Abstract
Modern life is not conceivable without the involvement of nanoparticles. Major aspects of science are dependent on nanotechnology for varied applications. The nanoscale size of nanoparticles, larger surface/volume ratio, characteristic structures, and similar dimensions to biomolecules led to their application in biomedical sciences. In the due course of development, nanoparticle synthesis has come a long way from conventional physical and chemical methods to latest method involving environment-friendly biological synthesis. Microorganisms like bacteria, fungi, blue-green algae, and yeast have been screened and adopted for biological synthesis of variety of metallic nanoparticles. This chapter discusses the detailed mechanism of microbe-mediated biosynthesis of nanoparticles, their applications in healthcare mainly as antimicrobials, and attempts at optimizing the factors influencing the quality of nanoparticles along with the newer dimensions and limitations of this arena.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanotechnology is the branch of science and engineering that involves the design, production, and application of structure, and systems by manipulating atoms and molecules at the nanoscale, i.e. having at least one dimension in the order of 1 nm to 100 nm. It is concerned with the creation of nanoparticles (NPs) that exhibits unique chemical properties, controlled monodispersity, and more reactivity than parent metal atom owing to their high surface-to-volume ratio (Sahoo et al. 2021). NP consists of three layers: a surface layer equipped with a wide range of molecules, surfactants, metal ions, and polymers; a shell layer, above the core, which is the central portion of NP; and the NP itself (Shin et al. 2016). These are classified into different types based on their morphology, size, and shape. Organic NPs include ferritin, micelles, dendrimers, and liposome. Being biodegradable and non-toxic, they are widely used in drug delivery. Inorganic NPs are biocompatible, hydrophilic, and non-toxic besides being more stable than organic ones, and include both metallic as well as non-metallic oxide NPs. Ceramic NPs are non-metallic solids having wide applications in the photodegradation of dyes, photocatalysis, catalysis, and imaging. Bio-nanoparticles are an aggregate of atoms/ions/molecules, which are prepared in the living system naturally (Ijaz et al. 2020).
NPs have been reported to improve crop yield, seed germination, and total biomass of crops (Ali et al. 2021). Metal-based NPs, in particular, have gained significance in the agriculture sector as nanobiofertilizers and nanobiopesticides. Different types of metallic NPs have been created as they exhibit unique physical, chemical, and biological properties and have broad-spectrum applications in areas such as drug delivery, gene transfer, management of insect pests and agriculture and as antibacterial agents (Ghosh et al. 2008; Rai et al. 2011).
There have been innumerable attempts on researching and analyzing the most economical and efficient ways to synthesize the NPs. These are synthesized by physical methods such as the ball milling method, pulsed wire discharge method, and pulse laser ablation. Chemical production of NPs is an expensive, harmful, time-consuming, and low-yielding process; whereas, physical methods require sophisticated and high power consuming equipment (Khodashenas and Ghorbani 2014). Recently, the focus has shifted towards reducing the production cost and to enhance the properties of NPs mediated by various biological systems, which are eco-friendly and safer to use. It involves the production of NPs using a living source, bacteria, fungi, plant extract, etc., and is termed a biological or ‘Green’ synthesis (Mukunthan and Balaji 2012). This chapter describes the biogenic synthesis of NPs via microorganisms, related aspects such as the mechanism taken up by them to reduce metal ions into its nano-form, and factors influencing the same. In addition, it cites recent developments happening in this particular field along with future prospects.
2 ‘Green’ Synthesis of NPs
Conventional physical and chemical methods of synthesizing the NPs are associated with their respective limitations. Physically synthesized NPs are formed by a top-down approach in which bulk material is broken down into nano-size particles. Although it is a fast manufacturing process, a high requirement of energy makes it unsuitable for large-scale production. It also leads to imperfection in surface structure, which affects other properties of the material (Kandasamy and Prema 2015). Chemical methods viz., chemical reduction, microemulsion, and solvothermal decomposition, employ a bottom-up approach using organic or inorganic chemicals acting as reducing (sodium citrate, sodium borohydride, and sodium hydroxide) and stabilizing/capping (PVA, PVP, polyethylene glycol, and polymethacrylic acid) agents (Satyanarayana and Reddy 2018). In the chemical and physical method of NPs synthesis, highly toxic, expensive, and hazardous chemicals are used which are harmful to the environment. The biological method is preferred, as it does not involve toxic chemicals for the synthesis of NPs. It is a non-toxic and energy-efficient method. Herein, NPs are formed by the bottom-up/constructive method by reducing and stabilizing metabolites present in the microbial supernatant/microbial cell or plant extract; thereby, there is no requirement of adding these agents separately (Salem and Fouda 2021). In addition to this, the biogenic method is environmentally safe, and is being seen as a more sustainable way of NPs production as compared to the other conventional methods in use.
