Abstract
The aviation sector is the largest producer of greenhouse gases, contributing 2% of global emissions. The finite supply of fossil fuels also emphasizes the need for sustainable energy sources in the aviation sector, which are producing significantly lower emissions as well as renewable resources. This chapter discusses the important production routes of biomass-derived fuels, also called biojet fuels (BJFs), which must meet the ASTM International specifications and are clean and complete substitutes for present-day jet fuels. The production of these fuels uses a wide range of biomass; consequently, the fuels produced have very different compositions. The performance characteristics of the fuels based on the physiochemical properties of their constituents are discussed elaborately. It has been observed that there is a direct association between the chemical composition of the biofuels produced and their performance characteristics. Many researchers have suggested that the properties of bio-aviation fuels are appropriate as per the specifications provided by the ASTM standards. The concentration of aromatic carbons is pivotal in influencing the characteristics of fuels. The blends of biofuel with conventional fuels are also studied to improve fuel performance. For biojet fuels to become 100% drop-in fuels in commercial aviation usage, some drawbacks such as the price of production, feedstock availability, energy intensity of the process, and storage stability need to be addressed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biojet fuels
- Performance characteristics
- Greenhouse gas emissions
- Aviation sector
- ASTM specifications
- Alternative jet fuels
- Aromatic content
5.1 Introduction
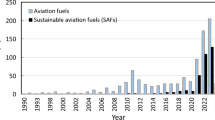
The Air Transport Industry plays a significant part in the world economy by allowing global connectivity; the Air Transport Action Group (ATAG) reported that 87.7 million jobs were provided worldwide by this industry, producing $961.3 billion of GDP per year, about 4.3 times higher than other jobs. Aviation is expected to continuously expand and contribute $1.7 trillion to world GDP by 2038. In 2019, 4.5 billion passengers were served by the airline industry (ATAG 2020). This increase in air travelers requires a considerably substantial quantity of aviation fuels, but the extended utilization of fuels in the past few years has developed a noticeable decline in the petroleum supply (Pavlenko and Kharina 2018).
The huge utilization of jet fuel provides a considerable volume of greenhouse gases (GHG), around 2.1% of all CO2 emissions that are generated by human activities and 12% of all aircraft emissions. The increasing demand for air transport results because there is aqua druple increase in the amount of emissions from 2015, which was 0.78 billion tons, and is expected to reach 3.1 billion tons of GHG emissions by 2050 (Doliente et al. 2020). Due to the effects of GHG on global warming, the airline industry is required to reduce 50% of CO2 emissions by 2050 compared to CO2 emissions in 2005 (ATAG 2020). The major challenge is to discover the most acceptable way to reduce GHG emissions to the determined target set at 50% less than the volume that was in 2005. The International Civil Aviation Organization (ICAO) and the International Air Transport Association (IATA) developed a few ways to accomplish the target: operational advancements, market-based measures, technological improvements, and sustainable jet fuel (SJF). Technological refinements are resulting in the reduction of GHG emissions. The improvements lower fuel utilization while traveling and give competency in mileage.
The majority of the reduction in GHG emissions can be achieved by substituting conventional jet fuel (CJF) with alternative jet fuel (AJF). The physiochemical properties of AJF must be similar to CJF like AJF should have 30,000 feet above elevation, lower carbon footprints than CJF, an adequate amount of energy density to fulfill the demands of long-haul flights, and temperature stability between −47 °C and 40 °C. Alternative jet fuel (AJF) like biofuel ensures immense reduction in GHG emissions (Doliente et al. 2020). Biomass-derived aviation fuels (biojet fuels) or BAF are used as an alternative to conventional jet fuels. The International Air Transport Association (IATA) recognized that biojet fuels are the guaranteed policy to bring down GHG release from the aviation sector. The aircraft that utilized BAF resulted in remarkably lower carbon emissions when weighed against CJF (Yang et al. 2019).
The American Society for Testing and Material (ASTM) D7566-10 is the international organization that decides the standard specifications for fuel quality. The fuel specification for the synthesized hydrocarbons as per this international body is that the fuel should contain up to 50% of any of the five types of synthesized paraffinic kerosene (SPK), which needs to be blended with CJF. In 2011, the ASTM approved one of the synthesized fuels called hydroprocessed esters and fatty acids (HEFA) that can be blended up to 50% with CJF. In 2015, FT-SPK combined with Aromatics (FT-SPK/A) became part of ASTM D7566 standards. The aromatics content is deliberately elevated up to the highest 20% in FT-SPK/A. At the same time, synthesized iso-paraffins (SIP) were also approved and certified in ASTM D7566, but the quantity of blends approved was up to only 10% with CJF. Alcohol-to-jet (ATJ) produces ATJ-SPK by utilizing C2-C5 alcohols, where iso-butanol (C4) and ethanol (C2) were approved in 2016 and 2018, respectively, with up to 50% blending permitted (Yang et al. 2019). In recent years, global interest in BAF production has escalated, showing the necessity of lowering GHG release by the jet industry through AJF.
The central theme of this chapter is the processes of conversion technologies of biomass to biofuels and the characteristics of BJFs.
5.2 Properties of Fuels Used in Aircraft
The ASTM-D16522 is an international institution that defines the basic characteristics of aviation fuels as shown in Table 5.1. Jet fuels are composed of stringent characteristics compared to land transportation fuels. As per the specifications declared by the ASTM, the jet fuel should basically be comprised of a complex mixture of C9–C16 range hydrocarbons. It should consist of a mixture of alkanes, which could be linear alkanes, slightly branched alkanes, cycloalkanes, and 20% arene hydrocarbons such as benzene and naphthalene. Moreover, other physiochemical properties, such as freeze point, energy density, flash point, viscosity, flammability limits, combustibility, sulfur content, density, and amount of hydrogen ions, are strictly adhered to for the purpose of operational certification. The carbon chain length and the amount of different kinds of alkanes should be maintained so as to match the guidelines of the jet fuels (Wang and Tao 2016).
Another significant attribute is the high flash points, which is the minimum temperature at which enough vapors of material are generated that can be ignited. The flash point of the jet fuel is required to be a minimum of 38οC as there is a low chance of fire hazards on board. The low freeze point is recommended such that at high altitudes it possesses good cold flow properties. The freezing point should be a maximum of −47οC, and it indicates the temperature at which wax that had been crystallized when the fuel was previously cooled completely melts when the fuel is rewarmed. The fuel is expected to have a high-energy density that aids in storage space. The proportion of aromatic hydrocarbons should be around 20% in the fuel. The aromatic hydrocarbons are shown to have negative effects on the combustion efficiency of fuel, but their presence in the fuel is unavoidable as they provide good sealing properties to the fuel that are required to avoid leakage (Kramer et al. 2022). The sulfur content of the fuel should also be controlled as it is involved in producing harmful emissions such as sulfur oxide. Most importantly, the exothermic release of energy, when the fuel is subjected to 100% combustion at constant pressure, is required to be at least 42.8 MJ/kg (ICAO 2018). This energy released is called net heat combustion, which should be kept high.
5.3 Different Production Technologies of Biojets
The idea behind designing the biojet fuel using more reliant renewable resources is to have the advantages of renewability, less dependence on petroleum, more sustainability, environment-friendly, and easy carbon dioxide recycling (Bozell et al. 2000). The waste biomass has garnered interest in its conversion to biojet fuels such as feedstocks having triglyceride-containing materials, lignocellulose-containing wastes, and sugar and starch wastes (Moreno-Gómez et al. 2020). The research on BJF production has been done using various raw materials, out of which jatropha, microalgae, and camelina have the most potential (Wei et al. 2019; Lim et al. 2021). The production methodology includes catalytic cracking, pyrolysis, trans-esterification, hydroprocessing, and fermentation. Different raw materials require different production processes and result in different final fuel properties. The production route also impacts the cost, its effect on the environment, and its ultimate composition (Shahid et al. 2021). Some of the methods of biojet manufacturing are discussed below.
5.3.1 Alcohol Oligomerization
This method is also called the alcohol-to-jet fuels (ATJ) route, which comprises three steps. For the purpose of biojet production, alcohol used is a short-chain fatty alcohol having C2 or C4 chain such as ethanol and butanol. Initially, the bio-alcohol is dehydrated to its olefin compound, for instance, ethanol yields ethylene upon dehydration, which is converted to its corresponding olefin derivative. Secondly, oligomerization of olefin is done to produce C9-C16 large-chain olefins; in the case of ethanol and dimerization of olefins, it is required if the raw material is butanol. Finally, the oligomerized olefin is subjected to hydrogenation to produce the saturated hydrogenated product, which has properties similar to jet fuel (Wang and Tao 2016).
One of the most important components of the ATJ route of biojet conversion is the use of a catalyst for the oligomerization and dehydration step, which enhances the rate at which the conversion of alcohol to biojet fuels takes place (Sundararaj and Kushari 2019). Some of the most efficient catalysts for this purpose are zeolite, aluminum (III) oxide, and heteropolyacid (HPAs) (Sundararaj and Kushari 2019). Over the years, with further research, the ATJ conversion route has been practiced using other acidic catalysts, specifically for dehydration and oligomerization steps. The ATF process is perfected by ByogyRenewables by means of a catalytic process, which involves the production of heterogeneous long-chain hydrocarbons from ethanol. The mixture produced is dissociated into aviation fuel and gasoline using a selective distillation process (Han et al. 2019). The production route is illustrated using a flowchart in Fig. 5.1. The Byogy fuel has ASTM approval for commercial flights, with an increase of 50% blend ratio. The other manufacturer of biojet fuels using the ATJ route is Gevo, in which higher alcohol is used instead of ethanol. It makes use of propanol and butanol and leads to the production of aromatics (Díaz-Pérez and Serrano-Ruiz 2020). The alcohol-to-jet synthetic paraffinic kerosene (ATJ-SPK) conversion technology was used to convert isobutanol feedstock to biojet fuel, and this technology was standardized in 2018 (Geleynse et al. 2018).
