Abstract
Objective
IL-17A is implicated in periodontitis pathogenesis. The roles of IL-17B–IL-17F and IL-17A/F are unknown. This study aimed to determine clinical associations between IL-17 family cytokines and periodontitis and to investigate the biological roles of IL-17A and IL-17E using in vitro model systems.
Materials and methods
Samples from 97 patients with periodontitis and 77 healthy volunteers were used in the study. Serum, saliva and gingival crevicular fluid (GCF) levels of IL-17 family cytokines were measured by ELISA. Oral keratinocytes were stimulated with a P. gingivalis biofilm, or IL-17A, in the presence and absence of IL-17E and the expression of IL-8 and CXCL5 were investigated by ELISA and real-time-PCR. NF-κB phosphorylation in similar experiments was also measured using a cell-based ELISA.
Results
Serum, saliva and GCF IL-17A levels were higher in periodontitis patients and correlated positively with clinical parameters of attachment loss, pocket depth and bleeding on probing. Serum IL-17E levels were lower in periodontitis patients and the serum IL-17A:IL-17E ratio correlated positively with clinical parameters. In vitro, IL-17E inhibited Porphyromonas gingivalis and IL-17A induced expression of chemokines by reducing phosphorylation of the NF-κB p65 subunit.
Conclusions
Serum IL-17A:IL-17E may be a marker of disease severity. IL-17E may have opposing roles to IL-17A in periodontitis pathogenesis. IL-17E can negatively regulate IL-17A and periodontal pathogen induced expression of chemokines by oral keratinocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The IL-17 family consists of IL-17A–IL-17F [1]. IL-17 family cytokines are secreted in a homodimeric form; although a functional IL-17A/IL-17F heterodimer also exists [2]. IL-17 cytokine signalling is propagated through homo- and hetero-dimeric receptor complexes of five known receptor subunits (IL-17RA–IL-17RE) [3]. IL-17 family cytokines have been demonstrated to be crucial in host defence against microbial organisms. However, they have also been shown to play prominent roles in the pathogenesis of chronic inflammatory diseases [1].
IL-17A was initially identified as a cytokine exclusively expressed by Th17 cells [4]. However, subsequent studies have identified numerous other cellular sources of IL-17A, including γδ T cells, natural killer cells, neutrophils, eosinophils, mast cells and macrophages [1]. Th17 cells have been detected in gingival tissues of periodontitis patients [5–8] and infiltration of IL-17 producing cells related to severity of inflammation in periodontitis lesions [9]. In addition, elevated levels of IL-17A have been detected in solubilised tissue [10], serum [11, 12], saliva [13] and gingival crevicular fluid (GCF) [14, 15] of patients with periodontitis. Interestingly, serum levels of IL-17A have been demonstrated to correlate with clinical attachment loss (CAL) in patients with aggressive periodontal disease [12]. Furthermore, both GCF levels of IL-17A in patients with periodontitis [15] and serum levels of IL-17A in patients with aggressive periodontitis [11] are reduced after non-surgical therapies. Therefore, these data suggest that IL-17A may play a role in the pathogenesis of periodontitis. Indeed, recent evidence has implicated polymorphisms in the IL-17 gene to be associated with disease [16–18].
In vivo models suggest that IL-17A plays a protective role in periodontitis. IL-17RA deficient mice show exacerbated bone loss in a Porphyromonas gingivalis induced model of periodontal disease [19]. However, an excessive IL-17A response promotes inflammatory alveolar bone loss in an ageing mouse model [20]. IL-17A signalling activates the receptor activator of nuclear factor kappa-B (NF-κB), which in turn induces expression of a variety of pro-inflammatory mediators and tissue destructive enzymes [21]. Furthermore, IL-17A can act in synergy with TNF-α and IL-1β and augment the expression of pro-inflammatory mediators from keratinocytes and fibroblasts [22, 23], further promoting a destructive inflammatory response. Moreover, IL-17A can modulate bone remodelling through induction of RANKL [24]. Therefore, an excessive IL-17A response can be hypothesised to play a key role in the destruction of the periodontal soft and hard tissues; the clinical hallmarks of periodontitis.
Literature describing the clinical associations between other members of the IL-17 family and periodontitis is sparse. There are no studies investigating IL-17B, IL-17C and IL-17D. IL-17F has been detected in periodontal tissues [25]. In addition, IL-17E, IL-17F and IL-17A/F have been detected in saliva and GCF [26, 27]. In patients with rheumatoid arthritis and periodontitis the concentrations of IL-17F and IL-17A/F were higher than in patients with periodontitis alone. Interestingly, although the serum and GCF concentrations of IL-17E did not differ between the two patient groups, the IL-17A:IL-17E ratio was significantly higher in serum and GCF of patients with rheumatoid arthritis and periodontitis [26]. Furthermore, GCF levels of IL-17E have been shown to negatively correlate with clinical markers of periodontitis in a study investigating the relationship between gingival inflammation and polycystic ovary syndrome (PCOS) [27].
IL-17E has been described as a ‘double edged sword’ and has been shown to down-regulate localised destructive inflammation and promote Th2 driven pathologies [28]. IL-17E induces activation and differentiation of Th2 cells and regulates their recruitment to sites of inflammation [29]. IL-17E also regulates Th9 cells [30] and activates multi-potent progenitor type 2 (MPPtype2) cells, innate type 2 helper (Ih2) cells, natural helper cells (NHCs) and nuocytes [31]. In contrast, IL-17E down-regulates Th1/Th17 and IL-17A responses [32, 33] and can directly inhibit toll-like receptor ligand driven expression of pro-inflammatory cytokines via p38 MAP kinase-driven SOCS-3 activation in human monocytes and intestinal CD14+ cells [34, 35]. Therefore, it is interesting to speculate that IL-17E may play a role in down regulating both periodontal pathogen and IL-17A induced inflammatory responses within the periodontium and protect against tissue destruction and alveolar bone loss.
