Abstract
Biomaterials refer to materials used in direct interaction with biological systems. Biocompatible and surface-modifiable metallic, polymeric, and ceramic biomaterials are currently used in various applications, allowing for adjustments to improve their performance while maintaining their bulk properties. Surface modification is done to enhance biocompatibility and come into contact as a bioactive substance for certain applications, such as protein surface modification on ceramics, polymers, metals, and composites. Surface modification techniques include calcium phosphate deposition, covalent binding of poly (ethylene glycol) and poly (heparin), and plasma polymerization. Analyzing the surface chemistry, structure, morphology, and topography of biomaterials is crucial in surface modification to improve interactions with blood, fight infection, interact with soft tissues, repair and regenerate nerve cells, manage stem cell growth and differentiation, and interact better with bone. Biomedical devices that can replace or repair damaged tissues and organs depend heavily on the usage of substances that can interact with people's bodies without triggering negative reactions. Although joint replacement surgery is a standard procedure, the inserted biomaterial may not last for a long time. Factors such as unfavorable immune system responses, the development of biofilms, or issues with the implants’ fabrication, biocompatibility, manufacturing processes, and their mechanical, chemical, or tri-biological processes may lead to their failure. Altering the surface of biomaterials can prevent these failures and improve the way the body responds to their implantation. Thus, the current chapter aims to show novel methodologies and applications of surface-modified biomaterials in the development of medical devices. It suggests novel studies on extending the lifespan of medical equipment and biomaterials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In various fields of medicine, there has been extensive use of biomaterials throughout a significant duration, and their perception has varied over time. Williams gave the first comprehensive explanation of biomaterials in 1987, describing them as “Non-living materials utilized in healthcare devices with a focus on interacting with biologically systems”. These substances had to be risk-free, non-carcinogenic, chemically and physiologically stable, and mechanically robust enough to withstand repeated loads during their existence [1]. As advancements in the applications and compositions of biomaterials progressed, these definitions were modified to incorporate these developments [2, 3]. The following examples illustrate the evolution of biomaterial definitions.
-
“Synthetic substance utilizes to modify a component of a living system or to interact closely with living tissue.”
-
“A pharmacologically and systemically inactive material intended for integration with or implantation among biological systems.”
-
“A nonviable substance employed in a medical apparatus meant to communicate with biological systems.”
-
“Materials that interact with biological fluids such as blood, tissues, and fluids have been developed that can be used in prosthetic devices, diagnostics, therapeutics, and storage without negatively impacting a living organism and its constituent parts. These materials come from both synthetically produced and naturally occurring substances.”
-
“Any chemical (apart from a medicine) or mixture of compounds, whether artificial or of natural origin, that can be utilized to treat, improve, or replace any tissue for any period of time, either in its overall terms or as a system component.”
Any substance designed by the manufacturer for a medical purpose, whether used alone or in conjunction, including a piece of equipment, an implant, a machine, a material, reagent, software, or other related item, is referred to as a medical device. Medical devices have become valuable tools in disease diagnosis, therapy, prevention and screening, and palliative care and are widely used in various settings, including at home by individuals, in remote clinics by paramedical staff and clinicians, by dentists and opticians, and in advanced medical facilities by healthcare professionals [4]. Recent advancements in manufacturing techniques have made it possible to produce medical devices using different materials (composites, metals, polymers, and ceramics). However, living tissues are sensitive to material surfaces which initiates several immune reactions (inflammation, rejection, and infection).
Long time ago understood that a medical device's success depends on how well it interacts with the host tissues. Significant advances in material science have been made as a result of extensive research into this interface issue, with the goal of enhancing the interface in biological and medicinal applications [5]. The ability to modify a surface of a device in order to give it particular physicochemical properties has emerged as a workable way to produce desired biological reactions. One major benefit of surface modification is that it only affects the device surface, resulting in minor modifications to the devices’ bulk physical and/or chemical properties and easier regulatory approval processes. Regardless of the materials’ classification, numerous techniques have been established to alter their surfaces to maintain control biological reactions and enhance device performance.
These techniques are utilized to lessen protein adsorption, slow down osseointegration, increase electrical conductivity, and improve wear and resistance to corrosion [1]. The two major types of surface modifications are (i) physicochemical modifications, which involve chemical reactions (oxidation, reduction, salinization, acetylation), etching, mechanical roughening, and changes to the surface's atoms, compounds, molecules, or topography, and (ii) surface coatings (noncovalent, covalent, and thin-film coating), which use materials other than the underlying support that bind the biomolecules [6]. The techniques of surface modification are widely recognized as being easily adopted by existing researchers and medical device companies due to its exciting multi-disciplinary opportunities.
This chapter's main emphasis is on surface modification of biomaterials utilized with devices for medical purposes, since it is currently increasing popularity as a flexible method of creating custom device interfaces. The chapter is structured to begin by going over the criteria for choosing biomaterials to have their surfaces modified and the techniques for doing so, then to look at how biomaterials and their surfaces interact, subsequently various approaches and thorough procedures for surface modification are described, along with legal and ethical concerns. The application of surface-modified biomaterials in medical devices is addressed in the final section, along with the current gaps and potential remedies.
2 Selection of Biomaterials for Surface Modification
Biomaterials have been chosen for surface modification in biomedical applications based on bulk characteristics such as non-toxicity, free from corrosion, minimal degradation, elastic modulus, and wear resistance. A functional group must be attached to the surface in order to change the surface's wettability, sealability, printability, dye uptake, glazing resistance, adherence to different materials, and interaction with the environment's biological organisms, among other biomaterial barrier qualities [7]. Due to the body's extremely sensitive chemical stability, implanted materials ought to possess bio-inert properties to avoid rusting and the unintended release of metallic ions. The selection of biomaterial in various therapies as shown in Table 1.
3 Provision for Biomaterial Surface Modification
Due to their higher affinity for a wider range of proteins than others, albumin, fibrinogen, IgG, fibronectin, and Von Willebrand factor make up the majority of plasma proteins found in utilized biomaterials. The process of protein adsorption and interaction can change the shape of the biomaterial that is produced. Especially fibrinogen, which becomes more adhesive when in contact with biomaterial or implant surfaces, can cause denaturation of biomaterials. In order to prevent neoepitope exposure after cell and tissue interactions and preserve the biomaterial for the duration of its intended duration of storage, it is crucial to change the biomaterial's surface.
Protein adhesion, cell disruption, and inflammatory response are all significantly influenced by the surface chemistry and structure of biomaterials. For instance, in vitro cell adsorption can be produced by changing the surface chemistry of a biomaterial. However, traditional chemistry is not a requirement for the in vivo mechanisms of externally applied biomaterials. Typically made of polymers, ceramics, metals (cobalt-chrome, titanium), and other materials, biomaterials have a variety of surface properties that affect how they react in vivo, ranging from hydrophilic to hydrophobic and tough to soft. In order to construct highly friendly biomaterial architectures, a variety of different techniques, including physical modifications, chemical alterations, and radiation, are employed to alter the surface of biomaterials.
Surface modification methods that significantly affect protein adsorption and biological responses in vitro include those that modify polymer chemistry, wetting capacity, and domain. Additionally, it has been demonstrated that protein adsorption and cellular responses are significantly influenced by the structure and design of biomaterials. Several topical experiments have made use of methods for changing surfaces that have been mentioned in the literature, such as plasma therapy, chemical graft modification, and self-assembled monolayer methods [14]. This instance of a foreign body reaction is typical of how cells in higher organisms react to artificial biomaterials that have been inserted.
4 Interaction of Biomaterial at the Surface
The method that tissues and the implant surface interact is always varying. Within the initial several seconds following implantation, dissolved ions, unbound biomolecules, and water are everywhere over the implant surface. Figure 1 illustrates how the bio fluid surrounding the wound changes in composition and a layer of biomolecules is adsorbing to the surface to begin the healing process. The adsorbed layer then controls how the cells behave once they reach the surface. Eventually, a tissue integration or fibrous capsule will form as the types of cells and their activity on the surface alter. The atomic, molecular, and higher-level physical textural properties operate as contact points for biological elements like proteins, cells, tissues, etc. At the same time, it's crucial to understand that the biomolecules react to the chemical activity of the implant surfaces.
