Abstract
Programmed cell death protein 1 (PD-1) and its ligand PD-L1 are critical for the regulation of T cell exhaustion and activity suppression. Tumor cells expressing immune checkpoints including PD-L1 escape monitoring of T cells from the host immune system. Checkpoint inhibitors are highly promising therapies that function as tumor-suppressing factors via modulation of tumor cell–immune cell interactions as well as boosting T cell-mediated anti-tumor immunity. Notably, PD-1 or PD-L1 monoclonal antibody (mAb) has demonstrated promising therapeutic effects in clinical studies of many types of cancer. These mAbs have caused significant tumor regression with impressive anti-tumor response rates as well as a favorable safety profile in cancer patients. Furthermore, the combination of PD-1/PD-L1 mAbs with other types of anti-tumor agents has also developed to boost the anti-tumor responses and enhance therapeutic effects in cancer patients. This review clarifies the mechanisms of PD-1/PD-L1-mediated anti-cancer immune responses and some clinical studies of mAbs targeting PD-1/PD-L1. The challenges and future of PD-1/PD-L1 blockade therapy are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In tumor microenvironments, tumors exploit a wide range of immune escape mechanisms to escape immune destruction, including induction of an immunosuppressive microenvironment and suppression of cytotoxic T-lymphocyte effector functions (Gajewski et al. 2013). Immunotherapy strategies are designed to activate anti-tumor responses and reverse tumor immune suppression (Zarour 2016). One of the most effective strategies is activating T cell-mediated anti-tumor responses, which is based on the regulation of the balance between co-stimulatory and co-inhibitory signals (Krogsgaard and Davis 2005). These signals are also called immune checkpoints, which are important for regulating self-tolerance, preventing autoimmunity, and protecting the host from tissue damage (Mellman et al. 2011; Sharma and Allison 2015; Topalian et al. 2015). The immune checkpoints include the receptors expressed by effector T cells and the regulatory T cells (Tregs) as well as their respective ligands expressed by antigen-presenting cells (APCs) and/or tumor cells, thereby assisting the invasion of the anti-tumor immune response. Immune checkpoints regulate immune cell activities and proliferation, thereby maintaining tolerance to self-antigens and ensuring the immune responses avoid chronic inflammation. However, in the tumor microenvironment, tumor cells express inhibitory receptors and block functions of effector T activity via impairing the ability of tumor-specific T lymphocytes and triggering the expression of immune checkpoints.
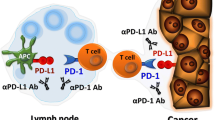
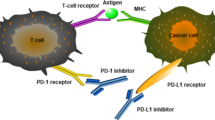
Monoclonal antibodies (mAbs) blocking immune checkpoint receptors have emerged as promising therapeutics. For example, mAbs against immune checkpoints including programmed cell death protein 1 (PD-1) or programmed death ligand 1 (PD-L1) have been widely applied to restore the function of effector T cells and enhance their anti-tumor abilities by blocking tumor cell triggered inhibitory signals in tumor-specific T cells (Fig. 1). Furthermore, mAbs with high specificity have also been shown to disrupt the function of immune checkpoints by blocking ligand-receptor interactions. Importantly, mAbs targeting immune checkpoints have demonstrated promising results in animal xenograft models as well as cancer patients in clinical studies, suggesting their potential as therapeutic candidates for cancer immunotherapy.
A series of immune checkpoints has been identified over the past few decades (Gajewski et al. 2013; Keir et al. 2006, 2008; Krummel and Allison 1995; Nishimura et al. 1999; Ocana-Guzman et al. 2016; Sledzinska et al. 2015). There has been a particular focus on cytotoxic T-lymphocyte protein 4 (CTLA-4) and PD-1 immune checkpoints, which are co-inhibitory molecules that regulate the immune function of T cells. CTLA-4 or PD-1 blockage activates T cell-mediated anti-tumor immunity, and some studies have shown anti-tumor efficiency by CTLA-4 or PD-1 blockage in animal xenograft models including non-small cell lung cancer (NSCLC), melanoma, and other types of cancer. To the best of our knowledge, the underlying molecular mechanisms of CTLA-4 and PD-1-mediated immune responses against cancer differ. CTLA-4 regulates the proliferation of T cells at an early stage of immune responses, whereas PD-1 is responsible for the proliferation of T cells at a later stage. Additionally, by competitively binding CD80/CD86, which is a ligand for CD28, CTLA-4 also inhibits the activation of T cells. PD-1 inhibits the proliferation and survival of T cells by interacting with PD-L1, impacting the production of cytokines including interleukin (IL)-2, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ ( Blank et al. 2004; Dong et al. 2002; Iwai et al. 2002; Leach et al. 1996).
Based on the understanding of the molecular mechanisms of CTLA-4 and PD-1 for the regulation of T cells, mAbs targeting CTLA-4 and PD-1 have been developed and approved for cancer immunotherapy. For instance, ipilimumab, the first anti-CTLA-4 mAb, was approved by the US Food and Drug Administration (FDA) in 2011. It is used to block CTLA-4 and induce sustained anti-tumor responses (Hodi et al. 2010). Additionally, some mAbs targeting PD-1 and PD-L1 have also been approved for cancer immunotherapy and demonstrated good therapeutic effects for a wide range of cancers (Brahmer et al. 2010; Hamid et al. 2013; Powles et al. 2014a; Zou et al. 2016). mAbs that target the PD-1 receptor include pidilizumab, nivolumab, pembrolizumab, PDR001, MEDI0680, and AMP-224. A combination of PD-1/PD-L1 blockage therapy with other anti-cancer drugs has also been widely investigated. In this review, the structures and interactions of PD-1 and PD-L1, molecular mechanisms of PD-1 and PD-L1-mediated anti-cancer immune responses, some clinical trials of mAbs targeting PD-1 and PD-L1, and current challenges and the future of PD-1 and PD-L1 blockage therapy are discussed.
Structures of PD-1 and PD-L1 and Their Roles in Cancer Immunotherapy
Structure and Interactions of PD-1 and PD-L1
PD-1, a member of the B7-CD28 receptor family, is a 55 kDa monomeric type I surface transmembrane glycoprotein (Chen 2004; Xia et al. 2016). It is composed of an extracellular IgV domain, a transmembrane domain, and an intracellular cytoplasmic domain (Butte et al. 2007; Viricel et al. 2015). PD-L1, a 40 kDa type I transmembrane protein, is composed of two side-by-side domains and extracellular IgV and IgC domains (Dong et al. 1999). Compared with PD-1, PD-L1 lacks intracellular signaling. The first murine Apo-PD-1 extracellular domain structure (PDB ID: 1NPU) was discovered by Zhang et al. (2004) as shown in Fig. 2a. Cheng et al. (2013) reported a human Apo-PD-1 extracellular domain structure that was identified by NMR. Lázár-Molnár et al. (2017) established the human Apo-PD-1 extracellular crystal structure (PDB ID: 3RRQ) though X-rays as shown in Fig. 2b. Figure 2c shows the overlap of 1NPU and 3RRQ. For PD-L1, several human Apo-PD-L1 extracellular crystal structures have been reported with high resolution (Chen et al. 2010; Lin et al. 2008; Zak et al. 2015; Zhang et al. 2017). The structures of two (PDB ID: 3FN3 and 5C3T) are shown in Fig. 2d, e and their overlap is shown in Fig. 2f.
