Abstract
Fish head, bones, and scales are the main solid fish waste or by-product, which are rich in calcium. The calcium obtained from these fish by-products can be used in feed, food, clinical, and biological materials after proper processing. This chapter first takes a glace of the fish by-products that can be used as calcium sources, with the focus on fish bones. The chemical composition, physicochemical characteristics, and bioavailability of the calcium sources are summarized. The main methods of extracting and processing the calcium source are introduced and compared by classifying chemical, enzymatic, and physical methods. The products derived from the calcium source are divided into fish bone foods, powder/paste/slurry, calcium supplements, and hydroxyapatite products. Finally, production lines for the calcium products made from fish by-products are depicted. This book chapter provides readers with a comprehensive knowledge of the calcium source made from fish by-product.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bioavailability
- Calcium
- Extraction
- Feed
- Fish bone
- Fish waste
- Fish by-product
- Hydroxyapatite
- Powder
- Paste
- Milling
1 Introduction
Thousands of tons of fish waste and by-product are produced during fish processing, including fish head, fish bones, fish scale, fish viscera, fish skin, etc. However, these by-product has not been effectively utilized, which not only causes environmental pollution, but also wastes a lot of valuable resources. Depending on the species, the head, bone, and scale account for about 30% of the total body weight of the fish. Fish head, bone, and scale can be used as a source of calcium. However, at present, most fish head and bone are combined in the solid waste stream and enter the fishmeal processing plant. In the fishmeal plant, all solid waste is heated/cooked and dried. Oil is separated before drying. Then the large bone is separated and ground as fish meal. Calcium in fish by-product is natural and has high bioavailability (Malde et al. 2010). Therefore, it has been the main direction and hot spot of scientific research to use calcium from fish by-product for the enhancement of food nutrition or to develop calcium health food to improve its economic added value (Nemati et al. 2016; Benjakul et al. 2017). Because of its good biocompatibility with human body, hydroxyapatite extracted from the fish by-product can be widely used as implant materials in plastic surgery, dentistry, and other fields (Boutinguiza et al. 2012). In addition, the hydroxyapatite can also be used as specific adsorption materials in the chromatographic columns (Ashokan et al. 2021).
The utilization of calcium from fish by-product usually goes through three stages: recovery, extraction, and product development. In the recovery stage of by-product that can be used as calcium source, different methods should be adopted by comprehensively considering the sanitary condition, recovery rate, and economic cost required for the development of different products. Most of the calcium in fish by-product exists in the form of hydroxyapatite. After hydroxyapatite is deposited on the surface of collagen fibers, the mineralized collagen fiber is formed, which is the basic unit of the hierarchical structure of fish bone and scale. In the development and utilization of calcium in fish by-product, two main schemes can be adopted. The first is to extract calcium ion or hydroxyapatite and then further process it; the other is to use it after crushing without extraction. Due to the complex hierarchical structure of fish bones and the extremely low solubility of hydroxyapatite, a combination of various processes is often used in the extraction of calcium ions and calcium compounds to improve the extraction rate. Fish bone is hard and tough, which makes it difficult to be crushed into desired size directly. Before crushing, pretreatments such as enzymatic hydrolysis and high-temperature cooking are often used to soften the bone to reduce the difficulty of crushing (Jafarpour et al. 2020). With the development of modern food processing technology, the ways of fish bone crushing have become diverse (Wu et al. 2012; Cui et al. 2021; Boutinguiza et al. 2012).
This chapter first reviews the recovery and characteristics of fish waste and by-product which possess the potential to be utilized as calcium source, and chemical composition, properties, and bioavailability of the recovered calcium compounds. Then, extraction and processing method, and applications of the calcium compounds from fish waste are summarized. Finally, production lines of several calcium products made from fish by-product are depicted.
2 Raw Materials as Calcium Source
2.1 Fish Bone
2.1.1 Backbone
The proportion of fish bones to total body weight is about 9%. The proportion of fish backbone in fish bones is the largest, about 80%. The fish backbone is composed of a number of vertebrae, which are connected one by one from the back of the head to the base of the tail to form a segmented column (Fig. 1). The basic function of fish backbone is to support the body and protect the spinal cord, internal organs, and main blood vessels. The backbone, ribs, skull, and scale of fish have multi-level hierarchical structure, which is formed by the mineralized collagen fibers of basic units through different arrangements (e.g., parallel, stagger, interweave, and enclosed arrangements). The mineralized collagen fibers are composed of hexagonal hydroxyapatite crystals and triple helix collagen (Fig. 1). The thermal and mechanical properties of bone in different parts are different, which may be mainly related to the mineralized form of collagen fibers and the arrangement structure of mineralized collagen fibers. The fish backbone is a typical trabecula, which is mainly composed of lamellar bundles. In the process of surimi production, the fish carcass is transported into the de-boning machine through the plastic belt. Under the extrusion of the belt and the stainless steel drum, the fish meat enters the drum through the pores of the drum and is separated from the fish backbone and other components (Shi et al. 2021). The fish backbone is easy to recover. In the processing of fish fillet, the backbone can be separated from the fish meat by a filleting machine. Generally, a residual part of fish meat is adhered to the fish backbone which can be removed by cooking, alkali, or enzymatic hydrolysis in the subsequent processing (Nemati et al. 2016).