The various bonding modalities connected to each of these biological units have an impact on how a surface is integrated hierarchically into its bonding environment. Chemical species that start distinct levels of bonding have an impact on adhesion qualities. A component experiences various chemical processes depending on the surroundings, which makes it more difficult to understand the specific nature of the interactions [15, 16].
5 Different Techniques Used for Surface Modification
Surface modification involves using physical, chemical, and mechanical techniques to modify or coat a material's surface, resulting in desired properties that differ from the original material. In regenerative medicine and the engineering of tissues, activating the surface is essential to allow for the binding of adhesive biomolecules to scaffolds. Achieving high binding efficiency requires selecting an appropriate technique to conjugate biomolecules or biomaterials onto scaffold surfaces, considering factors such as the presence of reactive functional groups, hydrophobicity, and hydrophilicity. Recent years have seen significant attention given to chemical and physical methods due to their effectiveness in achieving high binding efficiency, while mechanical modification techniques are widely used because of their strong impact on cell adhesion and growth. The various surface modification techniques and different types of cell adhesion molecules that can be used to modify scaffold surface characteristics [17].
Surface modification of biomaterials is performed to create a particular physical and chemical environment that favorably influences the biological response in either soft tissue or hard tissue. Macro, micro, and even nano-scale elements should all be present in the physical environment that promote cell adhesion, proliferation, and migration in situations when tissue integration is required. The functionality of various devices, such as articulating surfaces or cardiovascular apparatus, might occasionally be negatively impacted by textured surfaces, it is crucial to note [18]. The different techniques of surface modification are shown in Fig. 2.
5.1 Mechanical Methods
5.1.1 Blasting
Blasting is a common method utilized for sterilizing surfaces, roughen surfaces, or make surfaces smooth. It involves applying harsh, abrasive particles to metal surfaces. In biomedical devices, such as orthopedic implants, the blasted surfaces must be biocompatible. Therefore, ceramic particles like titanium, alumina, or hydroxyapatite are commonly used for blasting [19, 20]. Blasting can produce rough surfaces that strengthen the bond between implants and bone, promoting osseointegration. The chemical makeup of the surface may change as a result of blasting, which could have an impact on biocompatibility.
Medical device surfaces need to be handled carefully to keep them biocompatible after treatment. In addition, nano-rough surfaces can be created by blasting, which are far more resistant to corrosion, wear, and corrosive wear than conventionally grained or sandblasted surfaces. A further approach for producing micron, submicron, and nanostructures on metal surfaces is laser ablation. It has some benefits, including the ability to use it on geometrically difficult implants and a minimal risk of contamination. On titanium implants, laser ablation can make nanoscale grooves that greatly improve osseointegration and increase the extent to which bone develops when the treated implants were in connect with it. Additionally, surfaces that have been ablated by lasers may be extremely hydrophobic and selectively inhibit bacterial adhesion.
5.1.2 Grinding System
The expected electrical grinding system and the grinding's electrochemical operations. In this process, an electrode is positioned above a metallic bond grinding wheel that is conducting, with a gap of around 0.3 mm, or nearly 1/6 the size of the surface of the wheel. The electrode is given a negative potential, while the grinding wheel is given a positive potential. It employs an expert pulse generator. The potential during the process electrolytically breaks down the conductive alkaline machining fluid used in the grinding procedure, producing hydroxide ions. When the work piece receives the proper amount of positive potential, free hydroxide ions (OH-) are attracted to the surface being processed by substances, which are present in the machining fluid. This emphasizes the ions to the surface, which results in the formation of a stable oxidized layer. For our investigation, we used test pieces made of type 316 stainless steel and the Ti6AI-4V alloy, each of which is frequently used as metallic biomaterials. A grinding wheel with a metal-resin-hybrid bond (#8000, diamond abrasives, with a diameter of approximately 2 microns) was used. [21].
5.2 Chemical Methods
5.2.1 Sol-Gel Method
For the purpose of developing biomaterials for drugs, the sol-gel method is a straightforward, wet chemical process that doesn't necessitate a high pH or high sintering temperature. The term “sol-gel coating” refers to the colloidal dispersion of solid particles (1–500 nm) in a liquid solution. The techniques of spraying, dipping, spinning, and doctor-blading can be used to apply a sol to a substrate. To create the top layer, the substrate gel is dried or calcined. By immersing a metal sample in a calcium and phosphorus gel at lower temperatures, calcium phosphate layers are created on the sample using this approach. The covering coating is calcined at 400–600 °C depending upon the material since the layer produced is porous and less dense [22].
5.2.2 Anodization
Anodizing is a process that is widely used to treat titanium and its alloys. It is similar to acid etching. In this procedure, the substance is dissolved in an ionic solution of H2SO4, H3PO4, or acetic acid while being exposed to anodic voltage. The process results in the surface of titanium and its alloys developing a thicker oxide layer, increasing their corrosion resistance. However, because this oxide layer is porous, sealing is frequently needed. On titanium substrates, titanium nanotubular structures can develop when fluoride-containing electrolytes are employed. The voltage, electrolyte concentration, and reaction time are some of the anodization parameters that significantly affect the shape and structure of these nanotubular layers.
Researchers have looked into how these anodized structures affect bacterial adherence, cell function, and bone formation. Studies have demonstrated that compared to normal flat titanium substrates, anodized surfaces with titanium nanotubes of 40–60 nm in diameter significantly increase adhesion and proliferation of bone marrow cells. Additionally, calcium deposition on anodized substrates is significantly higher than on conventional titanium substrates. S. aureus and S. epidermidis are two bacteria that are impacted by the diameter of the nanotubes’ effects on bacterial adhesion to nanotubular titanium surfaces. The lowest bacterial adherence is seen on surfaces with 80 nm diameter nanotubes, which are less rigid than ordinary flat surfaces or nanotubular surfaces with tube diameters of 20, 40, and 60 nm.
5.2.3 Chemical Vapor Deposition (CVD)
Numerous commonly used formal techniques include CVD, low-pressure CVD, molecular beam epitaxy, and deposition of physical layer procedures like heat evaporation and laser ablation deposition. It is a technique used to deposit thin films onto substrates using gas-phase chemical processes. The more contemporary methods include sputtering, plasma atomic layer deposition, and plasma-enhanced CVD. In order to construct electronic devices that use electricity, magnetism, and photonics, including their utilization in biological contexts, these procedures are useful for depositing metal, semiconductor, and dielectric layers.
5.3 Mechanical Methods
5.3.1 Thermal Spray Technique
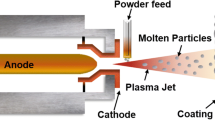
The thermal spray technology is rapidly evolving, with exciting developments focused on innovative uses of coatings. One area of particular interest is the application of coatings in cutting-edge energy generation processes, biomaterials, electronic-based features, and self-disinfecting surfaces enabled by photocatalysis, electrolysis, and various other applications. These advancements in thermal spray technique are driving progress in multiple directions and hold great promise for the future. [23].
5.3.2 Ion Implantation and Deposition
Ion implantation and deposition, a method that includes bombarding a substrate with high-energy ions to modify its surface properties. The deposition technique is a crucial step in micro- and nanofabrication. This process involves adding a layer of material to a bare silicon wafer to prepare it for subsequent lithography steps. Deposition can be performed using different methods, including plasma-based and non-plasma-based techniques.
5.3.3 Electrophoretic Deposition
The process of movement of charged particles in the electrolytic solution is necessary for the electrophoretic deposition (EPD) process, which has been intensively investigated. In an electric field, ceramic particles acquire charges whether they are in an aqueous or non-aqueous medium. When the initial surface oxide layer provides sufficient protection, depositing nanoparticles onto the surface of a material can enhance its resistance to wear and corrosion, particularly in the case of titanium implants. EPD system is used to achieve this, with a suspension containing uniformly dispersed powder particles in an aqueous or non-aqueous medium, as well as the appropriate anode and cathode electrodes. Hence, better deposition is achieved with a homogenous particle dispersion that possesses a suitable conductivity and medium dielectric constant [24].
6 Methods of Surface Modification of Biomaterial
Different methods with their advantages and disadvantages of biomaterials used in medical devices as shown in Table 2.