The structures of murine and human PD-1 and PD-L1. a The structure of the murine PD-1 extracellular domain (1NPU). b The structure of the human PD-1 extracellular domain (3RRQ). c The overlap of 1NPU and 3RRQ. d The structure of the human PD-L1 extracellular domain including both V-type and C2-type domains (3FN3). e The structure of the murine PD-L1 extracellular V-type domain (5C3T). f The overlap of 3FN3 and 5C3T
Before human PD-1/PD-L1 crystal structures were identified (Zak et al. 2015), the crystal structures of murine PD-1 with human PD-L1 were used to elucidate PD-1 and PD-L1’s interactions (Lin et al. 2008). However, human and murine PD-1 share only 64% of amino acid sequence identity, and amino acid sequence identity between human and murine PD-L1 is 77% (Konstantinidou et al. 2018), indicating that interactions between murine PD-1 and human PD-L1 are different from human PD-1/PD-L1 interactions. Figure 3a shows the crystal structure of human PD-1/PD-L1 (PDB ID: 4ZQK). Between the human Apo-PD-1 structure (PDB ID: 3RRQ) and human PD-1 (PDB ID: 4ZQK), an obvious structural arrangement of PD-1 can be observed (Fig. 3b). In addition, interaction analysis demonstrated that three hotspot pockets were identified that are responsible for the binding affinity between human PD-1 and PD-L1 (Fig. 3c).
The structure of human PD-1/PD-L1 complex and hotspot pockets between PD-1 and PD-L1. a The structure of human PD-1/PD-L1 complex (4ZQK); the red one is PD-1 and the blue one is PD-L1. b PD-L1 is shown at the surface and interacting with PD-1. c The key residues that bind with PD-L1 are shown in the sticks with residue numbers. Three hotspot pockets are shown in the red cycles
Mechanism of PD-1- and PD-L1-mediated Immune Responses
The PD-1/PD-L1 axis is a desirable target for cancer immunotherapy due to its higher efficiency, selectivity, lower toxicity, and broader spectrum of anti-tumor activities (Larkin et al. 2015a; Robert et al. 2015). PD-1 and its ligands including PD-L1 and PD-L2 regulate the inhibition and exhaustion of T cells. PD-L1 is broadly expressed in hematopoietic and non-hematopoietic cells, whereas PD-L2 is inducibly expressed in dendritic cells (DCs), macrophages, memory B cells, and bone-marrow-derived mast cells. Interestingly, when compared with PD-L2, the high expression of PD-L1 is more closely associated with tumor growth and metastasis as supported by several clinical studies. However, the high expression of PD-L2 is associated with a decrease in survival time but no statistical significance, indicating that the PD-1/PD-L1 axis is a more desirable target for cancer immunotherapy than the PD-1/PD-L2 axis (Fernandes and Brabek 2017).
PD-1 is widely expressed in B cells, T cells, and natural killer (NK) cells (Nurieva et al. 2006), while PD-L1 is expressed in DCs, macrophages, and tumor cells (Latchman et al. 2001). By binding with PD-1, PD-L1 triggers co-inhibitory signals and inhibits the function of T cells by modulating the T cell receptor (TCR)-meditated signaling pathways and counterbalancing the activation of co-stimulatory signals. Emerging evidence has also identified new functional roles of the PD-1/PD-L1 signaling axis (Alsaab et al. 2017; Wei et al. 2018).
The primary function of PD-1 is to inhibit the activation of TCR signaling. Therefore, cells highly expressing PD-L1, including APCs and tumor cells, interact with PD-1 overexpressed T cells, leading to dysfunction of T cells. TCR signaling is induced by the interactions between TCR on T cells and MHC-peptide loading complex on APCs. However, the activation of PD-1 inhibits the phosphorylation of the TCR signaling intermediates, leading to the termination of TCR signaling and inhibition of T cell proliferation (Patsoukis et al. 2012). PD-1 directly inhibits Ras, an enhancer of T cell inactivation (Patsoukis et al. 2012). There are two signaling motifs in the cytoplasmic tail of PD-1, the intracellular immunoreceptor tyrosine-based switch motif (ITSM) and the immunoreceptor tyrosine-based inhibitory motif (Chemnitz et al. 2004; Sheppard et al. 2004). By binding with PD-L1, ITSM is phosphorylated and recruits Src homology 2-containing tyrosine phosphatase (SHP-2), thereby inhibiting the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway (Hofmeyer et al. 2011; Parry et al. 2005; Yokosuka et al. 2012). PI3K/Akt signaling pathway blockage downregulates the mechanistic target of rapamycin and inhibits protein synthesis and cell growth. PI3K/Akt signaling pathway blockage also inhibits the degradation of transcription factor FoxO1, which in turn enhances the expression of PD-1 (Staron et al. 2014). Recent studies indicate that CD28 is also a primary target of PD-1-induced attenuation of T cell signaling. These studies utilized a cell-free membrane reconstitution model to examine functional relationships during T cell activation and reveal that PD-1 leads to preferential dephosphorylation of CD28 rather than the TCR, via recruitment of SHP-2. This suggests that PD-1, at least in part, acts through a similar molecular mechanism of attenuating CD28-mediated co-stimulation. Interestingly, recent findings indicate that SHP-2 is not essential for responses to anti-PD-1 therapy or induction of T cell exhaustion in vivo (Wei et al. 2018). This suggests the functional redundancy of the signaling pathways downstream of PD-1. This redundancy is most likely mediated through redundant phosphatases (for example, SHP1), but alternatively could be mediated through wholly distinct mechanisms.
PD-1 activation induces a decrease in B cell lymphoma extra-large, thereby impacting cell survival and proliferation. Basic leucine zipper transcription factor expression is also regulated by PD-1 activation, thereby weakening the functions of effector T cells and resulting to their dysregulation. Another study reported that PD-1 modulates cell metabolism by inhibiting glycolysis and promoting fatty acid oxidation (Patsoukis et al. 2015). The downstream effects mediated by PD-1/PD-L1 binding cause a decrease in several inflammatory cytokines including IL-2, TNF-α, and IFN-γ (Barber et al. 2006; Freeman et al. 2000; Latchman et al. 2001). Recent research reported that macrophage expression of PD-L1 may lead to the active eviction of T cells from the tumor microenvironment (Alsaab et al. 2017). This suggests that in addition to regulating T cell activation and cytolytic capacity, PD-1 signaling may also regulate T cell tracking and migration.
