Abstract
Background
Increased epicardial fat volume (EFV) has been shown to be associated with coronary atherosclerosis. While it is postulated to be an independent risk factor, a possible mechanism is local or systemic inflammation. We analyzed the relationship between coronary atherosclerosis, quantified by coronary calcium in CT, epicardial fat volume and systemic inflammation.
Methods
Using non-enhanced dual-source CT, we quantified epicardial fat volume (EFV) and coronary artery calcium (CAC) in 391 patients who underwent coronary computed tomography for suspected coronary artery disease. In addition to traditional risk factors, serum markers of systemic inflammation were measured (IL-1α, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10,IL-12, IL-13, IL-15, IL-17, IFN-γ, TNF-α, hs-CRP, GM-CS, G-CSF, MCP-1, MIP-1, Eotaxin and IP-10). In 94 patients follow-up data were obtained after 1.9 ± 0.5 years.
Results
The 391 patients had a mean age of 60 ± 10 years, and 69 % were males. Mean EFV was 116 ± 50 mL. Median CAC was 12 (IQR 0; 152). CAC and EFV showed a significant correlation (ρ = 0.37; P < 0.001). EFV and CAC were significantly correlated with the traditional risk factors like age, male gender, diabetes, smoking and hypertension. With regard to biomarkers, CAC was significantly associated (negatively) to G-CSF and IL-13. EFV (median binned) was significantly associated (positively) with IP-10 (P = 0.002) and MCP-1 (ρ = 0.037). In follow-up, EFV showed a mean annualized progression of 6 mL (IQR 3; 9) (P < 0.001); CAC progressed by a mean of six Agatston Units (IQR 0; 30). The progression of CAC was significantly correlated with the extent of EFV (P < 0.001) while there was no significant correlation between progression of EFV or CAC with systemic inflammation markers.
Conclusion
Epicardial fat volume and the baseline extent as well as progression of coronary atherosclerosis—measured by the calcium score—are significantly correlated. While both baseline EFV and CAC displayed significant correlations with systemic inflammation markers, biomarkers were not predictive of the progression of CAC or EFV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The major cause for coronary artery disease is atherosclerosis, which is a chronic inflammatory disease, and frequently accompanied by coronary calcification [1]. The involved mediators of inflammation are numerous [2–7]. The exact mechanisms of vascular calcification remain, however, unknown. Data from the Framingham Heart Study provided a correlation between epicardial fat volume with inflammatory markers [8]. The epicardial fat is frequently discussed to be closely involved in this inflammation progress [9–12] as it is a source of multiple bioactive factors and proinflammatory cytokines [13]. Several studies have shown that the epicardial fat produces multiple bioactive factors such as adiponectin, TNF-α, several interleukines and chemokines (e.g. MCP-1) [14–16]. These proinflammatory adipokines may locally interact with the coronary arteries [17–19]. IL-13 induces alternative macrophages differentiation and is a profibrotic mediator [20] which can even influence wound healing in infarction zones [21]. Furthermore, monocytes are important for the initiation and progression of coronary artery calcification [22, 23]. These cells are attracted by MCP-1, thereby initiating vascular inflammation [24]. It could be shown that MCP-1 was associated with risk factors for atherosclerosis and could, therefore, be a biomarker target for drug development [25]. On the other hand, the peri- or epicardial fat has been shown to correlate with coronary artery disease [26]. Patients with coronary artery disease show enhanced inflammation in their epicardial fat [9]. High epicardial fat represents the strongest risk factor for the presence of atherosclerosis [27]. It can be measured easily by cardiac CT. It uses the same raw data as it is used for so called calcium scanning. The CAC-Score or Agatston-Score is most commonly used to quantify the amount of calcium [28]. The amount of CAC is associated with the total amount of coronary atherosclerosis and is predictive of cardiovascular events [29].
We aimed to measure simultaneously coronary calcification, epicardial fat and a broad spectrum of biomarkers to examine potential associations among them.
Methods
Patients
391 patients with an enhanced risk for CAD underwent coronary calcium scoring for risk stratification. All patients gave written informed content and filled out a brief questionnaire. Blood was drawn prior to the examination and stored for <4 h at 4 °C before processing. Plasma aliquots were frozen at −80 °C until assays were performed. The study was approved by the institutional ethics committee for human subjects. Arterial hypertension was defined by taking either antihypertensive drugs prescribed by a physician or reported systolic blood pressures consistently higher than 140 mmHg. Hyperlipidemia was defined by a total cholesterol >200 mg/dL or the use of statins for hyperlipidemia at present or in the past. Smoking was defined positive for all current or former smokers. Diabetes was defined by the use of antidiabetic drugs.