6.1 Metallic Biomaterials
The widespread usage of metallic materials in biomedical technology is a result of their favorable mechanical and chemical characteristics. Stainless steels, cobalt-chrome, the metals titanium, and their alloys are among the most widely utilized. In load-bearing implant applications, these metals can be alloyed to tune attributes among the most often utilized metals are titanium and its alloys. to satisfy biological requirements. Surface modification techniques can be employed to enhance biocompatibility, reduce osseointegration time, and prevent corrosion without altering bulk properties.
Stainless steel (SS 316L), titanium alloy (Ti-6Al-4V), and cobalt-chromium (Co-Cr) alloy have attracted the most interest for orthopedic applications among the reported metallic biomaterials. It has been attempted to alter the bulk characteristics of metallic biomaterials to have mechanical properties similar to those of native bone in order to lessen stress shielding at the interface between tissue and metallic biomaterials. The recommended material for dental implants in dental applications is commercially pure titanium (CPTi). because of its better properties and the escalating cost of Pd. Titanium and its alloys have low Young's modulus, which is seen as a biomechanical advantage for replacing hard tissues. In cardiovascular implants, certain alloys, such as nickel-titanium alloy (Nitinol), have drawn increased interest in magnetic resonance imaging (MRI) due to their inert, robust, and non-magnetic qualities [25, 26].
6.1.1 Steel
Since stainless steel has a high level of corrosion resistance and strength, it is frequently utilized for biomedical implants. To keep the nickel content low, nickel-free stainless steel is used, and nitrogen is added to increase biocompatibility. Despite stainless steel's lower biocompatibility and corrosion resistance compared to titanium, temporary bone fracture therapies and implant studies frequently employ it since it is more affordable. Custom-sized stainless-steel implants can be created using 3D printing, and 3D dental implants can be created using liquid phase sintering.
Implants composed of pure metals, which had lesser corrosion resistance and mechanical strength, had been developed before stainless steel entered the biomedical business. The two types of stainless steel utilized in biomedical applications are standard stainless steel and Ni-free stainless steel, which is devoid of nickel. Stainless steel is widely used for bone fracture treatments, such as screws, nails, and fracture plates, and for fabricating durable implant trials. Compared to titanium, stainless steel has inferior biocompatibility, osseointegration, and corrosion resistance [27].
6.1.2 Titanium and Ti-Based Alloys
The Titanium and Ti-based alloys are well suited for usage with a high pace of loading than stainless steel for the reason that they have a higher strength-to-weight ratio. In comparison to steel, titanium implants have a significantly stronger bond with tissues because of the titanium dioxide layer's high dielectric constant, which forms quickly on the surface of bare titanium. It may increase the mechanical durability of titanium alloy by annealing, quenching, and thermal aging, and it can be further modified by alloying other elements like aluminum and niobium. For functionally demanding anatomically complex locations like cranio-maxillofacial surgery, 3D-printed titanium implants can be tailored.
Techniques for melting electron beams and directly sintering metal have been used to create customized titanium prostheses. To reduce stress shielding, porosity might be added to the implant's structure. To reduce stress shielding and encourage tissue regeneration or vascularization, porosity might be added to the implant's structure. The structure is rich in interconnected grooves and has 3D Ti-6Al-4V dental implants’ Young's modulus gradient changes from 104 GPa at the metal inner core to 77 GPa for the very porous outer shell when gradient porosity is generated utilizing the selective remain sintering procedure [28, 29].
6.1.3 Cobalt-Based Biomaterials
Since Co-Cr alloys have a higher wear resistance compared to Ti alloys, they frequently used in prosthetic hip joints. Although they are less biocompatible and less capable of osseointegration than Ti, their higher elastic modulus and stiffness result in higher stress shielding compared to Mg, Ti, and Ti alloys. Ti is frequently utilized in clinical settings for components higher stress shielding compared to Mg, Ti, and Ti alloys, while Co-Cr is the preferred material for components that do not come into contact with the bone [30]. At the point where Co-Cr and Ti come into contact, metal corrosion and shredding remain serious problems.
6.1.4 Bio Implants Based on Tantalum
Tantalum has been studied for usage in a range of applications where outstanding durability against corrosion even under acidic conditions and biocompatibility in biomaterials are required. Tantalum's ability to resist corrosion is because a protective coating of natural, stable Ta2O5 forms over the implant surface. The functioning of porous tantalum to connect with bone makes it a promising material for use in artificial joints as a polymeric matrix or includes a coating on titanium and implants are constructed from stainless steel to improve osseointegration and prevention from corrosion [31]. Tantalum has a density of 16.6 g/cm3 and an elastic modulus of more than 186 GPa Because they are significantly different from native cortical (12–18 GPa) and cancellous bone (0.1–0.5 GPa) in these respects, these features are detrimental when used in orthopedic implants.
It is challenging to produce this metal in large quantities due to its refractory properties, especially given its unusually high melting point of approximately 3017 °C. Due to Ta's high temperature conductivity, patients who undergo cranioplasty may also experience temperature-dependent headaches. To meet the growing need for orthopedic applications, metallic bioimplants have been developed using cutting-edge manufacturing techniques. An efficient method to create implants of the proper size may be discovered through a three-dimensional visualization of the patient and printing of the modified implant. Laser sintering is a technique that can be used to produce high-fidelity copies of pre-packaged implants using reasonably priced 316L stainless steel. Another use of liquid phase sintering is the fabrication of stainless-steel 3D dental implants.
6.1.4.1 Current Challenges with Metals
The utilization of 3D printing in numerous industries, including building, aerospace engineering, and clothing, has significantly advanced the discipline. The development of sophisticated multi-material scaffolds for tissue regeneration and the fulfillment of the needs of personalized medicine in the medical and healthcare sectors are all made possible by 3D printing, which has the potential to revolutionize tissue and organ engineering. [32]. It is clear that a key aspect of 3D printing is its capacity to produce implants that are exactly suited to the anatomy of the patient. This is especially true in surgeries to treat craniofacial ruptures or fractures, for children with dwarfism, or in cancer patients, when the implant is tailored to match the excised tissue and may lessen pressure exerted on the existing bone compared to a travail-customized implant.
However, combining these two approaches offers the most promise for personalized therapy [33]. The model is then used to provide data for the implant's high-accuracy quick prototyping, as well as to prescribe the implant's macroscopic features and give the material a desired structure. An example of this is the modification of porosity, in which the dimensions, orientation, size, and connectivity of the pores may be changed to promote the formation of capillaries, the ingrowth of tissues, and the supply of nutrients to support the developing tissues. Theoretically, printing many materials at once would allow for the construction of complex objects like tissues and organs utilizing a single technique. Metals, synthetic polymers, glass, ceramics, active molecules like proteins and factors, and living cells and organic materials can be found in one particular structure. [34].
The excessive structural rigidity of Co-Cr alloys can be mitigated by employing 3D printing to include nano- and micro-geometry into the bulk of the alloy. This lessens the stiffness differential between the alloy and bone and lowers the elastic modulus. He was able to effectively create his Co-Cr implants with the appropriate macro-geometry and interconnected pore architecture using his EBM, the preferred method for his 3D printing of Co-Cr alloys. After 26 weeks in adult sheep femurs, the implants were shown to have satisfactory overall bone-implant connection. The implant's surroundings gradually underwent tissue ingrowth and densification, and the generated bone's mineral crystallinity, apatite to collagen ratio, and carbonate to phosphate ratio differed between Co-Cr and Ti-6Al. It was in the range of 4 V implants [35].
Printing implants, both solid and mesh, is possible using EBM. Co-29Cr-6Mo alloy mesh-strut and foam-ligament microstructures both produced columnar-directed Cr23C6 precipitate architectures that were spaced about 2 m apart in the build direction to represent the directional solidification of solid cylindrical components during the assembly process in the melt pool. While similarly manufactured solid Ti-6Al-4V implants displayed acicular platelets in the - phase, mesh and foam implants largely displayed residual 0-martensitic phase. It displayed a fine cellular microstructure with enriched Mo and depleted Co grain boundaries when the CoCrMo implant was created using SLM. This provides the implant more corrosion resistance than a cast alloy by reducing carbide precipitation and the creation of the Martensitic phase on its surface. These characteristics, which also limit the discharge of metal ions into the peri-implant environment, lower the possibility of developing metallosis. Rates of ion release and corrosion were correlated with the number of laser melt pool borders [26].