The first aim of this study was to determine the clinical relationships between all members of the IL-17 family and periodontitis in a unique cohort of systemically healthy non-smokers. The clinical findings reported in this manuscript suggested that IL-17A and IL-17E may play opposing roles in periodontitis pathogenesis. This is commensurate with the proposed opposing immunological roles for these cytokines reported in the literature [32–35]. Oral keratinocytes are known to express an array of TLRs and release a variety of inflammatory mediators in response to stimuli. In vivo oral keratinocytes occupy the space adjacent to the dental biofilm on the tooth surface. Due to their proximity, oral keratinocytes will therefore be one of the first cells to encounter periodontal pathogens and co-ordinate host defence mechanisms accordingly. Therefore, the second aim of this study was to investigate whether IL-17E could inhibit both the P. gingivalis and IL-17A induced expression of neutrophil chemoattractant chemokines by oral keratinocytes.
Materials and methods
Sample collection
Serum, saliva and GCF samples were collected from patients with periodontitis, or healthy controls, in Glasgow and Newcastle. All participants in the study were systemically healthy non-smokers. The West of Scotland Research Ethics Service and the Sunderland Local Research Ethics Committee approved the conduct of this study and written informed consent was obtained from all those wishing to participate.
All patients had a thorough clinical periodontal examination with fullmouth six-point charting using the PCP12 probe (Hu-Friedy). The following data were obtained: number of teeth, number of pockets, loss of clinical attachment, sites with pockets of ≥5 mm, clinical probing depth (CPD), clinical attachment loss (CAL) from the cemento-enamel junction and bleeding on probing after 30 s.
Periodontal health was defined as BOP ≤ 15 %, no probing depth sites of >2 mm, no attachment loss and no bone loss. Periodontitis was defined as ≥6 sites with probing depths of ≥5 mm and evidence of loss of attachments and/or bone loss. Patients were excluded from the study if they had surgical periodontal therapy within the previous 12 months and if they were currently taking, or had previously taken, antibiotics or any other medication during the past 6 months. Females who were pregnant at the time of the study or had been in the previous year were also excluded.
GCF samples were obtained from the buccal aspects of up to four teeth with the greatest pocket depth/loss of attachment. These included single rooted teeth and/or the four 1st molars. GCF was collected over 30 s using Periopaper strips (Oraflow Inc, New York). The strips from each patient were placed into one polypropylene tube before freezing at −80 °C. Prior to assays, the periostrips were thawed on ice and placed into 200 µl ice cold PBS with 1 % BSA and rotated for 1 h at 4 °C. The strips were then removed and the eluate centrifuged at 350g for 60 min at 4 °C and then at 13,000g for 2 min at 4 °C.
Serum samples were obtained from blood collected from a peripheral vein in a coagulant tube. After clotting, samples were centrifuged (200g) and the serum isolated, aliquoted and stored at −80 °C.
Saliva samples were obtained by expectorating into polypropylene tubes the morning following an overnight fast during which participants were requested not to drink (except water) or chew gum [36]. The saliva samples were clarified by centrifugation (800g) for 10 min at 4 °C and immediately frozen and stored at −80 °C.
Enzyme-linked immunosorbent assays (ELISA)
IL-17A–IL-17F and IL-17A/F were assayed using commercially available ELISA kits, according to the manufacturer’s instructions. IL-17D was assayed using an optimised ELISA developed with 2 μg/ml rabbit anti-human IL-17D capture antibody (Peprotech, UK) and 0.5 μg/ml biotinylated anti-human IL-17D detection antibody (Peprotech, UK). CXCL5 (ENA-78) and CXCL8 (IL-8) concentrations were investigated by ELISA (CXCL5/ENA-78 Duoset, R&D Systems, UK and IL-8 Cytoset, Invitrogen, UK). The limits of detection were determined as two mean standard deviations higher than the mean baseline from six replicate standard curves: IL-17A = 1.9 pg/ml, IL-17B = 7.8 pg/ml, IL-17C = 7.8 pg/ml, IL-17D = 9.8 pg/ml, IL-17E = 1.9 pg/ml, IL17-F = 1.9 pg/ml, IL-17A/F = 2.5 pg/ml, IL-8 = 1.6 pg/ml and CXCL5 = 3.9 pg/ml.
Porphyromonas gingivalis biofilms
P. gingivalis ATCC 33277 was cultured under anaerobic conditions at 37 °C in Schaedler anaerobe broth (Oxoid, UK) for 2 days. The bacteria were then washed by centrifugation in PBS and standardised to 1 × 107 cells/ml in artificial saliva (AS) [37]. 500 μl of P. gingivalis suspension was cultured on Thermanox® plastic coverslips (NUNC, UK) at 37 °C in an anaerobic environment for 4 days, with AS replaced daily. After incubation, the non-adherent cells were removed and the biofilms were kept at −80 °C prior to use.
