Abstract
The Asthma, Bronchitis, Chronic obstructive pulmonary diseases (COPD) and Cystic fibrosis (CF) are observed now a day as the foremost cause for mortality worldwide. The lung organ having larger surface area and abundance vascular structure therefore the best site of choice for the various lung diseases treatment. The Anti-asthmatic and COPD drugs are developed for the treatment of COPD in variety of dosage forms namely Dry power inhaler, Metered dose inhaler and Nebulizers. To achieve the optimum distribution and absorption of the drug(s) its particle size must remain in the range of 1 to 5 µm. DPI dosage form formulated by various mixing techniques of drugs with the excipients preferably respiratory grade lactose. The MDI’s are the solution or suspensions filled in canisters with propellant such as Hydrofluoroalkanes. For both DPI and MDI dosage form inhaler devices are utilized for the proper administration of drugs. For paediatric patients nebulization therapy is extensively utilized for the asthma and COPD management. DPI and MDI product development is challenging process as the optimum deposition and specific target site of action is achieved with narrow particle size distribution. The analytical tests such as Assay, Impurities and degradants, delivered dose uniformity, aerodynamic particle size distribution, extraneous particles, net content, moisture content, microbial-load, leachable content, and inhaler device constituent part characteristics are performed for all the three dosage forms. The spray pattern and plume geometry are the additional analytical tests performed for MDI’s.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dry powder inhaler
- Metered dose inhaler
- Nebulizers
- Asthma
- COPD
- Analytical tests
- Spray pattern
- Plume geometry
1 Introduction to Pulmonary Drug Delivery Systems (PDDS)
Asthma, COPD and cystic fibrosis treatment by inhaler treatment is used since last many years. The drug or combination of drugs is directly reaches in the lung region with quick action, higher efficacy and lesser side effects when compared with the oral or parenteral formulations (Patton et al. 2004).
The DPI, MDI and nebulizer formulations are extensively prescribed for prophylaxis and managing of lung diseases, asthma and complication related with it for localized and systemic effects (Patton et al. 2004). The lung provides an optimum alveoli surface area which is rich in capillaries and acts as an excellent absorbing surface for drug administration (Newhouse and Corkery 2001).
Because of the treatment efficacy in the severe diseases, suggested twenty seven multidisciplinary approaches to delivery of drug to the targeting organ or tissues. The efficacy of drug is optimized with controlled pharmacokinetics, pharmacodynamics, immunogenicity and pattern of bio-recognition (Newhouse and Corkery 2001). The newer research and development studies are based on the polymeric sciences, advances pharmaceutical technologies; bio-conjugate chemistry and molecular biology are some of the strategies carrying out in PDDS. PDDS research and development is carried out scientific and biomedical importance to reduce the loss and degradation of drug, and to improve drug bioavailability (Corkery 2000). Since last 2 decades, to achieve optimum drug targeting and drug delivery the researchers mainly focusing on the nanotechnology (Georgitis 1999).
The PDDS is found to be advantageous because; only respiratory system is exposed to drugs therefore very few adverse effects are observed with quick onset of action and rapid relief (Georgitis 1999; Bennett et al. 2002). The viscous pharmaceutical formulation combinations with carrier molecules like lipid, water or lipid/water administration is possible in PDDS in the form of nebulizer dosage forms. The drug formulations containing liposomes are found to be stable if given in the form of nebulization (Georgitis 1999; Bennett et al. 2002). The drug particles with the help of respiratory grade carrier lactose as a carrier molecule reach the lungs and size having 1 to 5 µ is deposited at the site of action. Similarly, In MDI dosage form the particles of drug are solubilized or dispersed in the propellant (Bennett et al. 2002).
Limitations of PDDS include the high oropharyngeal deposition due to improper handling of inhaler devices by the patients (Dhand 2001). The delivered drug reproducibility is mainly dependant on the variables such as physiological and pharmaceutical variables. Drug retention in the lungs is reduced by the lungs clearance system, which may improve drug absorption (Beck-Broichsitter et al. 2009). The lungs are not an easy and accessible organ for delivery of drug; hence complicated delivery systems are needed for targeted delivery of drug, such as inhaler devices or other ancillaries. Respiratory Deposition Mechanisms Inhaled aerosol particle deposition in the respiratory system is caused by three processes: Inertial impaction, Brownian diffusion and Gravitational settling. For any breathing condition, a theory is established to estimate deposition and distribution of drug particle in the airway. The particles may deposit in several sites along the respiratory tract once it enters by the mouth or nose (Timsina et al. 994). The direction of airflow varies frequently when breathing in the nasal/mouth, pharynx, larynx, and airway bifurcations. In these areas, large particles (>0.5 μm) might be depositing through impaction because they are unable to monitor the air streamline. Infact, a significant percentage of the dose emitted by pMDI and DPI devices continues to be deposited by impaction in the oro-pharyngeal region (Beck-Broichsitter et al. 2010). The primary method of inhaled tiny particle deposition in the alveolar region and small airways is achieved by sedimentation.