There are many technological obstacles that are keeping 3D printing from developing. The presence of a vascular network poses the toughest challenge from a biological standpoint. An adequate supply of nutrients, correct gas exchange, and effective waste disposal are necessary for the growth of 3D tissues or organs during perfusion; these processes would not occur in the absence of such a network. As a result, the artificial organ's overall efficacy and cell longevity would be affected. However, there is still a significant obstacle to overcome before a system can successfully deliver nutrients, growth hormones, and oxygen to the cells without interfering with their metabolic activities [36].
Despite multiple attempts by researchers to develop vascular trees using computer models, it has not yet been possible to produce fractured veins with branched channels that are advantageous mechanically. For instance, electro-hydrodynamic inkjet printing (e-jetP) has the ability to generate prints with far higher resolution than traditional inkjet printing techniques. By utilizing an electric field to overcome surface tension, a metallic nanoparticle solution, such as Ag, Cu, Au, or Co, is evacuated as droplets in e-jetP [37]. Rapid layer-by-layer assembly with fine control over the form, dimension, and resolution of micro- and nano-scale features is made possible by accelerated solvent evaporation. Although there are currently few macroscopic implants that utilize this technology, these forms may be ideal for implantable electrodes and sensors [38].
6.1.4.2 Using Additive Manufacturing (AM) Surface Modifications on Metallic Biomaterials
For all metallic devices to perform their function efficiently and to endure for a long time, AM-based surface changes of metallic biomaterials are essential [39]. Passivating materials (Al, Cr, Ti) are added, as well as surface energy, topography, and crystalline structure to increase osseointegration, biocompatibility, and prevent corrosion without affecting the bulk properties of implants [40, 41]. Although including roughness to implant surfaces may reduce their mechanical properties, it can improve tissue integration [42].
It has been demonstrated that porous materials with purposeful topology promote adhesion and advantageous differentiation. Based on computer tomography (CT) images, AM is used to build biomimetic surface structures that have substantially identical surface characteristics to those found in living cells. The most prevalent technique for preventing corrosion is the incorporation of self-passivating materials (Ti, Cr, and AL). Implant of the lifespan is increased by the desirable wear qualities of some alloys, such as CoCrMo, as opposed to softer metals like titanium. But even these artificial limbs can become worn down gradually, releasing cobalt and chromium ions that can lead to metallosis and osteolysis. Degradation of UHMWPE, which leads to the creation of microparticles, might come from the incompatibility of hardness between the metal and the polymer.
6.2 Ceramic Biomaterials
Ceramics are utilized in various medical fields such as dentistry, orthopedics, and medication delivery. They are very helpful in the construction of bone scaffolds for the vital repair of fractures and other injuries in which the normal healing capacity of the bone is compromised. Ceramics are also used in dental procedures for enamel and root replacement, bone cement for minor fractures, ceramic-on-ceramic articulating surfaces for joints, and the hip, knee alignment, and shoulder area implants as coatings healing, and other drug release applications [43]. Ninety-nine percent of the calcium in the human body is found in bones and teeth, making it the fifth most common element in the body. The fact that many biomedical ceramics are calcium-based is therefore not surprising. Both bone and teeth are mostly made of ceramic materials, which have the right chemical makeup and crystallography for these uses.
Due to the similarities in chemical and crystal structure, as well as in micro- and nano topography, biomimetic devices are made that replicate the appearance and functionality of biological tissue, thereby preventing inflammation or rejection brought on by immune reactions to foreign objects [44]. The additional benefit of biomimicry is that it breaks down into ions and calcium phosphates the fact that the body is able to rapidly resorb or excrete. Whether used as a restorable material, degradation has a minor impact, and the rate of product absorption can be modified in accordance with the speed of healing of the tissue by altering the surface, the structure of the crystals, or the calcium phosphate phase. Calcium phosphate scaffolds have a high degree of elemental resemblance to actual bone in applications that come into touch with it, although they lack trace elements like Si4+, Mg2+, Sr2+, Zn2+, Cu2+, and Fe2+/Fe3+, among others. Recent studies have concentrated on ionic elements that are naturally present in bone that can be added to bone scaffolds. Further improving the biomimetic features of ceramics in biomedical applications are the ionic’ roles in angiogenesis, osteogenesis, and osteo induction [45]. Different ceramic materials used for surface modification of biomaterials are given below.
6.2.1 Alumina
Alumina is a bio-inert material with excellent immunological compatibility, good biocompatibility, and protection against rust. It is utilized in joint prosthesis, including the acetabulum and femoral heads in hip arthroplasty on worn surfaces due to its strong mechanical resistance to wear and polish ability. Two components are often polished together throughout the manufacturing process to achieve the best possible agreement between them. This lessens the production of harmful residues for the patient as well as friction between joint components. In order to attain lower wear values, it is also typical to attach an alumina femoral head with an ultra-high molecular polyethylene (UHMWPE) acetabular component.
6.2.2 Zirconia
With the use of proximal femur, zirconia was first examined as a biomaterial in the late 1960s. The combination of zirconia and yttrium, known as Tetragonal zirconia polycrystals, often known as TZP (tetragonal zirconia polycrystals), is the most common widespread after testing various chemical composition possibilities and is most frequently utilized in femoral heads [46]. It is one of the ceramic materials used in prosthetic equipment most frequently because of its mechanical and chemical qualities. It combines with oxygen and produces the biocompatible zirconia oxide (ZrO2) as a result. Due to their great biocompatibility, good flexural strength, and fracture resistance, ZrO2 implants are mostly employed in femoral heads for hip arthroplasty and dental implants.
6.2.3 Hydroxyapatite
Apatite crystals constitute the minerals found in bone tissues. The chief inorganic constituent responsible for the development of bones and teeth is a high hardness calcium phosphate salt, known as HA. Due to its chemical makeup and structural similarity with natural HA, HA ceramic exhibits exceptional biocompatibility.
6.2.4 Carbon
Carbon implants have an advantage over metallic implants in that they do not experience fatigue. However, because of their fragility and low tensile strength, they are not suitable for situations that necessitate support for substantial loads. Although it is legal to use it in blood-contact devices, it is still a good idea to double-check.
6.2.5 Bio-Glass and Vitro-Ceramic
A versatile and varied class of biomaterials, bio-glass has various capabilities depending on its composition. In order to generate a substance that might adhere to bone, Larry Hench originally developed it in 1969. A type of glass commonly known as “Bioglass” or “45S5” is composed of 46.1 mol% SiO2, 24.4 mol% Na2O, 26.9 mol% CaO, and 2.6 mol% P2O5. These biomaterials are used in prosthesis when highly hard tissues are required, such as in dentistry. Vitro-ceramic glass is ideal for application in dental implants. and bone development due to its strong mechanical qualities, good biocompatibility, bioactivity, and lack of toxicity. There currently exist a number of variations of the original 45S5 composition, each of which has a particular function or use as a biomaterial based on its chemical make-up.
6.2.5.1 Current Challenges with Ceramics
Because they perform better than metals and polymers in terms of compressive strength and corrosion resistance, additively produced ceramics are mostly used in dental applications. However, resorbable drug delivery systems are the main focus of surface modification of ceramics through AM. The low breakdown temperature of the additional material is one of the major difficulties, which makes it challenging to sinter an AM ceramic loaded with pharmaceuticals or biomolecules. The ceramic powder particles are held together by a binder, but they are nevertheless prone to bulk disintegration and the early release of loaded molecules.