5.3.2 Fermentation of Sugar and Platform Molecules
This process is called the fermentation to jet (FTJ) process and direct sugar-to-hydrocarbon (DSHC). The technology involves anaerobic fermentation for the synthesis of alkane-type fuels from sugars such as lignocellulosic sugar. Recently, attention has been paid to the use of simple sugars (sorghum, maize, sugarcane) or platform molecules (bio-derived molecules) as a feedstock for their ease of fermentation (Mawhood et al. 2015). The FTJ process is complex as the feedstock contains a variety of functional groups, is highly oxygenated, and contains a maximum of six carbon atoms. On the other hand, jet fuel comprises higher carbon atoms (C9-C16), is devoid of a variety of functional groups, and is less oxygenated (Díaz-Pérez and Serrano-Ruiz 2020). Therefore, there is a need for complex chemical reactions in the FTJ process such as dehydration, hydrogenation, and hydrogenolysis for the removal of oxygen, aldol condensation, ketonization, and oligomerization for C–C coupling reactions (Serrano-Ruiz et al. 2011).
Another procedure for obtaining biojet fuels through the fermentation of sugars and biomolecules is by using bioengineered microorganisms that are made compatible to feed on these sugars and produce biojet fuels. Producing such microorganisms through genetic engineering is difficult (Mawhood et al. 2015). Firstly, the hydrolytic catalysis of the preliminary treated biomass is required, followed by hydrosylate clarification. The engineering microorganisms are then introduced for the process of fermentation to occur. In the next step, the purification of the fermented products is carried out. Then, the hydrotreatment is given to the products before it is subjected to fractionation (Wang and Tao 2016). The steps involved in the synthesis of BJF using this method are explained in the flowchart in Fig. 5.2. The renewable fuel company, Virent, is involved in FTJ conversion for the production of sustainable fuels by BioForming (Díaz-Pérez and Serrano-Ruiz 2020).
5.3.3 Hydroprocessing
This method is involved in hydrocracking and hydrotreating of hydrogenated esters and fatty acids (HEFA) with the help of catalytic actions such as decarboxylation, hydrogenation, decarbonylation, cracking, and isomerization. This is a catalytic process and the intermediate biofuels produced using this route are called hydroprocessed renewable jet (HRJ). This technique is also known as oils-to-jet fuels.
The methodology involved in the production of HRJ using hydroprocessing involves the oils from vegetables as a feedstock. These oils (e.g., soybean, palm, corn, jatropha, camelina, and canola) are enriched with triglycerides (TG), which help in the synthesis of straight-chain alkanes (Morgan et al. 2012). The n-alkanes serve as a biojet fuel component because they have a good combustible tendency and high-energy density (Lin et al. 2020). In the first step, hydrogenation of TG leads to the production of free fatty acids and propane. In the subsequent reactions, oxygen is removed from the product by the process of hydrodeoxygenation (HDO) and hydrodecarbonylation/hydrodecarboxylation (HDC) to produce alkanes (Ng et al. 2021). The resultant alkane in the case of HDO reaction is n-alkane, whereas HDO produces n-1 alkanes, thereby yielding low carbon yield in comparison to HDO. In the second step, isomerization and hydrocracking are carried out to produce the hydrocarbons with the desired carbon chain length of C9-C16 (Sundararaj and Kushari 2019). High temperature and hydrogen pressure are necessary for this HEFA process, which converts oils into biofuels. It is carried out along with heterogeneous catalysts such as transition metals or their bimetallic composites (Monteiro et al. 2022). The conversion of oil to jet fuel is shown in the form of a flowchart in Fig. 5.3. The most appropriate airplane fuel is HRJ biojet. Some of the characteristics that make HRJ biofuels most suitable for being a drop-in fuel are lesser aromatic carbons, more calorific content, zero sulfur content, and low emissions (Sundararaj et al. 2019). The companies that produce HRF fuels and meet the ASTM standards are Neste Oil and Honeywell Universal Oil Products (Tao et al. 2017).
5.3.4 Hydrothermal Liquefaction
An alternative method to develop biojets from vegetable oils is hydrothermal liquefaction (HTL). It is also known as catalytic hydrothermolysis (CH). It produces biofuels with a very low content of oxygen by the liquefaction reaction. This method is capable of producing 100% sustainable aviation fuel (SAF) in 2019, which was named ‘ReadiJet.” This production pathway of SAF or biojets is developed and patented by Applied Research Associate Inc., which contains algal oil or vegetable oils as biomass. This production pathway begins with pretreatment of the biomass, which helps in treating the triglycerides and unsaturated fatty acids using catalytic reactions such as conjugation, cross-linking, and cyclization that are essential to enhance its molecular structure. The conversion of oil to hydrocarbon is explained in the flowchart in Fig. 5.4. The advantage of this technique is that the feedstock is not required to be dewatered. The reaction conditions are kept such that the water stays in the fluid state and pressure is maintained around ~100–350 bars such that water is at a dense supercritical state in order to produce biofuel with high-energy efficiency (Grande et al. 2021). Water and catalysts are used to facilitate the catalytic hydrothermolysis process. In the succeeding steps, the amount of unsaturation and oxygenated content of the product is reduced by a catalytic decarboxylation reaction. The resultant fuel has a variety of alkanes ranging from C6-C28 (Li et al. 2010).
5.3.5 Hydrotreated Depolymerized Cellulosic Jet (HDCJ)
It is a procedure that requires fast pyrolysis of biostock. Fast pyrolysis is the thermochemical treatment of the feedstock to convert it into liquid bio-oil, which is further processed to produce oils of biojet fuel standards. The procedure takes place in an oxygen-free environment at temperatures between 400 and 600 °C (Hu et al. 2020). The hydrotreatment steps of the process are carried out at mild conditions and with the presence of catalysts. Later, hydrogenation is performed under high temperatures. The treatment results in the synthesis of biofuels having less unacceptable properties as per the ASTM standards. The fuel production method is further improved by a three-step pathway, in which initially fast pyrolysis of biomass is carried out, which undergoes catalytic cracking; synthesis of aromatic hydrocarbons is the next step, followed by hydrogenation (Sundararaj and Kushari 2019). The bio-oil production from biomass using a fast pyrolysis method is depicted in Fig. 5.5. This technique is still in its initial stage, but various commercial groups, such as Ensyn, LLC, PNNL, UOP, and Tesoro, are dependent on this process for the production of biojet fuels (Abdullah and Battelle 2015).
5.3.6 Fischer–Tropsch (F-T) Synthesis
This method, also called gas to jet, is a catalytic process to transform biomass to jet fuel hydrocarbons with the intermediate step of gasification. The major advantage of producing bio-derived fuels using this method is that it can take any carbon-containing biomass as a feedstock, emits no net carbon dioxide upon combustion, and fits well with environmental regulations (Hu et al. 2012). The conversion process starts with the preliminary treatment of the biomass, which includes screening, drying, and reducing the particle size. It is essential for efficient heat transfer and depletion in the hydrogen content of the gas product. Some of the other pretreatment methods required for proper F-T synthesis are torrefaction, pyrolysis, and compression of the biomass to produce cylindrical pellets (Hu et al. 2012). Further, the gasification of the pretreated biomass is performed in the gasifiers and in the presence of gasification agents. The gasification method is dependent on biomass and the gasifier design. The syngas is produced at the end of gasification and carbon monoxide, hydrogen, carbon dioxide, nitrogen, and methane (Nwokolo et al. 2020). To obtain the required content of hydrogen and carbon monoxide, the optimization of gasification is needed. The remaining impurities are subjected to catalytic cracking and other reactions. Following this, the Fischer–Tropsch synthesis is carried out, which is a set of reactions that occur in the presence of a suitable catalyst to convert syngas to liquid hydrocarbons. The F-T process requires conditioned syngas so as to adjust its ratio of H2 and CO, which happens using a water gas shift (WGS) reaction. The flowchart containing the synthesis of BJF by utilizing F-T synthesis is described in Fig. 5.6. Ruthenium is the most efficient catalyst, which is also responsible for increasing the cost of the reaction. In comparison, iron is a cheaper alternative to the catalyst that can be used, but it comes with certain disadvantages such as catalyst agglomeration and low product selectivity (Ma and Dalai 2021). This method of production is being utilized to create biojet, which is blended with traditional fuels (SWAFEA 2011). The process does have the advantage of using a variety of feedstock, but it is the most expensive method of all the others discussed (Roberts 2008).
5.4 Performance Attributes of BJFs
The fuels are considered to be drop-in alternative jet fuels if they are produced from bio-hydrocarbon, function similarly to existing fuel, and are compatible with existing jets. The performance characteristics play a significant part in evaluating the viability of “drop-in” alternative jet fuels. The performance characteristics of biojet fuels need to be assessed in order to ensure fuel safety, dependability, compatibility with supporting aero-engines and airframe components, and conformity with the ASTM D7566-18 requirements. Here, with a solid grasp of the interplay between their physical and chemical properties, we address the performance attributes of BJFs. Although it becomes arduous to correctly estimate the fuel properties as biofuels are composed of different complex hydrocarbons (Wang et al. 2021). This chapter groups the BJFs’ performance characteristics into several physiochemical qualities that need to be examined in accordance with the ASTM guidelines. These characteristics and comparison with traditional gasoline are covered in detail in the rest of the chapter.