2 Respiratory Tract as a Target for PDDS
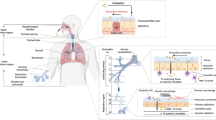
Human respiratory system is divided into 2 parts; nose, nasal cavity, pharynx and associated structures which known as upper respiratory tract and larynx, trachea, bronchi and the lungs which known as lower respiratory tract (Nyström and Fadeel 2012).
Nose divided into two; external nose comprises of bones, hyaline cartilage enclosed with muscle, skin and mucus membrane and two opening termed as nostrils. The internal Nose also called as nasal cavity it connects pharynx posteriorly through two openings known as internal choncae. Nose provides airways for respiration. Due to the high mucosal vascularity, the nose enables the breath to become warmed. The thick mucosa in the nose allows it to warm both the air being inhaled and released. The olfactory sensors are situated in this structure, which also helps as a resonating chamber for speech and aids in filtering inhaled air (Sharifi et al. 2012).
The pharynx is a funnel shaped tube like structure approximately 13 cm and divided into the three parts namely; the nasal cavity supplies air to the nasal cavity's apical section, called as nasopharynx. To balance the air pressure between the pharynx and middle ear, it also exchanges a moderate amount of air with auditory tubes (Schleh et al. 2011). Since it serves as a common pathway for air, food, and liquids, the oropharynx is the middle section that has both respiratory and digestive functions. The oropharynx has two pairs of tonsils; the palatine and lingual tonsils. The inferior part of the pharynx is called the laryngopharynx (Schleh et al. 2011).
Larynx creates sound with pitch, volume, tone and joins the laryngopharynx and trachea. It safeguards the lower respiratory system. Larynx upward motion occurs during swallowing. The epiglottis supports in larynx closure and ensures that food enters the oesophagus instead of into the respiratory airway (Possmayer et al. 2010). The larynx assists in keeping the pharynx and trachea's airway passage. It's a tube-shaped airway. It has a diameter of 2.5 cm and a length of 12 cm. It runs from larynx to fifth thoracic vertebra, and it is situated anterior to the oesophagus. It is made up of 16 to 20 hyaline cartilage rings that are incomplete and horizontal, resembling the letter C (Todo et al. 2004). One after the other, they are appearing as a sack like structure. Each C-shaped cartilage has an open portion that faces the oesophagus. The C-shaped cartilage supports the trachea semi-rigidly, helping to minimise airway blockage and the collapse of the tracheal wall during inhalation (Schleh et al. 2011).
The trachea or windpipe has tubular branches called bronchi that carry inhaled air to the lungs divided in the left and right primary bronchus at the fifth thoracic vertebra. The bronchi then split into bronchioles, which are progressively smaller branching (Labiris and Dolovich 2003).
On either side of the chest are two air-filled, spongy organs called as lungs. The pleura are a thin tissue layer that covers the lungs. The lungs slide easily as they expand and collapse with each breath due to a thin coating of fluid acting as a lubricant (Dhand 2001; Possmayer et al. 2010). The thoracic wall and superior face of the diaphragm are protected by a parietal pleura. The visceral pleura are a layer that spreads to cover the exterior lung surface and lining the fissures (Beck-Broichsitter et al. 2009).
Gas exchange takes place in the moist, thin-walled compartments known as alveoli. The alveolar walls are kept from adhering together and collapsing by a slightly oily surfactant. Alveoli and capillary walls make up the alveolus, which is the respiratory surface. The membrane lining the alveoli and capillary walls are found to be similar. Lungs along with muscle of respiration carry out the process of breathing, i.e. inhalation and exhalation (Todo et al. 2004; Labiris and Dolovich 2003). Principle muscle of respiration is diaphragm and intercostal muscles. The 12 pairs of ribs gaps are occupied by the 11 pairs of intercostal muscles. The external and internal intercostal muscles are arranged in two layers. Both muscles help in breathing by elevating the ribcage. A narrow dome shaped muscle called the diaphragm divides the thoracic compartment, which consists of heart and lungs, from the intestine, stomach, liver etc. for breathing and respiration, the diaphragm is essential (Schleh et al. 2011). It is engaged in breathing, pulling the chest inward during inhale and pushing it outward during exhalation. It also involved in non respiratory functions, facilitating to expelling faeces, urine and vomit, from the body by increase in the intra-abdominal pressure. The diaphragm and intercostal muscles contract during inspiration or inhalation, and they relax during exhale (Possmayer et al. 2010).
2.1 Respiration Physiology
Respiration is the term used to describe the gases exchange O2 and CO2 within the body. The pulmonary ventilation, external and internal respiration are the three fundamental stages of respiration. As part of breathing or pulmonary ventilation, ambient air is inhaled and carbon dioxide rich alveolar air is exhaled. Gases O2 and CO2 are then diffused across the alveolar membrane. The main sites of gas exchange are alveoli, where carbon dioxide is released as a result of oxygen use by cells during catabolic reactions, diffusion of oxygen and carbon dioxide takes place (Possmayer et al. 2010). Additionally, the blood and tissues gas exchange occurs. In these sites, oxygen and carbon dioxide are exchanged primarily based on pressure or concentration gradients by simple diffusion. Other significant variables that can impact the rate of diffusion include the solubility of the gases and the thickness of the membranes engaged in diffusion. Partial pressure, denoted as pO2 for oxygen and pCO2 for carbon dioxide is the pressure that each gas contributes to a mixture of gases. These two gases partial pressures in the atmospheric air were measured at their two sites of diffusion (Todo et al. 2004).
