Abstract
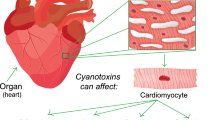
Cyanobacteria can bloom under the right circumstances, a process marked by the increase of algal biomass and the potential production of cyanotoxins in aquatic habitats. Many cyanobacterial toxins can have a serious negative impact on health or even result in death in both humans and animals. One of the most prevalent causes of cyanotoxin poisoning in humans is drinking contaminated water. Other common causes include skin contact and ingesting water while participating in recreational activities. This protocol demonstrates how to determine the lethal concentration (LC50) of acute toxicity, as well as the experimental design, growth parameters, histopathology, hematology, and serum biochemistry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Freshwater ecosystems are home to the majority of the photosynthetic microorganisms known as cyanobacteria. Cyanobacteria are generally known as blue-green bacteria. These are prokaryotic microorganisms that resemble bacteria, but unlike bacteria, they are oxygen-evolving photosynthetic organisms rather than chemosynthetic organisms. Because of their lengthy evolutionary history, they have evolved to cope with climatic, geochemical, and human-caused changes (Duy et al. 2000). Cyanobacteria play a critical role in preserving the ecological balance as well as the biodiversity of lower and higher order microbial communities. Moreover, they have the ability to colonize salty or brackish water and can withstand temperatures that are both very low and very high. The high growth of cyanobacteria in waterbodies is being intensified by human eutrophication and climate change, leading to a condition known as bloom that may impair the quality of drinking and recreational water. It has been noted that cyanobacteria create poisonous secondary metabolites known as cyanotoxins during blooms (Sanseverino et al. 2017). Due to the nutrient enrichment of the water throughout the summer, cyanobacterial blooms have a tendency to develop, and a thick oily coating is deposited on the water’s surface. These algal blooms give the water an unpleasant flavor and smell. The poisons are discharged into the water as this bloom degenerates, and the quantity of toxins may be too high for human consumption. The cyanobacterial species that produce toxins and are usually found in freshwaters are Lyngbya, Microcystis, Anabaena, Nodularia, Planktothrix, and Aphanizomenon. Cyanotoxins are classified based on their toxicological target and can affect humans, animals, and plants. These colonial or filamentous cyanobacteria produce a wide range of toxic substances that are diverse in their chemical and biological composition, including hepatotoxins (microcystin and nodularin), neurotoxins (anatoxins and saxitoxins), paralytic shellfish toxins (produced by Aphanizomenon), cytotoxins (cylindrospermopsin), and dermatotoxins (lipopolysaccharides) (Agrawal et al. 2012). The cytoplasm of cells, where the bulk of cyanotoxins are present, is intracellular. When an algal bloom decays, cyanobacteria often release the toxins contained inside their cells into the water, but in some species, living cells can also produce poisons (extracellular toxins). All of the poisons that are discharged into the water can bioaccumulate in the ecosystem and in creatures that are waterborne, where they can then be transferred to aquatic life and people. As poisons build up in fish, those who consume them may have symptoms that range from serious illnesses to death (Falfushynska et al. 2023). The invention of efficient extraction and analytical techniques capable of examining edible fish, shellfish, plants, and animals is required. To minimize health concerns and the frequency of blooms, national laws and guidelines that attempt to restrict cyanobacterial biomass in our rivers are required. In order to support regulatory policies and stop new risks to human and environmental health, it is also necessary to enhance early warning systems for cyanobacteria.
2 Material Required
2.1 Designing of the Experiment
-
1.
Fish
-
2.
Fiber, glass, or plastic tank
-
3.
Ph meter
-
4.
Purified water
2.2 Histopathology
-
1.
Formalin
-
2.
PBS
-
3.
Automatic Embedding Machine
-
4.
Microtome
-
5.
Paraffin wax
-
6.
Slide
-
7.
Microscope
2.3 Hematology and Serum Biochemistry
-
1.
Chloroform
-
2.
Centrifuge
-
3.
Syringe
-
4.
EDTA tube
-
5.
Freeze drier
3 Methods
3.1 Design of the Experiment
-
1.
Freshwater fish are collected from the fishpond.
-
2.
Transfer the fish into 1000-L fiber, glass, or plastic tanks where they are acclimatized and controlled diet is fed for 2 weeks.
-
3.
Maintain the photoperiod in this experiment 16 h light to 8 h dark.
-
4.
Check the water quality every 7 days during this experimental period.
-
5.
Change the water in the experimental tank every morning 20–30%
3.2 Acute and Chronic Toxicity
-
1.
Determine the lethal concentration (LC50) value of acute toxicity in 96 h period.
-
2.
A stratified random approach is used to different concentration cyanotoxin to assign fish to tanks at the beginning of each test among the five treatments and one control, each with two repetitions.
-
3.