In vitro experiments
OKF6/TERT-2 oral keratinocytes were a gift from the Rheinwald laboratory (Brigham and Women’s Hospital, Boston). OKF6/TERT-2 cells were cultured as previously described [38]. P. gingivalis ATCC 33277 biofilms were used in co-culture experiments with epithelial cells as described previously [39] to determine the effect of IL-17E on P. gingivalis induced chemokine expression. OKF6/TERT-2 cells were stimulated with a P. gingivalis biofilm for 4 and 24 h. In addition, cells were pre-incubated for 30 min with IL-17E, prior to stimulation with P. gingivalis. Unstimulated cells and cells stimulated with IL-17E alone were used as controls.
The effect of IL-17E on IL-17A induced chemokine expression was determined by stimulating OKF6/TERT-2 cells concomitantly with IL-17A (Peprotech, UK) alone or in combination with IL-17E (Peprotech, UK) for 24 h. In addition, cells were pre-incubated for 30 min with IL-17E, prior to stimulation with IL-17A. Unstimulated cells and cells stimulated with IL-17A and IL-17E alone were used as controls.
Real time PCR analysis
mRNA was isolated using the RNeasy® Kit (Qiagen, UK), and reverse transcribed using the High Capacity RNA-to-cDNA Master Mix (Applied Biosystem, UK), according to the manufacturer’s instructions. IL-8 and CXCL5 mRNA expression was analysed using SYBR® green Real Time PCR with the following primers, GAPDH: Fwd: CAAGGCTGAGAACGGAAG and Rev: GGTGGTGAAGACGCCAGT, IL-8: Fwd: CAGAGACAGCAGAGCACACAA and Rev: TTAGCACTCCTTGGCAAAAC and CXCL5: Fwd: CCCTGGGTTCAGAGACCTCCA and Rev: CCAGAAAATTTTGGACGGTGGAAACA. Relative expression of IL-8 and CXCL5 mRNA was calculated using the 2−ΔΔCT method [40].
Fast Activated Cell-based ELISA (FACE™) NF-κB p65 Profiler
Phosphorylation profiles of the NF-κB p65 subunit were investigated using the FACE™ NF-κB p65 Profiler (Active Motif, UK) in accordance with manufacturer’s instructions. The % of the total NF-κB p65 subunit phosphorylated at serine 468 or serine 536 was calculated in accordance with the manufacturer’s instructions.
Statistical analyses
Q–Q plot analysis showed the serum, saliva and GCF data was not normally distributed. With an estimated effect size = 1, group sizes n ≥ 34 were sufficient to give >80 % statistical power in non-parametric statistical tests. However, due to limitations in sample volume some analyses were underpowered and in consequence are described as exploratory in nature.
The Mann–Whitney U test was used to determine significant differences between health and disease. To adjust the analysis for significant age differences, a potential confounding factor between the two groups (Table 1), a weighted least squares regression t test analysis of natural log (Ln) transformed clinical data was performed.
Bivariate correlations were performed using Spearman correlation. Since there was a large spread in the ages of the participants in this study, which may influence both clinical parameters and cytokine levels, a partial correlation analysis weighted for age was performed on Ln transformed data.
For in vitro studies, after transformation (where necessary), variance of data was first assessed using Levene’s test and the appropriate independent t test or ANOVA was selected for analysis of differences between groups. Where multiple comparisons were performed a Holm-Bonferroni test was used as not all variables could be considered independent.
Results
Clinical parameters
77 healthy volunteers and 97 patients with periodontitis were enrolled in the study. The median age of the healthy subjects was significantly lower than periodontitis patients (Table 1). Commensurate with periodontal health, the healthy subjects had significantly lower median % of sites that bled on probing (BOP), lower median CPD and lower clinical attachment loss (CAL) compared to patients with periodontitis (Table 1).
Serum, gingival crevicular fluid and saliva levels of IL-17 family cytokines
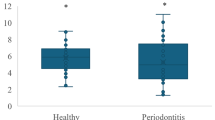
Serum levels of IL-17A and IL-17A/F were greater in patients with periodontitis compared to healthy volunteers (Fig. 1a). There were significantly lower serum levels of IL-17E in periodontitis patients compared to healthy volunteers. Weighted analyses of transformed data confirmed that none of these differences were due to differences in age of participants.
IL-17 family cytokine levels in serum, gingival crevicular fluid and saliva of periodontitis patients and healthy volunteers. The data shows the levels of IL-17 family cytokines in serum (a), GCF (b) and saliva (c) of periodontally healthy volunteers and patients with periodontitis expressed as pg/ml (a, c) or total pg/30 s collection for GCF (b). Box and whisker plots represent the median levels and quartiles. Outliers are shown by open circles. **P < 0.01, ***P < 0.001
Due to limitations in sample volume, GCF analysis was restricted to IL-17A, IL-17E, IL-17F and IL-17A/F. The median total amounts of IL-17A and IL-17A/F in GCF was elevated in patients with periodontitis (Fig. 1b). Weighted analysis of transformed data confirmed that these findings were not due to differences in age of the participants. However, the findings for IL-17F and IL-17A/F can only be interpreted as exploratory due to insufficient power.
Again, due to sample limitations, saliva analysis was restricted to IL-17A, IL-17E, IL-17F and IL-17A/F. Salivary levels of IL-17A, IL-17E and IL-17A/F were greater in periodontitis patients than healthy controls (Fig. 1c). Weighted analysis of transformed data confirmed these findings were not due to age differences.