2.2 Classification of Anti Asthmatic and COPD Drugs
2.2.1 Bronchodilators
-
B2 sympathomimetics- Salbutamol, Salmeterol, Formoterol, Terbutaline, Bambuterol
-
Methylxanthines- Theophylline, Aminophylline, Doxophylline
-
Anticholinergics- Tiotropium, Ipratropium
-
Leukotriene antagonists- Montelukast, Zafirlukast
-
Mast cell stabilizers- Sodium cromoglycate, Ketotifen
2.2.2 Corticosteroids
-
Systemic- Hydrocortisone, Prednisolone
-
Inhalation- Budesonide, Fluticasone propionate, Ciclesonide, Flunisolide
-
Anti-IgE antibody- Omalizumab
2.2.3 Fundamental Aspects of PDDS
Two main independent risk factors, which have an im pact on the drug deposition profile, are as follows:
-
(a)
Patient related factors
-
a.
Respiratory tract’s anatomy and physiology
-
b.
Inhalation air flow rate
-
c.
Inhalation mode
-
a.
-
(b)
Physical properties
-
a.
Drug properties
-
b.
Excipient properties carrier systems
-
c.
Formulation related factors
-
a.
3 Patient Related Factors
3.1 Respiratory Tract’s Anatomy and Physiology
To supply oxygen from lung to organ cell and removal of CO2 from the lungs are the main functions of respiratory system. There are two components in the respiratory tract consist of the upper respiratory tract consists of nose, nasal cavity and pharynx and the lower respiratory tract consists of larynx, trachea, bronchi and lungs. The trachea constitutes the essential respiratory path which connects the larynx, bronchi and at last to the lungs. The alveoli are the functional component for gas exchange. In respiratory system a steady reduction in diameter is observed in the direction of the alveoli. The alveolar deposition is therapeutically important as the alveoli have extensive surface area which is responsible for the rapid absorption of drugs. The drug deposition is considerably affected by the respiratory system geometry (Labiris and Dolovich 2003).
3.2 Inhalation Flow Rate
In DPI’s, the patients inspiration efforts is the driving force in the respiratory airway for drug deposition, the rate of inhalation flow is essential to attain standard particle disaggregation. Nevertheless the patient’s rate of inhalation flow cannot be controlled. The overall lung deposition is observed to be similar in both male and female patients. (Labiris and Dolovich 2003; Laube 2009) Female patients show high aerosol deposition in the trachea bronchial region and upper respiratory tract this might be due to the variation in airways in male and female patients; also it dependent on patients disease condition, gender, age, and height. In general in healthy human, the peak average inspiration flow rate found to be 300 L/min and patients suffering from asthma the average inspiration flow rate found to be 200 L/min or even less. Nature of airflow created inside the inhaler device that is laminar or turbulent also important in DPI dispersion. The turbulence in the air flow rate is optimum for particle dispersion of the DPI as compared to laminar airflow is proved (Laube 2009).
3.3 Inhalation Mode
Aerosol inhalation mode is responsible for the particle deposition in the airway. The inhalation mode comprises of airflow rate, air inhalation volume, and breath holding period (Lavorini et al. 2008).
4 Physical Properties
4.1 Properties of Pure Drug
The particle size reduction should be performed to produce the particles in the respirable fraction (i.e. >5 µm in diameter) therefore; milling, supercritical fluid and spray drying methods are the some methods to obtain the desired particle size for the drug products that we will discuss in detail in formulation aspects (Mishima 2008).
4.2 Properties of Excipient Carrier System
Excipients are used to enhance physicochemical stability of the active drug(s), also it enhance pharmaceutical and/or mechanical properties like permeation, dissolution etc. Newman and Busse (2002). In DPI formulations excipient used with main objective is carrier particles. In DPI few micrograms of active drug moiety needs to be delivered hence excipient provides bulk which is responsible for improved drug administration, drug distribution and quantification (Newman and Busse 2002, Nazrul and Ellen 2008). Excipient should also decrease the cohesiveness of drug by utilizing the higher energy sites of the drug particle to reach at pathological site in the lung (Nazrul and Ellen 2008). At present only lactose is the additive utilized in DPIs as it is safe and stable and easily available in numerous particle size and structural appearances. Its manufacturing process with controlled operating procedure can be estimated for purity and physical properties (Ren et al. 2008). Lactose is highly crystalline and has smooth surface morphology which results in improved flow ability which is necessary factor as a DPI carrier. Lactose is highly versatile and less hydroscopic when compared to other sugars but it is incompatible with drugs having primary amine moieties as lactose is a reducing sugar (Ren et al. 2008). In general, excipient’s can make upto 99% of the DPI drug product w/w; this makes them vital elements for complete performance of dry powder inhalers (Ren et al. 2008). No excipients are every time mandatory, Plumicort (budesonide), Terbuhaler (AstraZeneca) are examples of only drug formulation (free from excipient) (Sanjar and Matthews 2015).