2.1.2 Rib Bone
Fish rib bone accounts for about 15% weight of fish bones. The ribs are physiologically connected with the backbone (Fig. 1), and their main function is to protect visceral tissues. Fish ribs are mainly composed of densely arranged fibrous lamellar bone along the long axis (Jiao et al. 2020). During the production of fish fillets such as salmon, the ribs can be pulled out by a stinger and then collected.
2.1.3 Fine Bone
Fine bone accounts for about 5% of fish bones. Fine bones are generally distributed in the trunk muscles on both sides of the fish (Fig. 1). The trunk muscles on each side are divided into supra-axial and infra-axial muscles by the horizontal septum of connective tissue from the spine, namely, the dorsal and abdominal muscles. The fine bones are mainly located in the diaphragms of the supra-axial and infra-axial muscles (after the abdominal cavity). One end is close to the backbone, and the other end extends outward along the diaphragms. They are free and have no connection with the backbone. The number and arrangement of fine bones are related to the species of fish. Fine bones are commonly found in lower bony fishes, such as herring and carp fishes. The fine bones are generally difficult to be recovered alone.
2.2 Fish Head
Fish head accounts for about 16% of the total weight of the fish. The fish skull is the main part of the fish head (Fig. 1). The fish skull can be divided into two parts: the brain and the pharyngeal skull. The brain is located in the upper part of the whole skull, which is used to contain the brain, olfactory, visual, auditory, and other sensory organs. The pharyngeal skull is located in the lower part of the whole skull, which is arranged in an arc and surrounds both sides of the oropharyngeal cavity and the front of the esophagus. The fish skull is mainly composed of lamellar bones. The skull is mainly composed of a series of planar mineralized collagen fiber layers, including thick aligned fibrils and thin-layer oriented disordered fibrils. In addition, most collagen fibrils orthogonal to the lamellar plane are low mineralized (Atkins et al. 2015). In the processing, it can be removed by a decapitation machine. The fish head can be easily recovered and the integrity of raw materials can be maintained. The content of fat and connective tissue in fish head is high, so it is necessary to remove these components before extracting calcium.
2.3 Fish Scale
Fish scales are deformations of the dermis of fish skin (Fig. 1), accounting for 2%-5% of the weight of fish. Fish scales act as a protective barrier to prevent fish from being invaded by water microorganisms. The fish scale can be divided into two layers: the upper layer is the bone layer, the main component is hydroxyapatite, and some collagen fibers are scattered; The lower layer is a fibrous layer. The collagen fibers with a diameter of 70–80 nm are closely arranged in parallel in the same thin layer, and form an angle of 90° with the collagen fibers in the adjacent thin layers, thus forming an orthogonal splint structure composed of multiple thin layers (Feng et al. 2020). The method of obtaining fish scales generally adopts the mechanical descaling method, which applies a series of mechanical actions such as rubbing and stripping to separate the fish scales from the fish body. There is a large amount of mucus on the surface of fish scales, which should be cleaned with clean water before processing. At present, fish scales are mainly used as raw materials for processing collagen. The by-product of collagen production can be further used for calcium extraction and processing.
2.4 Fish Waste Mixture
In surimi processing, the fish carcass with bones is used for meat collection after the fish scales and heads are removed respectively. After the separation of bone and meat, the by-product which is the mixture of fish bone, fish skin, and other parts are obtained. In the process of surimi refine, the fish meat reaches the outside of the fine filter through the sieve hole, and the by-products of mixed connective tissue and fish fine bones are separated (Park et al. 2014). In the processing of small size fish, fish protein is extracted and separated by enzymatic hydrolysis, pH-shift, and other methods, and the mixed by-product containing fish bone and scale can be obtained.
3 Chemical Composition, Properties, and Bioavailability
3.1 Chemical Composition
3.1.1 Calcium Content
The calcium content in fish head, fish bone, fish scale, and mixture of fish processing by-product from different sources is shown in Table 1. The calcium content in these primary processing raw materials varies significantly. The calcium content in fish bone is the highest, which is 255.07–294.98 mg/g, and the calcium content in fish head is the lowest, which is 16.6–22.5 mg/g. The difference of calcium content in by-products from different sources is mainly related to the composition of mineral salt, collagen, and fat in raw materials. The mineral salt in fish head is mainly distributed in fish skull. However, the calcium content in other parts of fish head, such as fish eyes, fish brain, and fish lips, is very low. Therefore, the whole calcium content of fish head is the lowest. The calcium content of different fish species from the same source is also slightly different. Taking the calcium content in the backbone as an example, the calcium content in the backbone of cod is relatively high, while that of grass carp is relatively low, which is mainly related to the difference in the fat content in the backbone of fish.