Calcium scoring
Cardiac CT was performed using a MDCT Scanner (Somatom Definition, Siemens, Erlangen, Germany). According to a standard protocol, images were acquired with 1.2 mm collimation and reconstructed with 3.0 mm slice thickness. Images were acquired using prospective triggering in axial acquisition mode and image acquisition was triggered at 65 % of the cardiac cycle. All obtained scans were analyzed using an off-line workstation (Leonardo, Siemens Healthcare, Germany).
The software semiautomatically identifies calcified lesions with a densitiy of at least 130 Hounsfield units and a minimum area of 0.5 mm2. The Agatston score was calculated by multiplying the lesion area by a coefficient based on the peak density within the plaque. The total Agatston score (CAC score) was determined by summing individual lesion scores from each of the four main coronary arteries (left main, left anterior descending, circumflex and right).
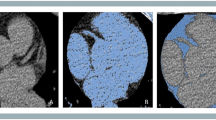
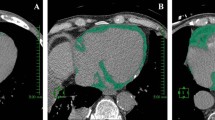
Epicardial fat measurement
Dedicated semi-automated software was used to quantify the volume of epicardial fat. In the non-contrast CT data sets, the pericardium was automatically segmented after estimating cardiac orientation and aligning a heart model [30]. If necessary, contours of the pericardium could be corrected by the reader. As a result, a volume of interest (VOI) delineating the pericardial sac was obtained. The fat content inside the VOI was then isolated using HU thresholding, which was visually adjusted by the user to ensure that all adipose tissue within the pericardial sac was included.
Luminex-based analysis
The simultaneous analysis of the cytokine concentrations was performed with the bead-based multiplexing technology using the assay from Linco Research (St. Charles, USA) and the Luminex platform. Briefly, standards, blanks, controls and the patients’ samples were applied in duplicate and incubated with the suspension of beads covered with antibodies specific for the analyzed molecules. Following the incubation and the washing steps, the cocktail of biotinylated detection antibodies was applied, followed by the incubation with the streptavidin–phycoerythrin solution. The fluorescence signal was read on the Luminex 100 IS reader.
Statistical analysis
All continuous data are expressed as mean ± SD with exception of non normally distributed data, for which median and interquartile range as 1st and 3rd quartile are reported. The cytokine data were presented in boxplots using logarithmic scale.
Univarible correlations were analyzed using Spearman’s rho correlation coefficient.
Two groups “High” and “Low” were built for EFV and its progression using the median. Differences across groups were tested by the independent samples Mann–Whitney U test. A multivariable logistic regression model including age, sex, cardiovascular risk factors and several cytokines binned by median was used to find independent factors for high epicardial fat volume. Non significant factors were stepwise excluded by the Wald algorithm.
Statistical analyses were performed using IBM® (New York) SPSS® Statistics (version 21.0) on Windows®.
Results
391 patients—272 males (69 %)—were included in the study (Table 1). Mean age was 58.8 ± 10.0 years, and mean BMI was 27.1 ± 3.9 kg/m2. Patients had at mean 2.2 ± 1.1 risk factors. Diabetes mellitus was present in 38 patients (9.7 %), arterial hypertension in 252 patients (64.5 %), hyperlipidemia in 248 patients (63.4 %) and familial history for CAD in 151 patients (38.6 %). At the baseline median CAC was 12 AU (IQR 0; 152) and EFV was 116 ± 50 mL. In our analysis CAC and EFV showed a significant correlation (ρ = 0.37; P < 0.001).
While overall CAC was at median 12 AU (IQR 0, 152), CAC in the Low-EFV group was 0 AU (O, 53) and 62 AU (4; 164) in the High-EFV group. High-EFV also correlated significantly with BMI (28.6 ± 3.9 for High-EFV vs. 25.6 ± 3.3 kg/m2 for Low-EFV; P < 0.001), male gender (155 vs. 117 patients, P < 0.001), diabetes (25 vs. 13 patients, P = 0.036), arterial hypertension (147 vs. 105 patients, P < 0.001) and smoking (100 vs. 65 patients, P < 0.001). All patients with EFV >115 mL had 2.5 ± 1.1 risk factors. Patients with EFV ≤115 mL had 1.9 ± 1.1 risk factors which was statistically significant (P < 0.001). Hyperlipidemia (P = 0.21) and familial history (P = 0.99) were not associated with baseline EFV in this study (Table 1).