4.3 Formulation Related Factors
Particle size: Appropriate particle size is found to be better for the pulmonary delivery and finally to the pharmacological effects. There are significant literatures in the field of Inhalation drug and pharmaceutical aerosol sciences which connects aerodynamic size and distribution of particle size to the deposition possibility in definite pathological site into the lungs (Sermet-Gaudelus et al. 2002). The desired particle size range less than 5 µm in diameter can be achieved by milling procedure. There are numerous mills however only limited are capable to mill powder size to the necessary size of particle ranging from 2 to 5 µm. the important three mills are as follows:-
-
Fluidized energy mill-Jet mill
-
High speed peripheral mill-Pin mill
-
Ball mill.
The basic designs (Refer Fig. 1).
The Jet milling or air attribution milling: It’s a most convenient technique; which utilizes high velocity particle–particle collision mechanism to reduce particle size. Particle size less than 1 µm in diameter can be achieved by jet milling (Hue et al. 2001). The pin mill utilizes the Phenomenon of mechanical impaction to grind material both by particle–particle collision as well as particle-solid collision. The pin mill yields 1 µm particle size but not as small as when compared to jet mill also, pin mills requires less power consumption than other mill (Hue et al. 2001). The ball mill is fundamentally a rolling cylindrical container consists of powder blend to be milled; ball like structure crush the drug amongst each other and tumbled into the milling chamber. Ball milling is very slow as compared to all other mills; hence ball mills are often used in trial scale only (Hue et al. 2001). Spray drying and supercritical fluid crystallization can also be utilized to obtain desired particle size (Hue et al. 2001).
Particle morphology: Particle Morphology may be defined as the shape, structure and exterior properties of a particle (Shoyele and Cawthorne 2006).
Aerodynamic diameter of the particle: The sphere particles, a constant density and a determined velocity that is similar to that of a no spherical shape particle (Shaikh et al. 2010). To describe the aerodynamic properties of a particle and deposition pattern of the drug in the respiratory system, the term Aerodynamic diameter frequently employed. The terminal velocity square root (VTS) is proportional to the particle aerodynamic diameter.
where de = diameter of predicted geometric particle, Pp = particle density, x = dynamic shape factor, ρ0 = density, da = aerodynamic particle diameter, η = gas viscosity, g = gravitational constant.
Mass median aerodynamic diameter: Some methods are used to measure invitro aerosol deposition, including ACI and NGI. The mainspring of all of this equipment is the cut off diameter on the individual collection stage, where fractional drug deposition takes place. MMAD is a form of sieve analysis, to put it simply. The cumulative mass of the collected powder quantity that is smaller than the specified dimensions of each individual impactor stage is used to compute the total quantity of drug recovered in the impactor (Shaikh et al. 2010). The MMAD can be determined by plotting the graph of cumulative quantity of powder against the effective cutoff diameter on a log scale. The key statistical variables are the geometric standard deviation (GSD) and the mass median aerodynamic diameter (MMAD) (Selroos et al. 2006). Particle size correlation with a cumulative sum of 50% can be used to determine the MMAD and the below formula can be utilized to determine the GSD.
where, X = the particle diameters related to 84% deposition of cumulative quantity and Y = the particle diameters related to 16% deposition of cumulative quantity (Selroos et al. 2006). Fine particle dose (FPD): It is the drug quantity in the prescribed dosage which is typically examined for the quantity required for entering into the pathological site in the lung i.e. respirable fraction; it is generally of 5 microns or less. The FPD is many times gets mislead by the term fine particle mass i.e. FPM. FPM is the drug quantity found after a single actuation of DPI, although the recommended drug dose may require several actuations. The FPD when expressed in terms of percentage of the drug dose delivered from an inhaler is termed as fine particle fraction (FPF) (Sermet-Gaudelus et al. 2002)
Inter-particle interactions: The interactions of particles, surface energy and the dispersion of powder are the three crucial factors affect the target of particle deposition in the respiratory system (Shoyele and Cawthorne 2006). The aggregate structure of particles affects the aerodynamic forces. The corresponding motion between the particle and the air stream is responsible for the generation of the aerodynamic forces (Shaikh et al. 2010). The powder dispersion in an airstream is carried out by the equilibrium within the aerodynamic force and the cumulative strength. When these forces are greater than the cumulative strength, deep lung deposition is attained and the powder particles are evenly disperse in an air stream (Shoyele and Cawthorne 2006). The cumulative strength determined as follows;
where \(\emptyset\) = ratio of the particle bulk density and particle true density; W (J/m2) = the adhesion work and dv = sphere shaped or equal particle diameter. The aggregate strength is affected by the adhesion. It is expected that non spherical particles can also achieve improved drug distribution profile than sphere-shaped particles; because of the lower average non-spherical particle curve (Dolovich et al. 2005) this is directly proportional to the spherical or equal particle diameter (dv).