Associations between individual IL-17 family cytokines, clinical parameters and age
Table 2 shows the correlations between the individual IL-17 family cytokines in serum, saliva, and GCF. Serum IL-17A showed significant positive correlations with IL-17B, IL-17C, IL-17F and IL-17A/F, but a negative correlation with IL-17E. Serum IL-17B levels showed significant positive correlations with IL-17C, IL-17D and IL-17A/F. Serum IL-17C showed significant positive correlations with IL-17D and IL-17F (Table 2a). GCF levels of IL-17A showed significant positive correlations with IL-17E, IL-17F and IL-17A/F (Table 2b). GCF levels of IL-17F showed significant positive correlations with IL-17E and IL-17A/F. Salivary levels of IL-17A showed significant positive correlations with IL-17E, IL-17F and IL-17A/F (Table 2c). Salivary levels of IL-17F showed significant positive correlations with IL-17E and IL-17A/F. Salivary levels of IL-17A/F showed significant positive correlations with IL-17E.
Table 3 shows correlations between serum, saliva and GCF levels of IL-17A, IL-17E, IL-17F and IL-17A/F and clinical parameters as well as age. Serum concentrations of IL-17A demonstrated a significant positive correlation with age, CPD, CAL and BOP. The positive correlations with clinical parameters remained after correction for age. In contrast, serum concentrations of IL-17E showed a significant negative correlation with CPD and CAL, but not with age or BOP. Partial bivariate correlation analysis demonstrated that after correction for age, significantly stronger negative correlations were seen for IL-17E with CPD (R = −0.275, P = 0.0021) and CAL (R = −0.317, P = 0.0007). Serum levels of IL-17A/F showed significant positive correlations with age, CPD, CAL and BOP and for the clinical parameters these correlations remained significant after correction for age (Table 3a).
GCF levels of IL-17A showed significant positive correlations with age, CPD, CAL and BOP. In addition, GCF levels of IL-17E showed a significant positive correlation with CPD, CAL and BOP, but not with age. Furthermore, GCF levels of both IL-17F and IL-17A/F positively correlated with age, CPD, CAL and BOP. Partial correlation analysis confirmed that for the clinical parameters none of these correlations were influenced by age (Table 3b).
Salivary levels of IL-17A showed significant positive correlations with age, CPD, and CAL, but not with BOP. In addition, salivary levels of IL-17E showed significant positive correlations with age, CPD, CAL and BOP. Salivary IL-17F concentrations also showed significant positive correlations with age, CPD and CAL, but not with BOP. Salivary levels of IL-17A/F only showed significant positive correlations with age. Partial correlation analysis confirmed that for the clinical parameters none of these correlations were influenced by age (Table 3c).
The IL-17A:IL-17E ratio
Previous studies have suggested the IL-17A:IL-17E ratio in biological fluids is related to the bi-directional links between periodontal disease and PCOS as well as rheumatoid arthritis [26, 27]. However, no study has investigated the direct relationship between the IL-17A:IL-17E ratio and periodontitis. Therefore, the IL-17A:IL-17E ratio was calculated for the serum, GCF and saliva samples of each participant.
The IL-17A:IL-17E ratio was significantly higher in serum of periodontitis patients than healthy controls, but not in GCF or saliva (Table 4a). This was confirmed not to be influenced by differences in the age of the participants using a weighted analysis. The serum IL-17A:IL-17E ratio exhibited moderate/strong correlations with CAL, CPD and BOP and a weak correlation with age. The GCF IL-17A:IL-17E ratio exhibited moderate correlations with CPD. In contrast, no correlations between the salivary IL-17A:IL-17E ratio and any of the parameters measured were identified (Table 4b). For clinical parameters, a weighted analysis confirmed that the correlations were not influenced by the differences in the age of the participants.
IL-17E inhibits the Porphyromonas gingivalis induced release of chemokines from oral keratinocytes
IL-17E has been demonstrated to inhibit the bacterially driven immune response of professional immune cells [34, 35]. Therefore investigations were performed to determine whether IL-17E could modulate the periodontal pathogen driven immune response of oral keratinocytes.
OKF6/TERT-2 cells were stimulated with a live P. gingivalis biofilm for 4 and 24 h in the presence and absence of IL-17E. No IL-17A or IL-17E was detected in any culture supernatants from unstimulated cells and cells stimulated with P. gingivalis alone by ELISA. In addition, no mRNA transcript was detected by real time PCR. This indicated that these cells do not express IL-17A or IL-17E (data not shown). Therefore, OKF6/TERT-2 derived IL-17A and IL-17E did not influence any of the findings reported.
Stimulation of OKF6/TERT-2 cells with a live P. gingivalis biofilm for 4 and 24 h induced a significant increase in IL-8 mRNA expression (P < 0.05) compared to unstimulated control cells, which was inhibited by 50 ng/ml IL-17E (P < 0.05, Fig. 2a). These changes in mRNA expression were mirrored by changes in protein expression. P. gingivalis significantly elevated release of IL-8 after 4 and 24 h (P < 0.05), and this was significantly inhibited by 50 ng/ml IL-17E at 24 h (P < 0.05, Fig. 2b).