6.2.5.2 Using AM Surface Modifications on Ceramics Biomaterials
In order to achieve consistent release profiles and ensure implant stability in vivo, surface modification techniques can be employed. One such approach is to introduce layers of polymeric coatings to the surface to prevent the sudden release of medication. Another technique involves coating ceramics with polymers and dosing them with desired medicine after calcination to regulate drug elution. Trace elements have also been utilized as additives or dopants to accelerate bone development and vascularization. When the drug delivery system (DDS) is subcutaneous or gastrointestinal, several binder formulations have been employed to create components with a bulk interior chemistry and a less soluble, polymer-enhanced outer surface chemistry. [47]. Mesoporous silica/bioglass scaffolds can be surface-modified to control the release profile of medications that have been adsorbed. The potential of surface-modified 3D-printed composite scaffolds was established in a study looking at bone replacement therapy for patients needing surgical bone removal due to osteoarticular pulmonary disease. These printed scaffolds are intriguing for further investigation in the area of bone treatment since they can compete with CaP scaffolds while maintaining a consistent drug release profile.
6.3 Polymeric Biomaterials
Polymers are extensively utilized as biomaterials in the field of biomedical engineering for various purposes, such as cartilage and bone repair, articulating surfaces for hips, knees, and shoulders, drug delivery systems, skin coatings for burn treatment, and optical applications. One of the most impressive qualities of polymers is their ability to biodegrade into components that can be processed by the body. Biodegradable polymers like polyethylene glycol/oxide (PEG/PEO), polylactic acid (PLA), polyglycolic acid (PGA), tri-methyl carbonate (TMC), and polydioxanone (PDS) are frequently employed for this purpose [48,49,50]. Medical applications utilize a variety of polymers, including polyethylene (PE), polyamide (PA), polymethyl methacrylate (PMMA), polyurethane (PU), polyethylene terephthalate (PET), synthetic rubber (SR), polytetrafluoroethylene (PTFE), polystyrene (PS), polylactic acid (PLA), and polyglycolide (PGA). The foundation for composites such as PEEK reinforced with glass or carbon or a variation like UHMWPE can be made from some of the above-mentioned polymers.
Latex, cellulose, gums, and starch are naturally occurring polymers, synthetic and semi-synthetic polymers with biodegradable or non-biodegradable polymers are the three categories of polymers now in use. Syringes and other disposable medical equipment, orthopedic uses, contact lens and corneas, sutures, cardiovascular devices, membranes, dental reconstruction, and adhesives are just a few of the medical fields where polymers are commonly used. It is also very important to consider the degree of crystallinity when choosing a polymer for medical purposes. The three states of polymers in this situation are amorphous, crystalline, and semi-crystalline [26].
6.3.1 Natural Polymers
Natural rubber latex is a colloidal system best recognized for its use in dental tools, condoms, and other medical supplies. Extensive research and documentation have been conducted on allergies to natural rubber latex. However, a recently developed manufacturing technique that avoids the use of carbamates or sulfur has resulted in a more biocompatible product. This development has opened up new possibilities for using the product as a delivery system for drugs, proteins, and nanoparticles, as well as for aiding in the controlled formation of bones and the healing of soft scar tissue [26].
6.3.2 Synthetic Polymers
Polyolefins are plastic resins that are produced from either propylene or ethylene, depending on the type of polyolefin. These plastics are hydrophobic and inert, meaning that they do not break down in the body. Polyethylene, or PE, is a type of polyolefin that comes in five different variants: high-density polyethylene (HDPE), low-density polyethylene (LDPE), linear low-density polyethylene (LLDPE), ultra-low-density polyethylene (ULDPE) and ultra-high-molecular weight polyethylene (UHMWPE). PE can be processed by various methods such as blowing, extrusion, and injection molding, utilized in sutures and netting and is physiologically inert. UHMWPE, which is one variant of PE, is commonly used for artificial joint surfaces because of its high impact strength, chemical stability, and good biocompatibility. However, the release of particles due to the friction of the components can cause reactions in the body that can lead to mechanical failure of the prostheses.
To resolve this issue, highly cross-linked polyethylene has been used, and the material is treated to gamma radiation to improve its biocompatibility and wear resistance. Antioxidants like vitamin E were added to the biomaterial to produce the second generation of cross-linked UHMWPE. Numerous medical fields, including orthopedics, cardiology, and neurology, use UHMWPE. Some examples of products made of UHMWPE are surgical cables for bone fractures, high-strength orthopedic sutures for soft tissue repair, catheters, stent grafts, heart valves, and disc replacements for spinal repair. Similar to polyethylene, polypropylene is a type of polyolefin that is biologically inert and used to manufacture nets and sutures.
6.3.3 PVC
It needs to be handled carefully when used in medical applications. The application of stabilizing and plasticizing agents causes the receptor to become poisonous in an unwanted way. It is typically utilized in catheters, blood bags, and tubes.
6.3.4 Silicones
These are hydrophobic, exhibit without additionally the application of plasticizers, biological stability and depending on their intended use, elicit completely different biological reactions in the host. It is frequently utilized in ophthalmological applications, breast implant encapsulation, and the induction of an inflammatory response in the synovial membrane in intra-articular implants. Hepatic cancers have been linked to silicone oil residues.
6.3.5 Acetal
It is a hard plastic substance made from formaldehyde that is highly durable and has a low coefficient of friction. This makes it a useful material for machining to create prototypes of medical devices and parts. Acetal is susceptible to the radiation used in sterilization, so it should be kept in mind that this could make the material brittle and easily break.
6.3.6 Polyamides
The most widely used synthetic polyamide used in medicinal uses is nylon. Due to its great tensile strength, it is utilized in suture lines. A composite material of nylon and PU with nylon strength and elasticity makes up the balloons of the catheters used in angioplasty. Recent studies have concentrated on synthetic polymers with assurance for use in soft and hard tissue regeneration, such as polycaprolactone (PCL), polypropylene fumarate (PPF), polyether ether ketone (PEEK), and functionalized polyurethanes [50, 51]. Furthermore, due to their attraction as naturally occurring biopolymers or their degrading properties, natural polymers including collagen and fibrin as well as polysaccharides like alginate, silk, hyaluronic acid, and chitosan have been investigated for biomedical uses.
Collagen, the most common protein and naturally occurring polymer in the ECM, is commonly used for surface modification. Cell mobility is supported and adhered to by collagen. The less biodegradable and even permanent polymers are polytetrafluoroethylene (PTFE) and poly (methyl methacrylate) (PMMA). PMMA, with its excellent optical properties, is used for contacts and intraocular lenses. However, foreign body reactions can occur, leading to the need for surface modification [52]. PMMA is used frequently in bone cement to anchor and secure implants. In particular, the difficulties of enhancing ocular compatibility and maintaining the duration of implantable ocular devices, the organ's shape, and the primary physiological roles of the component tissues influence the choice of biomaterials for ocular implants.
6.3.6.1 Current Challenges with Polymeric Biomaterials
Co-polymerization allows for greater customization of a polymer's chemical and mechanical properties, including biodegradability, toughness, and stiffness. To meet the requirements of each application, a different composition is used. Devices like sutures or bone screws, for instance, require stronger toughness or higher modulus, and include a rate of degradation which is comparable to the rate of tissue healing. Due to its excellent biocompatibility and ease of modifying its degradability, poly lactic-co-glycolic acid or PLGA), which degrades compared to formulations with a greater glycolide concentration, significantly more slowly, is one of the most often used polymers. Polymers frequently have high surface energies that make implants less wettable and without charges that promote cell and protein attachment. Its restricted bioactivity may hinder cell adhesion, prolonging the healing process, and perhaps causing the development of fibrotic tissue.
6.3.6.2 Using AM Surface Modifications on Polymeric Biomaterials
Polymeric biomaterials have limited biomedical applications due to the lack of surface characteristics. While AM techniques can modify hatch and strut dimensions, they minimal changes to surface energy. However, through nano- and micro-topography, crystallography and modifications to chemical composition including AM can be used to modify surface energy. For instance, type I collagen was found to promote more effective bone replacement than CaP or CaP/5% wt.% collagen scaffolds when it was added to the binder of a ceramic 3DP process [53]. Similarly, according to plasma or other chemical methods to attach functional groups can increase cell adhesion and proliferation, but are lengthy and require post-processing [54]. AM can create drug delivery systems with specific surface chemistries, topographies, and morphologies, which affect the rate of device resorption as well as the rate at which the drug dissolves into the tissue throughout it. The increased surface roughness can also increase the fixation of the implant [55].