Vander Waals forces: the deposition of particle and its profile of dispersion are both influenced by the interparticle forces; these complex forces include electrostatic forces and Vander Waals forces etc. The most powerful force influencing particle deposition and drug dispersion behavior is the Van der Waals force. All aerosol formulations experience the Vander Waals forces caused by the induced dipole dipole interactions (Hue et al. 2001).
Work of adhesion or cohesion: In DPI’s the cumulative strength is relying on the cohesive forces (originating from the interactions between drug-drug particles) or the adhesive forces (originating from the interactions between drug-excipient particles); also the induced dipole–dipole interactions are also dependent on Work of adhesion and cohesion. The effective interaction diameter (dv) is the difference between diameters of particles of drug(s) and excipient(s). Dv is determined as drug and additives mean of arithmetic diameters d1 and d2 respectively (Frijlink and De Boer 2004)
Electrostatic Interactions: The particle dispersion can also affected by electrostatic force and the associated Columbian forces. These interactions exhibit equally effective as Vander Waals force (Nolan et al. 2011). The electrostatic interactions between drug-drug and drug-excipient particles affect the quantity of particles of aerosol escaping the from the capsule or inhaler device. Agglomerate formation leads to the generation of differently charged particles (Nolan et al. 2011).
Aggregate strength: To determine the cumulative strength many theoretical procedures developed till date; and these methods are surely established on the particle surface dynamics. Nonetheless, in the estimation of the aggregate strength; the sum of interaction energies of drug particles must be taken into consideration. The particle dispersion, dipole–dipole and H2 bonding; these forces are the main reason for the drug particle interactions (Copley scientific manual Edition 2019). These forces depend on the parameter of solubility of hildebrand i.e. \(\delta {\text{A}}\) and \(\delta {\text{C}}\) (Pai and Nahata 2001). The correlation amongst the solubilization parameter, the cohesive strength \(\sigma {\text{C}}\) and adhesive strength \(\sigma {\text{A}}\) interactions are
where \(\theta\) = the drug molecules interaction parameter. Hence, it can be predicted that the work of adhesion and cohesion are proportionate to the drug-drug interactions and drug-excipient interactions strength. According to mentioned theoretical procedures, the conclusions made as follows:
-
The reduction in aggregate strength takes place with subsequent particle diameter (dv) increases (Sermet-Gaudelus et al. 2002).
-
Loose and weak aggregate structure is often produced by low bulk density powders.
-
The reduction in the particle surface energy is associated with the enhance powder dispersion behavior hence; in case of DPI’s coating is immensely suggested for precise cohesive strength (\(\sigma {\text{C}}\)) and adhesive strength (\(\sigma {\text{A}}\)) interaction (Shekunov et al. 2007).
-
Hollow porous micro particle formulations produce a comparatively higher arithmetic volume diameter when correlated to the aerodynamic diameter.
Span index: An ideal aerosol drug particle must have a small range distribution of particle size and quick dispersibility at comparatively lower aerodynamic force (Roberto and Dal Negro 2015a, b). The constricted span index shows narrow particle size distribution and is desired requirement for the DPI (Sanjar and Matthews 2001) The span index can be defined as the measures of distribution of particle size associated to its median diameter. Span index can be determined as follows:
where, D10 = 10% of the particles are less than 10 µm, D50 = 50% of the particles are less than 50 µm, and D90 = 90% of the particles are less than 90 µm.
The aerosol particles fraction settled in the upper respiratory tract i.e. mouth and throat; is termed as impaction loss; and it is high for powdered formulations having larger span index. The loss in the impaction is directly proportionate to the rate of airflow and drug particles diameter square and is affected by the poly-dispersity of particles (Roberto and Dal Negro 2015a, b). Large particles are governed by its mass and impacts upper respiratory tract because of its inertia. Oropharyngeal deposition indicates high inter and intra variation. Important force mechanisms in deposition of particles into the lungs are as follows (Refer Fig. 2).
-
Inertial impaction: The particle’s deposition into the exterior of respiratory airway. Inertial impaction takes place near to the respiratory tract at the branching of the conducting airways. In this high flow velocity and variations in airflow directions are observed (Roberto and Dal Negro 2015a, b).
-
Gravitational sedimentation: Gravitational sedimentation observed in the minor conducting airway in which the air velocity is relatively small and size of particle is <5 μm (Roberto and Dal Negro 2015a, b).
-
Diffusion: In small airway and alveoli at which low velocity of air and particle size <0.5 μm; diffusion mechanism takes place (Roberto and Dal Negro 2015a, b; Sanjar and Matthews 2015).
-
Interception: In Interception, deposition phenomenon may occur when a particle comes in contact with epithelial wall of respiratory tract; nevertheless its center of quantity may persist on the array of fluid. It is important only for particle aggregates (Roberto and Dal Negro 2015a, b).