PD-1 overexpression results in T cell exhaustion in mice and humans infected with chronic virus, which is supported by several studies (Barber et al. 2006; Hofmeyer et al. 2011; Ka et al. 2011; Lu et al. 2014; Quigley et al. 2010; Youngblood et al. 2011). Alternatively, PD-1 is upregulated in CD8+ T cells while PD-1 blockage reverses exhausted CD8+ T cells (Barber et al. 2006; Pauken and Wherry 2015). Exhausted T cells also exhibit insufficient protective immunologic response (Barber et al. 2006; Blackburn et al. 2009; Day et al. 2006). Studies have reported that PD-L1 expressed in tumor cells induces Treg proliferation by regulating Akt signaling pathways (Francisco et al. 2009; Haxhinasto et al. 2008). Treg has immunosuppressive functions, and part of its mechanism is to suppress the proliferation of effector T cells (Bettelli et al. 2006). Apart from binding with PD-1, PD-L1 interacts with CD80, which is mainly expressed in activated T cells, thereby inhibiting T cell-mediated immune responses (Butte et al. 2007; Park et al. 2010). Once PD-L1 is highly expressed in tumor cells, it signals T cells, thereby impacting their survival, but the underlying mechanisms are not fully understood (Azuma et al. 2008; Dong et al. 2003). Overall, the multiple effects initiated by PD-1/PD-L1 interactions including the inhibition of T cell proliferation, promotion of T cell exhaustion, and dampening of the functions of effector T cells lead to immune evasion of cancer cells.
mAbs targeting PD-1 and PD-L1 developed to block their binding, thereby shutting off the inhibitory signals and consequently leading to reactivation of anti-tumor immune response mediated by T cells (John et al. 2013; Mantovani 2010; Zang and Allison 2007). Numerous clinical studies have demonstrated that PD-1 and PD-L1 blockage therapies show promising anti-tumor activities for various cancer types (Ansell et al. 2015; Brahmer et al. 2010, 2012; Herbst et al. 2014; Le et al. 2015; Lipson et al. 2013; Powles et al. 2014a; Topalian et al. 2012). Herein, we discuss immune checkpoint blockage of PD-1 and PD-L1.
Immune Checkpoint Blockage of PD-1
mAbs target PD-1 by blocking PD-1/PD-L1 interactions, which in turn enhances the functions of effector T cells and simultaneously promotes the proliferation of T cells (Wong et al. 2007). In general, PD-1 inhibitors competitively bind to PD-1 and block PD-1/PD-L1 interactions, thereby inhibiting PD-1-mediated downstream events including the activation of NK cells and cytotoxic T cells. PD-1 inhibitors also regulate negative signals on the T cell surface and promote the activation and proliferation of T cells. In addition to the inhibitory effect on the immune checkpoint, PD-1 inhibitor AMP-224 also has antineoplastic activities. Currently, mAbs targeting PD-1 include pidilizumab, nivolumab, pembrolizumab, spartalizumab, MEDI0680, and AMP-224 (Table 1).
Pidilizumab
Pidilizumab (CT-011 and MDV9300) is the first humanized immunoglobulin (Ig) G1 mAb against PD-1. Pidilizumab has broader clinical activities for various cancer types including B cell lymphoma, colorectal cancer, hematologic malignancies, melanoma, and solid tumors.
In a phase I clinical trial, pidilizumab therapy was well tolerated and its total clinical benefit rate was approximately 33% in patients with hematologic malignancies including Hodgkin lymphoma, myeloid leukemia, and lymphocytic leukemia (Berger et al. 2008). In this study, the administration of pidilizumab (0.2–6.0 mg/kg) was well tolerated in patients with advanced hematologic malignancies. The median half-life of pidilizumab is up to 410 h, and an elevation of blood CD4+ lymphocytes is observed up to 21 days after pidilizumab therapy. We speculate that the clinical benefits may be related to the durable tumor-specific immune response induced by pidilizumab. The safety profile and clinical activities are supported by two phase II studies, in which a 55% overall response rate was observed in patients after autologous hematopoietic stem cell transplantation. Pidilizumab therapy also demonstrated safety profiles in these clinical studies (Armand et al. 2013; Westin et al. 2014). These results may be due to the lower dose and less frequent administration of pidilizumab. For instance, in one clinical trial of patients with relapsed follicular lymphoma, pidilizumab was dosed at 3 mg/kg intravenously every 4 weeks (Westin et al. 2014). Another phase II study initiated by Atkins et al. (2014) evaluated anti-tumor activity and the safety profile of pidilizumab in patients with metastatic melanoma. Overall, 45% of patients did not respond to pidilizumab therapy, although pidilizumab therapy was well tolerated and improved substantial survival in heavily pretreated patients (Atkins et al. 2014). Additionally, pidilizumab therapy was well tolerated in patients with diffuse intrinsic pontine glioma, demonstrating that pidilizumab might be a drug candidate for diffuse intrinsic pontine glioma therapy (Fried et al. 2018). Fried et al. (2016) evaluated the effects of pidilizumab on children with diffuse intrinsic pontine glioma. This was the first study conducted on pediatric patients. Administration of pidilizumab was well tolerated in 9 of 13 patients during the study period. However, two patients experienced grade 3 adverse events. Other adverse events included fatigue (50%), anorexia (17%), and hypophosphatemia (17%) but none were significant. However, the sample cohort was relatively small, limiting support of the final conclusions. Some phase III clinical trials of pidilizumab in other types of cancers are still underway.
Nivolumab
As a humanized IgG4 antibody targeting PD-1, nivolumab (BMS-936558) blocks interactions between PD-1 and its ligands including PD-L1 and PD-L2. In 2014, nivolumab was approved by the FDA for the treatment of refractory unresectable melanoma. In 2015, the FDA approved nivolumab for the treatment of NSCLC after progression on a platinum-based chemotherapy regimen (Brahmer et al. 2015). To date, nivolumab therapy has been approval for many cancers including advanced renal cancer, colorectal cancer, hepatoma, head and neck squamous cell carcinoma, and advanced urothelial cancer.
In the first phase I clinical trial, nivolumab therapy demonstrated therapeutic effects and favorable safety profiles in patients with metastatic melanoma, colorectal cancer (CRC), castrate-resistant prostate cancer (CRPC), NSCLC, and renal cell cancer (RCC). Although nivolumab was well tolerated with anti-tumor activity, one patient with metastatic melanoma experienced severe inflammatory colitis after nivolumab therapy following five doses (1 mg/kg) administered over 8 months (Brahmer et al. 2010). In this study, anti-tumor activity of nivolumab is most likely through immunologic mechanisms, because non-hematologic tumors do not express PD-1. Importantly, tumor regressions were seen in CRC and NSCLC patients, indicating that the capacity of nivolumab to enhance anti-tumor immunity extends beyond the tumor types of melanoma and RCC. Another phase I study evaluated nivolumab in patients with melanoma, NSCLC, and RCC but no responses were observed in patients with CRC or CRPC. Additionally, severe adverse effects were observed in 15% of patients and 6% of patients discontinued therapy (Antonia et al. 2014; Topalian et al. 2012).