6.4 Composite Biomaterials
A composite material is a material formed by combination of two or more resources with the aim of achieving properties that are greater than those of the individual constituents. The composite material consists of a base material, the reinforcement, and a matrix. For instance, carbon fibers can be added to a polymer to increase its hardness, mechanical strength, and fatigue resistance. Bioenergetics and bioactive ceramic materials are often used to produce composite materials that improve mechanical strength and bioactivity. For example, zirconia and hydroxyapatite (HA) can be used in combination to enhance HA's mechanical qualities while maintaining the ability to attach to bone tissue. Ceramic coatings are applied to metal-based composite implants to increase biocompatibility, strengthen the implant, and facilitate the growth of bone on its surface.
Ceramic reinforcements in polymer matrix implants, including HA, bio-glass, or calcium phosphates, can boost biocompatibility and raise the elastic modulus of the base substance, resulting in the mechanical properties of implants a closer resemblance to those of bone. This reduces the likelihood of stress shielding. Additionally, unlike metal implants, polymer-based composite materials have advantages over them, such as the lack of corrosion and toxicity brought on by the release of nickel or chromium metal ions, which could cause allergic reactions in patients. When compared to metal alloys and ceramic materials, polymer composites are suitable for diagnostic procedures like computerized tomography and magnetic resonance imaging (MRI), and do not obstruct radiographs [22].
6.4.1 Ceramic-Ceramic Composites
As comparison to HAP alone, biocomposites with a bioactive glass layer offer higher adhesion and mechanical strength while retaining the bioactivity of HAP. The Ti-6Al-4V with bioglass-apatite coatings have been the subject of numerous research, which have revealed better mechanical properties. Particularly, ZrO2 and Y2O3 composites on Ti-6Al-4V have shown enhancement on mechanical characteristics and increased bioactivity [42, 43]. It has also been discovered that bioceramic-free composites, like porcelain wollastonite, have improved mechanical characteristics. Also, an animal investigation using composites of HAP and bio-inert alumina demonstrated excellent osseointegration with bone [56, 57].
6.4.2 Polymer-Ceramic Composites
Metal oxides like ZnO, Al2O3, TiO3, and SiO2 are examples of inorganic materials that can be coupled with novel, high-performance materials. Thanks to research on polymer (organic)-inorganic composites. To enhance adhesion and microstructural homogeneity, a homogeneous PEEK/bioactive glass composite coating was created for NiTi. The PEEK polymer, which was present in the composite, provided the material with good resistance to chemical and tribological effects as well as high strength, according to the researchers [58]. The different materials used for manufacturing biomaterials for medical devices are as shown in Fig. 3.
7 Sterilization of Medical Devices and Biomaterials
Sterilization is a significant development in the production of many medical devices, as most devices must be free of living organisms and pyrogens to ensure patient safety. However, the choice of sterilization method is often not given due consideration during product development, and it is sometimes assumed that the process will be straightforward. There are many sterilization methods available, including traditional techniques such as autoclaving and ethylene oxide (EO) sterilization, as well as newer technologies like gamma irradiation and sterilization utilizing gas plasma at low temperatures. Every technique has both advantages and disadvantages, and no one approach works for all kinds of medical equipment [59].
Sterilization can affect the physical and chemical characteristics of biomedical polymers, and can lead to the production of toxic degradation products. Some sterilization methods, such as autoclaving, may not be suitable for certain types of biomedical polymers due to changes in mechanical characteristics or the creation of degradation products. EO sterilization is widely used in industry due to its ability to kill bacteria, spores, and viruses at low temperatures, but it has drawbacks such as residual gas and toxicity. Gas plasma sterilization is effective at sterilizing many materials, but it uses oxidative chemicals that can damage some biomedical elastomers [60]. In conclusion, the choice of sterilization method is an important consideration in the development of medical devices. Each method has its own benefits and drawbacks, and it is essential to carefully consider the sterilization's effect on chemical and physical characteristics of biomedical materials [61].
8 Regulatory Issues Regarding Biomaterial in Medical Device
The advancements in additive manufacturing (AM) technology, particularly in creating nanoscale features and interacting with the human body, have opened up a range of possibilities [62, 63]. However, the increasing use of nanomaterials in AM also raises new concerns related to safety and regulation. The safety risks associated with nanomaterials are still not fully understood, in particular when these materials come into interaction with membranes and body fluids. Nanoparticles have been found to pose a specific risk owing to their extensive surface area, which can significantly alter the means by which chemical reactions react with their surrounding atmosphere.
Additionally, these particles can pass through defenses like the blood-brain barrier because they are small enough. Regulatory restrictions are required for AM materials that come into touch with the human body in order to address these risks. The FDA has recognized the value of personalized medicine and the potential of AM in the next years. Devices developed using AM methods were to be subject to a set of regulatory standards to be established by the FDA. In May 2016, the FDA released a drafting guidance for 3D-printed medical devices that included its most recent opinions on device design and testing [64, 65].
The primary obstacle the business is now facing is acquiring FDA certification for the materials used in additive manufacturing. There needs to be minimal variance in mechanical attributes from build to build and testing criteria need to be established. There are now just three ASTM standards in use for testing materials made via additive manufacturing. Additionally, the FDA is responsible for ensuring that patient-specific devices, also known as objection-of-care products, adhere to regulatory standards. These devices, along with those used for pharmaceutical and cell treatment produced via AM, are not yet covered by the drafted guidelines [25].
9 Future Prospectives
Today's implantable devices, although being the ultimate achievement of biomaterial science and the outcome of numerous years of research, struggle with the same challenges that have only permitted implants to be implemented as a support to non-surgical or grafting approaches. Despite being in its early stages, additive manufacturing has already caught the attention of biomaterials researchers as a fresh and potent tool for creating new devices and modifying the basic manner that we approach design. The future prospects of surface modification of biomaterials for medical devices are extremely promising. There is a growing emphasis on developing novel approaches for functionalization by grafting techniques, which can improve the performance of medical devices at the interfaces of biomacromolecules, cells, tissues, and biomaterials.
One of the key areas of focus for future research is the development of surface modification methods that can enhance the biocompatibility of medical devices [66,67,68]. This is especially crucial in the case of implantable medical devices, where poor biocompatibility can lead to complications such as implant rejection or infection. Novel approaches such as plasma-induced graft polymerization and photo-induced graft polymerization hold great promise in this regard. Another important area of future research is the development of surface modification methods that can improve the antimicrobial properties of medical devices. This is particularly important given the growing problem of antibiotic resistance, which makes it difficult to treat infections that arise in the context of medical device implantation.
However, more in-depth studies are necessary to address several issues in the future. Novel approaches such as ozone graft polymerization and radiation-induced graft polymerization may offer potential solutions in this regard. In addition to the creation of novel surface modification approaches, future research in this field is likely to focus on achieving greater precision and control over the grafting process. This may involve the use of advanced analytical techniques to better understand the mechanisms underlying surface modification, as well as the invention of new materials and scaffolds that are more amenable to surface modification.
Overall, the future of surface modification of biomaterials in medical devices with the continued innovation and investment in this field, it is likely that we will see significant advancements in the design and performance of medical devices, leading to better patient outcomes and improved quality of life for individuals around the world. As global attention on medical implants and devices increases, the technology behind surface modification of polymeric biomaterials is expected to extend to an industrial level, opening up a wider range of biomedical applications [69, 70]. With continuous progress in this field, it is reasonable to believe that various biomedical applications will finally be achieved. However, more in-depth studies are necessary to address several issues in the future.
10 Conclusion
In conclusion, while implantable devices have come a long way through years of research in biomaterial science, they still have drawbacks that limit their application in comparison with non-surgical or grafting techniques. However, the emerging technology of additive manufacturing (AM) has the capacity to revolutionize the way we think about design and develop novel devices. By allowing for direct control over both bulk and surface characteristics in the three-dimensional space, AM enables the use of materials that were previously unsuitable for certain applications. Additionally, surface modification methods such as nanometer-scale electrochemical anodizing have the potential to improve cellular interactions and protein adhesion in biomedical applications. With AM, we can look forward to personalized implants being created for each patient that are reliable, match their demands, and become the standard treatment for the many people throughout the world who are afflicted with illness and strategy that leverages.