Fish bone can be processed into fish bone powder and fish bone paste after crushing. The content of calcium in the fish bone powder prepared from the backbone of silver carp was 236.90 mg/ g, while the content of calcium in the fish bone paste was 391.09 mg/g. The difference of calcium content between the two products may be related to the different pretreatment process before crushing. After high-energy ball milling, the particle size of fish bone powder and fish bone paste can even reach nanometer level. According to Yin et al. (2015), the calcium content in nano fish bone was 236 mg/g.
3.1.2 Form and Crystalline Structure
Hydroxyapatite and calcium carbonate are the dominate calcium in fish by-product. And the content of calcium carbonate is generally less than 1%. Calcium in fish by-product raw materials and processed products mainly exists in the following forms: inorganic calcium (hydroxyapatite, calcium carbonate, calcium chloride, calcium citrate, etc.), organic calcium (peptide chelated calcium) (Fig. 2). In order to improve the bioavailability of calcium, calcium chloride and calcium citrate with high solubility are often prepared by adding hydrochloric acid and citric acid (Fig. 1). In recent years, peptide chelated calcium has become a research hotspot because of its high bioavailability. After identification, one of the peptide sequences with high calcium chelating capacity is Val-Leu-ser-Gly-Gly-thr-thr met-ala-met-Tyr-thr-Leu-Val (Peng et al. 2017). For the peptide-chelated calcium, the amino, carboxyl, and side chain groups of the peptide react with calcium ions. The carbonyl and imino groups in the peptide chain may also participate in the coordination of calcium ions, so it has high coordination rate and stability.
Hydroxyapatite (HAP), also known as hydroxyapatite, basic calcium phosphate, with the molecular formula of (Ca10(PO4)6(OH)2). The crystal structure is hexagonal system (Fig. 1), which belongs to L6PC symmetric type and P63/M space group. Its cell parameters are a = b = 0.9418 nm, c = 0.6884 nm, and theoretical ca/p = 1.67. Calcium carbonate is a white fine crystalline powder, tasteless and odorless. There are amorphous and crystalline forms. The crystalline forms can be divided into orthorhombic and hexagonal systems (anhydrous calcium carbonate is a colorless orthorhombic crystal, and hexahydrate calcium carbonate is a colorless monoclinic crystal, which is columnar or rhombic.
3.2 Physiochemical Properties
3.2.1 Solubility
Calcium compounds (mainly hydroxyapatite) in fish by-product are difficult to dissolve in water, acid, and alkaline solutions at room temperature, mainly because calcium in fish by-product is mainly deposited in the network structure formed by collagen fibers in the form of hydroxyapatite. The solubility of hydroxyapatite is 0.4 ppm, which is most stable at 25 ℃ and pH > 4.2. Its dissolution characteristics are affected by many factors, including the weight percentage of hydroxyapatite and water, the specific surface of hydroxyapatite, the pH value of the solution, and the grain defects and impurity content of hydroxyapatite.
3.2.2 Particle Size
The particle size of calcium containing particles in fish by-product is related to the way how they are crushed. Through coarsely crushing by a bone paste mill, the particle size range is 74 ~ 833 μm. The particle size ranges from 2 to 20 μm after dry ball milling. Yin et al. (2015) reported that the average particle size range of fish bone crushed by wet high-energy ball milling was 100 ~ 700 nm. The particle size determines its bioavailability and application range.
3.2.3 Potential
The zeta potential of calcium containing particles from fish by-product is negatively charged, and its value is related to the particle size. When the absolute value of zeta potential is greater than 30, the particles can be dispersed stably. It is reported that the absolute value of zeta potential in nano fish bone increases significantly from 7 to 14 mV with the decrease of particle size from 481 to 111 nm, which may be related to the release of positively charged calcium ions in fish bone particles during ball milling (Yin et al. 2015). Yin et al. (2018) prepared pure nano-hydroxyapatite particles by hydrothermal synthesis, with an absolute potential of 23.2 MV.
3.2.4 Affinity
Calcium in fish by-products mainly exists in the form of hydroxyapatite. Because of the regular hexagonal structure of the hydroxyapatite crystal plane, it can distinguish small differences in the geometric arrangement of atoms on the surface of adsorbed molecules. According to its absorptivity, it can be used as the filling material of chromatographic column to separate a variety of proteins. There are two different adsorption sites C-site and P-site on the surface of hydroxyapatite crystal. The adsorption mechanism is mainly as follows: when the OH- near the Ca ion on the crystal surface falls off instantaneously, the local area is positively charged, forming a C-site; when the local Ca ion on the surface is vacant at a certain moment, the Ca position connected with six O atoms forms a P-site. When hydroxyapatite interacts with proteins, acidic proteins are mainly adsorbed at c-sites, and basic proteins are mainly adsorbed at p-sites (Ashokan et al. 2021).