5.4.1 Low-Temperature Fluidity
The major characteristic of drop-in fuel is that it should be able to maintain its fluidity even at high altitudes, where the temperature is very low, or at places with extreme climates. Failing to do so, the fuel flow to the engine will be poor or equal to zero. The freezing point and the kinematic viscosity of the fuel are the two parameters that control the low-temperature fluidity of biojet fuels. These two factors are reliant on intermolecular forces between the components of the fuels and, hence, on their molecular structure. To meet the need for proper fluidity of biojet fuels at very low ambient temperatures in high altitudes and low freezing points, kinematic viscosity of the fuel is required to make certain the flow of the fuel in the turbine engine is not affected (Benavides et al. 2021).
5.4.1.1 Freezing Point
The lowest temperature at which a certain fuel does not form hydrocarbon crystals and maintains enough fluidity to allow unobstructed fuel flow from the aviation system’s tanks to the engine is referred to as the fuel’s freezing point (Benavides et al. 2021). The fuel’s freezing point is one factor that affects how biojet fuels behave at low temperatures. The ASTM D2386-19 standard test procedure for aviation fuel freezing point measurement is used to measure it. Differential scanning calorimetry (DSC) is used to calculate the crystallization onset temperature (Tco) (Benavides et al. 2021). The synthesized iso-paraffins (SIP) technique of producing biofuels has a maximum freezing point of −60 °C, while other biofuels created using FT-SPK, HEFA, FT-SPK/A, and ATJ-SPK have a maximum freezing point of −40 °C (Yang et al. 2019). The other two parameters that are used to determine biofuel fluidity are the pour point and the cloud point. The pour point is defined as the measure of the propensity of the fuel to gain viscosity and cease to flow when the temperature is low and the cloud point is explained as the temperature at which the paraffin in biojet fuels begins to separate and becomes cloudy at cold conditions (Demirbas 2009). The freezing point for biojet fuels is found to be lower than the ranges of the pour point (−35 to −15 °C) and cloud points (−15 to 5 °C) that are often used to assess the fluidity of diesel and biodiesel (Yang et al. 2019).
The composition of biojet fuels is majorly responsible for their freezing point. Components having higher viscosity have lower freezing points and, therefore, better fluidity at low temperatures (Pires et al. 2018). In addition to the length of the carbon chain of bio-paraffins, the amount of iso-paraffins and alkylated aromatics in the fuel also affects the freezing point. The appearance of a large amount of branched paraffin contributes to the very low freezing point, such as Sasol FT-SPK, which has a freezing point of about < −77 °C (Renninger et al. 2010). On the contrary, the presence of branched alkanes such as farnesane in SIP fuel contributes to an even lower freezing point of −90 °C (Renninger et al. 2010). The various production routes also result in different composition of biojets, which alters their characteristics. For instance, the freezing point of coconut HEFA-1 and HEFA-2 is higher than −40 °C, and in the event of isomerization, the freezing point of HEFA-2 (−18.5 °C) is lower than that of HEFA-1 (9.5 °C). Similarly, the freezing point of −80 °C is lower for biofuels containing branching cyclohexane that are made from furfural alcohols and aromatic oxygenates via alkylation and hydroxygenation (Han et al. 2017).
There are many other approaches for the production of biofuels, other than those mentioned in this chapter. In one such method, called the H2SO4 catalytic one-pot method, the liquid pretreatment and saccharification take place in one vessel. In this process, cyclic alcohols such as cyclohexanol and cyclopentanol, along with branched cycloalkanes like methylcyclohexane and methylcyclopentane, are utilized to produce branched decalins (also called decahydronaphthalene) at room temperature. Branched decalins are excellent components of jet fuel, with properties like high density, high thermal stability, and low freezing points, but their availability by fossil resources is finite (Nie et al. 2018). With a freezing point of less than −51 °C and a high heating value of ∼42 MJ/kg, the decalin fuel is a potential jet fuel mixing (Nie et al. 2018). Furthermore, some constituents, like highly branched diamyl ether (DAE), have a freezing point as low as −92 °C. It has the capability of blending with fossil fuels like QAV-1 in various proportions, which results in an adequate freezing point. This DAE can be produced from the thermal cracking of iso-amyl alcohol and C5 hydrocarbon using an insulated bioreactor with minimum heat transfer (Cataluna et al. 2018).
According to reports, alkylated aromatics have an effect on the freezing point of biojet fuels because propylbenzene reduces the freezing point of HEFA proportionate to the volume injected (Hong et al. 2013). The range of raw materials, including acidified oil, waste cooking oil, soyabean oil, and rubber seed oil, are utilized to create biojet fuels with a freezing point of −37 °C. A significant amount (60–77%) of linear C8–C15 hydrocarbons was obtained by pyrolyzing the source material at 350–450 °C with 5% base catalyst weight. Because of a higher freezing point (−40 °C) than HEFA, HZSM-5 zeolites convert linear hydrocarbons into aromatics at 350 °C for 6 hours, and then aromatics are converted into cycloalkanes using PD/AC for 6 hours at 200 °C. The finished mixture has a freezing point of −47 °C (Li et al. 2018).
As previously indicated, in addition to their composition, the carbon chain’s length in bio-paraffins significantly affects the freezing point of created BJFs. Fuels with short carbon chain lengths exhibit desirable low freezing points. The generated hydrocarbons must be hydrocracked in order to reduce the length of the carbon chain (Monteiro et al. 2022). As seen in an example, a biojet fuel substitute carrying short carbon chain limonene (C10) has a freezing point of −97 °C, whereas farnesane (C15) has a much higher freezing point of −40 °C (Yang et al. 2019). In a similar example, bio-kerosene produced as an end product of the catalytic distillation of triglyceride-based oils showed characteristics that were not up to the requirements, especially with regard to the freezing point. Research reported that the freezing point of coconut bio-kerosene is −10 °C and palm kernel bio-kerosene is −15 °C. The probable reason for high freezing points could be due to the carbon chain length without proper hydrocracking (Llamas et al. 2012). Upon blending 20% of palm kernel bio-kerosene with Jet A-1, the freezing point (−41.5 °C) higher than the ASTM D7566-18 specifications was obtained, which was not satisfactory (ElGalad et al. 2018).
The concentrations of iso-paraffins, alkylated aromatics, and the carbon chain length of bio-paraffins are all positively correlated with the freezing point of BJFs, according to a summary of the relationship between the composition of biofuels and their freezing points. Higher alkylated aromatics and iso-paraffin content led to a lower freezing point. Biojet fuels with a short carbon chain composition have a lower freezing point; hydrogenated algal oil had to be hydrocracked in order to lessen the carbon chain length.
5.4.1.2 Kinematic Viscosity at −20 °C
Kinematic viscosity at −20 °C is another criterion that typically characterizes the low-temperature fluidity of aviation gasoline. Kinematic viscosity (KV) is usually described as the internal resistance of the fuel under the effect of gravitational force. It is associated with chain length and degree of saturation of carbon chains (Gouveia et al. 2017). In spite of the fact that ASTM D7566 standards did not specify the limitations of kinematic viscosity of synthesized hydrocarbon fuels, the KV value of 8 mm2/s at −20 °C is required to be maintained for a blended jet fuel to be considered as a drop-in fuel (Chuck and Donnelly 2014). The kinematic viscosity of fuel should not be very high because it causes various complications like poor atomization, pumping difficulties, incomplete combustion, and the blocking of fuel injectors. The viscosity of the blended jet fuel at a low blend level is suitable for aviation kerosene (Chuck and Donnelly 2014).
At −20 °C, the blended biofuels exhibited kinematic viscosities of less than 8 mm2/s. However, certain bio-kerosenes, which are obtained through the catalytic distillation of triglyceride-based oils, had high viscosities. According to the research, at −20 °C, the viscosities of the castor HEFA and its even equal blended jet fuel were 5.3 mm2/s and 3.3 mm2/s, respectively (Liu et al. 2015). At −20 °C, the kinematic viscosity of the FT-SPK mix, even when blended equally with Jet A-1, is 4.65 mm2/s (Lobo et al. 2011). Another research shows that ATJ-SPK fuel had a kinematic viscosity of 4.795 mm2/s at −20 °C. However, SIP fuel shows more kinematic viscosity than FT-SPK, HEFA, and ATJ-SPK, which is 14.28 mm2/s at −20 °C. The 50 volume % blends of SIP fuel with Jet A-1 had a viscosity of 8.37 mm2/s and 20 volume % had a viscosity of 5.66 mm2/s at −20 °C, respectively (Scheuermann et al. 2017).
There is insubstantial information that highlights the association of kinematic viscosity and chemical compositions of biojet fuels. In a study by Chuck and Donnelly (2014), the kinematic viscosities of a few biofuels, such as methyl linolenate, farnesane, n-butanol, butyl levulinate, limonene, butyl butyrate, n-hexanol, ethyl octanoate, and ethyl cyclohexane, were measured at temperatures between −30 °C and 40 °C. The researchers concluded that the viscosities of biofuels increased with decreasing temperature in a manner similar to an ideal fluid and that n-butanol and n-hexanol had high viscosities at −20 °C, 12.84 mm2/s, and 36.21 mm2/s, respectively, likely because of hydrogen bonding between alcohol groups. Butyl butyrate (C8) and ethyl octanoate (C10) show beneficial viscosities that are less than 8 mm2/s at −20 °C, whereas methyl linolenate (C18) had a viscosity of 20.68 mm2/s and its blended fuel had viscosity of 12.77 mm2/s at −20 °C (Chuck and Donnelly 2014). This illustrates that at the temperature of −20 °C, a reduced carbon chain length results in a lesser kinematic viscosity.