3 Microbe-Mediated Biogenic Synthesis of NPs and Their Applications
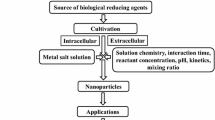
Production of NPs through microorganism is considered one of the most efficient ways of synthesizing NPs, as they are less toxic and covers a wider area of applications. Different types of microbes utilize different kinds of mechanisms to synthesize NPs (Rani et al. 2022). Reducing enzymes, mainly reductase, and stabilizing or capping agents present in microbes help in the conversion of metal into its nanoscaled particles (Rao et al. 2017). Stabilizing and capping agents prevent aggregation, therefore, lead to the formation of stable NPs. On the basis of the location of NPs formation, it can be differentiated as intracellular and extracellular synthesis. The reduction of metal ions into their nanoform within the cell is referred to as the intracellular mode of synthesis. It starts by binding oppositely charged metal ions to the carboxylate groups of the metabolites like polypeptides, and enzymes, present on the microbial cell wall (Zhang et al. 2011). This binding is necessary to facilitate the trapping of metal ions inside the cell, which are reduced to their atomic form by the reducing agents such as NADH-dependent reductase, present in the microbial cell. Then, these atomic nuclei undergo growth leading to the formation of aggregates and ultimately NPs after being stabilized by amino acids such as tryptophan, cysteine, tyrosine, and proteins/peptides (Iravani 2014; Shedbalkar et al. 2014). NPs are found to accumulate in the cytoplasm as well as cell wall (Mukherjee et al. 2001; Yusof et al. 2019). The extracellular mode of synthesis is facilitated by the extracellular enzymes that are either located on the cell membrane or released into the growth medium and mediate the reduction of metals from their ionic to atomic form followed by nucleation to form NPs. A few extracellular proteins also acts as capping agents and stabilize the NPs thus formed (Yusof et al. 2019). A number of microbial metabolites have been found to involve in the extracellular production of NPs (Ovais et al. 2018a, b; Ghosh et al. 2022). Barabadi et al. (2017) and Qamar and Ahmad (2021) discussed a few compounds that are involved in the synthesis of NPs from bacteria and fungi. Chemical structures of some of them have been depicted in Fig. 29.1.
There have been a number of attempts at synthesizing the metallic NPs from the microorganisms in recent times, some of which have been discussed below. Copper NPs are well known for their anti-microbial, anti-oxidant, and catalytic activity (Reddy Mallem 2022). Shantkriti and Rani (2014) reported the synthesis of CuNPs by incubating cell-free culture supernatant of non-pathogenic bacteria P. fluorescens with CuSO4 (Copper sulfate) solution for 90 min. Lv et al. (2018) used Shewanella loihica PV-4 to produce CuNPs having antibacterial activity against E. coli. Noman et al. (2020) studied the production of CuNPs from the bacteria Escherichia sp. within a size range of 22.3 to 39 nm that acts as a photo catalyst during dye degradation. ZnONPs, widely used in antibacterial creams, lotions, ointments, mouthwashes, and paints, also act as antimicrobial agents and biofilm growth inhibitors (Mandal 2016). Spherical Zinc NPs within a size range of 14.39–37.85 nm were reported to be synthesized from the supernatant of a microalgae, Cladophora glomerata (Abdulwahid et al. 2019). A yeast, Pichia kudriavzevii, was also employed to prepare hexagonal zinc NPs by Moghaddam et al. (2017). Nadeem et al. (2018) reported bacterial strains, Bacillus subtilis, and Lactobacillus sp., as a source for the bio-production of TiO2 NPs. Khan et al. (2021) reported that iron oxide magnetic nanoparticles synthesized by Pseudomonas aeruginosa are used in many applications, such as magnetic resonance imaging, diagnostics, and therapeutics. Magnetic NPs with narrow-size distribution help in maintaining the temperature needed as per the exact calculations for cancer treatment. Iron NPs synthesized using Proteus vulgaris ATCC-29905 proved to be excellent anticancer and antimicrobial agents (Nadeem et al. 2021). Spherical AgCl (silver chloride) NPs with a centered cubic crystal structure and a mean particle size of around 10–50 nm synthesized by cell-free culture supernatants of Pantoea agglomerans and Raoultella planticola exhibited antimicrobial activity against Staphylococcus aureus, Streptococcus pyogenes, Salmonella, and Bacillus amyloliquefaciens (Ghiuta et al. 2021). Alsamhary (2020) studied the antibacterial efficacy of Bacillus subtilis-mediated silver NPs to treat MDR (multidrug-resistant) microorganisms. Jebur and Abd (2021) synthesized MgO (magnesium oxide) NPs having different average diameters by two Streptococcus sp., S. salivarius and S. mutans. These NPs showed antibacterial efficacy against MDR, Gram-positive bacterium, Acinetobacter baumannii with a MIC (minimum inhibitory concentration) of 250 μg/mL. In a similar study, Fouda et al. (2021) investigated the antimicrobial potential of MgO-NPs (Source-Penicillium chrysogenum) which exhibited a zone of inhibitions at 200 μg/mL against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans.