In a phase II clinical trial, nivolumab therapy was administered to patients with metastatic renal cell carcinoma and a 20–22% objective response rate (ORR) and an 18.2- to 25.5-month prolonged overall survival rate were observed (Motzer et al. 2015b). In this study, a total of 168 patients were randomly treated with nivolumab at doses of 0.3, 2, or 10 mg/kg intravenously once every 3 weeks. Interestingly, no dose–response relationship was observed as measured by the survival rate. Overall, nivolumab therapy demonstrated a manageable safety profile in all groups, consistent with some phase I clinical trials. Similarly, another phase II clinical trial showed that nivolumab therapy demonstrated a 21% ORR and a 35% 3-year overall survival rate in patients with advanced RCC (McDermott et al. 2015). Nivolumab also has therapeutic effects for hepatoma patients with a manageable adverse event profile supported by a prolonged 6-month overall survival rate after nivolumab therapy (Hamanishiet al. 2015). In addition to a wide spectrum of cancer types, nivolumab therapy also shows promising activities compared with other traditional chemotherapies. For instance, in a phase III trial of patients with refractory melanoma, nivolumab therapy demonstrated a higher ORR than chemotherapy (32% vs. 11%) (Rexer 2015). In other phase III trials for NSCLC, advanced squamous cell lung cancer, advanced RCC, and recurrent head and neck squamous cell carcinoma, nivolumab therapy demonstrated better survival benefits than some traditional therapies (Brahmer et al. 2015; Borghaei et al. 2015; Motzer et al. 2015a). For instance, in advanced NSCLC patients, the median overall survival was 9.2 months with nivolumab vs. 6.0 months with docetaxel. Moreover, the safety profile of nivolumab was more favorable than that of docetaxel. Importantly, the survival benefit of nivolumab therapy was observed independent of tumor PD-L1 expression levels (Brahmer et al. 2015).
Pembrolizumab
As a humanized IgG4 mAb against PD-1, pembrolizumab (MK-3475) has drawn considerable attention in recent years. In 2014, pembrolizumab was initially approved by the FDA for the treatment of refractory unresectable melanoma. In 2017, pembrolizumab was further approved for the treatment of unresectable or metastatic solid tumors with mismatch repair deficiency (Syn et al. 2017). Notably, this was the first anti-cancer drug approved by the FDA based on tumor genetics. In 2018, pembrolizumab was approved by the FDA for the treatment of advanced cervical cancer and refractory or relapsed primary mediastinal large B cell lymphoma.
In a phase I trial, evaluating the safety and anti-tumor activity of pembrolizumab in patients with primary mediastinal B cell lymphoma, the ORR was 41% (7/17) and 13 out of 16 patients (81%) showed decreases in target lesions. Additionally, 11 patients (61%) experienced drug-related adverse events (mostly grade 1–2) and no treatment-related deaths were observed (Zinzani et al. 2017). In another phase I clinical trial, pembrolizumab was used for the treatment of ten patients with advanced solid tumors. Pembrolizumab was administered as an intravenous infusion at 2 or 10 mg/kg every 2 weeks until unacceptable toxicity. Therefore, it did not have a definite maximum-tolerated dose (Patnaik et al. 2015). The response rate across all cohorts was 38% and adverse events (grade 3 or 4) were reported in 13% of patients. This study also determined the PD-L1 expression levels in tumor tissue. However, the anti-tumor activities of pembrolizumab were independent of tumor PD-L1 expression levels. This may have been due to the limited number of patients. An expansion clinical trial was further applied to evaluate the anti-tumor activities of pembrolizumab in 173 patients with malignant melanoma previously treated with ipilimumab or inhibitors for proto-oncogene B-Raf (BRAF) and/or mitogen-activated protein kinase. Pembrolizumab therapy (2 or 10 mg/kg every 3 weeks) was well tolerated with no drug-related deaths. The ORR (41/157) was 26% and adverse events (grade 3 or 4) were reported in five patients (Robert et al. 2014). In addition to its therapeutic effects against melanoma, pembrolizumab also demonstrated promising activities in Hodgkin lymphoma. In a phase I clinical trial, pembrolizumab therapy achieved 65% ORR in relapsed or refractory Hodgkin lymphoma (Armand et al. 2016). In the subsequent phase II clinical trial, pembrolizumab therapy achieved 69% ORR and adverse events (grade 3 or 4) were reported in 4.4% of patients (Chen et al. 2017). Except for its safety profile, pembrolizumab demonstrated superior overall survival than ipilimumab (55% vs 43%) in a phase III clinical trial of patients with advanced melanoma (Schachter et al. 2017). This study conducted a head-to-head comparison of pembrolizumab vs ipilimumab for advanced melanoma. A total of 834 patients were enrolled and randomly assigned to receive pembrolizumab every 2 weeks (n = 279), pembrolizumab every 3 weeks (n = 277), or intravenous ipilimumab every 3 weeks (n = 278). The 24-month overall survival rate was 55% in the 2-week group, 55% in the 3-week group, and 43% in the ipilimumab group. These data support that the use of pembrolizumab is more beneficial for advanced melanoma. The difference may be due to their different mechanisms. Two additional phase III clinical trials of pembrolizumab for the treatment of patients with advanced hepatocellular carcinoma or metastatic head and neck cancer are ongoing (Abou-Alfa et al. 2018; Cohen et al. 2015).
Spartalizumab
Spartalizumab (PDR001) is another humanized anti-PD-1 IgG4 mAb with a subnanomolar affinity with PD-1. The first clinical trial of spartalizumab treated advanced solid tumors. Spartalizumab was well tolerated with a safety profile similar to other anti-PD-1 antibodies. A total of 58 patients were treated with Spartalizumab and only one developed grade 3 autoimmune colitis (Naing et al. 2016a). Additionally, spartalizumab maintained patients with anaplastic thyroid cancer in a stable stage for 7 months in another phase I study. Spartalizumab was also used in an expansion cohort of anaplastic thyroid cancer and a 27% overall disease control rate was achieved. Spartalizumab also had a favorable safety profile as no unexpected side effects were reported (Wirth et al. 2018). Spartalizumab was consistently well tolerated and demonstrated a manageable safety profile in another phase I/II clinical trial of advanced melanoma and NSCLC. It is worth noting that the ORR was higher in PD-L1 positive patients in certain tumor types including melanoma and NSCLC (Lin et al. 2018). Overall, these data support the potential anti-tumor activities of spartalizumab. The therapeutic effects of spartalizumab in other types of cancer have not yet been determined. Spartalizumab demonstrated synergistic anti-tumor effects with other agents in a clinical trial.
MEDI0680
MEDI0680 (AMP-514) is a humanized IgG4κ antibody that blocks the binding between PD-1 and PD-L1/PD-L2. The isotype information on MEDI0680 is not yet available. In 2016, the first phase I clinical trial of MEDI0680 was conducted in patients with advanced solid tumors to assess its safety profile and anti-tumor activity and define its highest tolerable dose (Naing et al. 2016b). In 51 patients, 9 (18%) had an objective response including 1 (2%) complete response (renal cancer) and 14 (28%) had stable disease as their best response. No unexpected adverse events were observed. These results suggested that MEDI0680 has preliminary signs of efficacy with an acceptable safety profile. Naing et al. (2019) conducted a phase I study of MEDI0680 in patients with advanced solid malignancies. In 58 patients, eight had objective responses (14%) including five with kidney cancer and three with melanoma. MEDI0680 showed a manageable safety profile as no treatment-related deaths were observed and most adverse effects were mild to moderate. This study also showed that MEDI0680 therapy enhanced CD4+ and CD8+ T cell proliferation in tumors and promoted plasma IFN-γ (Naing et al. 2019). Another phase I clinical trial of MEDI0680 in patients with advanced malignancies is ongoing (Infante et al. 2015).