The effect of IL-17E on P. gingivalis biofilm induced IL-8 expression by OKF6/TERT-2 cells. The data shows the effect of P. gingivalis alone, IL-17E alone or IL-17E (50 ng/ml; added 30 min prior) in combination with a P. gingivalis biofilm on the expression of IL-8 at the mRNA (a) and protein level (b) and CXCL5 at the mRNA (c) and protein (d) level. Changes in mRNA expression were determined by real time PCR and protein release by ELISA. For the real time data mRNA levels were normalised against GAPDH (housekeeping gene) and fold induction calculated by the 2−ΔΔCT method. Each bar represents the mean (±standard deviation). For the ELISA data, each bar represents the mean (±standard deviation). All data is derived from duplicate wells of four independent experiments performed on separate days (n = 4). *P < 0.05. Ns not significant. ±Statistical significance in comparison to unstimulated control, #statistical significance in comparison to cells stimulated with P. gingivalis biofilm only
Stimulation of OKF6-TERT2 cells with a live P. gingivalis biofilm for 4 and 24 h also induced a significant increase in CXCL5 mRNA expression (P < 0.05) compared to unstimulated control cells, which was inhibited by 50 ng/ml IL-17E (P < 0.05, Fig. 2c). These changes in mRNA expression were mirrored by changes in protein expression. The P. gingivalis-induced CXCL5 release was inhibited by 50 ng/ml IL-17E at 4 and 24 h (P = <0.05) (Fig. 2d).
None of the observed effects were related to cell viability as determined by a lactate dehydrogenase (LDH) activity assay (data not shown).
IL-17E inhibits the IL-17A-induced release of IL-8 from oral keratinocytes
Preliminary investigations suggested that oral keratinocytes expressed IL-17RA, IL-17RB and IL-17RC and thus were a target for both IL-17A and IL-17E (data not shown). As IL-17E had previously been shown to inhibit the P. gingivalis-induced release of IL-8 from oral keratinocytes; it was interesting to speculate whether it could also inhibit the IL-17A driven expression of this chemokine.
Stimulation of OKF6/TERT-2 cells with IL-17A alone for 24 h induced significant release of IL-8 compared to unstimulated control cells (P = <0.01, Fig. 3a). In contrast, stimulation with IL-17E alone had no effect. There was a dose-dependent decrease in IL-17A-induced IL-8 release from OKF6/TERT-2 cells when stimulated concomitantly with varying concentrations of IL-17E, which was significant only with 400 ng/ml IL-17E (P < 0.01). However, 30 min of pre-incubation with IL-17E resulted in greater inhibition of IL-17A-induced IL-8 release. Once again, there was a dose-dependent decrease in IL-17A-induced IL-8 release from OKF6/TERT-2 cells. However, on this occasion the inhibition was significant with 10, 50, 100, 200 and 400 ng/ml IL-17E (Fig. 3b). The inhibitory effect of IL-17E was mediated at the transcriptional level. Stimulation of OKF6/TERT-2 cells with 10 ng/ml IL-17A alone induced a significant 7-fold increase in IL-8 mRNA expression (P = <0.01, Fig. 3c). Furthermore, 30 min pre-stimulation with 10 and 50 ng/ml IL-17E reduced the IL-17A-induced up-regulation of IL-8 mRNA expression. However, this reduction was only significant with 50 ng/ml IL-17E (P = <0.01, Fig. 3c).
The effect of IL-17E on IL-17A induced IL-8 expression by OKF6/TERT-2 cells. a Mean ± standard deviation IL-8 release from OKF6/TERT-2 cells stimulated concomitantly with combinations of IL-17A and IL-17E (see x axis for concentrations) (n = 3). Statistical analysis was performed by log transforming the data followed by an ANOVA with a post hoc Bonferroni correction (**P < 0.01). b Mean ± standard deviation IL-8 release from OKF6/TERT-2 cells pre-incubated with IL-17E for 30 min prior to stimulation with IL-17A (see x axis for concentrations) (n = 3). Statistical analysis was performed by log transforming the data followed by an ANOVA with a post hoc Bonferroni correction (*P = <0.05, **P < 0.01). c Fold induction change (±standard deviation) in IL-8 mRNA expression by OKF6/TERT-2 cells pre-incubated with IL-17E for 30 min prior to stimulation with IL-17A (see x axis for concentrations) (n = 3). Statistical analysis of real time PCR data was performed by a Levene’s test of homoscedasticity on the natural log transformed 2−∆CT values followed by an independent t test to compare two means (**P = <0.01). ±Statistical significance in comparison to unstimulated control, #statistical significance in comparison to cells stimulated with 10 ng/ml IL-17A only
IL-17E inhibits the IL-17A-induced activation of NF-κB in oral keratinocytes
IL-17A signalling activates the NF-κB transcription factor which in turn drives the expression of pro-inflammatory mediators [21]. Therefore, the ability of IL-17E to inhibit IL-17A driven NF-κB activation was investigated in vitro.
Exposure of OKF6/TERT-2 cells to 10 ng/ml IL-17A or 50 ng/ml IL-17E alone (or in combination) had no significant effect on total NF-κB p65 subunit levels compared to unstimulated control cells (data not shown). Stimulation of OKF6/TERT-2 cells with 10 ng/ml IL-17A did however cause significantly elevated phosphorylation of the NF-κB p65 subunit at serine 468 and 536 (P < 0.05) compared to unstimulated control cells. Addition of 50 ng/ml IL-17E to cultures 30 min prior to addition of IL-17A significantly decreased IL-17A-induced NF-κB P65 serine 468 and 536 phosphorylation (P < 0.05, Fig. 4a, b).