4 Factors Affecting the Microbial Synthesis of NPs and Optimization Studies
Microbial species, the concentration of metallic precursor solution, incubation and reaction time, varying concentration of supernatant, pH, and temperature, are the factors that influence the quantity and quality of NPs significantly (Patra and Baek 2014). Not just the metabolites composition of each microbial species is different, but the pathways taken up to synthesize the NPs are also distinct. HR-TEM analysis revealed variations in the size and shape of NPs when the same microbe took up extracellular and intracellular modes of synthesis (Mohd Yusof et al. 2020). Different precursors of similar metals are used along with its varying concentration that influences the characteristics of NPs viz., shape and size. Therefore, a number of studies have been taken up to optimize these parameters in order to obtain NPs of desired quality and yield. One factor at a time (OFAT) is one of the most common approaches to carry this out that involves varying one factor at a time while keeping other factors as standard. Ebadi et al. (2019) optimized the synthesis of ZnONPs from the cell extract of cyanobacterium Nostoc sp. EA03. The maximum quantity of ZnONPs was obtained at a slightly alkaline pH (9) and 1000 μL cell extract concentration. Similarly, Bukhari et al. (2021) optimized the parameters that came out to be the concentration of Cu metal precursor solution (5 mM), reaction time (60 min.), filtrate to substrate ratio (1:1), and pH (7), from marine Streptomyces sp. Table 29.1 depicts various metallic NPs that have been optimized for different parameters affecting their biological synthesis from the microbes.
5 Bimetallic NPs: A New Dimension of Microbe-Based Nanotechnology
Bimetallic NPs, composed of two metals, are prepared by reducing the ions of two different metals simultaneously. These have been reported to improve the catalysts property of the original single metal that creates a new property (Ramsurn and Gupta 2013). The synergy existing between these metals is the major cause of their multi-functionality. They act as effective catalyzers in a variety of reactions owing to their large surface area. Bimetallic nanostructures comprise segregated and mixed nanostructures. Two different metals possess ordered and random arrangements of atoms, in the case of mixed structures like intermetallic and alloy nanoparticles, respectively. Segregated nanostructures, on the other hand, usually involve the formation of an initial structure of one metal type followed by the addition of the second metal. Thus, these are further classified as subcluster structures (separate distribution of two metals with a shared interface), core–shell structures (metal core is surrounded by a shell of second metal), multishell core–shell structures (shells possess alternative arrangement forming a shape like onion rings), and multiple core materials coated by a single shell (Ferrando et al. 2008; Srinoi et al. 2018).