-
Electrostatic attraction: Improved drug deposition in consequences of electrostatic charges by expanding the attractive forces toward the airway (Roberto and Dal Negro 2015a, b).
5 Lung Diseases and Its Treatment by Various Dosage Forms Such as DPI’s, MDI’s and Nebulizers
5.1 Asthma
The chronic inflammatory disorder of the airways is called as asthma. Chronic inflammation increases the airways hyper responsiveness, which frequently results in episodes of coughing, chest tightness, wheezing and dyspnea, especially in the morning or at night. Their signs and indications are comparable to those of other illnesses with restricted airflow, such as chronic bronchitis (Corkery 2000; Beck-Broichsitter et al. 2009). In wheezing a whistling sound made while breathing is known as wheezing. It happens as a result of air being pushed through the restricted and smaller air channel. One of the most typical symptoms of asthma is coughing. The cough can be dry or wet, and it may get worse at night, in the early morning or after activity. Breathlessness is the sensation that one breath is almost about to expire when another one is essential. It is referred as “air hunger.” Due to mucus production many asthmatics produce an excessive amount of mucus, which can clog the airways and cause coughing. Chest constriction; this may feel as if something is pressing against or resting on the chest. The patient may experience a feeling of chest tightening because of the muscles surrounding the airway gets constrict.
5.2 Types of Asthma
Extrinsic asthma often known as allergic asthma: It occurs when an allergen specific immune reaction causes the symptoms. The IgE based type I immediate hypersensitivity reactions.
Intrinsic asthma often known as non-allergic asthma: It is brought on by airborne irritants that are not connected to allergens. These irritants cause inflammation and bronchoconstriction by stimulating parasympathetic nerve cells in these irritants cause inflammation and bronchoconstriction by stimulating parasympathetic nerve cells in the airways. These irritants cause inflammation and bronchoconstriction by stimulating parasympathetic nerve cells in the airways.
-
Mixed asthma is occurring by both allergic and non-allergic symptoms are known as “mixed asthma” It’s a most typical type of asthma.
-
Cough variant asthma prolonged dry cough is the only symptom observed; the typical asthmatic symptoms such as wheezing and breathlessness are not observed.
-
Physical activity triggered asthma affects the human during or after exercise.
-
Nocturnal asthma is gets worsen at night is known as nocturnal asthma. Anyone with nocturnal asthma may feel indications at any time.
-
Occupational asthma is triggered by stimuli present at person's place of employment, such as farming, woodworking, textile industry and chemical industry etc.
5.3 Pathophysiology of Asthma
See Fig. 3.
5.4 Diagnosis
-
Spirometer—This instrument measures the amount of air we can exhale and the force with which we can do so. One could take the test both before and after inhaling the medication. An effective technique to measure how much breathing is affected during an asthmatic episode.
-
Peak Expiratory Flow Determination—Measure the capacity to force air out of the lungs or the flowrate at air is expelled using the peak expiratory flow method. A peak flow meter is used in this test, which the patient can carry out at home to check lung function.
-
Blood tests for allergies and chest X-rays can also be useful for diagnosis of asthma.
5.5 Chronic Obstructive Pulmonary Disease
The terms chronic obstructive pulmonary disease (COPD) or chronic obstructive airway disease (COAD) refer to the pathological conditions in which there is a persistent, incomplete or total airflow hindrance at several stages, from the trachea to the smallest airway, which results in the functional impairment of lungs. Smoking is one causative factor that is present in all kinds of COPD (Timsina et al. 1994; Beck-Broichsitter et al. 2009). The clinical complications falls under the COPD are emphysema, chronic bronchitis, bronchial asthma, bronchiectasis, and bronchiolitis etc. A group of respiratory disorders is known as COPD. It is challenging to breath and get worst over the period of time. The lung airway passage and air sacs are often elastic. The airways transport air to the air sacs during inhalation. Like a little balloon, the air sacs expand as they fill with air. The air sacs collapse as a person exhales, letting the air out. People with COPD have less airflow into and out of their airways due to a several problems; the walls separating the air sacs are damaged, the airway walls get thicken, enlarged and produce more mucus than normal. The lung airways and air sacs also turn into less elastic (Timsina et al. 1994).
5.6 Types of COPD
There are two types of COPD.
-
Emphysema—Lung air sacs and their interstitial walls are both impacted by emphysema. They deteriorate and lose their elasticity.
-
Chronic bronchitis—the airway lining is continuously inflamed and irritated in chronic bronchitis. The lining swells and produces mucus.
These are some of the COPD risk factors. The biggest risk factor is smoking upto 75% of patients with COPD smoking habits. Exposure for a long period of time to irritants like chemical fumes, dusts and air pollution causes COPD risks. Age is the majority of COPD patients are at least 40 years old when their symptoms first appear. A genetic condition that comes into this class is alpha-1 antitrypsin deficiency. Also, if there is a family history of COPD and smokers who develop COPD are more likely to do so. Chest tightness and short breath, especially after intense physical activity, wheezing or noisy sound when patient breath, recurrent dry cough or wet coughing is observed commonly.