Likewise, SIP (UQJ-1) fuel had a higher kinematic viscosity of 7.714 mm2/s at −20 °C when 90 volume % of farnesane (C15) and 10 volume % of limonene (C10) are blended with fuel. But when the SIP fuel contains 97.1 volume % of short-chain limonene, then kinematic viscosity is 3.818 mm2/s at −20 °C. Rather than hydrocarbon classes, the molecular mass of chemical compounds determines the degree of viscosity of propellant. Due to the higher likelihood of high molecular weight molecules missing the viscosity test for biojet fuel, diaromatics predominated over monoaromatics (Scheuermann et al. 2017).
Most of the biojet fuels like FT-SPK, HEFA, and ATJ-SPK had acceptable kinetic viscosities except SIP; due to the presence of long-chain farnesane (C15) content, the kinetic viscosity was relatively high.
5.4.2 Stability During Thermal Oxidation
In biojet fuels, thermal oxidation stability is categorized into two different aspects, thermal stability and oxidation stability. One crucial performance attribute needed in fuels is the biojet fuel’s capacity to withstand thermal oxidation at the aircraft’s operating temperature. Thermal oxidation stability should be high. The quartz crystal microbalance (QCM) is a suitable technique for measuring the thermal equilibrium of aircraft fuel (Corporan et al. 2011).
5.4.2.1 Thermal Stability
The capacity of biojet fuel to tolerate high temperatures under operating conditions without experiencing noticeable degradation is known as thermal stability, and it may be measured by the amount of deposits that accumulate in the engine fuel system (Lin and Tavlarides 2013). To estimate the thermal stability of biojet fuels, the jet fuel thermal oxidation stability test (JFTOT) is performed, which is standardized under the ASTM D3241 (Christison et al. 2019). Jet fuel deposit formation can be evaluated using two metrics provided by JFTOT: the surface deposit on the test tube and the pressure drop following fuel degradation (Jia et al. 2020). The ASTM D7566-18 standards provide information on these metrics to guarantee the thermal stability of BJFs. The JFTOT test requires that the pressure decrease after 2.5 hours is less than 25 mm Hg and that the surface deposit on the test tube is less than 3 at a temperature of 325 °C (Yang et al. 2019).
In general, biojet fuel has superior thermal stability than traditional jet fuels; nevertheless, there has not been much research done to estimate this thermal stability (Corporan et al. 2011). Fully synthesized jet fuel (FSJF) had very good thermal stability at more than the standard temperature, that is, 360 °C. HEFA had less than the standard value at tube deposit metrics, where almost no pressure drop was detected at 325 °C after 2.5 hours (Amara et al. 2016).
As we know, the thermal stability of biojet fuel is better compared to current in-use jet fuels; this notion is supported by the literature stating that biojet fuels are not much deteriorated under high temperatures than the JP-8, and also, the fully synthetic jet fuels are more resistant to deposit formation under high temperatures in comparison to conventional jets (Corporan et al. 2011). The existence of heteroatom-containing hydrocarbons accounts for contemporary jet fuel’s reduced tolerance to high-temperature stress. Benzothiophenes (C8H6S) with cyclic sulfur structures may be the reason for the bad thermal stability of conventional jet fuel. As benzothiophene (C8H6S) was not present in FSJF, as a result, it shows better thermal stability than conventional jet fuel. Some researchers also concluded that the omission of heteroatom-containing compounds results in better thermal stability (Westhuizen et al. 2011).
Moreover, the presence of aromatic compounds also affects the thermal stability of the biojet fuels. Biojet fuels are almost devoid of aromatics as these are mainly composed of n-paraffins, iso-paraffins, and cyclo-paraffins, whereas conventional jet fuel contains about 10–20 weight % of aromatics. The constituents of biojet fuel such as paraffinic compounds were not shown to have the desirable capability of forming deposits at high temperatures, thereby improving their thermal stability. Amara et al. conducted experiments to evaluate the thermal stability of HEFA with the addition of several aromatic compounds, such as xylene, 1-methyl-naphthalene, and tetralin. The results showed that the addition of aromatic compounds in fuels had an effect on pressure drop, with the addition of 1-methyl-naphthalene resulting in a greater than threefold increase in deposit rate (Amara et al. 2016).
To conclude the findings on the comparison of the thermal stability of biojet fuel and conventional fuels, conventional jet fuels have poor thermal stability due to the presence of heteroatom-containing compounds and aromatic compounds. The biojet fuels show better performance in this regard.
5.4.2.2 Oxidative Stability
The term oxidative stability signifies the propensity of the fuel to react with oxygen at moderate temperature. In other words, it is the quantification of the resistance of a fuel to oxidize in the availability of oxygen at a temperature range between 100 and 160 °C (Jia et al. 2020). To measure the extent of fuel degradation by oxidation, the induction period (IP) of the fuel is calculated, which is the time when the fuel achieves the highest oxidation rate (Ben Amara et al. 2014). The thermal oxidative stability of propellant is determined by the physical conditions of the fuel as well as its chemical composition. It is dependent on ambient temperature, the amount of oxygen in the physical environment, the hydrocarbon molecular structure of its compositions, and the concentration of heteroatomic compounds (Odziemkowska et al. 2018). The oxidative stability of biodiesel at a temperature of 110 °C is more than 3 h IP according to the ASTM D6751 standards, whereas it is more than 8 h IP according to the EN 14214 (Moser and Vaughn 2010).
IP for HEFA was around 60 minutes at 140 °C and 7 bar of oxygen pressure. Because of the inclusion of aromatic chemicals, the IP for Jet A-1 was approximately 2.3 hours. To enhance the oxidative stability of HEFA, they blend it with Jet A-1 containing aromatic compounds. Before addition, they evaluate the effect of molecular structure on IP. Now blend of 25 volume % of Jet A-1 with HEFA gives an IP of about 3 hours. It is observed that diaromatic compounds like 1-methyl-naphthalene show higher IP values than monoaromatics compound and hydrocarbons show lower IP values than aromatic compounds (Amara et al. 2016). Further, it is noticed that the oxidative stability of HEFA was enhanced from 1 hour to 8 hours by blending 5 volume % of 1-MN. HEFA’s oxidative stability shows average improvement by blending monoaromatics compounds, whereas cyclic alkane shows no improvement in HEFA’s oxidative stability. Apart from this, FT-SPK and HEFA had better oxidative stability than conventional JP-8 due to the high oxygen consumption rate and lack of aromatics compounds in FT-SPK and HEFA. Besides, fossil jet fuels contain phenolic antioxidants that also lead to the low oxidative stability of BJFs (Tomar et al. 2023).
In conclusion, compared to commercial jet fuels, the oxidative resistance of biojet fuels was worse because they lacked antioxidant and aromatic components.
5.4.3 Combustion Characteristics
Fuel combustion characteristics are computed in order to examine the impact of biojet fuels, particularly with regard to their effect on climate change and rising greenhouse gas emissions (GHG). In the research led by Sundararaj et al., the biofuels containing camelina and jatropha have better emission characteristics, once tested against fossil fuel-based fuels. For the purpose of assessing how well biofuels burn, several gaseous emissions are taken into account, including carbon monoxide, soot, nitrogen oxides, and unburned hydrocarbons. The study involving the blends of biofuels suggested that the more the amount of camelina in the blend, the lesser the emission of these gases. The release of nitrogen oxides is also dependent on combustion temperature; therefore, there is an increase of nitrogen oxide emission with increasing camelina. Whereas jatropha-based biofuel blends do not follow the same trend and give mixed values (Sundararaj et al. 2019). In an aviation turbine engine, biojet fuel ignites and vaporizes with rapid hot air. Incomplete combustions are the outcome of particulates and unburned hydrocarbons. If the concentration of particulates is high, then it will be seen as smoke or soot. The metrics used to assess the BJF’s combustion properties include the derived cetane number (DCN), smoke point, particle matter (PM), carbon dioxide (CO2), and monoxide (CO) emissions (Yang et al. 2019).
5.4.3.1 Smoke Point
The temperature at which a particular fuel starts to produce smoke is known as its “smoke point.” A fuel with a high smoke point is thought to have a low tendency to produce smoke. The fuel’s smoke point is measured with the specific wick-fed test lamp, where the height of the highest flame produced (in millimeters) is checked, which is given off without soot breakthrough (Jiao et al. 2015), thereby assessing the combustion properties of the fuel. For instance, the smoke point of fossil jet fuels is 25 mm in height of flame without smoke production (Saffaripour et al. 2011).
The smoke produced is influenced by the amount of heavy hydrocarbon particles present in fuel. The lesser the concentration of aromatic hydrocarbons, the greater its smoke point, therefore, the better its burning quality. FT-SPK and HEFA show remarkable combustion performance with smoke points higher than 40 mm. The currently used jet fuel JP-8 had a smoke point of 25 mm and FT-SPK had a higher smoke point than JP-8, which is more than 50 mm. The difference between the smoke points of FT-SPK and JP-8 is due to the presence of aromatic contents. The soot-forming capacity is higher in aromatic compounds; thus, JP-8 shows a lower smoke point, whereas FT-SPK, which is free from aromatic compounds, shows a higher smoke point. Blending conventional aviation fuel with biojet fuel raised the smoke point of the fuel. By adding 20 volume % of bio-kerosene (palm kernel biodiesel) into Jet A-1, there is a minute increase in smoke point from 27.1 to 29.1 mm (Corporan et al. 2007). All the biojet fuel blends have high smoke points because of the least aromatic content, low density, and higher hydrogen concentration than conventional fuels (Sundararaj et al. 2019). If we consider the example of biofuel blend 3, which is made of 90% universal oil products – synthetic paraffinic kerosene (UOP-SPK) and 10% Van-Sol 53, its chemical composition is composed of the least aromatic content, lowest density, and highest hydrogen content of all of the blends possible, consequently having high smoke point (Sundararaj et al. 2019).