Exhibiting higher activity, selectivity, and stability, they have wide applications in imaging and biomedical devices including nano-medicines (Erkey 2011; Loza et al. 2020). Bimetallic nanocomposites act as sensors and help in the early diagnosis of the disease. They have the ability to detect even 2–3 cancerous cells present in the body and are emerging as a bright option in the diagnostics field (Sharma et al. 2019). They also demonstrate greater antioxidant activity and dye removal efficacy used for wastewater treatment as compared to monometallic NPs (Riaz et al. 2022). Merugu et al. (2021) used the toddy palm extract to synthesize copper-zinc bimetallic NPs having anti-bacterial activity against Alcaligenes faecalis, S. aureus, K. pneumoniae, and Clostridium perfringens. Similarly, Riaz et al. (2022) took up the synthesis of monometallic as well as bimetallic/alloy NPs of zinc and copper from the aqueous extract of Mirabilis Jalapa. An equal volume of the plant extract was added to the aqueous solution of zinc chloride and copper sulfate (precursor salts of both metals) at varying concentrations. The continuous change in color from royal blue to sea green indicated the formation of nanoparticles. Fawzy et al. (2021) suspended cell pellet as well as cell-free supernatant of Pseudomonas fluorescens (PS1and PS2) into ZnSO4 and CuSO4 solution wherein color change indicated the formation of bimetallic (Cu-Zn) NPs. Iron-manganese NPs synthesized using bacteria-fermented supernatant containing auxin complex compounds (indole-3-acetic acid) as reducing agents were evaluated as plant nanofertilizer. Spherical NPs showed a positive impact on seed germination, root development, and fresh weight of Zea mays (de França Bettencourt et al. 2020). They also act as promising antimicrobial agents besides displaying their tendencies in several industries affected by microbes such as water, food, textiles, and oil and gas (Arora et al. 2020).
6 Conclusion
Microbial synthesis of NPs is expected to take a leap forward in the near future owing to its environment friendliness, lesser cost of production, and biocompatibility provided it address a few limitations (Ovais et al. 2018a, b). A slower reduction process, reproducibility issues, and requirements of entirely aseptic infrastructure, besides unelucidated mechanisms underlying the same, can halt the synthesis as well as the application process (Rani et al. 2022). Therefore, besides focusing on exploring various microbial taxa for the synthesis of metallic NPs, researchers should also focus on the details of the reduction and stabilization process. In addition, the procedures involved in the extraction and purification of NPs from microbes needs to be studied in order to facilitate the full-fledged use of this application.
References
Abdulwahid KE, Dwaish AS, Dakhil OA (2019) Green synthesis and characterization of zinc oxide nanoparticles from Cladophora glomerata and its antifungal activity against some fungal isolates. Plant Arch 19:3527–3532. http://plantarchives.org/19-2/3527-3532(5932).pdf. Accessed 26 Nov 2021
Ali A, Shah T, Ullah R, Zhou P, Guo M, Ovais M, Rui Y et al (2021) Review on recent progress in magnetic nanoparticles: synthesis, characterization, and diverse applications. Front Chem 9:629054
Alsamhary KI (2020) Eco-friendly synthesis of silver nanoparticles by Bacillus subtilis and their antibacterial activity. Saudi J Biol Sci 27(8):2185–2191
Arora N, Thangavelu K, Karanikolos GN (2020) Bimetallic nanoparticles for antimicrobial applications. Front Chem 8:412
Barabadi H, Ovais M, Shinwari ZK, Saravanan M (2017) Anti-cancer green bionanomaterials: present status and future prospects. Green Chem Lett Rev 10(4):285–314
Bukhari SI, Hamed MM, Al-Agamy MH, Gazwi HS, Radwan HH, Youssif AM (2021) Biosynthesis of copper oxide nanoparticles using Streptomyces MHM38 and its biological applications. J Nanomater 2021:1