5.7 Treatment of COPD
Adopt lifestyle adjustments, such as giving up smoking and adopting a nutritious diet. Bronchodilator, inhalation steroids helps to expand airways and ease breathing by relaxing the muscles around them.
Since COPD patients are more likely to acquire the flu and pneumococcal pneumonia, vaccination is vital for the management of both illnesses. If you get a viral or bacterial lung infection, antibiotics are prescribed along with oxygen therapy to prevent the condition from getting severe. Exercise may be a part of a pulmonary rehabilitation programme, which is carried out to improve the patient's health and treat chronic respiratory problems.
Exercise may be a part of a pulmonary rehabilitation programme, which is carried out to improve the patient's health and treat chronic breathing issues. Surgery is typically used as a last option for patients with severe symptoms that have not responded to medical treatment. Emphysema is the main cause of COPD, which can be treated surgically by removing damaged lung tissue and the big air spaces (bullae) which develop when air sacs are damaged. The bullae could make difficulty in breathing; severe COPD could need to have their lungs transplant.
7 Analytical Testing of DPI’s, MDI’s and Nebulizers
Common tests of DPI, MDI and Nebulizers include the identification test and other physicochemical tests such as Assay, related substances, fill weight or net content or average weight of the content, DDU and APSD etc. Tong et al. (2006). Valve Delivery per Actuation (Shot weight), Number of delivery per Canister, Leak Rate, Moisture Content by KF Coulometric Titration, content per canister (CPC), fine Particle Dose are other tests of MDI dosage forms (Tong et al. 2006; Usmani 2012). The SP and PG are the additional analytical tests performed for MDI’s in which the tests are carried out on the basis of laser diffraction techniques (Usmani 2012; Copley scientific manual Edition 2019).
For generic product submission into the regulatory market it is foremost that the respiratory dosage forms needs to comply with the innovator sample for an approval.
That’s why the respiratory dosage forms are termed as complex generics (Usmani 2012; Copley scientific manual Edition 2019).
8 Conclusion
The chapter explains about inhalation therapy is utilized for the treatment of Asthma, Cystic fibrosis and COPD. The advantages of PDDS with anatomy and physiology of respiratory tract, physiology of respiration, mechanisms of respiratory deposition for PDDS is summarized. The detailed classification of antiasthmatic and COPD drugs is illustrated. The fundamental aspects of PDDS including patient related factors and physical properties of drug and carrier or vehicle molecule is taken into consideration.
The chapter also illustrated the important force mechanisms which are involved in drug deposition into the lungs. Lung diseases and its treatment by various dosage forms such as DPI’s, MDI’s and Nebulizers is narrated. The asthma and COPD its types, diagnosis and problems associated with it are explained in detail. Treatment of Asthma and other lung diseases with DPI’s, MDI’s and Nebulizers including the advantages and disadvantages of particular dosage forms with the analytical testing associated with the DPI’s, MDI’s and Nebulizers are summarized. The chapter is useful to have a quick glance of pulmonary drug delivery system with its anatomy physiology, its formulation and analytical aspects.
References
Beck-Broichsitter M, Gauss J, Gessler T, Seeger W, Kissel T, Schmehl T (2010) Pulmonary targeting with biodegradable salbutamol loaded nanoparticles. J Aerosol Med Pulm Drug Deliv 23(1):47–57
Beck-Broichsitter M, Gauss J, Packhaeuser CB, Lahnstein K, Schmehl T, Seeger W, Kissel T, Essler T (2009) Pulmonary drug delivery with aerosolizable nanoparticles in an ex vivo lung model. Int J Pharm 367(1–2):169–178
Bennett WD, Brown JS, Zeman KL, Hu SC, Scheuch G, Sommerer K (2002) Targeting delivery of aerosols to different lung regions. J Aerosol Med 15(2):179–188
Copley scientific manual Edition (2019)
Corkery K (2000) Inhalable drugs for systemic therapy. Respir Care 45(7):831–835
Dhand R (2001) Future directions in aerosol therapy. Respir Care Clin N Am 7(2):319–335
Dolovich MB, Ahrens RC, Hess DR (2005) Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest 127(1):335–371
Frijlink HW, De Boer AH (2004) Dry powder inhalers for pulmonary drug delivery. Expert Opin Drug Deli 1:67–86
Georgitis JW (1999) The 1997 Asthma management guidelines and therapeutic issues relating to the treatment of asthma. Natl Hear, Lung, Blood Inst Chest 115(1):210–217
Hue G, Otaki H, Watanuki K (2001) Optimization of grinding performance of tumbling ball mill. Int J Ser C-Mech Syst Mach Elem Manuf 44:267–274