Regarding biomarkers EFV was significantly associated with MCP-1 (P = 0.037) and IP-10 (P = 0.002) (Fig. 1; Table 2) while CAC was significantly correlated with G-CSF (P = 0.016) and IL-13 (P = 0.041) (Table 2). Other biomarkers showed no associations with EFV and CAC in this study. In multivariable analysis IP-10 (values >150 pg/mL) remained as the only predictive biomarker for High-EFV with an odds ratio of 1.68 (95 % CI 1.05; 2.68; P = 0.029). Regarding traditional risk factors, obesity (OR 5.08; CI 2.65; 9.75; P < 0.001), male gender (OR 3.25 CI 1.92; 5.51; P < 0.001), hypertension (OR 2.71; CI 1.63; 4.51; P < 0.001), age >60 years (OR 1.95, CI 1.19; 3.18; P = 0.008) and smoking (OR 1.93; CI 1.20; 3.11, P = 0.007) were significantly associated with high epicardial fat in multivariable analysis (Fig. 2).
For 94 patients follow-up data could be obtained after 1.9 ± 0.5 years. The overall annualized progression of CAC was at median 4 AU (IQR 0;13). High-EFV at baseline was significantly correlated with the progression of CAC. While patients with Low-EFV at baseline showed no progression of CAC, patients with High-EFV at baseline had a progression of 13 AU (IQR 2; 47) at follow-up which was statistically highly significant (P < 0.001). Regarding the annualized relative difference of EFV, associations were found for IP-10 (P = 0.049) and MCP-1 (P = 0.05) (Fig. 3).
Discussion
In our study epicardial fat and coronary artery calcification showed a high significant correlation. This information corresponds well to results from previous studies like the Framingham Heart Study [31] or the Multi-Ethnic Study of Atherosclerosis (MESA) [32]. We could further show that high epicardial fat volume at baseline is significantly correlated with the progression of coronary atherosclerosis. While patients with low epicardial fat at baseline showed no progression of CAC, patients with high epicardial fat volume at baseline had a significant extent of coronary heart disease. Epicardial fat is also significantly correlated to the traditional cardiovascular risk factors such as male gender, age, hypertension, diabetes and smoking; this is a result of our study which confirms previous trials [33].
Regarding biomarkers, CAC was significantly associated (negatively) with G-CSF and IL-13. The contribution of G-CSF could potentially be due to its increased stimulatory effects on the mobilization of CD34 +/KDR + cells [34]. A correlation of higher CAC and G-CSF had been showed for subgroups in other studies as well [7, 34], e.g. in patients with acute myocardial infarction the combination of G-CSF and GM-CSF induced a CD14 +/KDR + cell population with potential pro-angiogenic properties [35]. In our study G-CSF—like the BMI—is differently associated with EFV as compared to the association pattern of CAC. Plasma levels of the anti-inflammatory cytokine IL-13 were significantly reduced in patients with higher calcification, which has already been predescribed as an independent predictor for higher CAC [7].
These findings suggest that there may be a future role for these cytokines in the diagnostic and therapeutic pathway for CAD. MCP-1 and IP-10 were significantly associated with higher epicardial fat volume, and IP-10 even remained as an independent risk factor for EFV in our multivariable analysis. MCP-1 has already been reported to be a potential biomarker target for drug development [11] as it plays a central role in vascular inflammation. Both IP-10 and MCP-10 have been reported to play a pivotal role in the progression of atherosclerosis [36].
In conclusion, IL-13, G-CSF, MCP-1 and especially IP-10 could be shown to be involved in the inflammatory progress of high EFV and CAC and may play a role in the diagnostic work up. Beside the confirmation of significant correlation of epicardial fat volume with traditional risk factors we could show that high basal epicardial fat volume is associated with progression of coronary atherosclerosis while low basal epicardial fat is not.
Abbreviations
- 95 % CI:
-
95 % Confidence interval
- AU:
-
Agatston units
- BMI:
-
Body mass index
- CAC:
-
Coronary artery calcification
- CAD:
-
Coronary artery disease
- CT:
-
Computed tomography
- CVD:
-
Cardiovascular disease
- EFV:
-
Epicardial fat volume
- HU:
-
Hounsfield units
- IQR:
-
Interquartile range (lower; upper quartile)
- mSv:
-
Millisievert
- VOI:
-
Volume of interest
References
Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26.
Burdon KP, Langefeld CD, Beck SR, Wagenknecht LE, Carr JJ, Rich SS, et al. Variants of the CD40 gene but not of the CD40L gene are associated with coronary artery calcification in the Diabetes Heart Study (DHS). Am Heart J. 2006;151:706–11.