3.3 Calcium Bioavailability
3.3.1 In Vitro Test
Bioavailability of calcium products made from different fish by-products and evaluated by various methods are summarized in Table 2. Nemati et al. (2016) reported that the in vitro bioavailability of calcium in tuna bone powder (TBP) fortified biscuit and tricalcium phosphate (TCP) fortified biscuit were 38.9% and 39.5% respectively. Benjakul et al. (2017) found that the bioavailability of calcium in powder extracted from pre-cooked tuna bones decreased from 8.57% to 4.63% after calcining at 900 °C.
3.3.2 Cell Test
Idowu et al. (2019) reported that the solubility of salmon frame powders (Bio-cal-H, Bio-cal-L) and CaCO3 in the gastrointestinal tract were 7.65%, 8.41%, and 0.62% respectively. The calcium transport capacity (calcium bioavailability) through CaCo-2 monolayer was 43.02%, 38.18%, and 21.48% respectively. The results showed that salmon skeleton biological calcium could be used as a potential source of calcium supplement. Surya et al. (2021) determined different concentrations (10, 50, 100, 250 μg/ml) of n-HAP on the activity of human osteoblast (MG-63) cells. The cell proliferation rates at each concentration were 105%, 124%, 141.3%, and 125.3%, respectively. And they found n-HAP with concentrations at 50 and 100 μg/ ml obviously promoted the mineralization of osteoblast MG-63 cells.
3.3.3 Animal Test
Peng et al. (2017) studied the bioavailability of Pacific cod bone peptide-chelated calcium in rats. It was found that the apparent calcium absorption rate, calcium retention rate, and femoral calcium content were significantly higher than those of the model group and CaCO3 group, while the serum ALP was significantly lower than that of the model group. Yang et al. (2020) prepared tuna bone calcium powder by microwave method. The study showed that the calcium absorption rate of mice in the microwave fish bone powder group was 59.62%, and the calcium retention rate 58.31%, which was significantly higher than that of common fish bone meal group, calcium carbonate group, and low calcium control group. Xie et al. (2014) prepared fish bone powder and fish protease hydrolysate mixtures with low, medium, and high particle sizes. According to their results, the evaluation indexes of all calcium bioavailability of the silver carp bone powder-fish protein hydrolysate mixture were significantly higher than those of other experimental groups and calcium carbonate control and low calcium control groups. And the apparent calcium absorption rate, calcium retention rate, and bone calcium content of the mixture with the smallest particle size were the highest (47.84%, 44.94%, and 24.18%, respectively). The above results showed that the bioavailability of calcium products made from fish by-products is high. And the calcium bioavailability dependent on processing method, particle size, and composition, etc.
3.3.4 Clinic Test
Malde et al. (2010) evaluated the calcium bioavailability of cod bone, salmon bone, and CaCO3 in young healthy men by 47Ca whole-body counting method. The bioavailability of cod bone, salmon bone, and CaCO3 was 21.9%, 22.5%, and 27.4%, respectively (Table 2). The results showed that the bones of Atlantic salmon and Atlantic cod were suitable as natural calcium sources, which can be utilized as functional foods or supplements.
4 Extraction and Processing Method
4.1 Chemical Method
4.1.1 Acid Solution
The extraction and processing methods of calcium in fish by-products are summarized in Table 3. At present, acid extraction is the most widely used method, which can dissolve calcium from fish by-products. Generally, food grade acids are used, including hydrochloric acid, lactic acid, acetic acid, citric acid, malic acid, etc. Sriuttha et al. (2014) extracted calcium from tilapia bone powder using acetic acid, and obtained an extract with a concentration of 2,376 mg/ L. The factors that affect the extraction of calcium from fish by-products include the type of acid, acid concentration, temperature, time, and the ratio of by-product to acid. In addition, the size of by-product particles is also a key factor affecting the extraction efficiency. Before acid dissolution, reducing the particle size can increase the extraction efficiency. The main reason may be that the crushing process destroys the structure of collagen fiber network and hydroxyapatite crystal, and increases the specific surface area of fish bone powder, which is conducive to the release of calcium ions (Li et al. 2020).
4.1.2 Alkaline Solution
The use of an alkaline solution to remove organic molecules, particularly protein and lipids, is a useful procedure. The ash/protein ratio is an essential criterion for determining the purity of bone powder. As a result, alkaline aid solubilization may be the most practicable way for producing a high-purity fish bone powder (Hemung 2013). Nemati et al. (2016) extracted Tuna bone powder (TBP) using an alkaline process and found that the TBP was tiny in particle size, white in color, and odorless. These characteristics are ideal for use in the food fortification process.
4.2 Enzyme Method
Enzymatic hydrolysis is the preferred method over chemical hydrolysis for various reasons, including moderate reaction conditions, fewer unwanted products, and good product quality and yield. Alcalase has been discovered to be a very effective enzyme in the hydrolysis of fish proteins, because it may achieve a high degree of hydrolysis in a short amount of time under moderate circumstances. A few investigations on the enzymatic hydrolysis of fish by-products utilizing alcalase enzyme, such as pacific whiting solid waste, catfish frames, and sardinella by-product have been undertaken (Table 3). In addition to alkaline protease, papain, flavor protease, and neutral protease are also used alone or in combination to process calcium products from fish by-products. According to Jafarpour et al. (2020), enzymatic hydrolysis of cod frame (MCF) was performed using alcalase and neutrase, either singly or consecutively, to generate phosphorus and calcium-rich bone powder. After enzymatic hydrolysis, significant levels of phosphorus and calcium (330 and 583 g/kg, respectively) were recovered from the cod frame.