In addition, the threshold sooting index (TSI) assesses the soot-forming capacity of conventional jet fuels and biojet fuels, which is also used to test the combustion characteristics of fuel. The TSI is linearly associated with the density of the fuel and the smoke point. The TSI of biojet fuels is relatively lower; for example, if we consider the TSI of Shell FT-SPK (9.11), Sasol FT-SPK (17.28), camelina HEFA (11.99), and tallow HEFA (11.58), whereas conventional jet fuels JP-8 have TSI of 19.28. After this study, a new fuel oil substitute will be created using an advanced optimization methodology to measure composition that satisfies sooting capacity, physiochemical properties, and optimized mole fraction for decalin (0.1449), toluene (0.2591), iso-octane (0.0195), iso-cetane (0.2059), and n-dodecane (0.3706) (Yu et al. 2018).
5.4.3.2 Particulate Matter (PM) Emissions
Particulate matter emissions are caused by noncombustible fuel components that have the potential to produce smog, which is harmful to both human health and the environment (Tiwari et al. 2023). The PM emissions in alternative jet fuel depend on the amount of aromatics compounds present. As FT-SPK has extremely low aromatics content, there is 52% of PM number reduction. Hence, the reduction in PM number and PM mass is achieved by blending FT-SPK with jet fuels. When PM emissions from aircraft are assessed, it is found that blended fuels, such as a camelina HEFA blend with Jet A, lower mass and PM emissions (Moore et al. 2017). Biojet fuels producing PM have a particle size smaller than fossil jet fuels. Farnesane showed a low potential from soot intermediates in the kinetic modeling of its burning, whereas p-cymene produces comparatively more naphthalene (Oßwald et al. 2017). This implies that low PM emissions of biojet fuels are due to the absence of aromatic content.
5.4.3.3 Gaseous Emissions
During the different phases of flight, such as take-off, climb, and cruise, the emission from the burnt fuel contains different concentrations of gases. Some of the gases released from jet fuel are carbon monoxide, carbon dioxide, nitrogen oxides (NOx), and unburned hydrocarbons (UHC) (Gaspar and Sousa 2016). In the different studies conducted, it was seen that the gaseous emission of biojet fuel blends was less than fossil-derived jet fuels. In an experiment conducted by Timko et al., it was observed that FT-SPK when blended with conventional jet fuels in a ratio of 1:1 emits 5% gaseous emission, whereas pure jet fuel produces 10% of NOx, in particular. There is a slight reduction in CO emission also in comparison to standard jet propellant (Timko et al. 2011). A study conducted by Corporan et al. suggests that the emission of NOx and CO2 is similar in both biojets (tests conducted in biofuels produced using FT-SK and HEFA routes) and JP-8 (with no blends with biofuel). Although a 10–25% reduction in CO and UHC emission index was seen, owing to the fact that lesser aromatic hydrocarbons were present in biofuels produced by this method (Corporan et al. 2011).
The data emphasizes the fact that there is a moderately lower emission of gases upon combustion of biojet fuels in contrast with jet fuels, which is not very significant. The explanation for this result could be the improper mixing of the fuel blends, the difference in viscosity and density of fuel blends, and the high fuel-to-air ratio (Sundararaj et al. 2019).
5.4.3.4 Derived Cetane Number (DCN)
DCN constitutes characteristics of ignited fuels considering the minimum standards that are set by various countries (Prak et al. 2021). With more combustion of fuel, there is an increase in DCN value, which also indicates that there is a decrease in ignition delay time. Therefore, higher DCN specifies better combustion performance, in addition to lower harmful emissions. The DCN number in the fuel is affected by the amount of aromatic hydrocarbons present in the fuel. The DCN value of Jet A fuel is calculated to be 49.35, which is comparatively lesser than FT-SPK and HEFA, which have a DCN value of 33.46 (Hui et al. 2012).
5.4.4 Consistency with the Current Aviation Fueling Infrastructure
Biojet fuels are functionally equal to or better than fossil-derived jet fuels as they reflect the excellent characteristics mentioned above. Nonetheless, it is crucial to take into account how well biojet fuels and elastomers work together. Also, 10–20% of conventional jet fuel contains aromatic compounds; biojet fuels do not contain aromatic compounds, which can cause fuel leakage because they cause O-ring seals to harden and shrink. A blend of biojet fuel and conventional jet fuel permits enough quantity of aromatic compounds to ensure the purity of engine seals (IRENA 2017). We go into great detail regarding the volume swell of sealant and lubricity in this section.
5.4.4.1 Volume Swell of Seal Materials
Alternative fuel enhancement is limited by the volume swell of seal material compatibility. It is necessary to evaluate whether BJF is suitable with engine seals prior to commercialization. The low density of biojet fuel due to the lack of aromatic compounds causes shrinkage in the seal. Because of this seal shrinking, we have seal failure, which further causes damage to the system. The two primary parameters in the aircraft system that determine the volume swell of sealant are the strength of the interaction between aircraft fuel and seal materials. Because of their large molecular weight, aromatic chemicals, such as naphthalene, have excellent interaction with seal polymers.
The three most commonly used seals in aircraft engines are fluorocarbon, fluorosilicone, and nitrile seals. Nitrile rubber is usually used as an O-ring seal in aircraft engines because it shows a greater response toward aromatic compounds than fluorosilicate and fluorocarbon seals (Moses 2008). Leakage in the hydraulic system and engine is prevented by the elastomers, like O-ring seals. The sealing function of O-ring elastomer is because of deformation when it is crushed between two parts of the engine (Qin et al. 2019).
In the O-ring, two effects that are usually seen are swelling and shrinking of the O-ring; an increase in seal volume is defined as swelling of the O-ring, here elastomer absorbs chemical components of fuel that result in swelling, whereas a decrease in seal volume is defined as shrinking of the O-ring, here the O-ring degrades when some components are released into the fuel and absorbed by the seal. The seal defiance with regard to fuel is indicated by swelling of the O-ring (Liu and Wilson 2012).
However, adding aromatic chemicals to biojet fuels improves their compatibility and may also lead to an increase in PM emissions. Both concentrations of aromatic compounds and types of aromatic compounds used are correlated to the PM emissions and volume swell. The concentration of aromatic compounds is directly proportional to the PM emissions and volume swell. An increase in the molecular weight of aromatic compounds causes an increase in PM emissions (DeWitt et al. 2008).
Less than 10% of aromatic compounds with greater molecular weight and more than 10% of arene compounds with a lower molecular weight must be added in order to produce biojet propellant that meets the required output standards for volume swell and PM emissions (Yang et al. 2019).
5.4.4.2 Lubricity
Lubricity is the capacity of the fuel to reduce wear or friction between two surfaces of engine components in relative motion. Good lubricity of fuel is important for the engine to run smoothly. The lubricity of a substance or fuel depends on the fuel composition, and it is not an intrinsic property. In ASTM D7566-18, the synthesized hydrocarbons do not have any specific lubricity limits (Elkelawy et al. 2022). The presence of polar compounds in biojet fuels is directly associated with the lubricity of the fuel (Hari et al. 2015).
The main disadvantage of biojet fuel production approaches of BJFs is that they comprise various steps of hydrotreatment processes due to which the compounds containing oxygen, nitrogen, and sulfur are removed from synthesized hydrocarbons, thus ensuing lubricity of below standard (Hari et al. 2015). This limitation of BJF is withdrawn by making blends of BJFs with suitable conventional jet fuels as it contains 700 ppm (parts per million) sulfur or adding additives like fatty acid methyl ester (FAME), which is used as an additive in HEFA fuel to enhance the lubricity. The amount of FAME in HEFA is limited due to its poor low-temperature fluidity as according to the ASTM D7566 standards it should be less than 5 ppm (McNutt 2016).
As per the ASTM D7566 standards, the compatibility and characteristics of BJF combustion are balanced by blending about 8 weight % of aromatic compounds in the final blend (Lahijani et al. 2022). This implies that biojet fuels show poor lubricity due to the absence of naturally occurring compounds like oxygen, nitrogen, and sulfur and the absence of compounds with polarity.
5.4.5 Volatility of Fuel
Fuel volatility is defined as the fuel’s ability to evaporate quickly. Fuel volatility is caused by two key features, which are covered in the sections that follow: the distillation property and the flash point.
5.4.5.1 Distillation Property
The distillation property describes the percentage of fuel vaporized with the increase in temperature, that is, it tells us about the percentage of recovery fraction when fuel is burnt (del Coro Fernández-Feal et al. 2017). The distillation property is determined by the concentration of volatile substances present in the fuel and the amount of residue left after the combustion. This can be tested using a distillation test (ASTM D1160 2015). The temperature of the boiling point (BP) of the fuel has an impact on its vaporization and combustion (Kook and Pickett 2010). The BP is defined as initial BP, mid-BP, and final BP. The initial BP is the temperature at which the fuel starts to evaporate, mid-BP is the temperature at which half of the fuel has been vaporized, and final BP is the temperature at which 100% of the fuel sample is evaporated (Sundararaj et al. 2019). The fuel with the low BP has the advantage of vaporizing readily and thus complete combustion of the fuel (Maly et al. 2007). The complete combustion leads to low PM emissions. Although the complete combustion of fuels with low BP leads to low PM emission, there is a release of more nitrogen oxide (NOx). The reason for NOx emission is that the quick evaporation of the fuel causes more of the fuel to get mixed with the air before the actual combustion starts, which increases the volume of the flammable mixture and, thus, more heat emission (Kook and Pickett 2010). The higher final BP is linked with more smoke and PM release. This distillation property needs to be critically considered to understand energy penetration so that product optimization is done during fuel production (Acosta-Solórzano et al. 2016).