AMP-224
AMP-224 was the first anti-PD-1 fusion protein composed of the extracellular domain of PD-L2 and the Fc region of human IgG1. By binding with PD-1 on chronically stimulated T cells, AMP-224 triggers cytotoxic T cell activation and immune response against tumors. AMP-224 exhibits distinctive safety and efficacy compared to other PD-L1 inhibitors because of different components and mechanisms. In 2013, the first clinical trial showed that AMP-224 was well tolerated up to 30 mg/kg in patients with advanced solid tumor. A total of 42 patients were treated with AMP-224 at doses of 0.3, 1, 3, 10, or 30 mg/kg intravenously at day 1 and day 15. No drug-related inflammatory adverse events were identified and one patient at 30 mg/kg AMP-224 developed flu-like symptoms. This study also demonstrated that AMP-224 specific inhibits population of PD-1+ CD4 and PD-1+ CD8 T cells in a dose-dependent manner (LoRusso et al. 2013). Another pilot study demonstrated that AMP-224 has a manageable safety profile in colon cancer patients with radiation therapy. A total of 17 patients were enrolled and intravenously administered 10 mg/kg AMP-224 at day 1. However, no objective responses were observed in this study. This may have been due to the lower efficacy of one single injection of AMP-224 and the relatively small sample size (Duffy et al. 2016). The therapeutic effects of AMP-224 in other types of cancer have not yet been determined.
Immune Checkpoint Blockage of PD-L1
Pre-clinical studies have shown that the expression of PD-L1 on tumor cells suppresses T cell activation and promotes tumor cell escape from the host immune system (Dong et al. 2002; Hirano et al. 2005). Binding of PD-L1 to its receptor inhibits T cell migration, proliferation, secretion of cytotoxic mediators, and restriction of cell killing. Therefore, the blockage of PD-L1 with specific mAbs provides an alternative method of activating T cell-mediated immune response. Anti-PD-L1 antibodies specifically disturb PD-1/PD-L1 interactions but do not block PD-1/PD-L2 interactions, which makes anti-PD-L1 antibodies less toxic as PD-1/PD-L2 interactions are important for maintaining peripheral tolerance. Four anti-PD-L1 mAbs are currently approved. Herein, we briefly discuss current clinical trials of anti-PD-L1 mAbs including BMS-986559, atezolizumab, durvalumab, and avelumab (Table 1).
BMS-936559
BMS-936559 (MDX-1105) is a human IgG4 mAb against PD-L1. Pre-clinical studies have demonstrated the anti-tumor activities of BMS-936559 in animal tumor models. Phase I trials of BMS-936559 were conducted in 207 patients with different refractory malignancies, including melanoma (n = 55), NSCLC (n = 75), colorectal (n = 18), ovarian (n = 17), renal cell (n = 17), pancreatic (n = 14), and breast cancer (n = 4). The patients were intravenously administered BMS-936559 at doses of 0.3, 1, 3, or 10 mg/kg on days 1, 15, and 29 in a 6-week cycle. Patients received BMS-936559 for up to 16 cycles until an unacceptable toxic effect was reported. The median duration of therapy was 12 weeks. Fifteen patients had objective responses (14%) including nine with melanoma, two with renal cancer, five with NSCLC, and one with ovarian cancer. Grade 3 or 4 adverse effects occurred in 9% of patients. Overall, BMS-936559 was well tolerated and the ORR was 6–17% in patients with different cancers; however, no response was found in patients with CRC or pancreatic cancer (Brahmer et al. 2012). Additionally, two clinical trials (NCT01455103 and NCT01452334) were withdrawn prior to enrollment (Li et al. 2016).
Atezolizumab
Atezolizumab (MPDL3280A and RO5541267) is human Fc-engineered anti-PD-L1 antibody. In 2015, atezolizumab was approved by the FDA for the treatment of NSCLC. In 2016, it was approved by the FDA for the treatment of urothelial carcinoma. However, in another phase III clinical trial, atezolizumab failed as the second-line treatment for urothelial carcinoma. In 2019, it was approved by the FDA for the treatment of advanced triple-negative breast cancer. Overall, atezolizumab was well tolerated in several clinical studies as no unexpected side effects were reported. In a phase I clinical trial, atezolizumab was well tolerated for several advanced solid tumors with no maximum-tolerated dose including CRC, melanoma, RCC, NSCLC, and gastric cancer. The median duration of therapy was 127 days. The ORR was 39% in patients with PD-L1+ tumors, whereas patients with PD-L1– tumors had an ORR of 13%. Grade 3 or 4 adverse effects including hepatitis, rash, and colitis occurred in 39% of patients (Herbst et al. 2013). In another phase II clinical trial, 119 patients received ≥ 1 dose of atezolizumab. The median duration of therapy was 15 weeks. Grade 3 or 4 treatment-related effects occurred in 19 patients and one grade 5 treatment-related effect was observed. Interestingly, atezolizumab achieved higher ORR in patients with PD-L1-positive tumors than in patients with PD-L1-negative tumors (18% vs. 15%) (Balar et al. 2017). These results supported that atezolizumab therapeutic efficiency is associated with PD-L1 expression. Atezolizumab also showed anti-tumor activities in metastatic urothelial bladder cancer. A total of 31 urothelial bladder cancer patients were treated with atezolizumab for a median duration of 43 days. The ORR was 26% and grade 3 or 4 treatment-related adverse effects occurred in 3.2% of patients (Powles et al. 2014b). Some clinical trials of atezolizumab to explore its anti-tumor activities in other tumors are ongoing.
Durvalumab
Durvalumab (MEDI4736) is an Fc-optimized humanized anti-PD-L1 IgG1κ mAb. It was approved by the FDA for the treatment of bladder cancer patients who had progressed after treatment with platinum. Anti-tumor activity of durvalumab is associated with PD-L1 expression. In a phase I/II open label study of patients with urothelial bladder cancer, a total of 61 patients (40 with PD-L1+ and 21 with PD-L1–) were enrolled. A 44.6% ORR in the PD-L1-positive group was achieved after treatment with durvalumab compared to a 0% ORR in the PD-L1-negative group (Massard et al. 2016). Grade 3 or 4 treatment-related adverse effects occurred in three patients. These results supported the promising anti-tumor activities of durvalumab in PD-L1+ patients with a manageable safety profile. In another phase I clinical trial of patients with NSCLC or melanoma, durvalumab therapy showed an ORR of 23% in a PD-L1+ group and a 14% ORR in all patients. Only grade 1 or 2 treatment-related adverse effects occurred in 43% of patients. A phase III clinical trial of patients with locally advanced NSCLC is ongoing (Brahmer et al. 2014).
Avelumab
Avelumab (MSB0010718C) is a humanized IgG1 antibody directly against PD-L1. It was approved by the European Medicines Agency (EMA) for the treatment of gastric cancer in 2017. The FDA and EMA also approved it in 2017 for the treatment of Merkel cell carcinoma, a highly aggressive skin cancer (Kim 2017).
