, Kim H, Kim SY, Noh HS, Kang SS, Cho GJ, Choi WS, Suk K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 2003;17:1943–4.
Huang GC, Chow JM, Shen SC, Yang LY, Lin CW, Chen YC. Wogonin but not Nor-wogonin inhibits lipopolysaccharide and lipoteichoic acid-induced iNOS gene expression and NO production in macrophages. Int Immunopharmacol. 2007;7:1054–63.
Piao HZ, Choi IY, Park JS, Kim HS, Cheong JH, Son KH, Jeon SJ, Ko KH, Kim WK. Wogonin inhibits microglial cell migration via suppression of nuclear factor-kappa B activity. Int Immunopharmacol. 2008;8:1658–62.
Chen CC, Hung TH, Wang YH, Lin CW, Wang PY, Lee CY, Chen SF. Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-kappaB signaling after experimental traumatic brain injury. PLoS One. 2012;7:e30294.
Wang F, Song X, Zhou M, Wei L, Dai Q, Li Z, Lu N, Guo Q. Wogonin inhibits H2O2-induced vascular permeability through suppressing the phosphorylation of caveolin-1. Toxicology. 2013;305:10–9.
Huynh-Delerme C, Huet H, Noel L, Frigieri A, Kolf-Clauw M. Increased functional expression of P-glycoprotein in Caco-2 TC7 cells exposed long-term to cadmium. Toxicol In Vitro. 2005;19:439–47.
Lopez-Posadas R, Ballester I, Mascaraque C, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F. Flavonoids exert distinct modulatory actions on cyclooxygenase 2 and NF-kappaB in an intestinal epithelial cell line (IEC18). Br J Pharmacol. 2010;160:1714–26.
Sina C, Arlt A, Gavrilova O, Midtling E, Kruse ML, Muerkoster SS, Kumar R, Folsch UR, Schreiber S, Rosenstiel P, Schafer H. Ablation of gly96/immediate early gene-X1 (gly96/iex-1) aggravates DSS-induced colitis in mice: role for gly96/iex-1 in the regulation of NF-kappaB. Inflamm Bowel Dis. 2010;16:320–31.
Richter JM, Schanbacher BL, Huang H, Xue J, Bauer JA, Giannone PJ. LPS-binding protein enables intestinal epithelial restitution despite LPS exposure. J Pediatr Gastroenterol Nutr. 2012;54:639–44.
Stahl M, Ries J, Vermeulen J, Yang H, Sham HP, Crowley SM, Badayeva Y, Turvey SE, Gaynor EC, Li X, Vallance BA. A novel mouse model of Campylobacter jejuni gastroenteritis reveals key pro-inflammatory and tissue protective roles for Toll-like receptor signaling during infection. PLoS Pathog. 2014;10:e1004264.
Chen L, Lin MJ, Zhan LL, Lv XP. Analysis of TLR4 and TLR2 polymorphisms in inflammatory bowel disease in a Guangxi Zhuang population. World J Gastroenterol. 2012;18:6856–60.
Lin YC, Huang DY, Chu CL, Lin YL, Lin WW. The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci Signal. 2013; 6:ra71.
Madera-Salcedo IK, Cruz SL, Gonzalez-Espinosa C. Morphine prevents lipopolysaccharide-induced TNF secretion in mast cells blocking IkappaB kinase activation and SNAP-23 phosphorylation: correlation with the formation of a beta-arrestin/TRAF6 complex. J Immunol. 2013;191:3400–9.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
W. Wang and T. Xia contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, W., Xia, T. & Yu, X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm. Res. 64, 423–431 (2015). https://doi.org/10.1007/s00011-015-0822-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0822-0