The standard distillation range selected is as temperature at 10% recovery (T10) should be less than 205 °C and final BP should be less than 300 °C. Upon investigation of the distillation property of alternative jet fuels, it was seen that FT-SPK and HEFA have a distillation range within the set standard range (Wierzbicki et al. 2014). The distillation range of some of the BJFs is mentioned in Table 5.2.
The fuel’s constituents have an effect on the fuel’s distillation range as well. For example, ethyl cyclohexane (C8H16) has a lower boiling point than Jet A-1 because its carbon chain is shorter. The HEFA fuel and its blends have distillation temperatures at all fractions because of their higher chain length (C17) (Scheuermann et al. 2017).
5.4.5.2 Flash Point
The lowest temperature at which a liquid’s vapors are concentrated enough to create an ignitable vapor in the presence of an ignition source is known as the flash point. It represents fuel volatility (Kong et al. 2003). Fuels can be classified as combustible, flammable, or gasoline based on their flash point. It is commonly used to evaluate the handling as well as hazards of flammable substances during storage and shipping (Hassan et al. 2023). Fuels are classified as combustible when their flash point exceeds 37.8 °C and flammable fuels when their flash point falls below 37.8 °C (Kong et al. 2003). According to the ASTM D7566-18 standards, FT-SPK, HEFA, FT-SPK/A, and ATJ-SPK should have a minimum flash point of 37.8 °C. But because farnesane (C15) has a long carbon chain and a high flash point, synthetic iso-paraffins (SIP) fuel needs a minimum flash temperature of 100 °C. Pensky Martens Flash Point Tester is the equipment used to measure the flash point of BJFs (Hristova 2013). The flash points of various alternative fuels were studied, and it was concluded that biofuels with low-BP aliphatic components have low flash points, in contrast to the fuels with high-BP aromatic compounds, which have high flash points (Scheuermann et al. 2017). Thus, the fuel’s flash points are likewise influenced by the chemical components’ BJFs.
5.4.6 Fuel Metering and Aircraft Range
The fuels in its liquid state are not combustible. Correct air and fuel mixture are required for the proper and complete combustion of fuel. The fuel metering system is a device that allows the proper fuel flow while maintaining the air/fuel ratio required for the clean combustion of the fuel at existing engine operating conditions (Hideg 1982). The jet load and jet range are very well impacted by the density of the fuel. Since the fuel occupies the engine of the aircraft volumetrically, the density of the fuel is the major criterion in deciding the flow calculations, adjusting the fuel metering device, and calculations with respect to thermal expansions of the fuel (Vozka et al. 2019). The amount of heat energy produced upon the combustion of fuel is directly proportional to fuel density, fuel volume, and net heat of combustion. The fuel with higher energy extent allows more aviation range and higher payload. Besides, the reduced thermal energy generated by the full combustion of fuel leads to a significant increase in fuel consumption, which raises the expense of jet operations (Yang et al. 2019). The fuel density and composition of fuel that contribute to their densities, along with the net heat of combustion of BJFs, are elaborated below.
5.4.6.1 Density of Fuel at 15 °C
As per the standard density values for the BJFs to be a drop-in fuel, ASTM D7566-18 has decided the density range of 730–770 kg/m3 at 15 °C (Green et al. 2020). Table 5.3 displays the densities of a few biojet fuels at 15 °C.
The densities of FT-SPK, HEFA, and ATJ-SPK are all within the ideal range for them to generate a considerable amount of heat energy when they burn. Whereas SIP does not have the optimum density because of the presence of a large content of long-chain farnesene. The blends of SIP also do not provide a satisfactory density range. The best-suited SIP fuel with optimum density at 15 °C is a blend of 90 volume % farnesene from SIP and 10 volume % limonene (Chuck and Donnelly 2014).
The biojet fuel with a relatively higher amount of aromatics provides even more high fuel density. Therefore, the blends of biojet fuels with current fuels are expected to have better densities in terms of enhancing their energy content. Scheuermann et al. in 2017 tested the fuel density of blends of ATJ-SPK/A with 15.8 volume % of aromatics resulting in a higher density of 785.9 kg/m3. These findings suggested that the concentration of aromatics in the biofuels is directly related to the density of the fuel and eventually to the heat energy production of fuel upon combustion.
5.4.6.2 Net Heat of Combustion
For both conventional and blended fuels, the ASTM D7566-18 specifies that the net heat combustion value must be greater than 42.8 MJ/kg. The biofuels are also known to have optimum net heat combustion. The net heat of combustion of various biofuels is SIP has 43.93 MJ/kg (Brennan et al. 2012), farnesene has 47 MJ/kg (Rude and Schirmer 2009), FT-SPK and HEFA have 44 MJ/kg (Hui et al. 2012), and 50 volume % FT-SPK/Jet A-1 blend fuel had lower net heat of combustion (43.7 MJ/kg), in comparison to pure FT-SPK (Timko et al. 2011). There is a slight dip in the net heat of combustion when biofuels are used as blends as the conventional jet fuels have availability of aromatics in their composition. The decrease in the ratio of H/C (hydrogen/carbon content) has reportedly shown lower net heat of combustion and aromatics having a lesser H/C ratio as it contains one or more double bonds (Lobo et al. 2011).
5.5 Challenges and Future Look
This chapter has thoroughly described the characteristics of biofuels and made the comparison of biofuels with fossil-based fuels. Consequently, it can be stated that biojet fuels are the better choice for the selection of fuel as they are technologically advanced and ecologically sustainable. There are a few areas in which biojet fuel needs improvement to overcome the small challenges it faces to replace fossil fuel-based aviation fuels completely. Using the techniques outlined in this chapter to generate BJFs for commercialization is expensive and currently unable to satisfy fuel demand. The cost of the production is affected by the feedstock used for the production of biojet fuels. The selection of the production route and raw material feedstock can be worked upon to reduce the cost involved. Furthermore, it has been tested that not all production routes and choice of feedstock are capable of reducing greenhouse gas emissions (GHG) (Cui et al. 2018).
Less information is available with regard to the correct amount of constituents present in the biojet fuels, and thereby, their relation with the performance characteristics of the fuel. More research and proper characterization are required to deeply understand the role of each component present in fuels (ElGalad et al. 2018). Not all of the characteristics of biojet fuels are covered in the already published work, such as the presence of gum, corroding properties, water separation trait, and electrical conductivity. These properties are not readily studied while selecting a fuel, but these also impact the performance characteristics of the fuel in a great way. For example, studying the gum-existent feature of the fuel gives information about the contamination of high-BP oils and particulate matter in the fuel. Moreover, the gum in the fuel makes it difficult to store (Yang et al. 2019).
Further research on the fuels’ characteristics, such as soot generation paths, combustion species profiles, laminar flame speeds, and extinction limits, is necessary before considering biojet fuels. Research is required in this direction as the long-term combustion of biojets and blends is not documented much (Yang et al. 2019).
There is an insufficiency of effective government policy incentives to promote the switch from traditional fuels to biofuel. Moreover, there are strict guidelines to be followed, which pose difficulty in the production of BJFs. The field is also facing a lack of investments owing to the fact that the returns expected from biojet fuels are uncertain. There is also a negative perception associated with safety while using biojet (Lim et al. 2023). Lastly, some of the undesirable properties are witnessed with the synthesis of biojet fuels through the methods mentioned, such as constituents of fuel with long-chain carbon atoms or fuels with oxygen in distillate having properties that do not adhere to the guidelines. The evaluation of each constituent is therefore important to understand the performance characteristics of the fuel designed. Alternative approaches to producing BJFs are being adopted. One such approach is the catalytic synthesis of high-density BJFs using bio-derived furfurals as biomass. This process uses alkylation, aldol condensation, and hydrodeoxygenation (Han et al. 2017). These alternative methodologies are the main scope of biojet fuels, which needs to be characterized more.
5.6 Conclusion
With an increased demand for aircraft travelers, emission reduction has become the main area of research nowadays. The application of biojet fuels has become quintessential to dealing with deficient fossil fuel supply and environmental problems that come with fuels. Many production routes have been optimized for the production of biofuels such as FT-SPK, HEFA, FT-SPK/A, SIP, and ATJ-SPK as specified in the ASTM D7566-18. The evaluation of performance characteristics suggested that the chemical composition of the fuel is highly influencing its performance. The BJFs demonstrated acceptable low-temperature fluidity in fuels with more levels of iso-paraffins, short-chain paraffins, and alkylated aromatic content. Moreover, high kinematic viscosity is also observed in SIP fuels having high farnesane content, thereby increasing the low-temperature fluidity. Biofuels have relatively greater thermal stability, but the oxidative thermal stability is still questionable due to the presence of high paraffin in biofuels. Less particle emission, gaseous release, a high smoke point, and derived cetane number are among the combustion properties of the BJFs that also meet the required standards. However, while blending with the current aviation fueling system, BJFs’ lubricity and compatibility are unsatisfactory. The amount of aromatics that is near zero is not compatible with the volume swell of seal materials and thus can lead to shrinkage and leakage. Due to the ideal chain length of the components that make up these fuels, the distillation property of the BJFs is adequately good. The flash point of these is also fitting within the range due to the presence of low-BP aliphatic components. The fuel metering and aircraft range are acceptable as per specified standards.