The anti-tumor activities of avelumab were investigated in a large-scale clinical trial in which more than 1,700 patients were recruited with different types of tumors including head and neck cancer, gastric cancer, bladder cancer, adrenocortical cancer, renal cancer, ovarian cancer, melanoma, breast cancer, and NSCLC. Avelumab was well tolerated in different cancer patients with acceptable side effects (below grade 3 adverse effects) (Boyerinas et al. 2015). Avelumab also showed anti-tumor activities in patients with metastatic urothelial cancer in a phase 1 trial. A total of 249 patients (82 patients with PD-L1+ and 124 patients with PD-L1–) were enrolled. The patients were intravenously administered 10 mg/kg avelumab every 2 weeks until unacceptable toxicities occurred or other protocol-specified criteria for withdrawal. The median duration of therapy was 12 weeks. The ORR was 17% including 6% complete responses and 11% partial responses. Interestingly, the ORR was 24% in patients with PD-L1+ tumors, whereas patients with PD-L1– tumors had an ORR of 13% (Rao and Patel 2019). In a phase 2 trial of patients with Merkel cell carcinoma, 88 patients were enrolled and received at least one dose of avelumab by intravenous infusion every 2 weeks. Grade 3 treatment-related adverse effects occurred in 5% of patients and a 31.8% ORR was achieved indicating the potential of avelumab for the treatment of difficult malignancies (Kaufman et al. 2016). In a phase I clinical trial of advanced or metastatic breast cancer, avelumab therapy achieved only a 5.4% ORR in the entire cohort. Interestingly, among all patients with PD-L1 expression, 33.3% had polygenic risk scores. In the PD-L1 positive group, four of nine patients with triple-negative breast cancer had polygenic risk scores compared with one of 39 patients with triple-negative breast cancer who had polygenic risk scores in the PD-L1-negative group (Dirix et al. 2018). These results support that the anti-tumor activities of avelumab are associated with PD-L1 expression in tumors. Clinical trials of avelumab in patients with other types of cancers are ongoing.
Current Challenges and the Future of PD-1/PD-L1 Blockade Therapy
To date, considerable research has been devoted to cancer immunotherapy strategies based on immune checkpoint blockade. Regulation of T cell-mediated anti-tumor immune response is one of the most frequently used cancer immunotherapy strategies. The PD-1/PD-L1-mediated pathways play critical roles in suppressing T cell immunity. Therefore, mAbs targeting PD-1 or PD-L1 have been developed for the treatment of cancer and clinical trials are ongoing. According to the clinical data, many PD-1/PD-L1 mAbs have exhibited tolerance, high response rates, durable responses, and acceptable toxicity profiles. However, some obstacles persist in PD-1/PD-L1 blockade therapy including unpredicted efficacy of PD-1/PD-L1 inhibitors and the emergence of resistance to PD-1/PD-L1 blockade.
One of the major obstacles to cancer immunotherapy is unpredicted efficacy of immune checkpoint inhibitors. According to the clinical data, approximately 20–30% of cancer patients respond to PD-1 or PD-L1 inhibitors, whereas 70–90% of patients report adverse events (Fernandes and Brabek 2017). Additionally, to date, some anti-PD-1 or PD-L1 mAbs have demonstrated efficiency only in patients with specific types of cancers. One reason for variabilities in patients’ responses to treatment with PD-1 or PD-L1 inhibitors is that additional immune checkpoints, such as CTLA-4, are also crucial for the regulation of anti-cancer immune responses. Therefore, combination therapy has been used to overcome this obstacle. Indeed, the combination of various anti-tumor therapeutic agents with PD-1/PD-L1 pathway blockade has offered new therapeutic options for patients with advanced cancers. Combination therapy not only enhances the total anti-tumor responses in patients but also significantly improves the therapeutic efficiency (Larkin et al. 2015a; Postow et al. 2015; Robert et al. 2015; Wolchok et al. 2013). For instance, in a phase III clinical trial of melanoma, nivolumab combined with ipilimumab prolonged the median progression-free survival to 11.5 months compared to 2.9 months in an ipilimumab-treated group and 6.9 months in an nivolumab-treated group. However, combination therapy also leads to a higher incidence of adverse events. In a combination therapy group, a 55% rate of adverse events was reported compared to the nivolumab-treated group (16.3%) and ipilimumab-treated group (27.3%) (Larkin et al. 2015b). Therefore, exploration of various combination strategies with high efficiency and minimum toxicity might be an option for cancer immunotherapy.
The emergence of resistance to PD-1/PD-L1 blockade is another major hurdle. Although some promising clinical results have been achieved by PD-1/PD-L1 pathway blockage strategies, few patients with advanced cancers respond to single immune checkpoint blockade. It is known that tumors apply multiple strategies for immune evasion (Taube et al. 2012). Therefore, to promote an anti-tumor response in patients with no response to single PD-1/PD-L1 blockage therapy, a combination of anti-PD-1/PD-L1 mAbs with other therapeutic agents has been used. Various candidates have been applied in combination with anti-PD-1 or PD-L1 mAbs including chemotherapy drugs, small molecule compounds, and radiation drugs (Dovedi et al. 2014). For instance, in a phase II clinical trial, pidilizumab was combined with rituximab (anti-CD20 antibody) for the treatment of follicular lymphoma. A 52% complete response rate was reported and no grade 3 or 4 adverse events were observed in the combination group (Westin et al. 2014). In another clinical trial of patients with advanced solid tumors, a combination of atezolizumab with VEGF-specific mAb bevacizumab exhibited favorable anti-tumor effects with minimum toxicity (Lieu et al. 2014). Various combination strategies are still ongoing. Favorable therapeutic results achieved by these strategies will likely improve cancer immunotherapy.
Conclusion
Considerable research has been devoted to the field of cancer immunotherapy. Recovering T cell-mediated anti-tumor immunity is one of the most frequently used strategies to date. The PD-1/PD-L1 pathway plays a crucial role in the regulation of T cell activities and is the most studied immune checkpoint for cancer immunotherapy. Numerous mAbs have been developed against PD-1 or PD-L1 for cancers and some relevant clinical trials are still underway. According to the clinical data we reviewed, some PD-1/PD-L1 mAbs have exhibited good tolerance and therapeutic outcomes for some types of cancer. The toxicity profiles are also reasonable with acceptable adverse event rates. Furthermore, the combination of anti-tumor therapeutic agents targeting other pathways with PD-1/PD-L1 pathway blockade has been developed to provide a more effective therapeutic option for cancer patients. Armed with the understanding of the molecular mechanisms of immune checkpoints, cancer microenvironments, and the discovery of novel targeting immune checkpoints, cancer immunotherapy will achieve more promising results against cancer.
Taken together, we clarified the mechanisms of PD-1/PD-L1-mediated anti-cancer immune responses and some clinical studies of mAbs targeting PD-1/PD-L1. Based on the clinical data we reviewed, some PD-1/PD-L1 mAbs have demonstrated good tolerance, promising therapeutic outcomes, and acceptable toxicity profiles for some types of cancer. We believe that the combination of anti-tumor therapeutic agents targeting other pathways with PD-1/PD-L1 pathway blockade will improve cancer immunotherapy strategies.
Data Availability
All of the data analyzed in this study were included in the final published article.