de França Bettencourt GM, Degenhardt J, Torres LAZ, de Andrade Tanobe VO, Soccol CR (2020) Green biosynthesis of single and bimetallic nanoparticles of iron and manganese using bacterial auxin complex to act as plant bio-fertilizer. Biocatal Agric Biotechnol 30:101822
Ebadi M, Zolfaghari MR, Aghaei SS, Zargar M, Shafiei M, Zahiri HS et al (2019) A bio-inspired strategy for the synthesis of zinc oxide nanoparticles (ZnO NPs) using the cell extract of cyanobacterium: Nostoc sp. EA03: from biological function to toxicity evaluation. RSC Adv 9:23508–23525. https://doi.org/10.1039/c9ra03962g
El-Saadony MT, Saad AM, Taha TF, Najjar AA, Zabermawi NM, Nader MM, AbuQamar SF, El-Tarabily KA, Salama A (2021) Selenium nanoparticles from lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J Biol Sci 28(12):6782–6794
Erkey C (2011) Synthesis of nanostructured composites of metals. In: Supercritical fluid science and technology, vol 1. Elsevier, Amsterdam, pp 79–120
Fawzy H, Mohamed Mahmoud S, Ahmed Hanafy M, Hassan Bakr M, Mohamed Mahmoud AE, Abdel-Alim Ali M, Sayed Barakat O (2021) Production of zinc and copper as nanoparticles by green synthesis using Pseudomonas fluorescens. Pak J Biol Sci 24(4):445–453
Ferrando R, Jellinek J, Johnston RL (2008) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108(3):845–910
Fouda A, Hassan SED, Abdel-Rahman MA, Farag MM, Shehal-deen A, Mohamed AA, Azab MS et al (2021) Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr Res Biotechnol 3:29–41
Ghiuta I, Croitoru C, Kost J, Wenkert R, Munteanu D (2021) Bacteria-mediated synthesis of silver and silver chloride nanoparticles and their antimicrobial activity. Appl Sci 11(7):3134
Ghosh P, Han G, De M, Kim CK, Rotello VM (2008) Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 60(11):1307–1315
Ghosh S, Sarkar B, Kaushik A, Mostafavi E (2022) Nanobiotechnological prospects of probiotic microflora: synthesis, mechanism, and applications. Sci Total Environ 838:156212
Hammed A, Polunin Y, Voronov A, Pryor S (2022) Tubular electrosynthesis of silica-coated magnetite and evaluation of magnetic nanobiocatalyst efficacy during biomass hydrolysis. Bioprocess Biosyst Eng 45(8):1311–1318
Ijaz I, Gilani E, Nazir A, Bukhari A (2020) Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett Rev 13(3):223–245
Iravani S (2014) Bacteria in nanoparticle synthesis: current status and future prospects. Int Sch Res Not 2014:1–18. https://doi.org/10.1155/2014/359316
Jebur YM, Abd FG (2021) Biosynthesis of MgO nanoparticles by using streptococcus species and its antibacterial activity. Biochem Cell Arch 21:2557
Kandasamy S, Prema RS (2015) Methods of synthesis of nano particles and its applications. J Chem Pharm Res 7(3):278–285
Khan AA, Khan S, Khan S, Rentschler S, Laufer S, Deigner HP (2021) Biosynthesis of iron oxide magnetic nanoparticles using clinically isolated Pseudomonas aeruginosa. Sci Rep 11(1):1–10
Khodashenas B, Ghorbani HR (2014) Synthesis of copper nanoparticles: an overview of the various methods. Korean J Chem Eng 31(7):1105–1109
Krishnamoorthi R, Bharathakumar S, Malaikozhundan B, Mahalingam PU (2021) Mycofabrication of gold nanoparticles: optimization, characterization, stabilization and evaluation of its antimicrobial potential on selected human pathogens. Biocatal Agric Biotechnol 35:102107
Kumar M, Upadhyay LS, Kerketta A, Vasanth D (2022) Extracellular synthesis of silver nanoparticles using a novel bacterial strain Kocuria rhizophila BR-1: process optimization and evaluation of antibacterial activity. BioNanoScience 12(2):423–438
Loza K, Heggen M, Epple M (2020) Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Adv Funct Mater 30(21):1909260
Lv Q, Zhang B, Xing X, Zhao Y, Cai R, Wang W, Gu Q (2018) Biosynthesis of copper nanoparticles using Shewanellaloihica PV-4 with antibacterial activity: novel approach and mechanisms investigation. J Hazard Mater 347:141–149
Mandal BK (2016) Scopes of green synthesized metal and metal oxide nanomaterials in antimicrobial therapy. In: Nanobiomaterials in antimicrobial therapy. William Andrew Publishing, Norwich, NY, pp 313–341