The effect of IL-17E on IL-17A induced phosphorylation of the NF-κB p65 subunit at serine 468 and serine 536 by OKF6/TERT-2 cells. a Mean (±standard deviation) angular transformed % of NF-κB p65 subunit phosphorylated at serine 468 pre-incubated with IL-17E for 30 min prior to stimulation with IL-17A (see x axis for concentrations) (n = 3). b Mean (±standard deviation) angular transformed % of NF-κB p65 subunit phosphorylated at serine 536 pre-incubated with IL-17E for 30 min prior to stimulation with IL-17A (see x axis for concentrations) (n = 3). Statistical analysis was performed by ANOVA and a post hoc Holm–Bonferroni (H–B) corrected least significant difference test (*P < 0.05/H–B). ±Statistical significance in comparison to unstimulated control, #statistical significance in comparison to cells stimulated with 10 ng/ml IL-17A only
None of the observed effects were related to cell viability as determined by a lactate dehydrogenase (LDH) activity assay (data not shown).
Discussion
Previous studies investigating the clinical relationships between serum, saliva and GCF levels of IL-17 family cytokines and periodontitis have predominantly focused on IL-17A. In agreement with these studies this manuscript confirms that serum, saliva, and GCF levels of IL-17A are elevated in periodontitis patients and correlate with clinical parameters of periodontitis and age [2–6]. The unique aspect of this study was the investigation of the clinical associations between all members of the IL-17 family and periodontitis in systemically healthy non-smokers. Despite IL-17B, IL-17C, IL-17D, IL-17F and IL-17A/F all being reported to have pro-inflammatory functions [1], only serum levels of IL-17A/F were significantly elevated in periodontitis patients (Fig. 1) and these levels correlated with clinical parameters (Table 3). However, serum levels of IL-17A did show positive correlations with levels of the majority of the other pro-inflammatory IL-17 family members; with the exception of IL-17D (Table 2a). This is therefore suggestive of a relationship between circulating levels of IL-17A and other pro-inflammatory family members. However, the biological significance of these findings and their relationship to periodontitis pathogenesis remains to be determined.
In contrast to other IL-17 family members, IL-17E has been suggested to have anti-inflammatory functions. Indeed, IL-17E has been suggested to play a protective role in chronic inflammatory pathologies [28] and can negatively regulate both bacterially driven [34, 35] and IL-17A driven immune responses [32, 33]. Given that periodontitis has a bacterial aetiology [41], and that previous studies have suggested that IL-17A drives pathogenesis [20], it is interesting to speculate toward an anti-inflammatory role for IL-17E in disease pathogenesis. In this study, periodontitis patients presented with decreased serum levels of IL-17E (Fig. 1), which negatively correlated with CPD and CAL (Table 3) and serum levels of IL-17A (Table 2). Given the proposed opposing biological functions of IL-17A and IL-17E, previous studies have suggested a relationship between the serum IL-17A:IL-17E ratio and the link between periodontitis and both RA and PCOS [26, 27]. In this study, a direct association between the serum IL-17A:IL-17E ratio and periodontitis is reported. Indeed, the serum IL-17A:IL-17E ratio was higher in patients with periodontitis than healthy controls (Table 4a). Furthermore, the IL-17A:IL-17E ratio showed significant positive correlations with clinical parameters of periodontitis (Table 4b). This therefore suggests that the serum IL-17A:IL-17E ratio may be a predictive marker of disease.
In contrast to the findings in serum, the clinical associations between IL-17 family cytokine levels in GCF and saliva and periodontitis, particularly with respect to IL-17E, revealed telling differences. In GCF, no significant differences between levels of IL-17E were determined between health and disease (Fig. 1b). In contrast, salivary levels of IL-17E were significantly elevated in patients with periodontitis compared to healthy controls (Fig. 1c). Furthermore significant correlations between the IL-17A:IL-17E ratio and clinical paramters in these biological fluids were limited to a correlation between the GCF ratio and CPD (Table 4b). The differences between the serum and GCF and saliva findings could possibly be explained by the anatomical location from which these biological fluids are derived [41]. GCF and saliva are derived from sites close to the site of periodontal inflammation and therefore are more representative of the events occurring during the disease process [41]. Interestingly, in RA, increased expression of IL-17E occurs only in later stages of disease [42]. Therefore, it is interesting to speculate that late stage IL-17E expression occurs in an attempt to down-regulate damaging pro-inflammatory responses within tissues. Given that our disease cohort present with clinical hallmarks of periodontitis; they can be categorised as being in the later stages of pathogenesis. In addition, real time PCR analysis of gingival tissues revealed that IL-17E expression is elevated locally in diseased patients (data not shown). Therefore, it is interesting to speculate that elevated gingival expression of IL-17E is a mechanism by which tissues attempt to regulate localised chronic inflammation. As locally expressed mediators present in both GCF and saliva, this may explain why IL-17E levels were elevated in these fluids and thus the differences in the IL-17A:IL-17E ratio from those reported in serum.
The in vitro studies reported in this manuscript begin to support an anti-inflammatory role for IL-17E in periodontal disease pathogenesis. IL-17E has previously been shown to regulate the innate immune responses of bacterial LPS stimulated myeloid cells [35]. These studies demonstrate that a similar phenomenon occurs in oral keratinocytes. Interestingly, the data presented demonstrate that IL-17E can inhibit the periodontal pathogen-induced expression of neutrophil chemotactic chemokines; IL-8 and CXCL5. Neutrophils are the most abundant leukocyte within periodontal tissues in early and chronic periodontal lesions and drive the destructive inflammation associated with periodontitis [43]. The ability of IL-17E to regulate chemokine expression are suggestive of a role in modulating neutrophil infiltration. IL-17A has also been demonstrated to promote chemokine expression and neutrophil recruitment in a mouse model of periodontitis [20]. Therefore, the ability of IL-17E to regulate IL-8 expression, as described in this manuscript, further supports a role for IL-17E in negatively regulating neutrophil infiltration and subsequently periodontal disease pathogenesis.