4.3 Physical Method
4.3.1 Calcination
Compared with chemical method and enzymatic method, calcination method is more environmentally friendly, simpler, and cheaper to process. At high temperature, inorganic substances in by-products, mainly hydroxyapatite, remain after calcination. The calcination conditions have an effect on the crystal form of hydroxyapatite. Boutinguiza et al. (2012) reported that material obtained at 600 °C is a B type hydroxyapatite. At 950 °C a biphasic material was found: biological hydroxyapatite/beta-TCP in a 87/13 ratio.
4.3.2 Grinding and Milling
Milling and grinding are common methods to prepare calcium containing products by processing fish by-products. In the process of milling and grinding, raw materials are crushed under the force of collision, extrusion, and friction. The crushing efficiency depends on the amount of energy transferred to the material per unit time. The smaller the particle size of fish bone particles obtained by crushing, the higher the calcium release rate, the smaller the adverse sensory effects, and the wider the application range (Yin et al. 2017). In the physical crushing of fish bones, there must be a limit value of the actual particle size of raw materials, which mainly depends on the tendency of product particles to re-accumulate and the dynamic balance established between accumulation and crushing. Therefore, after the particle size of raw material particles reaches the limit value, increasing the crushing time can not further reduce the particle size, but can only increase energy consumption. With the gradual decrease of the particle size of the material, the van der Waals force increases significantly, the small particles gather among the powders, and the particle size of the powders even increases with the extension of the processing time (Yin et al. 2016a). The ultimate particle size of fish bone particles is affected by the crushing mode. As so far, the particle size of fish bone crushed by wet ball milling is the smallest. Recently, Yin et al. (2015) described a method for producing Nano fish bone (NFB) slurry with an average particle size of 110 nm utilizing high-energy wet medium milling.
4.3.3 Steam Explosion
Steam explosion is an environmentally friendly and revolutionary technology. It enables high-temperature, high-pressure reactions in which steam penetrates the interior space of raw materials, followed by rapid decompression for physical treatment. In a recent study, a steam explosion pretreatment technique was developed to prepare tuna bone powder (Cui et al. 2021). Their results indicated that the median particle size (D50) of steam explosion pretreated tuna bone powder was much lower than that of normal biological calcium tuna bone powder.
5 Calcium Products and Application
5.1 Fish Bone Foods
In Southeast Asia, it is one of the important ways for people to supplement calcium source by eating small whole fish with bones, such as deep fried juvenile sardine. The backbone of larger fish (such as Pseudosciaena crocea) is cut into sections, pickled with seasoning, wrapped with bread bran and fried, which can be prepared into golden and crisp snack. These products maintain the natural form of raw materials and do not need deep processing. As a result, increasing the value of fish bones by turning them into nutritious foods is a plausible method.
5.2 Powder/Paste/Slurry
Fish by-products are dried and crushed into powder, then mixed with other main and auxiliary materials and granulated into fish or animal feed, which is the main way of processing at present. After the clean fish backbone is ground by dry method and wet method, the edible fish bone powder or fish bone paste can be obtained. Furthermore, fish bone powder or paste can be prepared into nano-scale fish bone slurry by high-energy wet ball milling (Yin et al. 2015). Fish bone powder, paste, and slurry are intermediate products of fish by-products, which can be added to fish sausage, baked foods, snack foods, tofu, and other foods. In addition to supplementing calcium in food, calcium ion released from fish bone intermediate products can also improve the texture of food by forming ionic bonds and activating endogenous transglutaminase activity (Yin and Park 2014; Khoder et al. 2020).
5.3 Calcium Supplements
After deep processing, fish by-products can be prepared into chewable tablets, soft capsules, and other calcium supplements. Their core components are generally acid soluble calcium or peptide chelated calcium. Calcium chelated with peptides is thought to be a good choice for improving calcium absorption. Several calcium-chelating peptides have recently been discovered in hoki frame, tilapia scale, and other sources. Salmon ossein oligopeptides (SOOP) were made from the bones of Atlantic salmon (Salmo salar L.). According to the report by Liu et al. (2015) that SOOP with 22 peptide sequences has a calcium-chelating capability of 52.47%.
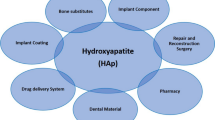
5.4 Hydroxyapatite Products
The main component of mineral salt in fish bone is hydroxyapatite (HAP). Because of its excellent characteristics, such as biocompatibility with human tissue, bone conductivity, bone induction, no inflammation and immune response, HAP is widely used as an implant material in plastic surgery, dentistry, and other fields (Boutinguiza et al. 2012). Hydroxyapatite prepared from fish by-products can also be used to make chromatographic column filler or additive because of its porous structure and special surface properties, which can nonspecifically adsorb bioactive macromolecules (Ashokan et al. 2021).