Merugu R, Gothalwal R, Deshpande PK, De Mandal S, Padala G, Chitturi KL (2021) Synthesis of Ag/Cu and Cu/Zn bimetallic nanoparticles using toddy palm: investigations of their antitumor, antioxidant and antibacterial activities. Mater Today Proc 44:99–105
Moghaddam AB, Moniri M, Azizi S, Rahim RA, Ariff AB, Saad WZ et al (2017) Biosynthesis of ZnO nanoparticles by a new Pichia kudriavzevii yeast strain and evaluation of their antimicrobial and antioxidant activities. Molecules 22:872. https://doi.org/10.3390/molecules22060872
Mohd Yusof H, Abdul Rahman NA, Mohamad R, Zaidan UH, Samsudin AA (2020) Biosynthesis of zinc oxide nanoparticles by cell-biomass and supernatant of lactobacillus plantarum TA4 and its antibacterial and biocompatibility properties. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-76402-w
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI et al (2001) Bioreduction of AuCl4- ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew Chem Int Ed 40:3585–3588. https://doi.org/10.1002/1521-3773(20011001)40:19<3585::AID-ANIE3585>3.0.CO;2-K
Mukunthan KS, Balaji S (2012) Cashew apple juice (Anacardium occidentale L.) speeds up the synthesis of silver nanoparticles. Int J Green Nanotechnol Biomed 4:71–79. https://doi.org/10.1080/19430892.2012.676900
Nadeem M, Tungmunnithum D, Hano C, Abbasi BH, Hashmi SS, Ahmad W, Zahir A (2018) The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem Lett Rev 11(4):492–502
Nadeem M, Khan R, Shah N, Bangash IR, Abbasi BH, Hano C, Celli J (2021) A review of microbial mediated iron nanoparticles (IONPS) and its biomedical applications. Nanomaterials 12(1):130
Noman M, Shahid M, Ahmed T, Niazi MBK, Hussain S, Song F, Manzoor I (2020) Use of biogenic copper nanoparticles synthesized from a native Escherichia sp. as photocatalysts for azo dye degradation and treatment of textile effluents. Environ Pollut 257:113514
Ovais M, Khalil AT, Ayaz M, Ahmad I, Nethi SK, Mukherjee S (2018a) Biosynthesis of metal nanoparticles via microbial enzymes: a mechanistic approach. Int J Mol Sci 19(12):4100
Ovais M, Khalil AT, Islam NU, Ahmad I, Ayaz M, Saravanan M, Shinwari ZK, Mukherjee S (2018b) Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl Microbiol Biotechnol 102(16):6799–6814
Patra JK, Baek KH (2014) Green Nanobiotechnology: factors affecting synthesis and characterization techniques. J Nanomater 2014:219. https://doi.org/10.1155/2014/417305
Qamar SUR, Ahmad JN (2021) Nanoparticles: mechanism of biosynthesis using plant extracts, bacteria, fungi, and their applications. J Mol Liq 334:116040
Rai M, Gade A, Yadav A (2011) Biogenic nanoparticles: an introduction to what they are, how they are synthesized and their applications. In: Metal nanoparticles in microbiology, pp 1–14. https://doi.org/10.1007/978-3-642-18312-6_1
Rajkumar R, Ezhumalai G, Gnanadesigan M (2021) A green approach for the synthesis of silver nanoparticles by Chlorella vulgaris and its application in photocatalytic dye degradation activity. Environ Technol Innov 21:101282
Ramsurn H, Gupta RB (2013) New and future developments in catalysis: Chapter 15. In: Hydrogenation by nanoparticle catalysts. Elsevier, Amsterdam
Rani S, Kumar P, Dahiya P, Dang AS, Suneja P (2022) Biogenic synthesis of zinc nanoparticles, their applications, and toxicity prospects. Front Microbiol 13:824427
Rao BG, Mukherjee D, Reddy BM (2017) Novel approaches for preparation of nanoparticles. In: Nanostructures for novel therapy. Elsevier, Amsterdam, pp 1–36
Reddy Mallem SP (2022) Nano/micro and bio-inspired materials on wide-bandgap-semiconductor-based optoelectronic/power devices. Crystals 12(1):67
Riaz T, Assey N, Javed M, Shahzadi T, Zaib M, Shahid S, Fatima U (2022) Biogenic plant mediated synthesis of monometallic zinc and bimetallic copper/zinc nanoparticles and their dye adsorption and antioxidant studies. Inorg Chem Commun 140:109449