Observe the fish tank for the occurrence of dead fish and removed from tanks at 12-h intervals.
-
4.
Find out the LC50 value to fix the higher concentrations and lower concentrations for the chronic toxicity experiment (Adelman et al. 2009).
-
5.
The chronic toxicity assay is performed for the period of 30 days.
-
6.
To examine the toxin accumulation in the fish, every 10 days, dissect the fish and collected the gills, intestine, liver, kidney, and muscle tissue and store in 10× PBS for histology, hematology examination.
3.3 Growth Rate
-
1.
Growth evaluations is calculated the test chemical concentration that impacted fish development during a 30-day period.
-
2.
During measurement, food is withheld for 24 h prior to the fish.
-
3.
Fish are given 100 mg/L of chloroform to make them unconscious for the measurement.
-
4.
Using the scale to measure the length and weight balance to measure the weight of the fish.
3.4 Histopathology
-
1.
Collect the organs and store the tissue using 10% formalin.
-
2.
The organ is fixed in paraffin wax using automatic embedding equipment.
-
3.
Keep the blocks in the freezer for at least 24 h before cutting; use a microtome to cut the block in the size (5 μm).
-
4.
Use hematoxylin-eosin stain in the organs and fix the slide.
-
5.
After preparation of slide, use microscope to examine the organ for histology (Martins et al. 2018).
3.5 Hematology
-
1.
Anesthetize fish using chloroform at a concentration of 100 mL/L.
-
2.
Use 1 mL syringe to collect the blood from the caudal vein of the fish
-
3.
Anticoagulant 10% ethylene diamine tetra-acetate (EDTA) tube is used to collect blood.
-
4.
Analyze the number of white blood cells (WBC), red blood cells (RBC), hematocrit (Hct), hemoglobin (HB), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC) (Abdel-Aziz et al. 2020).
3.6 Serum Biochemistry
-
1.
Anesthetize fish using chloroform at a concentration of 100 mL/L.
-
2.
Use 1 mL syringe to collect the blood from the caudal vein of the fish.
-
3.
After collection wait for 10 min for clotting of blood, then centrifuge at 9000 rpm for 10 min.
-
4.
Collect the serum and freeze dry in −20°C for analyzing the biochemical test.
-
5.
Test the serum to identify the total protein (g/dL), albumin (g/dL), serum globulin, plasma glucose, and creatinine using colorimetric method (Abdel-Aziz et al. 2020).
4 Observation
The Histopathology study shows normal in the control and experimental due to toxicity, the liver’s architecture change. Hepatocytes frequently detached after 20–30 days of treatment. Parenchyma cells are damaged after the long exposure of toxicity.
Evaluation of growth rate is measured using the following equation:
References
Abdel-Aziz M, Bessat M, Fadel A, Elblehi S (2020) Responses of dietary supplementation of probiotic effective microorganisms (EMs) in Oreochromis niloticus on growth, hematological, intestinal histopathological, and antiparasitic activities. Aquac Int 28:947–963
Adelman IR, Kusilek LI, Koehle J, Hess J (2009) Acute and chronic toxicity of ammonia, nitrite, and nitrate to the endangered Topeka shiner (Notropis topeka) and fathead minnows (Pimephales promelas). Environ Toxicol Chem 28(10):2216–2223
Agrawal M, Yadav S, Patel C, Raipuria N, Agrawal MK (2012) Bioassay methods to identify the presence of cyanotoxins in drinking water supplies and their removal strategies. Eur J Exp Biol 2(2):321–336
Duy TN, Lam PK, Shaw GR, Connell DW (2000) Toxicology and risk assessment of freshwater cyanobacterial (blue-green algal) toxins in water. In: Reviews of environmental contamination and toxicology: continuation of residue reviews, pp 113–185
Falfushynska H, Kasianchuk N, Siemens E, Henao E, Rzymski P (2023) A review of common cyanotoxins and their effects on fish. Toxics 11(2):118
Martins ML, Cardoso L, Furtado W, Tancredo KR, Lehmann NB, Figueiredo AB, Steckert LD, Silva KAG, Padua SB, Ferreira TH (2018) Histopathology guide for freshwater fish. Editora UFSC, Florianopolis
Sanseverino I, António DC, Loos R, Lettieri T (2017) Cyanotoxins: methods and approaches for their analysis and detection. Joint Research Centre
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kumar, R., Dhanasekaran, D. (2023). Detection of Cyanotoxin Toxicity in Fish. In: Thajuddin, N., Sankara narayanan, A., Dhanasekaran, D. (eds) Protocols for Cyanobacteria Sampling and Detection of Cyanotoxin . Springer, Singapore. https://doi.org/10.1007/978-981-99-4514-6_29
Download citation
DOI: https://doi.org/10.1007/978-981-99-4514-6_29
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-4513-9
Online ISBN: 978-981-99-4514-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)