6 Production Lines of Calcium Products
6.1 Calcium Supplements
6.1.1 Calcium Capsule
The production process flow diagram of calcium soft capsule is depicted in Fig. 3. Fresh or thawed fish bones are washed to remove impurities such as lipids and blood. The fish bones are cut into about 3 cm long sections, immersed in water, and transferred to a high-pressure cooking pot. It is thermally treated for 1 h at 120 ℃ and 0.1 MPa. After that, the fish bones are rinsed with tap water 3 times to remove the adhered flesh and floating lipids. The fish bones are coarsely crushed using a grinder with a sieve plate aperture of 2 mm. The obtained bone particles are milled the first time under the conditions of plate gap 1 mm and rotating speed of 1500 rpm, and then the second time under the conditions of plate gap of 0.1 mm and rotating speed of 2000 rpm (Yin et al. 2016b).
After adjusting the water content to 90%, the fish bone paste is crushed to an average particle size of 110 nm using a high-energy media mill. The particle size of fish bone particles in the obtained slurry can be adjusted by controlling the mixing speed, media particle diameter, media filling rate and other parameters (Yin et al. 2015). Ball mill is the core equipment for processing nano fishbone. The nano fish bone slurry was mixed with other ingredients and sterilized under ultra-high pressure. The calcium soft capsule is obtained by aseptic filling with a capsule filling machine.
6.1.2 Calcium Tablet
The production process flow diagram of calcium tablet is depicted in Fig. 3. The same as above, fish bone paste is obtained after being treated by a grinder and a millstone miller. Then the paste is added with 3 times weight of water. The paste is hydrolyzed with alkaline protease (2500 U/g fish bone) at 45 ℃ for 3 h, followed by flavor protease (1000 U/g fish bone) at 40 ℃ for 3 h. The solid residue and hydrolysate are separated by centrifugation. After drying, the solid residue was superfine crushed to obtain fish bone powder with an average particle size of less than 300 nm; The hydrolysate is concentrated under vacuum pressure, freeze-dried, and superfine crushed to obtain peptide powder. The fish bone powder and peptide powder are thoroughly mixed with casein phosphopeptide powder, vitamin D3, dextrin, mannitol, sodium carboxymethyl cellulose, etc. After that, the mixture is pressed to tablet and package in plastic bottle.
6.2 HAP Products
6.2.1 HAP for Chromatographic Column
The production process flow diagram of HAP for chromatographic column is depicted in Fig. 4. First, the preparation of fish bone powder is required. Fish bones are put into a continuous steam cooker and heat at 100 ℃ for 15 ~ 30 min to remove the residual meat, oil, blood, etc. The fish bones are separated and crushed into small fish bone particles, and then immersed in a soaking tank with a temperature of 50 ℃ and a concentration of 1 M Na2CO3 solution for 5 h to completely remove the residual oil and protein in the bones. The soaked fish bone particles are transferred to the bubble cleaner through the elevator, fully cleaned to neutral pH, and then dried in an oven at 45 ~65 ℃ for 6 ~8 h. Finally, the dried fish bone particles are subjected to grinding to below 100 mesh sieve to obtain fish bone powder.
The prepared fish bone powder and water are evenly mixed in a stirring tank, and then pumped into the spray dryer through the feed pump to prepare a particle size of 0 ~15 μM hydroxyapatite microspheres. In spray drying, the particle size of the product can be controlled by adjusting the spray air pressure and the solid content of the slurry. Hydroxyapatite microspheres less than 2 μm are deposited at the bottom of a sedimentation tank to avoid plugging during subsequent column filling. Finally, the high-pressure homogenization column filling machine is used to fill the hydroxyapatite microsphere homogenate into the chromatographic column through the high-pressure pump.
6.2.2 HAP for Medical Treatment
The preparation of polylactic acid/hydroxyapatite composite powder, which is used for bone tissue scaffold, is depicted in Fig. 4. The fish bone powder prepared the same as above is calcined in a muffle furnace. It is first quickly heated up to 300 ℃ for 60 min, up to 450 ℃ for 60 min, then heat up to 700 ℃ for 60 min, and then cooled down to room temperature to obtain hydroxyapatite particles (Boutinguiza et al. 2012). The purpose of segmented calcination is to gradually remove water and organic matter from fish bone powder and increase its specific surface area. Then, polylactic acid particles and methylene chloride are added into the reactor. After the solid is dissolved, the hydroxyapatite particles are added and fully stirred, and pumped into the spray dryer by the feed pump to obtain 5 ~ 11 μm porous polylactic acid / hydroxyapatite composite microspheres.
7 Conclusion and Prospect
Fish by-products that can be used as calcium sources include fish bones, fish heads, fish frame, fish scale, and mixtures. The size, proportion, composition, recovery difficulty, and hygiene level of these by-products vary significantly, which determines their suitable uses and types of processed products. There are many reports on the content, physicochemical properties, and bioavailability of calcium compounds in fish head and fish bone, which provide a scientific basis for its application. Reducing the particle size of fish bone or calcium compounds to the nanometer level can improve their functional properties, which has become a research hotspot in recent years. The safety of these nanoparticles has gradually attracted the attention of scientists and government regulators. However, there are few related studies.