Rosyidah A, Nantapong N, Chudapongse N, Weeranantanapan O, Limphirat W (2021) Optimization of silver nanoparticles synthesis by the green method using Streptomyces sp. SSUT88A and their antimicrobial activity against Pseudomonas aeruginosa. In: IOP conference series: earth and environmental science, vol 948. IOP Publishing, Bristol, p 012085
Sahoo SK, Panigrahi GK, Sahoo A, Pradhan AK, Dalbehera A (2021) Bio-hydrothermal synthesis of ZnO–ZnFe2O4 nanoparticles using Psidium guajava leaf extract: role in waste water remediation and plant immunity. J Clean Prod 318:128522
Saied E, Eid AM, Hassan SED, Salem SS, Radwan AA, Halawa M, Saleh FM, Saad HA, Saied EM, Fouda A (2021) The catalytic activity of biosynthesized magnesium oxide nanoparticles (Mgo-nps) for inhibiting the growth of pathogenic microbes, tanning effluent treatment, and chromium ion removal. Catalysts 11(7):821
Salem SS, Fouda A (2021) Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res 199:344–370. https://doi.org/10.1007/s12011-020-02138-3
Satyanarayana T, Reddy SS (2018) A review on chemical and physical synthesis methods of nanomaterials. Int J Res Appl Sci Eng Technol 6(1):2885–2889
Shantkriti S, Rani P (2014) Biological synthesis of copper nanoparticles using Pseudomonas fluorescens. Int J Curr Microbiol App Sci 3(9):374–383
Sharma G, Kumar A, Sharma S, Naushad M, Dwivedi RP, ALOthman ZA, Mola GT (2019) Novel development of nanoparticles to bimetallic nanoparticles and their composites: a review. J King Saud Univ Sci 31(2):257–269
Sharma B, Kumari N, Mathur S, Sharma V (2022) Kinetics and optimization of azo dye Decolorisation using green synthesized iron-oxide nanoparticles: a pilot scale study. Int J Environ Res 16(4):1–18
Shedbalkar U, Singh R, Wadhwani S, Gaidhani S, Chopade BA (2014) Microbial synthesis of gold nanoparticles: current status and future prospects. Adv Colloid Interface Sci 209:40–48. https://doi.org/10.1016/j.cis.2013.12.011
Shin WK, Cho J, Kannan AG, Lee YS, Kim DW (2016) Cross-linked composite gel polymer electrolyte using mesoporous methacrylate-functionalized SiO2 nanoparticles for lithium-ion polymer batteries. Sci Rep 6(1):1–10
Siddiqui T, Khan NJ, Asif N, Ahamad I, Yasin D, Fatma T (2022) Screening, characterisation and bioactivities of green fabricated TiO2 NP via cyanobacterial extract. Environ Sci Pollut Res 29(26):39052–39066
Singh P, Pandit S, Jers C, Joshi AS, Garnæs J, Mijakovic I (2021) Silver nanoparticles produced from Cedecea sp. exhibit antibiofilm activity and remarkable stability. Sci Rep 11(1):1–13
Srinoi P, Chen YT, Vittur V, Marquez MD, Lee TR (2018) Bimetallic nanoparticles: enhanced magnetic and optical properties for emerging biological applications. Appl Sci 8(7):1106
Wang D, Xue B, Wang L, Zhang Y, Liu L, Zhou Y (2021) Fungus-mediated green synthesis of nano-silver using aspergillus sydowii and its antifungal/antiproliferative activities. Sci Rep 11(1):1–9
Yu X, Li J, Mu D, Zhang H, Liu Q, Chen G (2021) Green synthesis and characterizations of silver nanoparticles with enhanced antibacterial properties by secondary metabolites of Bacillus subtilis (SDUM301120). Green Chem Lett Rev 14(2):190–203
Yusof NAA, Zain NM, Pauzi N (2019) Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against gram-positive and gram-negative bacteria. Int J Biol Macromol 124:1132–1136. https://doi.org/10.1016/j.ijbiomac.2018.11.228
Zhang X, Yan S, Tyagi RD, Surampalli RY (2011) Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 82:489–494. https://doi.org/10.1016/j.chemosphere.2010.10.023
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rani, S., Kumar, P., Dahiya, P., Priya, Dang, A.S., Suneja, P. (2023). Synthesis of Nanoparticles by Microbes. In: Sobti, R., Kuhad, R.C., Lal, R., Rishi, P. (eds) Role of Microbes in Sustainable Development. Springer, Singapore. https://doi.org/10.1007/978-981-99-3126-2_29
Download citation
DOI: https://doi.org/10.1007/978-981-99-3126-2_29
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-3125-5
Online ISBN: 978-981-99-3126-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)