Abstract
To promote sustainability in the field of organic wastewater treatment, this research paper provides an insight on the usage of photocatalysts like TiO2 to degrade Methylene Blue dye (MB) in wastewater while relying on a sustainable source of energy—Ultraviolet Ray from sunlight. However, existing research indicates that despite its common usage for its favourable characteristics like high chemical stability, low cost, non-toxicity and eco-friendliness, TiO2 has limited photocatalytic activity. To solve the problem, this paper explores the usage of a cheap and non-toxic resource, activated carbon, to be doped with TiO2 to boost its photocatalytic activity. TiO2 in anatase form was mixed with activated carbon in mass percentages of 0.5%, 1.0%, 2.0% and 4.0%, then dissolved in 96% ethanol to form a paste. The prepared pastes were then applied separately onto glass slides using the doctor blade technique and calcined at 450°C to make photocatalytic films. The films were used in photodegradation experiments with MB to evaluate the photocatalytic activity of the films. Our research findings show an increase in the photodegradation efficiency as the concentration of C present in the photocatalysts increases up to 1.0%, but a decrease in photodegradation efficiency as the concentration of C rises beyond 1.0%. The photocatalysts containing 1.0% C-TiO2 presented the greatest degradation efficiency of 92.7% and hence had the greatest photocatalytic activity. This finding can be explained by band gap narrowing, Burstein-Moss effect as well as the structure and nature of activated carbon. These research findings may potentially contribute to the field of sustainable organic wastewater treatment as it offers a comprehensive reference for future research on improving the degradation efficiency of photocatalysts and provides promising opportunities for such efficient and sustainable photocatalysts to be implied in the treatment of other types of organic wastewater. This study also examines the potential improvement of the functionality and reusability of the photocatalysts.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Sustainability
- Photocatalysts
- TiO2
- Methylene Blue dye (MB)
- Photocatalytic activity
- Activated carbon
- Photodegradation
1 Introduction
The presence of man-made contaminants or pollutants within natural water resources is a very pressing issue in the field of sustainability. It not only affects the availability of potable water, but also leads to the destruction of biodiversity where water pollution depletes aquatic ecosystems and endangers the wildlife inhabiting there. Such water contaminants include organic pollutants like pesticides, pharmaceuticals and dyes.
Chemical dyes are used in various aspects like textile, paper, leather, inks and many more. Approximately 50,000 tons of dye are being discharged into global water systems from textile industries on their own [1]. They are characterised by their chemically stable and less biodegradable properties, causing them to resist degradation and last in the environment over long periods of time [2]. All these result in chemical dyes becoming one of the main contributors to water pollution worldwide.
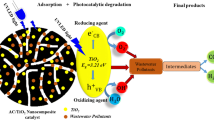
In order to prevent the detrimental impacts of chemical dye pollution on the environment, one of the advanced solutions include photocatalytic degradation. Photocatalysts have been of great interest due to its potential in degrading toxic dyes sustainably. Photocatalysts are semiconductors which utilise UV light or visible light to create electron and hole pairs. The electrons from the valence band (VB) gain energy, get excited and reach a higher energy state to the conduction band (CB), leaving behind positively charged holes at the VB. Oxygen accepts electrons from the CB and gets reduced, forming superoxide anions which are powerful reducing agents. Meanwhile, H2O molecules can donate an electron to the holes in the VB and get oxidised, forming hydroxyl radicals which are powerful oxidising agents. Both the superoxide anions and the hydroxyl radicals can then degrade various types of pollutants into smaller, non-toxic molecules during redox reactions [3].