Islam N, Gldaki E (2008) Mini review Dry Powder Inhalers (DPIs)—a review of device reliability and innovation. Int J Pharm 360:1–11
Labiris NR, Dolovich MB (2003) Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol 56(6):600–612
Laube BL (2009) The expanding role of aerosols in systemic drug delivery, gene therapy, and vaccination. Respir Care 50(9):1161–1176
Lavorini F, Magnan A, Dubus JC (2008) Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med 102(4):593–604
Mishima K (2008) Biodegradable particle formation for drug and gene delivery using supercritical fluid and dense gas. Adv Drug Deliv Rev 60(3):411–432
Newhouse MT, Corkery KJ (2001) Aerosols for systemic delivery of macromolecules. Respir Care Clin N Am 7(2):261–275
Newman SP, Busse WW (2002) Review evolution of dry powder inhaler design, formulation, and performance. Respir Med 96:293–304
Nolan LM, Li J, Tajber L, Corrigan OI, Healy AM (2011) Particle engineering of materials for oral inhalation by dry powder inhalers II-Sodium cromoglicate. Int J Pharm 405:36–46
Nyström AM, Fadeel B (2012) Safety assessment of nanomaterials: implications for nanomedicine. J Control Release 161(2):403–408
Pai VB, Nahata MC (2001) Efficacy and safety of aerosolized tobramycin in cystic fibrosis. Pediatr Pulmonol 32:314–327
Patton JS, Fishburn CS, Weers JG (2004) The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc 1(4):338–344
Possmayer F, Hall SB, Haller T, Petersen NO, Zuo YY, Bernardino de la Serna J, Postle AD, Veldhuizen RAW, Orgeig S (2010) Recent advances in alveolar biology: Some new looks at the alveolar interface. Respir Physiol Neurobiol 173(SUPPL.):S55–S64
Ren Y, Yu C, Meng K, Tang X (2008) Influence of formulation and preparation process on ambroxol hydrochloride dry powder inhalation characteristics and aerosolisation properties. Drug Dev Ind Pharm 34(9):984–991
Roberto W, Dal Negro (2015a) Dry powder inhalers and the right things to remember: a concept review. Dal Negro Multidiscip Respir Med 10(13):2–4
Roberto W, Dal Negro (2015b) Dry powder inhalers and the right things to remember: a concept review. Dal Negro Multidiscip Respir Med 10(13):2–4
Sanjar S, Matthews J (2001) Treating systemic diseases via the lung. J Aerosol Med: off J Int Soc Aerosols Med 14(Suppl 1):S51-58
Sanjar S, Matthews J (2015) Treating systemic diseases via the lung. J Aerosol Med: Off J Int Soc Aerosols Med 14(Suppl1):S71–S78
Schleh C, Rothen-Rutishauser B, Kreyling WG (2011) The influence of pulmonary surfactant on nanoparticulate drug delivery systems. Eur J Pharm Biopharm 77(3):350–352
Selroos O, Borgstrom L, Ingelf J (2006) Performance of Turbuhaler in patients with acute airway obstruction and COPD, and in children with asthma: understanding the clinical importance of adequate peak inspiratory flow, high lung deposition, and low in vivo dose variability. Treat Respir Med 5:305–315
Sermet-Gaudelus I, LE cocguic Y, Ferroni A (2002) Nebulized antibiotics in cystic fibrosis. Paediatr Drugs 4(7):455–467
Shaikh S, Nazim S, Khan T, Shaikh A, Zameeruddin M, Quazi A (2010) Recent advances in pulmonary drug delivery system: a review. Int J Appl Pharm 2(4):27–31
Sharifi S, Behzadi S, Laurent S, Laird Forrest M, Stroeve P, Mahmoudi M (2012) Toxicity of nanomaterials. Chem Soc Rev 41(6):2323–2343
Shekunov B, Chattopadhyay P, Tong HY, Chow AL (2007) Particle size analysis in pharmaceutics: principles. Methods Appl Pharm Res 24(2):203–227
Shoyele SA, Cawthorne S (2006) Particle engineering techniques for inhaled biopharmaceuticals. Adv Drug Deliv Rev 58(9–10):1009–1029
Timsina MP, Martin GP, Marriott C, Ganderton D, Yianneskis M (1994) Drug delivery to the respiratory tract using dry powder inhalers. Int J Pharm 101(1–2):1–13
Todo H, Okamoto H, Iida K, Danjo K (2004) Improvement of stability and absorbability of dry insulin powder for inhalation by powder-combination technique. Int J Pharm 271(1–2):41–52
Tong HHY, Shekunov BY, York P, Chow AH (2006) Predicting the aerosol performance of dry powder inhalation formulations by interparticulate interaction analysis using inverse gas chromatography. J Pharm Sci 95(1):228–233
Usmani OS (2012) Treating the small airways. Respiration 84:441–453
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gondhale-Karpe, P. (2023). Novel Techniques in Pulmonary Drug Delivery Systems. In: Kulkarni, S., Haghi, A.K., Manwatkar, S. (eds) Novel Technologies in Biosystems, Biomedical & Drug Delivery. Springer, Singapore. https://doi.org/10.1007/978-981-99-5281-6_9
Download citation
DOI: https://doi.org/10.1007/978-981-99-5281-6_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-5280-9
Online ISBN: 978-981-99-5281-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)