This information is helpful in understanding the practicality of biojet production. Further research such as the life cycle study of the fuel is necessary to comprehend the carbon footprints and efficiency of biojets. Efforts are required to improve the performance characteristics as well as ensure the storage stability of the fuels.
References
Abdullah Z, Battelle MI (2015) Upgrading of biomass Fast Pyrolysis Oil (Bio-oil)
Acosta-Solórzano AD, Guerrero-Farfán O, Ramírez-Márquez C, Gómez-Castro FI, Segovia-Hernández JG, Hernández S et al (2016) Controllability analysis of distillation sequences for the separation of Bio-Jet fuel and Green diesel fractions. Chem Eng Technol 39(12):2273–2283
Amara AB, Kaoubi S, Starck L (2016) Toward an optimal formulation of alternative jet fuels: enhanced oxidation and thermal stability by the addition of cyclic molecules. Fuel 173:98–105
ASTM D1160 (2015) Standard test method for distillation of petroleum products at reduced pressure. Annual Book of Standards
ATAG (2020). Air Transport Action Group- Facts & figures. https://atag.org/facts-figures.
Ben Amara A, Lecointe B, Jeuland N, Takahashi T, IIda Y, Hashimoto H, Bouilly J (2014) Experimental study of the impact of diesel/biodiesel blends oxidation on the fuel injection system. SAE Int J Fuels Lubr 7. https://doi.org/10.4271/2014-01-2767
Benavides A, Benjumea P, Cortés FB, Ruiz MA (2021) Chemical composition and low-temperature fluidity properties of jet fuels. PRO 9(7):1184
Bozell, J. J., Moens, L., Wang, Y., Neuenswander, G. G., Fitzpatrick, S. W., Bilski, R. J., & Jarnefeld, J. L. (2000). The use of renewable feedstocks for the production of chemicals and materials. National Renewable Energy Laboratory 1617 Cole Boulevard Golden, CO, 8040
Brennan TC, Turner CD, Krömer JO, Nielsen LK (2012) Alleviating monoterpene toxicity using a two-phase extractive fermentation for the bioproduction of jet fuel mixtures in Saccharomyces cerevisiae. Biotechnol Bioeng 109(10):2513–2522
Cataluna R, Shah Z, Venturi V, Caetano NR, da Silva BP, Azevedo CM et al (2018) Production process of di-amyl ether and its use as an additive in the formulation of aviation fuels. Fuel 228:226–233
Christison KM, Lorenz RM, Xue L, Sparkman OD (2019) Exploring the molecular origin of jet fuel thermal oxidative instability through statistical analysis of mass spectral data. Energy Fuel 33(2):830–836
Chuck CJ, Donnelly J (2014) The compatibility of potential bioderived fuels with jet A-1 aviation kerosene. Appl Energy 118:83–91
Corporan E, DeWitt MJ, Belovich V, Pawlik R, Lynch AC, Gord JR, Meyer TR (2007) Emissions characteristics of a turbine engine and research combustor burning a Fischer− Tropsch jet fuel. Energy Fuel 21(5):2615–2626
Corporan E, Edwards T, Shafer L, DeWitt MJ, Klingshirn C, Zabarnick S et al (2011) Chemical, thermal stability, seal swell, and emissions studies of alternative jet fuels. Energy Fuel 25(3):955–966
Cui Q, Li Y, Lin JL (2018) Pollution abatement costs change decomposition for airlines: an analysis from a dynamic perspective. Transp Res A Policy Pract 111:96–107
del Coro Fernández-Feal MM, Sánchez-Fernández LR, Sánchez-Fernández B (2017) Distillation: basic test in quality control of automotive fuels. In: Distillation innovative applications and modeling, p 77
Demirbas A (2009) Characterization of biodiesel fuels. Energy Sources Part A 31(11):889–896
DeWitt MJ, Corporan E, Graham J, Minus D (2008) Effects of aromatic type and concentration in Fischer− Tropsch fuel on emissions production and material compatibility. Energy Fuel 22(4):2411–2418
Díaz-Pérez MA, Serrano-Ruiz JC (2020) Catalytic production of jet fuels from biomass. Molecules 25(4):802
Doliente SS, Narayan A, Tapia JFD, Samsatli NJ, Zhao Y, Samsatli S (2020) Bio-aviation fuel: a comprehensive review and analysis of the supply chain components. Front Energy Res 8:110
ElGalad MI, El-Khatib KM, Abdelkader E, El-Araby R, ElDiwani G, Hawash SI (2018) Empirical equations and economical study for blending biofuel with petroleum jet fuel. J Adv Res 9:43–50
Elkelawy M, Bastawissi HAE, Radwan AM, Ismail MT, El-Sheekh M (2022) Biojet fuels production from algae: conversion technologies, characteristics, performance, and process simulation. In: Handbook of Algal biofuels. Elsevier, pp 331–361
Gaspar RMP, Sousa JMM (2016) Impact of alternative fuels on the operational and environmental performance of a small turbofan engine. Energy Convers Manag 130:81–90
Geleynse S, Brandt K, Garcia-Perez M, Wolcott M, Zhang X (2018) The alcohol-to-jet conversion pathway for drop-in biofuels: techno-economic evaluation. ChemSusChem 11(21):3728–3741
Gouveia L, Oliveira AC, Congestri R, Bruno L, Soares AT, Menezes RS, Tzovenis I (2017) Biodiesel from microalgae. In: Microalgae-based biofuels and bioproducts. Woodhead Publishing, pp 235–258
Grande L, Pedroarena I, Korili SA, Gil A (2021) Hydrothermal liquefaction of biomass as one of the most promising alternatives for the synthesis of advanced liquid biofuels: a review. Materials 14(18):5286
Green R, Cooper S, McManus M (2020) Fuel standards summary. University of Bath
Han P, Nie G, Xie J, Xiu-tian-feng E, Pan L, Zhang X, Zou JJ (2017) Synthesis of high-density biofuel with excellent low-temperature properties from lignocellulose-derived feedstock. Fuel Process Technol 163:45–50
Han GB, Jang JH, Ahn MH, Jung BH (2019) Recent application of bio-alcohol: Bio-jet fuel, vol 1. IntechOpen, pp 109–121
Hari TK, Yaakob Z, Binitha NN (2015) Aviation biofuel from renewable resources: routes, opportunities and challenges. Renew Sust Energ Rev 42:1234–1244
Hassan SH, Attia NK, El Diwani GI, Amin SK, Ettouney RS, El-Rifai MA (2023) Catalytic hydrocracking of jatropha oil over natural clay for bio-jet fuel production. Sci Rep 13(1):13419
Hideg L (1982) U.S. Patent no. 4,357,923. U.S. Patent and Trademark Office, Washington, DC
Hong TD, Soerawidjaja TH, Reksowardojo IK, Fujita O, Duniani Z, Pham MX (2013) A study on developing aviation biofuel for the tropics: production process—experimental and theoretical evaluation of their blends with fossil kerosene. Chem Eng Process Process Intensif 74:124–130
Hristova M (2013) Measurement and prediction of binary mixture flash point. Cent Eur J Chem 11:57–62
Hu J, Yu F, Lu Y (2012) Application of Fischer–Tropsch synthesis in biomass to liquid conversion. Catalysts 2(2):303–326
Hu Y, Bassi A, Xu CC (2020) Energy from biomass. In: Future Energy. Elsevier, pp 447–471
Hui X, Kumar K, Sung CJ, Edwards T, Gardner D (2012) Experimental studies on the combustion characteristics of alternative jet fuels. Fuel 98:176–182
ICAO Environment (2018) Sustainable aviation fuel guides. Version 2
IRENA (2017) Renewable Energy Statistics 2017. The International Renewable Energy Agency, Abu Dhabi
Jia T, Gong S, Pan L, Deng C, Zou JJ, Zhang X (2020) Impact of deep hydrogenation on jet fuel oxidation and deposition. Fuel 264:116843
Jiao Q, Anderson JE, Wallington TJ, Kurtz EM (2015) Smoke point measurements of diesel-range hydrocarbon–oxygenate blends using a novel approach for fuel blend selection. Energy Fuel 29(11):7641–7649
Kong D, am Ende DJ, Brenek SJ, Weston NP (2003) Determination of flash point in air and pure oxygen using an equilibrium closed bomb apparatus. J Hazard Mater 102(2–3):155–165
Kook S, Pickett LM (2010) Effect of fuel volatility and ignition quality on combustion and soot formation at fixed premixing conditions. SAE Int J Engines 2(2):11–23
Kramer S, Andac G, Heyne J, Ellsworth J, Herzig P, Lewis KC (2022) Perspectives on fully synthesized sustainable aviation fuels: direction and opportunities. Front Energy Res 9:782823
Lahijani P, Mohammadi M, Mohamed AR, Ismail F, Lee KT, Amini G (2022) Upgrading biomass-derived pyrolysis bio-oil to bio-jet fuel through catalytic cracking and hydrodeoxygenation: a review of recent progress. Energy Convers Manag 268:115956
Li L, Coppola E, Rine J, Miller JL, Walker D (2010) Catalytic hydrothermal conversion of triglycerides to non-ester biofuels. Energy Fuel 24(2):1305–1315
Li F, Jiang J, Liu P, Zhai Q, Wang F, Hse CY, Xu J (2018) Catalytic cracking of triglycerides with a base catalyst and modification of pyrolytic oils for production of aviation fuels. Sustainable Energy Fuels 2(6):1206–1215
Lim JHK, Gan YY, Ong HC, Lau BF, Chen WH, Chong CT et al (2021) Utilization of microalgae for bio-jet fuel production in the aviation sector: challenges and perspective. Renew Sust Energ Rev 149:111396