One of the most widely investigated semiconductors is TiO2 due to its non-toxicity, high chemical stability and low cost [4]. Despite favourable properties of TiO2, it has certain drawbacks which impedes its efficiency in photodegradation. These include its wide energy band gap and high rates of electron-hole recombination [5, 6]. As a semiconductor, TiO2 possesses a relatively wide band gap of ~ 3.1 eV and usually requires UV excitation to promote an electron from the VB to the CB, generating a short-lived electron-hole pair that can take part in redox reactions [7]. There is generally low solar efficiency [8] due to the need for high energy photon activation [4]. With environmental pollution being a pressing issue, photocatalysts have to be upgraded in order to better utilise the whole solar spectrum. This can be done by doping the semiconductors to alter their chemical properties favourably. This includes causing its energy band gap to decrease and having lower rates of electron-hole recombination.
Doping TiO2 with various elements results in the formation of new energy levels near the CB, narrowing the band gap [8]. While transition metal ion-doping effectively narrows the band gap of TiO2, the metal ions may also serve as recombination centres for electrons and holes [6]. As the recombination rate increases, the overall photocatalytic activity decreases. In comparison, non-metal doping has shown to slow down the recombination of electron-hole pairs, in addition to lowering the band gap energy (Ev) [5].
Recently, activated carbon has become widely used in the treatment of wastewater from various sources as it is a low cost material, non-toxic and hence, environmentally friendly and is known to possess the ability to effectively purify and remove a vast array of contaminants from wastewaters. This particular characteristic is attributed to its physical and chemical properties. Activated carbon is porous, maximising its surface area to remove contaminants through adsorption. It has the largest useful surface area per gram in comparison to other materials available for physical adsorption [9]. In terms of its chemical properties, activated carbon is able to form instantaneous dipole-induced dipole interactions with water contaminants, allowing it to effectively adhere the pollutants to its surface while causing the pollutants to precipitate out of the solution to the carbon’s nano-sized pores or adsorption spaces. This is referred to as premature condensation, facilitated by the activated carbon [10].
As activated carbon has been effective in purifying water, doping it with TiO2 for the photodegradation of dye for water purification purposes is apt and can potentially increase its photodegradation characteristics to treat wastewater. In addition, carbon is known to be able to be easily doped with TiO2 and enhance its photocatalytic activity [5]. Hence, this study aims to find out the effects of activated carbon-doping on the degradation efficiency of TiO2. It is hypothesised that doping TiO2 with activated carbon will enhance its photodegradation efficiency, creating a more effective sustainable source in treating dye-containing wastewater.
2 Methodology
2.1 Preparation of Photocatalytic Films
Fluorine-doped tin oxide (FTO) glass slides were washed down with ethanol before being coated with different pastes of photocatalysts containing different concentrations of C. The pastes were prepared by mixing TiO2 in anatase phase, activated carbon and 96% ethanol as solvent. The pastes were then magnetically stirred for 90 min at room temperature to obtain a homogeneous consistency. The constituents of the photocatalysts are presented in Table 1.
As the percentage of C by mass in the photocatalyst paste had been increased, the mixture had become increasingly visibly dark in colour. Hence, the maximum percentage of C by mass in the photocatalyst had been chosen to be 4% as a greater percentage of C in the mixture led to a very dark mixture. This would prevent sufficient UV light from being absorbed by the semiconductor for UV light irradiation to occur.
The prepared pastes were then applied separately onto the glass slides using the doctor blade technique [8]. This method is easily employable as a fast and non-energy consuming technique for mass production of thin films with good uniformity and reproducible properties. The surface area of 1.5 cm2 was defined using masking tape, which acted as a spacer to obtain films of approximately the same thickness. Finally, the photocatalyst pastes were calcined at 450 °C for 1 h. This calcination temperature and period were used as it had been obtained as the most ideal through a series of experimentation. At this set of calcination conditions, the calcined photocatalytic films were found to be the most well adhered to the glass slide with minimal disintegration when placed in the MB solution.
Photodegradation Experiments
The photocatalytic efficiency of the photocatalysts was evaluated through their degradation of Methylene Blue dye (MB).