Most fish by-products exist in the form of mixtures, such as mixture containing fish bones, fish skin which is generated during the de-boning process of surimi production. However, there is little research on these mixtures. In addition, it is necessary to carry out a comparative study on the characteristics of calcium in fish by-products and calcium from other sources.
At present, the calcium compounds in fish by-products are mainly used for feed processing, which can not effectively improve the added value. Fish bone food, calcium supplements, medical and biochemical materials are the main high value-added products processed with calcium compounds in fish by-products. In the future, it is necessary to develop more high value-added products with calcium compounds in fish by-products as raw materials and realize commercialization, which would make our fisheries environmentally more sustainable and profitable.
References
Ashokan A, Rajendran V, Kumar T, Jayaraman G (2021) Eggshell derived hydroxyapatite microspheres for chromatographic applications by a novel dissolution - precipitation method. Ceram Int 47:18575–18583
Atkins A, Reznikov N, Ofer L, Masic A, Weiner S, Shahar RJAB (2015) The three-dimensional structure of anosteocytic lamellated bone of fish, 13, 311–323
Benjakul S, Mad-Ali S, Senphan T, Sookchoo PJJoFB (2017) Biocalcium powder from precooked skipjack tuna bone: Production and its characteristics’, e12412
Boutinguiza M, Pou J, Comesaa R, Lusquios F, Science BLJM, CE (2012) Biological hydroxyapatite obtained from fish bone, 32(3), 478–486
Cui Y, Yang L, Lu W, Yang H, Zhang Y, Zhou X, Ma Y, Feng J, Shen Q (2021) Effect of steam explosion pretreatment on the production of microscale tuna bone power by ultra-speed pulverization. Food Chem 347:129011
Feng H, Li X, Deng X, Li X, Guo J, Ma K, Jiang BJRA (2020) The lamellar structure and biomimetic properties of a fish scale matrix. RSC Advance 10:875–885
Hemung B-O (2013) Properties of tilapia bone powder and its calcium bioavailability based on transglutaminase assay. Int J Biosci, Biochem Bioinform 3:306–309
Huang CH, Zeng BP, Dong JB (2008) Comparison of nutrients in the head of black carp, grass carp, silver carp and bighead carp. J Hunan Univ Arts Sci (Nat Sci Ed), 20(3), 46–48,57
Huo J, Deng S, Tong G (2010) Studies on preparation of haddock calcium tablet and its biological utilization. J Fish China 34(3):382–387
Idowu AT, Benjakul S, Sae-Leaw T, Sookchoo P, Kishimura H, Suzuki N, Kitani Y (2019) Amino acid composition, volatile compounds and bioavailability of biocalcium powders from salmon frame as affected by pretreatment. J Aquat Food Prod Technol 28(1):1–9
Jafarpour A, Gomes RM, Gregersen S, Sloth JJ, Srensen A (2020) Characterization of Cod (Gadus morhua) frame composition and its valorization by enzymatic hydrolysis. J Food Compos Anal 89:103469
Jiao YY, Okada M, Hara ES, Xie SC, Nagaoka N, Nakano T, Matsumoto T (2020) Micro-architectural investigation of teleost fish rib Inducing pliant mechanical property. Materials 13:5099
Khoder RM, Yin T, Liu R, Xiong S, You J, Hu Y, Huang Q (2020) Effects of nano fish bone on gelling properties of tofu gel coagulated by citric acid. Food Chem 332:127401
Kołodziejska I, Skierka E, Sadowska M, Kołodziejski W, Niecikowska C (2008) Effect of extracting time and temperature on yield of gelatin from different fish offal. Food Chem 107(2):700–706
Lee D-Y, Oh J-H, Uhm J-T, Kim I-H, Park M-J, Moon S-H (2020) Impact of acidity regulator and excipient nutrients on digestive solubility and intestinal transport of calcium from calcium phosphate and carbonate. Food Funct 11:10655–10664
Li J, Yin T, Xiong S, Huang Q, You J, Hu Y, Liu R, Li YJ (2020) Mechanism on releasing and solubilizing of fish bone calcium during nano-milling. J Food Process Eng 43:13354
Li Y, Xiong S, Yin T, You J, Hu Y (2017) Comparative study on the physicochemical properties of six types of nano-sized fish bones. Mod Food Sci Technol 33(7):125–133
Liu Q, Liu C, Wang C (2000) Scale nitriernt composition and effect on serum lipids in experimental hypercholesterolemic rats. J J Fish Sci China 7(4):56–59
Liu W-Y, Lu J, Gao F, Gu R-Z, Lin F, Ren D-F, Cai M-Y (2015) Preparation, characterization and identification of calcium-chelating Atlantic salmon (Salmo salar L.) ossein oligopeptides. Eur Food Res Technol 241(6):851–860