Oei HH, Sayed-Tabatabaei FA, Hofmann A, Oudkerk M, van Duijn CM, Witteman JC. The association between angiotensin-converting enzyme gene polymorphism and coronary calcification: the Rotterdam Coronary Calcification Study. Atherosclerosis. 2005;182:169–73.
Reilly MP, Wolfe ML, Dykhouse J, Reddy K, Localio AR, Rader DJ. Intercellular adhesion molecule 1 (ICAM-1) gene variant is associated with coronary artery calcification independent of soluble ICAM-1 levels. J Investig Med. 2004;52:515–22.
Khera A, de Lemos JA, Peshock RM, Lo HS, Stanek HG, Murphy SA, et al. Relationship between C-reactive protein and subclinical atherosclerosis: the Dallas Heart Study. Circulation. 2006;113:38–43.
Wang TJ, Larson MG, Levy D, Benjamin EJ, Kupka MJ, Manning WJ, et al. C-reactive protein is associated with subclinical epicardial coronary calcification in men and women: the Framingham Heart Study. Circulation. 2002;106:1189–91.
Raaz-Schrauder D, Klinghammer L, Baum C, Frank T, Lewczuk P, Achenbach S, et al. Association of systemic inflammation markers with the presence and extent of coronary artery calcification. Cytokine. 2012;57:251–7.
Tadros TM, Massaro JM, Rosito GA, Hoffmann U, Vasan RS, Larson MG, et al. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity. 2010;18:1039–44.
Hirata Y, Kurobe H, Akaike M, Chikugo F, Hori T, Bando Y, et al. Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int Heart J. 2011;52:139–42.
Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses apathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1.
Deo R, Khera A, McGuire DK, Murphy SA, Meo Neto Jde P, Morrow DA, et al. Association among plasma levels of monocyte chemoattractant protein 1, traditional cardiovascular risk factors and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–8.
Konishi M, Sugiyama S, Sato Y, Oshima S, Sugamura K, Nozaki T, et al. Pericardial fat inflammation correlates with coronary artery disease. Atherosclerosis. 2010;213:649–55.
Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metabo Res. 2008;40:442–5.
Iacobellis G, Pistilli D, Gucciardo M. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–5.
Mazurek T, Zhang L, Zalewski A. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6.
Kremen J, Dolinkova M, Krajickova J. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91:4620–7.
Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–43.
Iacobellis G, Pond CM, Sharma AM. Different “weight” of cardiac and general adiposity in predicting left ventricle morphology. Obesity. 2006;14:1679–84.
Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–17.
Gordon S. Do macrophage innate immune receptors enhance atherogenesis? Dev Cell. 2003;5:666–8.
Hofmann U, Knorr S, Vogel B, Weirather J, Frey A, Ertl G, et al. Interleukin-13 deficiency aggravates healing and remodeling in male mice after experimental myocardial infarction. Circ Heart Fail. 2014;7:822–30.
Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes Implications for plaque rupture. Circulation. 1994;90:775–8.
Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:23844–50.
Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res. 1999;19:91–104.
Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:78–86.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32.
Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging Council on Clinical Cardiology. Circulation. 2006;114:1761–91.
Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45.
Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as ‘‘low risk’’ based on Framingham risk score: the multiethnic study of atherosclerosis (MESA). Arch Intern Med. 2007;167:2437–42.
Zheng Y, Higuera FV, Zhou SK Fast and automatic heart isolation in 3d ct volumes: optimal shape initialization. IMLMI 2010: 84–91.
Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–13.
Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499–504.
Krueger K, Klocke R, Kloster J, Nikol S, Waltenberger J, Mooren FC. Activity of dialy living is associated with circulating CD34 +/KDR + cells and granulocyte colony-stimulating factor levels in pateints after myocardial infarction. J Appl Physiol. 2014;116:532–7.
Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–79.
Bruno S, Bussolati B, Scacciatella P, Marra S, Sanavio F, Tarella C, et al. Combined administration of G-CSF and GM-CSF stimulates monocyte-derived pro-angiogenic cells in patients with acute myocardial infarction. Cytokine. 2006;34:56–65.
Klinghammer L, Urschel K, Cicha I, Lewczuk P, Raaz-Schrauder D, Achenbach S, et al. Impact of telmisartan on the inflammatory state in patients with coronary atherosclerosis—influence on IP-10, TNF-α and MCP-1. Cytokine. 2013;62:290–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Gauss, S., Klinghammer, L., Steinhoff, A. et al. Association of systemic inflammation with epicardial fat and coronary artery calcification. Inflamm. Res. 64, 313–319 (2015). https://doi.org/10.1007/s00011-015-0809-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0809-x