Aqueous MB solution was made by mixing deionized water with solid MB. The initial MB solution was found to have a maximum light absorbance of 1.068 at 658 nm. The 5 photocatalytic films were placed into 15 ml of the MB solution in clear beakers, after which were placed at an equal distance from a UV lamp. One additional beaker containing the same amount of MB solution that underwent UV light irradiation without any photocatalyst served as a control set up. The degradation efficiency of each of the photocatalysts can be determined by the rate of degradation of the MB dye by the photocatalytic films which in turn, can be determined by the rate of decrease of MB concentration in the samples. The intensity of light absorbed by the solutions was measured using a spectrophotometer at time intervals of 0.5 h, 1 h, and 2 h. This data was used to determine the percentage change in concentration of MB in the solutions as according to the Beer-Lambert law, the concentration of MB in the solution is directly proportional to the light absorbance by the solution.
The following equation can be derived:
where [MB0] represents the initial concentration of MB in the sample, [MBt] represents the concentration of MB in the sample after t hours of reaction, A0 represents the initial light absorbance of the sample, and At is the light absorbance of the sample after t hours of reaction.
3 Results and Discussion
From Figs. 1 and 2, it can be derived that after 2 h of UV light irradiation, the samples with photocatalysts had a lower MB concentration than the sample without, hence proving the functionality of all the photocatalysts. The calculated degradation efficiency of the photocatalysts is summarised in Table 2.
Based on Fig. 1, the MB solution degraded by 1.0% C-TiO2 (MB/C-TiO2) had the lowest light absorbance after UV light irradiation for 0.5 h, 1 h and 2 h respectively. It had the lowest light absorbance of 0.078 after 2 h of UV light irradiation. These indicate that 1.0% C-TiO2 has the highest photocatalytic efficiency.
Based on Fig. 2, as the concentration of C present in the photocatalysts increases up to 1.0%, the photodegradation efficiency of the photocatalyst increases. However, as the concentration of C rises beyond 1.0%, the photodegradation efficiency decreases. 4% C-TiO2 has a lower degradation efficiency than pure TiO2, with a degradation efficiency of 76.217% compared to 80.150% respectively.
As experimental factors that affect photocatalytic activity such as pH, temperature, oxygen concentration, humidity, amount of photocatalyst, surface area of the photocatalytic films, initial concentration of MB and intensity of UV light provided were kept constant for all the set-ups, the difference in degradation efficiency would be solely due to the different concentrations of C present in the photocatalysts.
Improved degradation efficiency of 0.5% C-TiO2 and 1.0% C-TiO2 from pure TiO2 can be explained as carbon doping of TiO2 modifies the band gap of the semiconductor. Many researchers have attempted to reason this. It has been proposed that the introduction of an impurity, activated carbon, provides a new impurity energy level higher than the VB but lower than the CB, resulting in a significant narrowing of the band gap and lowering of band gap energy values [4, 11]. Based on our findings, we propose that the increase in the concentration of C present in the photocatalyst allows for the presence of more energy levels, resulting in a broader VB and CB.
The new impurity energy formed is due to the activated carbon causing distortion to the initial giant ionic lattice structure of TiO2, causing carbon defects like the new impurity energy level to be introduced between the band gap of TiO2 [3]. This results in a lower amount of energy required to excite the valence electrons and promote them from the impurity level to the CB, allowing for more photogenerated electron-hole pairs to be produced.
Moreover, the high porosity of activated carbon, resulting in its high adsorbent characteristic, has potentially allowed more of the MB to agglomerate on the surface of the doped photocatalyst. This allows the photocatalyst to effectively photodegrade the MB under UV light irradiation due to the accumulation of the dye onto the surface of the photocatalyst.