Abbreviations
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed death ligand 1
- mAbs:
-
Monoclonal antibodies
- Tregs:
-
Regulatory T cells
- APCs:
-
Antigen-presenting cells
- CTLA-4:
-
Cytotoxic T-lymphocyte protein 4
- NSCLC:
-
Non-small cell lung cancer
- IFN-γ:
-
Interferon-γ
- TNF-α:
-
Tumor necrosis factor-α
- IL-2:
-
Interleukin-2
- DC:
-
Dendritic cells
- NK:
-
Natural killer
- SHP-2:
-
Src homology 2
- PI3K:
-
Phosphatidylinositol 3-kinase
- MHC:
-
Major histocompatibility complex
- CRC:
-
Colorectal cancer
- CRPC:
-
Castrate-resistant prostate cancer
- RCC:
-
Renal cell cancer
- ORR:
-
Objective response rate
- BRAF:
-
Proto-oncogene B-Raf
References
Abou-Alfa GK, Qin S, Ryoo BY et al (2018) Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 29:1402–1408
Alsaab HO, Sau S, Alzhrani R et al (2017) PD-1 and PD-L1 Checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 8:561
Ansell SM, Lesokhin AM, Borrello I et al (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372:311–319
Antonia SJ, Brahmer JR, Gettinger S et al (2014) Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 90:S2
Armand P, Nagler A, Weller EA et al (2013) Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 31:4199–4206
Armand P, Shipp MA, Ribrag V et al (2016) Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 34:3733–3739
Atkins MB, Kudchadkar RR, Sznol M et al (2014) Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma. J Clin Oncol 32:9001
Azuma T, Yao S, Zhu G et al (2008) B7–H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 111:3635–3643
Balar AV, Galsky MD, Rosenberg JE et al (2017) Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389:67–76
Barber DL, Wherry EJ, Masopust D et al (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687
Berger R, Rotem-Yehudar R, Slama G et al (2008) Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14:3044–3051
Bettelli E, Carrier Y, Gao W et al (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238
Blackburn SD, Shin H, Haining WN et al (2009) Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29–37
Blank C, Brown I, Peterson AC et al (2004) PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res 64:1140–1145
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639
Boyerinas B, Jochems C, Fantini M et al (2015) Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 3:1148–1157
Brahmer JR, Drake CG, Wollner I et al (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28:3167–3175
Brahmer JR, Tykodi SS, Chow LQ et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465
Brahmer JR, Rizvi NA, Lutzky J et al (2014) Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol 32:8021
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135
Butte MJ, Keir ME, Phamduy TB et al (2007) Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity 27:111–122
Chemnitz JM, Parry RV, Nichols KE et al (2004) SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 173:945–954
Chen L (2004) Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nat Rev Immunol 4:336–347
Chen Y, Liu P, Gao F et al (2010) A dimeric structure of PD-L1: functional units or evolutionary relics? Protein Cell 1:153–160
Chen R, Zinzani PL, Fanale MA et al (2017) Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 35:2125–2132
Cheng X, Veverka V, Radhakrishnan A et al (2013) Structure and interactions of the human programmed cell death 1 receptor. J Biol Chem 288:11771–11785
Cohen EE, Machiels JPH, Harrington KJ et al (2015) KEYNOTE-040: a phase III randomized trial of pembrolizumab (MK-3475) versus standard treatment in patients with recurrent or metastatic head and neck cancer. J Clin Oncol 33:TPS6084
Day CL, Kaufmann DE, Kiepiela P et al (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354
Dirix LY, Takacs I, Jerusalem G et al (2018) Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 167:671–686
Dong H, Zhu G, Tamada K et al (1999) B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5:1365–1369
Dong H, Strome SE, Salomao DR et al (2002) Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800
Dong H, Strome SE, Matteson EL et al (2003) Costimulating aberrant T cell responses by B7–H1 autoantibodies in rheumatoid arthritis. J Clin Invest 111:363–370
Dovedi SJ, Adlard AL, Lipowska-Bhalla G et al (2014) Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 74:5458–5468
Duffy AG, Makarova-Rusher OV, Pratt D et al (2016) A pilot study of AMP-224, a PD-L2 Fc fusion protein, in combination with stereotactic body radiation therapy (SBRT) in patients with metastatic colorectal cancer. J Clin Oncol 34:560
Fernandes M, Brabek J (2017) Cancer, checkpoint inhibitors, and confusion. Lancet Oncol 18:e632
Francisco LM, Salinas VH, Brown KE et al (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 206:3015–3029
Freeman GJ, Long AJ, Iwai Y et al (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034
Fried I, Lossos A, BenAmi T et al (2016) HG-02A phase 1/2 trial of the antibody pidilizumab (MDV9300) in pediatric diffuse intrinsic pontine glioma. Neuro-Oncology 18(Suppl 3):iii48
Fried I, Lossos A, Ben Ami T et al (2018) Preliminary results of immune modulating antibody MDV9300 (pidilizumab) treatment in children with diffuse intrinsic pontine glioma. J Neurooncol 136:189–195
Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14:1014–1022
Hamanishi J, Mandai M, Ikeda T et al (2015) Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 33:4015–4022
Hamid O, Robert C, Daud C et al (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369:134–144
Haxhinasto S, Mathis D, Benoist C (2008) The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med 205:565–574
Herbst RS, Gordon MS, Fine GD et al (2013) A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J Clin Oncol 31:3000
Herbst RS, Soria JC, Kowanetz M et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–567
Hirano F, Kaneko K, Tamura H et al (2005) Blockade of B7–H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65:1089–1096
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Hofmeyer KA, Jeon H, Zang X (2011) The PD-1/PD-L1 (B7–H1) pathway in chronic infection-induced cytotoxic T lymphocyte exhaustion. J Biomed Biotechnol 2011:451694
Infante JR, Goel S, Tavakkoli F et al (2015) A phase I, multicenter, open-label, first-in-human study to evaluate MEDI0680, an anti-programmed cell death-1 antibody, in patients with advanced malignancies. J Clin Onclol 33:TPS3088
Iwai Y, Ishida M, Tanaka Y et al (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99:12293–12297
John LB, Devaud C, Duong CP et al (2013) Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res 19:5636–5646
Ka C, Oestreich KJ, Paley MA et al (2011) Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol 12:663–671
Kaufman HL, Russell J, Hamid O et al (2016) Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 17:1374–1385
Keir ME, Liang SC, Guleria I et al (2006) Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 203:883–895
Keir ME, Butte MJ, Freeman GJ et al (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Kim ES (2017) Avelumab: first global approval. Drugs 77:929–937
Konstantinidou M, Zarganes-Tzitzikas T, Doemling A (2018) Immune checkpoint PD-1/PD-L1: is there life beyond antibodies? Angew Chem Int Ed Engl 57:4840–4848
Krogsgaard M, Davis MM (2005) How T cells “see” antigen. Nat Immunol 6:239–245
Krummel MF, Allison JP (1995) CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 182:459–465
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015a) Combined Nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–34
Larkin J, Hodi FS, Wolchok JD (2015b) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:1270–1271
Latchman Y, Wood CR, Chernova T et al (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2:261–268
Lázár-Molnár E, Scandiuzzi L, Basu I et al (2017) Structure-guided development of a high-affinity human programmed cell death-1: Implications for tumor immunotherapy. EBioMedicine 17:30–44
Le DT, Uram JN, Wang H et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509–2520
Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734–1736