IL-17A has been shown to drive IL-8 expression via activation of the NF-κB transcription factor [44]. In this manuscript we report that IL-17A promotes IL-8 expression via phosphorylation of the NF-κB p65 subunit at serine 536 and serine 468. Phosphorylation of serine 536 of the NF-κB p65 subunit has been demonstrated to be important in transcription of the IL-8 gene [45], while phosphorylation at serine 468 has been shown to have an important role for NF-κB ubiquitination and degradation [46]. However, despite their opposing functions, phosphorylation at serine 536 and 468 occurs simultaneously [47]. The data in this manuscript suggest that IL-17E inhibits the IL-17A-induced phosphorylation events which promote NF-κB p65 subunit activation. Previous studies have demonstrated that IL-17E exhibits anti-inflammatory properties by the promotion of SOCS3 activation. There is evidence for a role of SOCS3 in inhibiting NF-kB activation [48] and therefore this could be hypothesised to be occurring in the in vitro studies described in this manuscript. However, it is also interesting to speculate that regulation may be occurring at the receptor level. Indeed, the IL-17RA subunit has been demonstrated to be required for both IL-17A and IL-17E signalling [3]. Therefore, it could be hypothesised that IL-17E competes with IL-17A for IL-17RA and binding to this subunit inhibits IL-17A binding and signal activation. At present, however, the exact mechanism by which IL-17E inhibits both IL-17A and P. gingivalis-induced immune responses remains unknown and is an area of ongoing study.
IL-17A and IL-17F have a high degree of sequence similarity and share many biological properties [1]. In addition, a heterodimeric IL-17A/IL-17F cytokine has been shown to exist [2]. Interestingly, the ability of IL-17A, IL-17F and IL-17A/F to activate an innate immune response varies subtly due to differential affinities for IL-17RA [2, 49, 50]. Hence, competition for the same receptor and differential binding affinities may be a mechanism by which activity of IL-17 family cytokine activities are regulated. Given the close relationship between these members of the IL-17 family it is unsurprising perhaps that on the whole there are significant correlations between IL-17A, IL-17F and IL-17A/F in serum, saliva and GCF. However, no significant correlation was observed between IL-17F and IL-17A/F in serum. The biological reason for this finding remains unknown.
In conclusion, these data implicate a role for IL-17 family cytokines in periodontitis. Interestingly, the data suggests that IL-17A and IL-17E may have opposing functions in disease pathogenesis; particularly as IL-17E can abrogate P. gingivalis and IL-17A induced expression of neutrophil chemotactic chemokines in vitro. However, at present, the cellular sources, exact signalling mechanisms and biological functions of IL-17 family cytokines in the periodontium remain to be fully characterised. Therefore, further robust in vitro and in vivo studies are required to fully delineate the biological functions of these cytokines and their role in periodontitis before extensive conclusions can be made.
References
Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64(2):477–85.
Wright JF, Guo Y, Quazi A, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4 + T cells. J Biol Chem. 2007;282(18):13447–55.
Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–67.
Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155(12):5483–6.
Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, Silva JS. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24(1):1–6.
Adibrad M, Deyhimi P. Ganjalikhani Hakemi M, Behfarnia P, Shahabuei M, Rafiee L. Signs of the presence of Th17 cells in chronic periodontal disease. J Periodontal Res. 2012;47(4):525–31.
Beklen A, Ainola M, Hukkanen M, Gurgan C, Sorsa T, Konttinen YT. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dent Res. 2007;86(4):347–51.
Konermann A, Beyer M, Deschner J, Allam JP, Novak N, Winter J, Jepsen S, Jager A. Human periodontal ligament cells facilitate leukocyte recruitment and are influenced in their immunomodulatory function by Th17 cytokine release. Cell Immunol. 2012;272(2):137–43.
Allam JP, Duan Y, Heinemann F, et al. IL-23-producing CD68(+) macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. J Clin Periodontol. 2011;38(10):879–86.
Honda T, Aoki Y, Takahashi N, et al. Elevated expression of IL-17 and IL-12 genes in chronic inflammatory periodontal disease. Clin Chim Acta. 2008;395(1–2):137–41.
Duarte PM, da Rocha M, Sampaio E, Mestnik MJ, Feres M, Figueiredo LC, Bastos MF, Faveri M. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: a pilot study. J Periodontol. 2010;81(7):1056–63.
Schenkein HA, Koertge TE, Brooks CN, Sabatini R, Purkall DE, Tew JG. IL-17 in sera from patients with aggressive periodontitis. J Dent Res. 2010;89(9):943–7.
Ozcaka O, Nalbantsoy A, Buduneli N. Interleukin-17 and interleukin-18 levels in saliva and plasma of patients with chronic periodontitis. J Periodontal Res. 2011;46(5):592–8.
Vernal R, Dutzan N, Chaparro A, Puente J. Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32(4):383–9.
Buduneli N, Buduneli E, Kutukculer N. Interleukin-17, RANKL, and osteoprotegerin levels in gingival crevicular fluid from smoking and non-smoking patients with chronic periodontitis during initial periodontal treatment. J Periodontol. 2009;80(8):1274–80.
Kadkhodazadeh M, Baghani Z, Ebadian AR, Youssefi N, Mehdizadeh AR, Azimi N. IL-17 gene polymorphism is associated with chronic periodontitis and peri-implantitis in Iranian patients: a cross-sectional study. Immunol Invest. 2013;42(2):156–63.