Photocatalysts with higher C concentration were visibly darker in colour. Hence, as the percentage of C increases above the optimal concentration of 1.0%, it is proposed that the amount of light being absorbed by the semiconductor reduces significantly despite the lowered band gap energy. This results in a decreased production of electron-hole pairs. Another possible reason for the observation is that increasing the dopant concentration above the optimal level could have resulted in an increased rate of recombination of electrons from the conduction band to the wider and higher impurity energy level due to the very small energy gap. This reduces the rate of formation of electron-hole pairs, decreasing the rate of redox reactions occurring for the degradation of MB [10].
Additionally, the decrease in photocatalytic activity can be explained with the Burstein-Moss effect. It suggests that the optical band gap of degenerately doped semi- conductors increases when all states close to the CB get populated due to shifting of an absorption edge to higher energy [12, 13]. Due to initial effect or band gap narrowing, more electrons are being promoted to the CB. This only increases as the concentration of C increases. After the concentration of C increases beyond the optimal, the Burstein-Moss effect becomes more significant and competes with the band gap narrowing. The optical band gap of 4.0% C-TiO2 had increased beyond that of pure TiO2 and hence overall resulted in less electrons being promoted to the CB. With fewer electron-hole pairs being generated, there is a slower rate of redox processes taking place, resulting in a slower rate of photodegradation.
A similar research was conducted by another group of researchers [10] under a slightly different set of conditions and reported a similar trend. They tested the photodegradation efficiency of different concentrations of carbon-doped TiO2. 5 photocatalyst samples were synthesised with different concentrations (0, 0.5, 1, 2 and 4 atomic %) of carbon using glucose (C6H12O6) as source material was used for doped TiO2 nanoparticles. The photocatalysts were prepared using a different methodology and were tested under solar light to photodegrade MB solutions. The research results demonstrated that all the C-TiO2 composite samples exhibited greater photocatalytic activity than pure TiO2. The carbon content influences the photocatalytic activity markedly under solar light. The photocatalytic activity of the catalyst increases with the increase in content of carbon and then decreases as carbon concentration increased beyond 2%. This concentration is the optimum condition to achieve the synergism between carbon content and TiO2 and is due to small crystallite size and low recombination rate of electrons and holes at the surface of TiO2.
4 Conclusion
This study evaluates the photocatalytic activity of C-TiO2 with different concentrations of activated carbon through the photodegradation of MB. From this study, we have found that the optimal percentage of activated carbon to dope TiO2 is 1.0% as it shows the highest degradation efficiency of 92.697% after 2 h of reaction. There is an increasing trend of degradation efficiency as C concentration increased up to 1.0% but a decreasing trend beyond the optimal concentration. 4.0% C-TiO2 showed a lower degradation efficiency than pure TiO2.
The photodegradation experiments were carried out under UV light irradiation. Future works can be carried out to explore the functionality of the photocatalysts under sunlight irradiation in order to assess the effects of the alterations being made to the band gap energy on photon absorption. Improvements to photocatalysts can be done through a change in preparation methods which may further affect the band gap as well as morphology.
Additionally, we had decided to create photocatalytic films instead of using powder, as we had considered the practicality of removal of photocatalyst when being used to photodegrade large amounts of dye for water purification purposes. Carbon doped photocatalytic films with improved photodegradation efficiency would allow for the process of water purification to be quicker and more efficient. The photocatalytic films can be used in industrial settings to purify contaminated water before disposal into the environment, reducing the water pollution. Future work should improve the making of photocatalytic films to allow for reusability and minimal disintegration.