Malde MK, Bügel S, Kristensen M, Malde K, Graff IE, Pedersen JI (2010) Calcium from salmon and cod bone is well absorbed in young healthy men: a double-blinded randomised crossover design. Nutr MetabIsm 7(1):61
Nawaz A, Xiong Z, Xiong H, Chen L, Wang PK, Ahmad I, Hu C, Irshad S, Ali SW (2019) The effects of fish meat and fish bone addition on nutritional value, texture and microstructure of optimised fried snacks. Int J Food Sci Technol 54(4):1045–1053
Nemati M, Kamilah H, Huda N, Ariffin FJIJoFS, Nutrition (2016) In vitro calcium availability in bakery products fortified with tuna bone powder as a natural calcium source, 67(5), 535–540
Park JW, Graves D, Draves R, Yongsawatdigul J (2014) Manufacture of surimi: harvest to frozen block. In: Park JW (ed) Surimi and surimi seafood, 3rd edn. CRC Press, Boca Raton, FL, pp 55–100
Peng Z, Hou H, Zhang K, Li BJFC (2017) Effect of calcium-binding peptide from Pacific cod ( Gadus macrocephalus ) bone on calcium bioavailability in rats, 221, 373–378
Shi L, Yin T, Huang QL, You J, Hu Y, Jia D, Xiong SB (2021) Effects of filleting methods on composition, gelling properties and aroma profile of grass carp surimi. Food Sci Human Wellness 10:307–314
Sriuttha M, Chantarangsee M, Hemung BO (2014) Characteristics of tilapia bone powder and calcium xxtraction by acid solution. Asian J Chem, 26(Supp.), S103-S106
Surya P, Nithin A, Sundaramanickam A, Sathish M (2021) Synthesis and characterization of nano-hydroxyapatite from Sardinella longiceps fish bone and its effects on human osteoblast bone cells. J Mech Behav Biomed Mater 119(2021):1–9
Wang X, Wang X, Huang Z, Liu Z, Huang T (2019) Element distribution analysis of crucian carp, silver carp and snakehead. China Brewing 38(8):163–167
Wiriyaphan C, Chitsomboon B, Yongsawadigul J (2012) Antioxidant activity of protein hydrolysates derived from threadfin bream surimi byproducts. Food Chem 132(1):104–111
Wu G, Zhang M, Wang Y, Mothibe KJ, Chen W (2012) Production of silver carp bone powder using superfine grinding technology: Suitable production parameters and its properties. J Food Eng 109(4):730–735
Xie WW, Yin T, Zhang J, Liu R, Zhao SM, Xiong SB (2014) Effects of fish bone powder particle size on calcium bioavailability of fish bone powder-fish protein hydrolysate mixture. Food Sci (In Chin) 35(7):211–216
Yang L, Wang H, Zhou X, Ma Y, Wang J, Shen Q (2020) Microwave-assisted preparation and calcium bioavailability evaluation of fishbone flour of tuna (Thunnus albacares). Food Sci 41(4):235–242
Yin ML, Zhao SK, Zheng Y, Shi SD, Sun YQ (2018) Preparation, characterization and calcium supplement efficiency of hydroxyapatite nanocrystals. Chemical Reagents 40(3):212–216
Yin T, Du H, Zhang J, Xiong S (2016a) Preparation and characterization of ultrafine fish bone powder. J Aquat Food Prod Technol 25(7):1045–1055
Yin T, Park JW (2014) Effects of nano-scaled fish bone on the gelation properties of Alaska pollock surimi. Food Chem 150:463–468
Yin T, Park JW, Xiong S (2015) Physicochemical properties of nano fish bone prepared by wet media milling. LWT Food Sci Technol 64(1):367–373
Yin T, Park JW, Xiong S (2017) Effects of micron fish bone with different particle size on the properties of silver carp (Hypophthalmichthys molitrix) surimi gels. J Food Qual 2017:1–8
Yin T, Shi L, Zhang J, Xiong SB, You J, Hu Y (2016b) Effects of processing methods on nutrition of the micro sized fish bone pastes. J Huazhong Agric Univ 35(6):124–128
Zeng SK, Zhang CH, Jiang ZH (2002) Study on the comparation of the food nutrient contents between the muscle and head of Muraenesox Cinereus. Mar Sci 26(5):13–15
Zhang J, Duan R, Chao YE, Konno K (2010) Isolation and characterzation of collagens from scale of solver carp (Hypophthalmichthys molitrix). J Food Biochem 34(6):1343–1354
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yin, T., Khalifa, I., You, J., Peng, L., Khoder, R.M. (2024). Fish Waste and By-Product as a Source of Calcium. In: Maqsood, S., Naseer, M.N., Benjakul, S., Zaidi, A.A. (eds) Fish Waste to Valuable Products. Sustainable Materials and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-99-8593-7_10
Download citation
DOI: https://doi.org/10.1007/978-981-99-8593-7_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8592-0
Online ISBN: 978-981-99-8593-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)