Lim M, Luckert MM, Qiu F (2023) Economic opportunities and challenges in biojet production: a literature review and analysis. Biomass Bioenergy 170:106727
Lin R, Tavlarides LL (2013) Thermal stability and decomposition of diesel fuel under subcritical and supercritical conditions. J Supercrit Fluids 75:101–111
Lin CH, Chen YK, Wang WC (2020) The production of bio-jet fuel from palm oil derived alkanes. Fuel 260:116345
Liu Y, Wilson CW (2012) Investigation into the impact of n-decane, decalin, and isoparaffinic solvent on elastomeric sealing materials. Advances in Mechanical Engineering 4:127430
Liu S, Zhu Q, Guan Q, He L, Li W (2015) Bio-aviation fuel production from hydroprocessing castor oil promoted by the nickel-based bifunctional catalysts. Bioresour Technol 183:93–100
Llamas A, García-Martínez MJ, Al-Lal AM, Canoira L, Lapuerta M (2012) Biokerosene from coconut and palm kernel oils: production and properties of their blends with fossil kerosene. Fuel 102:483–490
Lobo P, Hagen DE, Whitefield PD (2011) Comparison of PM emissions from a commercial jet engine burning conventional, biomass, and Fischer–Tropsch fuels. Environ Sci Technol 45(24):10744–10749
Ma W, Dalai AK (2021) Effects of structure and particle size of iron, cobalt and ruthenium catalysts on Fischer–Tropsch Synthesis. Reactions 2(1):62–77
Maly RR, Schaefer V, Hass H, Cahill GF, Rouveirolles P, Röj A et al (2007) Optimum diesel fuel for future clean diesel engines. SAE Trans:28–47, Vol116
Mawhood RK, Gazis E, Hoefnagels R, De Jong S, Slade R (2015) Technological and commercial maturity of aviation biofuels: emerging options to produce jet from lignocellulosic biomass
McNutt J (2016) Development of biolubricants from vegetable oils via chemical modification. J Ind Eng Chem 36:1–12
Monteiro RR, dos Santos IA, Arcanjo MR, Cavalcante CL Jr, de Luna FM, Fernandez-Lafuente R, Vieira RS (2022) Production of jet biofuels by catalytic hydroprocessing of esters and fatty acids: a review. Catalysts 12(2):237
Moore RH, Thornhill KL, Weinzierl B, Sauer D, D’Ascoli E, Beaton B et al (2017) Biofuel blending reduces aircraft engine particle emissions at cruise conditions. Nature 543(7645):411
Moreno-Gómez AL, Gutiérrez-Antonio C, Gómez-Castro FI, Hernández S (2020) Production of Biojet fuel from waste raw materials: a review. In: Process systems engineering for biofuels development, pp 149–171
Morgan T, Santillan-Jimenez E, Harman-Ware AE, Ji Y, Grubb D, Crocker M (2012) Catalytic deoxygenation of triglycerides to hydrocarbons over supported nickel catalysts. Chem Eng J 189:346–355
Moses CA (2008) Comparative evaluation of semi-synthetic jet fuels. Contract 33415(02-D):2299
Moser BR, Vaughn SF (2010) Coriander seed oil methyl esters as biodiesel fuel: unique fatty acid composition and excellent oxidative stability. Biomass Bioenergy 34(4):550–558
Ng KS, Farooq D, Yang A (2021) Global biorenewable development strategies for sustainable aviation fuel production. Renew Sust Energ Rev 150:111502
Nie G, Zhang X, Pan L, Wang M, Zou JJ (2018) One-pot production of branched decalins as high-density jet fuel from monocyclic alkanes and alcohols. Chem Eng Sci 180:64–69
Nwokolo N, Mukumba P, Obileke K (2020) Gasification of eucalyptus wood chips in a downdraft gasifier for syngas production in South Africa. Int J Renewable Energy Res (IJRER) 10(2):663–668
Odziemkowska M, Czarnocka J, Wawryniuk K (2018) Study of stability changes of model fuel blends. In: Improvement trends for internal combustion engines, pp 1–17
Oßwald P, Whitside R, Schäffer J, Köhler M (2017) An experimental flow reactor study of the combustion kinetics of terpenoid jet fuel compounds: Farnesane, p-menthane and p-cymene. Fuel 187:43–50
Pavlenko N, Kharina A (2018) Policy and environmental implications of using HEFA+ for aviation. IntCounc Clean Transp 9:1–9
Pires AP, Han Y, Kramlich J, Garcia-Perez M (2018) Chemical composition and fuel properties of alternative jet fuels. Bioresources 13(2):2632–2657
Prak DL, Cooke J, Dickerson T, McDaniel A, Cowart J (2021) Cetane number, derived cetane number, and cetane index: when correlations fail to predict combustibility. Fuel 289:119963
Qin K, Zhou Q, Zhang K, Feng Y, Zhang T, Zheng G et al (2019) Non-uniform abrasive particle size effects on friction characteristics of FKM O-ring seals under three-body abrasion. Tribol Int 136:216–223
Renninger NS, Newman JD, Reiling KK (2010) U.S. Patent no. 7,854,774. U.S. Patent and Trademark Office, Washington, DC
Roberts WL (2008) Bio jet fuels. ile 4(4.00):3–75
Rude MA, Schirmer A (2009) New microbial fuels: a biotech perspective. Curr Opin Microbiol 12:274–281
Saffaripour M, Zabeti P, Kholghy M, Thomson MJ (2011) An experimental comparison of the sootingbehavior of synthetic jet fuels. Energy Fuel 25(12):5584–5593
Scheuermann SS, Forster S, Eibl S (2017) In-depth interpretation of mid-infrared spectra of various synthetic fuels for the chemometric prediction of aviation fuel blend properties. Energy Fuel 31(3):2934–2943
Serrano-Ruiz JC, Luque R, Sepúlveda-Escribano A (2011) Transformations of biomass-derived platform molecules: from high added-value chemicals to fuels via aqueous-phase processing. Chem Soc Rev 40(11):5266–5281
Shahid MK, Batool A, Kashif A, Nawaz MH, Aslam M, Iqbal N, Choi Y (2021) Biofuels and biorefineries: development, application and future perspectives emphasizing the environmental and economic aspects. J Environ Manag 297:113268
Sundararaj RH, Kushari A (2019) A review of Bio-jet production pathways. Recent Adv Petrochem Sci 7(1):555702
Sundararaj RH, Kumar RD, Raut AK, Sekar TC, Pandey V, Kushari A, Puri SK (2019) Combustion and emission characteristics from biojet fuel blends in a gas turbine combustor. Energy 182:689–705
Sustainable Way for Alternative Fuels and Energy in Aviation (SWAFEA) (2011) formal report, European Commission
Tao L, Milbrandt A, Zhang Y, Wang WC (2017) Techno-economic and resource analysis of hydroprocessed renewable jet fuel. Biotechnol Biofuels 10:1–16
Timko MT, Herndon SC, De La Rosa Blanco E, Wood EC, Yu Z, Miake-Lye RC et al (2011) Combustion products of petroleum jet fuel, a Fischer–Tropsch synthetic fuel, and a biomass fatty acid methyl ester fuel for a gas turbine engine. Combust Sci Technol 183(10):1039–1068
Tiwari R, Mishra R, Choubey A, Kumar S, Atabani AE, Badruddin IA, Khan TY (2023) Environmental and economic issues for renewable production of bio-jet fuel: a global prospective. Fuel 332:125978
Tomar M, Abraham A, Kim K, Mayhew E, Lee T, Brezinsky K, Lynch P (2023) Bio-derived sustainable aviation fuels—on the verge of powering our future. In: Combustion chemistry and the carbon neutral future. Elsevier, pp 521–598
van der Westhuizen R, Ajam M, De Coning P, Beens J, de Villiers A, Sandra P (2011) Comprehensive two-dimensional gas chromatography for the analysis of synthetic and crude-derived jet fuels. J Chromatogr A 1218(28):4478–4486
Vozka P, Modereger BA, Park AC, Zhang WTJ, Trice RW, Kenttämaa HI, Kilaz G (2019) Jet fuel density via GC× GC-FID. Fuel 235:1052–1060
Wang WC, Tao L (2016) Bio-jet fuel conversion technologies. Renew Sust Energ Rev 53:801–822
Wang X, Jia T, Pan L, Liu Q, Fang Y, Zou JJ, Zhang X (2021) Review on the relationship between liquid aerospace fuel composition and their physicochemical properties. Trans Tianjin Univ 27:87–109
Wei H, Liu W, Chen X, Yang Q, Li J, Chen H (2019) Renewable bio-jet fuel production for aviation: a review. Fuel 254:115599
Wierzbicki TA, Lee IC, Gupta AK (2014) Performance of synthetic jet fuels in a meso-scale heat recirculating combustor. Appl Energy 118:41–47
Yang J, Xin Z, Corscadden K, Niu H (2019) An overview on performance characteristics of bio-jet fuels. Fuel 237:916–936
Yu W, Tay K, Zhao F, Yang W, Li H, Xu H (2018) Development of a new jet fuel surrogate and its associated reaction mechanism coupled with a multistep soot model for diesel engine combustion. Appl Energy 228:42–56
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Arora, P., Mishra, S. (2024). Characteristics of Biojet Fuel. In: Kuila, A. (eds) Biojet Fuel: Current Technology and Future Prospect. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-99-8783-2_5
Download citation
DOI: https://doi.org/10.1007/978-981-99-8783-2_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8782-5
Online ISBN: 978-981-99-8783-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)