Li Y, Li F, Jiang F et al (2016) A mini-review for cancer immunotherapy: molecular understanding of PD-1/PD-L1 pathway & & translational blockade of immune checkpoints. Int J Mol Sci 17:1151
Lieu C, Bendell J, Powderly J et al (2014) 1049Osafety and efficacy of mpdl3280a (anti-pdl1) in combination with bevacizumaB (BEV) and/or chemotherapy (CHEMO) in patients (PTS) with locally advanced or metastatic solid tumors. Ann Oncol 25(suppl 4):iv361
Lin DY, Tanaka Y, Iwasaki M et al (2008) The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci USA 105:3011–3016
Lin C, Taylor M, Boni V et al (2018) Phase I/II study of spartalizumab (PDR001), an anti-PD1 mAb, in patients with advanced melanoma or non-small cell lung cancer. Oncology PRO 29:400–441
Lipson EJ, Sharfman WH, Drake CG et al (2013) Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res 19:462–468
LoRusso PM, Powderly J, Burris HA et al (2013) AACR Abstract LB-193: Phase I study of safety, tolerability, pharmacokinetics, and pharmacodynamics of AMP-224 (B7-DC Fc fusion protein) in a regimen containing cyclophosphamide (CTX) in patients with advanced solid tumors. Cancer Res 73(8 Suppl):LB-193
Lu P, Youngblood BA, Austin JW et al (2014) Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J Exp Med 211:515–527
Mantovani A (2010) The growing diversity and spectrum of action of myeloid-derived suppressor cells. Eur J Immunol 40:3317–3320
Massard C, Gordon MS, Sharma S et al (2016) Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 34:3119–3125
McDermott DF, Drake CG, Sznol M et al (2015) Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 33:2013–2020
Mellman I, Coukos G, Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480:480–489
Motzer RJ, Escudier B, McDermott DF et al (2015a) Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813
Motzer RJ, Rini BI, McDermott DF et al (2015b) Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 33:1430–1437
Naing A, Gelderblom H, Gainor JF et al (2016a) A first-in-human phase I study of the anti-PD-1 antibody PDR001 in patients with advanced solid tumors. J Clin Oncol 34:3060
Naing A, Goel S, Curti B et al (2016b) A Phase 1 first-in-human study of MEDI0680, an anti-PD-1 monoclonal antibody (mAb) in adult patients (pts) with advanced tumors. Ann Oncol 27:367
Naing A, Infante J, Goel S et al (2019) Anti-PD-1 monoclonal antibody MEDI0680 in a phase I study of patients with advanced solid malignancies. J Immunother Cancer 7:225
Nishimura H, Nose M, Hiai H et al (1999) Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11:141–151
Nurieva R, Thomas S, Nguyen T et al (2006) T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J 25:2623–2633
Ocana-Guzman R, Torre-Bouscoulet L, Sada-Ovalle I (2016) TIM-3 regulates distinct functions in macrophages. Front Immunol 7:229
Park JJ, Omiya R, Matsumura Y et al (2010) B7–H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 116:1291–1298
Parry RV, Chemnitz JM, Frauwirth KA et al (2005) CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25:9543–9553
Patnaik A, Kang SP, Rasco D et al (2015) Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 21:4286–4293
Patsoukis N, Brown J, Petkova V et al (2012) Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal 5:ra46
Patsoukis N, Bardhan K, Chatterjee P et al (2015) PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 6:6692
Pauken KE, Wherry EJ (2015) Overcoming T cell exhaustion in infection and cancer. Trends Immunol 36:265–276
Postow MA, Chesney J, Pavlick AC et al (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006–2017
Powles T, Eder JP, Fine GD et al (2014a) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558–562
Powles T, Vogelzang NJ, Fine GD et al (2014b) Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC). J Clin Oncol 32:5011
Quigley M, Pereyra F, Nilsson B et al (2010) Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med 16:1147–1151
Rao A, Patel MR (2019) A review of avelumab in locally advanced and metastatic bladder cancer. Ther Adv Urol 11:1756287218823485
Rexer H (2015) Therapy of untreated local advanced or metastatic renal cell carcinoma. Phase III, randomized, open-label study of nivolumab combined with ipilimumab versus sunitinib monotherapy in subjects with previously untreated, local advanced or metastatic renal cell carcinoma (CheckMate 214 - AN 36/15 of the AUO). Urologe A 54:1443–1445
Robert C, Ribas A, Wolchok JD et al (2014) Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384:1109–1117
Robert C, Schachter J, Long GV et al (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532
Schachter J, Ribas A, Long GV et al (2017) Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390:1853–1862
Sharma P, Allison JP (2015) Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161:205–214
Sheppard KA, Fitz LJ, Lee JM et al (2004) PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett 574:37–41
Sledzinska A, Menger L, Bergerhoff K et al (2015) Negative immune checkpoints on T lymphocytes and their relevance to cancer immunotherapy. Mol Oncol 9:1936–1965
Staron MM, Gray SM, Marshall HD et al (2014) The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity 41:802–814
Syn NL, Teng MWL, Mok TSK et al (2017) De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol 18:e731–e741
Taube JM, Anders RA, Young GD et al (2012) Colocalization of inflammatory response with B7–h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4:127137
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27:450–461
Viricel C, Ahmed M, Barakat K (2015) Human pd-1 binds differently to its human ligands: a comprehensive modeling study. J Mol Graphics Modelling 57:131–142
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8:1069–1086
Westin JR, Chu F, Zhang M et al (2014) Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 15:69–77
Wirth LJ, Eigendorff E, Capdevila J et al (2018) Phase I/II study of spartalizumab (PDR001), an anti-PD1 mAb, in patients with anaplastic thyroid cancer. J Clin Oncol 36:6024
Wolchok JD, Kluger H, Callahan MK et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133
Wong RM, Scotland RR, Lau RL et al (2007) Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol 19:1223–1234
Xia Y, Medeiros LJ, Young KH (2016) Signaling pathway and dysregulation of PD1 and its ligands in lymphoid malignancies. Biochim Biophys Acta 1865:58–71
Yokosuka T, Takamatsu M, Kobayashi-Imanishi W et al (2012) Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 209:1201–1217
Youngblood B, Oestreich KJ, Ha SJ et al (2011) Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity 35:400–412
Zak KM, Kitel R, Przetocka S et al (2015) Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure 23:2341–2348
Zang X, Allison JP (2007) The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res 13(18 Pt 1):5271–5279
Zarour HM (2016) Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res 22:1856–1864
Zhang X, Schwartz JCD, Guo X et al (2004) Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 20:337–347
Zhang F, Wei H, Wang X et al (2017) Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade. Cell Discov 3:17004
Zinzani PL, Ribrag V, Moskowitz CH et al (2017) Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 130:267–270
Zou W, Wolchok JD, Chen L (2016) PD-L1 (B7–H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 8:328rv324
Funding
This work was supported by the Social Development of Science and Technology Bureau of Zhangjiagang City (Grant No. ZKS1734) and Project of Diagnosis and Treatment Technology for Key Clinical Diseases of Suzhou (Grant No. LCZX201617).
Author information
Authors and Affiliations
Contributions
YY, LZ, YZ, and HQ wrote the manuscript. CL contributed to the English assessment and manuscript revision. All of the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yan, Y., Zhang, L., Zuo, Y. et al. Immune Checkpoint Blockade in Cancer Immunotherapy: Mechanisms, Clinical Outcomes, and Safety Profiles of PD-1/PD-L1 Inhibitors. Arch. Immunol. Ther. Exp. 68, 36 (2020). https://doi.org/10.1007/s00005-020-00601-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00005-020-00601-6