References
Person.: Fabric dyeing = death water bodies: Hazardous effects of textile dyes. Fabric of the World. Retrieved December 25, 2022, from https://www.fabricoftheworld.com/post/fabric-dyeing-death-water-bodies-hazardous-effects-of-textile-dyes (2020, Feb 12)
Olusakin Oladoyeab, P., Oladiran, T., Oyinkansola, A.E.,Olusola, O., Joel, O.: Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results in Engineering. Retrieved December 31, 2022, from https://www.sciencedirect.com/science/article/pii/S2590123022003486 (2022, Sep 30)
Khan, I., Saeed, K., Zekker, I., Zhang, B., Hendi, A. H., Ahmad, A., Ahmad, S., Zada, N., Ahmad, H., Shah, L. A., Shah, T., Khan, I.: Review on methylene blue: Its properties, uses, toxicity and photodegradation. MDPI. Retrieved December 31, 2022, from https://www.mdpi.com/2073-4441/14/2/242 (2022, January 14)
Piątkowska, A., Janus, M., Szymański, K., Mozia, S.: C-,N- and S-Doped TiO2 photocatalysts: a review. Catalysts 11(1), 144 (2021). MDPI AG. https://doi.org/10.3390/catal11010144
Akhter, P., Arshad, A., Saleem, A., Hussain, M.: Recent development in non-metal-doped titanium dioxide photocatalysts for different dyes degradation and the study of their strategic factors: a review. Catalysts 12(11), 1331 (2022). https://doi.org/10.3390/catal12111331
Yalçın, Y., Kılıç, M., Çınar, Z.: The role of non-metal doping in TiO2 photocatalysis. J. Adv. Oxidation Technol. 13(3) (2010). https://doi.org/10.1515/jaots-2010-0306
Pitre, S.P., Yoon, T.P., Scaiano, J.C.: Titanium dioxide visible light photocatalysis: Surface Association enables photocatalysis with visible light irradiation. Chemical communications (Cambridge, England). Retrieved December 31, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5657382/#:~:text=Titanium%20dioxide%20is%20an%20inexpensive,(400%E2%80%93700%20nm (2017, April 13)
Sampaio, M.J., Silva, C.G., Marques, R.R.N., Silva, A.M.T., Faria, J.L.: Carbon nanotube–TiO2 thin films for photocatalytic applications. Catal. Today 161(1), 91–96 (2011). https://doi.org/10.1016/j.cattod.2010.11.081
Khlyustova, A., Sirotkin, N., Kusova, T., Kraev, A., Titov, V., Agafonov, A.: Doped TiO2 The effect of doping elements on photocatalytic activity. Materials Advances. Retrieved December 31, 2022, from https://doi.org/10.1039/D0MA00171F (2020, June 15)
Nowicki, H.: (n.d.). The basics of activated carbon adsorption | water technology. The basics of activated carbon adsorption. Retrieved December 29, 2022, from https://www.watertechonline.com/wastewater/article/15549902/the-basics-of-activated-carbon-adsorption
Hussain, S., Khan, N., Gul, S., Khan, S., Khan, H.: Contamination of water resources by food dyes and its removal technologies. IntechOpen. Retrieved December 26, 2022, from https://www.intechopen.com/chapters/70455 (2022)
Alkorbi, A.S., Javed, H.M.A., Hussain S., Latif, S., Mahr, M.S., Mustafa, M.S., Alsaiari, R., Alhemiary, N.A.: (n.d.). (rep.). Solar light-driven photocatalytic degradation of methyl blue by carbon-doped TiO2 nanoparticles
Gahlawat, S., Singh, J., Yadav, A.K., Ingole, P.P.: Exploring Burstein-Moss type effects in nickel doped hematite dendrite nanostructures for enhanced photo-electrochemical water splitting. Phys. Chem. Chem. Phys. 21(36), 20463–20477 (2019). https://doi.org/10.1039/C9CP04132J
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Safa, F.T., Goh, S. (2023). Investigating Photocatalytic Activity of Carbon-Doped TiO2 in the Treatment of Dye-Containing Wastewater. In: Lu, J., et al. Proceedings of the 9th IRC Conference on Science, Engineering, and Technology. IRC-SET 2023. Springer, Singapore. https://doi.org/10.1007/978-981-99-8369-8_46
Download citation
DOI: https://doi.org/10.1007/978-981-99-8369-8_46
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8368-1
Online ISBN: 978-981-99-8369-8
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)