Abbreviations
- CVD:
-
Chemical vapor deposition
- EPD:
-
Electrophoretic Deposition
- Co-Cr:
-
Cobalt-Chromium
- MRI:
-
Magnetic Resonance Imaging
- CT:
-
Computer Tomography
- UHMWPE:
-
Ultra-High Molecular Polyethylene
- DDS:
-
Drug Delivery System
- PEG/PEO:
-
Polyethylene glycol/oxide
- PLA:
-
Polylactic acid
- PGA:
-
Polyglycolic acid
- TMC:
-
Tri-Methyl Carbonate
- PDS:
-
Polydioxanone
- PE:
-
Polyethylene
- PA:
-
Polyamide
- PMMA:
-
Polymethyl methacrylate
- PU:
-
Polyurethane
- PET:
-
Polyethylene terephthalate
- SR:
-
Synthetic Rubber
- PTFE:
-
Polytetrafluoroethylene
- PS:
-
Polystyrene
- PLA:
-
Polylactic acid
- PGA:
-
Polyglycolide
References
Patel, N.R., Gohil, P.P.: A review on biomaterials: scope, applications & human anatomy significance. Int. J. Emerg. Technol. Adv. Eng. 2(4), 91–101 (2012)
Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E.: Biomaterials Science: An Introduction to Materials in Medicine. Elsevier (2004)
Kulinets, I.: Biomaterials and their applications in medicine. In: Regulatory Affairs for Biomaterials and Medical Devices, pp. 1–10. Woodhead Publishing (2015)
Martin, J.L., Norris, B.J., Murphy, E., Crowe, J.A.: Medical device development: the challenge for ergonomics. Appl. Ergon. 39(3), 271–283 (2008)
Madalina Mihai, M., Maria Holban, A., Giurcaneanu, C., Gabriela Popa, L., Mihaela Oanea, R., Lazar, V., Carmen Chifiriuc, M., Popa, M., Ioan, P.M.: Microbial biofilms: impact on the pathogenesis of periodontitis, cystic fibrosis, chronic wounds and medical device-related infections. Curr. Top. Med. Chem. 15(16), 1552–1576 (2015)
Agarwal, R., García, A.J.: Surface modification of biomaterials. In: Principles of Regenerative Medicine, pp. 651–660). cademic Press (2019)
Fabbri, P., Messori, M.: Surface modification of polymers: chemical, physical, and biological routes. In: Modification of Polymer Properties, pp. 109–130. William Andrew Publishing (2017)
Merola, M., Affatato, S.: Materials for hip prostheses: a review of wear and loading considerations. Materials 12(3), 495 (2019)
Abraham, A.M., Venkatesan, S.: A review on application of biomaterials for medical and dental implants. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 237(2), 249–273 (2023)
Li, S., Wang, S., Liu, W., Zhang, C., Song, J.: Current strategies for enhancement of the bioactivity of artificial ligaments: a mini-review. J. Orthop. Transl. 1(36), 205–215 (2022)
Milinkovic, D.D., Zimmermann, F., Balcarek, P.: Medial patellofemoral ligament reconstruction using nonresorbable sutures yields comparable outcomes to reconstruction with a pedicled quadriceps tendon autograft when performed in addition to bony risk factor correction. Knee Surg. Sports Traumatol. Arthrosc. 31(1), 264–271 (2023)
Wang, Y., Li, G., Yang, L., Luo, R., Guo, G.: Development of innovative biomaterials and devices for the treatment of cardiovascular diseases. Adv. Mater. 34(46), 2201971 (2022)
Bui, H.T., Khair, N., Yeats, B., Gooden, S., James, S.P., Dasi, L.P.: Transcatheter heart valves: a biomaterials perspective. Adv. Healthcare Mater. 10(15), 2100115 (2021)
Raval, N., Kalyane, D., Maheshwari, R., Tekade, R.K.: Surface modifications of biomaterials and their implication on biocompatibility. In: Biomaterials and Bionanotechnology, pp. 639–674. Academic Press (2019)
Kurella, A., Dahotre, N.B.: Surface modification for bioimplants: the role of laser surface engineering. J. Biomater. Appl. 20(1), 5 (2005)
Steckbeck, S.: Experimental Analysis of Interactions between Biomolecules and Inorganic Surfaces. Doctoral dissertation, Bremen, Universität Bremen, Diss. (2014)
Amani, H., Arzaghi, H., Bayandori, M., Dezfuli, A.S., Pazoki-Toroudi, H., Shafiee, A., Moradi, L.: Controlling cell behavior through the design of biomaterial surfaces: a focus on surface modification techniques. Adv. Mater. Interfaces 6(13), 1900572 (2019)
Bose, S., Robertson, S.F., Bandyopadhyay, A.: Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 15(66), 6–22 (2018)
Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P.: Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications. Springer, Berlin (2001)
Ahirwar, H., Zhou, Y., Mahapatra, C., Ramakrishna, S., Kumar, P., Nanda, H.S.: Materials for orthopedic bioimplants: modulating degradation and surface modification using integrated nanomaterials. Coatings 10(3), 264 (2020)
Ohmori, H., Katahira, K., Nagata, J., Mizutani, M., Komotori, J.: Improvement of corrosion resistance in metallic biomaterials using a new electrical grinding technique. CIRP Ann. 51(1), 491–494 (2002)
Callister, W.D.: Fundamentals of Materials Science and Engineering. Wiley, London (2000)
Priyadarshini, B., Rama, M., Chetan, V.U.: Bioactive coating as a surface modification technique for biocompatible metallic implants: a review. J. Asian Ceram. Soc. 7(4), 397–406 (2019)
Gibson, I.R., Bonfield, W.: Preparation and characterization of magnesium/carbonate co-substituted hydroxyapatites. J. Mater. Sci. Mater. Med. 13(7), 685–693 (2002)
Kulkarni, M., Mazare, A., Schmuki, P., Iglič, A., Seifalian, A.: Biomaterial surface modification of titanium and titanium alloys for medical applications. Nanomedicine 111(615), 111 (2014)
Festas, A.J., Ramos, A., Davim, J.P.: Medical devices biomaterials—a review. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 234(1), 218–228 (2020)
Lara, N., Neto, A.F., da Mota, A.J., de Souza, A.E., Brito, A.G., Da Silva, A.C., Martinez, A.C., Nozaki, A.P., da Silva, A.A., de Vasconcelos Ferraz, A., Moraes, Â.M.: Engenharia de Materiais: materializando o futuro. Pimenta Cultural (2022)
Soni, R., Pande, S., Kumar, S., Salunkhe, S., Natu, H., Hussein, H.M.: Wear characterization of laser cladded Ti-Nb-Ta alloy for biomedical applications. Crystals 12(12), 1716 (2022)
Su, Y., Luo, C., Zhang, Z., Hermawan, H., Zhu, D., Huang, J., Liang, Y., Li, G., Ren, L.: Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 1(77), 90–105 (2018)
Devgan, S., Sidhu, S.S.: Evolution of surface modification trends in bone related biomaterials: a review. Mater. Chem. Phys. 15(233), 68–78 (2019)
Bandyopadhyay, A., Mitra, I., Shivaram, A., Dasgupta, N., Bose, S.: Direct comparison of additively manufactured porous titanium and tantalum implants towards in vivo osseointegration. Addit. Manuf. 1(28), 259–266 (2019)
Hojjatzadeh, S.M., Parab, N.D., Yan, W., Guo, Q., Xiong, L., Zhao, C., Qu, M., Escano, L.I., Xiao, X., Fezzaa, K., Everhart, W.: Pore elimination mechanisms during 3D printing of metals. Nat. Commun. 10(1), 3088 (2019)
Ni, J., Ling, H., Zhang, S., Wang, Z., Peng, Z., Benyshek, C., Zan, R., Miri, A.K., Li, Z., Zhang, X., Lee, J.: Three-dimensional printing of metals for biomedical applications. Mater. Today Bio. 1(3), 100024 (2019)
Sing, S.L., Tey, C.F., Tan, J.H., Huang, S., Yeong, W.Y.: 3D printing of metals in rapid prototyping of biomaterials: techniques in additive manufacturing. In: Rapid Prototyping of Biomaterials, pp. 17–40. Woodhead Publishing (2020)
Liu, X., Chu, P.K., Ding, C.: Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R. Rep. 47(3–4), 49–121 (2004)