Correa JD, Madeira MF, Resende RG, et al. Association between polymorphisms in interleukin-17A and -17F genes and chronic periodontal disease. Mediators Inflamm. 2012;2012:846052.
Saraiva AM. Alves e Silva MR, Correia Silva Jde F, da Costa JE, Gollob KJ, Dutra WO, Moreira PR. Evaluation of IL17A expression and of IL17A, IL17F and IL23R gene polymorphisms in Brazilian individuals with periodontitis. Hum Immunol. 2013;74(2):207–14.
Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109(9):3794–802.
Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13(5):465–73.
Koenders MI, Lubberts E, Oppers-Walgreen B, et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167(1):141–9.
Koenders MI, Marijnissen RJ, Devesa I, et al. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1beta, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum. 2011;63(8):2329–39.
Iyoda M, Shibata T, Kawaguchi M, Hizawa N, Yamaoka T, Kokubu F, Akizawa T. IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-alpha and IL-1beta. Am J Physiol Renal Physiol. 2010;298(3):F779–87.
Kotake S, Yago T, Kawamoto M, Nanke Y. Role of osteoclasts and interleukin-17 in the pathogenesis of rheumatoid arthritis: crucial ‘human osteoclastology’. J Bone Miner Metab. 2012;30(2):125–35.
Konermann A, Winter J, Novak N, Allam JP, Jager A. Verification of IL-17A and IL-17F in oral tissues and modulation of their expression pattern by steroid hormones. Cell Immunol. 2013;285(1–2):133–40.
Gumus P, Buduneli E, Biyikoglu B, Aksu K, Sarac F, Nile C, Lappin D, Buduneli N. Gingival crevicular fluid, serum levels of receptor activator of nuclear factor-kappa B ligand, osteoprotegerin, interleukin-17 in rheumatoid arthritis and osteoporosis patients with periodontal disease. J Periodontol. 2013;84(11):1627–37.
Ozcaka O, Buduneli N, Ceyhan BO, Akcali A, Hannah V, Nile C, Lappin DF. Is IL-17 involved in the interaction between polycystic ovary syndrome and gingival inflammation? J Periodontol. 2013;83(12):1529–37.
Monteleone G, Pallone F, Macdonald TT. Interleukin-25: a two-edged sword in the control of immune-inflammatory responses. Cytokine Growth Factor Rev. 2010;21(6):471–5.
Tamachi T, Maezawa Y, Ikeda K, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118(3):606–14.
Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. 2010;11(3):250–6.
Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010;31(11):407–13.
Owyang AM, Zaph C, Wilson EH, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203(4):843–9.
Kleinschek MA, Owyang AM, Joyce-Shaikh B, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204(1):161–70.
Caruso R, Sarra M, Stolfi C, et al. Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology. 2009;136(7):2270–9.
Caruso R, Stolfi C, Sarra M, Rizzo A, Fantini MC, Pallone F, MacDonald TT, Monteleone G. Inhibition of monocyte-derived inflammatory cytokines by IL-25 occurs via p38 Map kinase-dependent induction of Socs-3. Blood. 2009;113(15):3512–9.
Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–7.
Pratten J, Smith AW, Wilson M. Response of single species biofilms and microcosm dental plaques to pulsing with chlorhexidine. J Antimicrob Chemother. 1998;42(4):453–9.
Ramage G, Jose A, Coco B, Rajendran R, Rautemaa R, Murray C, Lappin DF, Bagg J. Commercial mouthwashes are more effective than azole antifungals against Candida albicans biofilms in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(4):456–60.
Sherry L, Millhouse E, Lappin DF, Murray C, Culshaw S, Nile CJ, Ramage G. Investigating the biological properties of carbohydrate derived fulvic acid (CHD-FA) as a potential novel therapy for the management of oral biofilm infections. BMC Oral Health. 2013;13:47.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8.
Buduneli N, Kinane DF. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol. 2011;38(Suppl 11):85–105.
Yamada H. Current perspectives on the role of IL-17 in autoimmune disease. J Inflamm Res. 2010;3:33–44.
Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontology. 2000;1997(14):33–53.
Hata K, Andoh A, Shimada M, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282(6):G1035–44.
Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004;279(53):55633–43.
Geng H, Wittwer T, Dittrich-Breiholz O, Kracht M, Schmitz ML. Phosphorylation of NF-kappaB p65 at Ser468 controls its COMMD1-dependent ubiquitination and target gene-specific proteasomal elimination. EMBO Rep. 2009;10(4):381–6.
Mattioli I, Sebald A, Bucher C, Charles RP, Nakano HT, Kracht M, Schmitz ML. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J Immunol. 2004;172(10):6336–44.
Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7(6):454–65.
Liang SC, Long AJ, Bennett F, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179(11):7791–9.
Kuestner RE, Taft DW, Haran A, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179(8):5462–73.
Acknowledgments
We are very grateful to the patients and volunteers who agreed to participate in the study. The project was supported by Funding from Tenovus Scotland (Registered charity number SC009675) and The Oral and Dental Research Trust (Registered charity number 800234).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Mauro Teixeira.
Rights and permissions
About this article
Cite this article
Azman, R., Lappin, D.F., MacPherson, A. et al. Clinical associations between IL-17 family cytokines and periodontitis and potential differential roles for IL-17A and IL-17E in periodontal immunity. Inflamm. Res. 63, 1001–1012 (2014). https://doi.org/10.1007/s00011-014-0776-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-014-0776-7