Mondy, W.L., Cameron, D., Timmermans, J.P., De Clerck, N., Sasov, A., Casteleyn, C., Piegl, L.A.: Computer-aided design of microvasculature systems for use in vascular scaffold production. Biofabrication 1(3), 035002 (2009)
Setti, L., Fraleoni-Morgera, A., Ballarin, B., Filippini, A., Frascaro, D., Piana, C.: An amperometric glucose biosensor prototype fabricated by thermal inkjet printing. Biosens. Bioelectron. 20(10), 2019–2026 (2005)
Farahani, R.D., Dubé, M., Therriault, D.: Three-dimensional printing of multifunctional nanocomposites: manufacturing techniques and applications. Adv. Mater. 28(28), 5794–5821 (2016)
Stevens, M.M., George, J.H.: Exploring and engineering the cell surface interface. Science 310(5751), 1135–1138 (2005)
Elfick, A.P., Green, S.M., Krikler, S., Unsworth, A.: The nature and dissemination of UHMWPE wear debris retrieved from periprosthetic tissue of THR. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Japan. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 65(1), 95–108 (2003)
Roy, M., Balla, V.K., Bandyopadhyay, A., Bose, S.: MgO-doped tantalum coating on Ti: microstructural study and biocompatibility evaluation. ACS Appl. Mater. Interfaces 4(2), 577–580 (2012)
Bai, L., Gong, C., Chen, X., Sun, Y., Zhang, J., Cai, L., Zhu, S., Xie, S.Q.: Additive manufacturing of customized metallic orthopedic implants: materials, structures, and surface modifications. Metals 9(9), 1004 (2019)
Darsell, J., Bose, S., Hosick, H.L., Bandyopadhyay, A.: From CT scan to ceramic bone graft. J. Am. Ceram. Soc. 86(7), 1076–1080 (2003)
Bose, S., Fielding, G., Tarafder, S., Bandyopadhyay, A.: Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 31(10), 594–605 (2013)
Tarafder, S., Bose, S.: Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: in vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. ACS Appl. Mater. Interfaces 6(13), 9955–9965 (2014)
Khaled, S.A., Burley, J.C., Alexander, M.R., Roberts, C.J.: Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 461(1–2), 105–111 (2014)
Song, S.W., Hidajat, K., Kawi, S.: Functionalized SBA-15 materials as carriers for controlled drug delivery: influence of surface properties on matrix−drug interactions. Langmuir 21(21), 9568–9575 (2005)
Stallard, C.P., Solar, P., Biederman, H., Dowling, D.P.: Deposition of non-fouling PEO-like coatings using a low temperature atmospheric pressure plasma jet. Plasma Processes Polym. 13(2), 241–252 (2016)
Gloria, A., Causa, F., Russo, T., Battista, E., Della Moglie, R., Zeppetelli, S., De Santis, R., Netti, P.A., Ambrosio, L.: Three-dimensional poly (ε-caprolactone) bioactive scaffolds with controlled structural and surface properties. Biomacromol 13(11), 3510–3521 (2012)
Puppi, D., Chiellini, F., Piras, A.M., Chiellini, E.: Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 35(4), 403–440 (2010)
Kurtz, S.M., Devine, J.N.: PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 28(32), 4845–4869 (2007)
Dawson, J.I., Wahl, D.A., Lanham, S.A., Kanczler, J.M., Czernuszka, J.T., Oreffo, R.O.: Development of specific collagen scaffolds to support the osteogenic and chondrogenic differentiation of human bone marrow stromal cells. Biomaterials 29(21), 3105–3116 (2008)
Inzana, J.A., Olvera, D., Fuller, S.M., Kelly, J.P., Graeve, O.A., Schwarz, E.M., Kates, S.L., Awad, H.A.: 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 35(13), 4026–4034 (2014)
Yu, D.G., Branford-White, C., Ma, Z.H., Zhu, L.M., Li, X.Y., Yang, X.L.: Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int. J. Pharm. 370(1–2), 160–166 (2009)
Lee, K.Y., Mooney, D.J.: Alginate: properties and biomedical applications. Prog. Polym. Sci. 37(1), 106–126 (2012)
Balamurugan, A., Balossier, G., Michel, J., Ferreira, J.M.: Electrochemical and structural evaluation of functionally graded bioglass-apatite composites electrophoretically deposited onto Ti6Al4V alloy. Electrochim. Acta 54(4), 1192–1198 (2009)
Stojanovic, D., Jokic, B., Veljovic, D., Petrovic, R., Uskokovic, P.S., Janackovic, D.: Bioactive glass–apatite composite coating for titanium implant synthesized by electrophoretic deposition. J. Eur. Ceram. Soc. 27(2–3), 1595–1599 (2007)
Boccaccini, A.R., Peters, C., Roether, J.A., Eifler, D., Misra, S.K., Minay, E.J.: Electrophoretic deposition of polyetheretherketone (PEEK) and PEEK/Bioglass® coatings on NiTi shape memory alloy wires. J. Mater. Sci. 41, 8152–8159 (2006)
Lerouge, S., Guignot, C., Tabrizian, M., Ferrier, D., Yagoubi, N., Yahia, L.H.: Plasma-based sterilization: effect on surface and bulk properties and hydrolytic stability of reprocessed polyurethane electrophysiology catheters. J. Biomed. Mater. Res. 52(4), 774–782 (2000)
Hooper, K.A., Cox, J.D., Kohn, J.: Comparison of the effect of ethylene oxide and γ-irradiation on selected tyrosine-derived polycarbonates and poly (L-lactic acid). J. Appl. Polym. Sci. 63(11), 1499–1510 (1997)
Simmons, A.: Future trends for the sterilisation of biomaterials and medical devices. In: Sterilisation of Biomaterials and Medical Devices, pp. 310–320. Woodhead Publishing (2012)
Srivastava, M., Rathee, S., Maheshwari, S., Kundra, T.K.: Additive Manufacturing: Fundamentals and Advancements. CRC Press (2019)
Guddati, S., Kiran, A.S., Leavy, M., Ramakrishna, S.: Recent advancements in additive manufacturing technologies for porous material applications. Int. J. Adv. Manuf. Technol. 105, 193–215 (2019)
Morrison, R.J., Kashlan, K.N., Flanangan, C.L., Wright, J.K., Green, G.E., Hollister, S.J., Weatherwax, K.J.: Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices. Clin. Transl. Sci. 8(5), 594–600 (2015)
Schuh, J.C., Funk, K.A.: Compilation of international standards and regulatory guidance documents for evaluation of biomaterials, medical devices, and 3-D printed and regenerative medicine products. Toxicol. Pathol. 47(3), 344–357 (2019)
Harawaza, K., Cousins, B., Roach, P., Fernandez, A.: Modification of the surface nanotopography of implant devices: a translational perspective. Mater. Today Bio. 1(12), 100152 (2021)
Douglass, M., Garren, M., Devine, R., Mondal, A., Handa, H.: Bio-inspired hemocompatible surface modifications for biomedical applications. Prog. Mater. Sci. 17, 100997 (2022)
Zhang, D., Chen, Q., Shi, C., Chen, M., Ma, K., Wan, J., Liu, R.: Dealing with the foreign-body response to implanted biomaterials: strategies and applications of new materials. Adv. Func. Mater. 31(6), 2007226 (2021)
Punj, S., Singh, J., Singh, K.: Ceramic biomaterials: properties, state of the art and future prospectives. Ceram. Int. 47(20), 28059–28074 (2021)
Alghamdi, H.S., Jansen, J.A.: The development and future of dental implants. Dent. Mater. J. 39(2), 167–172 (2020)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Soni, B., Shivgotra, R., Kaur, M., Thakur, S. (2023). Surface-Modified Biomaterials in Medical Device Development. In: Malviya, R., Sundram, S. (eds) Engineered Biomaterials. Engineering Materials. Springer, Singapore. https://doi.org/10.1007/978-981-99-6698-1_15
Download citation
DOI: https://doi.org/10.1007/978-981-99-6698-1_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6697-4
Online ISBN: 978-981-99-6698-1
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)