Abstract
Food safety is a worldwide problem and ensuring food safety requires strict monitoring of toxins, antibiotics, and bacteria. Thus, sensitive methods of analysis to determine the types and residues of harmful components in food are needed. Point-of-care sensors have been abundantly developed over the last two decades and are proven to be innovative to analyze the contaminants present in food samples both quantitatively and qualitatively. In this book chapter, an overview of aptamer-based optical methods for monitoring food safety is provided. This chapter will focus on optical biosensing techniques such as UV-visible spectroscopy, fluorescence spectroscopy, surface-enhanced Raman spectroscopy, and photonic crystals. The principle, mechanism, advantages, and limitations of each technique are described with an ample number of examples.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Food safety is extremely important for better nutrition, good health, and good quality of life. There is a general propensity to link food and health, to boost well-being and prevent diseases. Any spoilage, deterioration, contamination, pathogenic infestation, or adulteration of food not just leads to degrading its nutritional value, but also consuming these contaminated foods steers foodborne diseases. Foodborne illnesses are a global health problem that contributes to social and economic burden of many countries. According to the World Health Organization (WHO), there are more than 200 food-related diseases that are caused by ingesting contaminated or spoiled food. Approximately 600 million people get sick by contaminated food, of which 4.2 lakhs die every year. Children under 5 years of age are worse affected with 40% of all illnesses and 1.25 lakh deaths every year (World Health Organization 2022). Besides this, foodborne diseases account for economic losses worth billions of dollars around the world.
In the last 30 years, there is an enormous growth in the food sector to fulfill the need of big population and to adapt to changes in lifestyles. Increasing consumption of ready-to-eat food items leads to many socioeconomic and health-related impacts. Many microbiological and chemical changes occur throughout the food processing, distribution, and storage that affects the shelf life and quality of the food, which in turn negatively affects consumer health. Escalating regulatory requirements to control the presence of unwanted or harmful molecules in food leads to increasing food safety concerns. Scientific groups working in food science are constantly required to provide the sufficient answers to the users, whose awareness and concerns for food safety are accelerating (Arduini et al. 2016). Existing methods to assess food safety are chromatographic techniques like gas chromatography, thin-layer chromatography, or high-performance liquid chromatography and advanced techniques such as quantitative real-time polymerase chain reaction (qPCR) and enzyme-linked immunosorbent assay (ELISA). These techniques are costly, time-consuming, laborious, and complex and need heavy instruments and experienced personnel. All these limitations make them difficult to apply in rural areas or resource-limited places where the foodborne diseases are prevalent (Choi et al. 2019). To overcome these problems, biosensors can be a solution (Akhtar et al. 2018, Chandra et al. 2010, and Chandra and Prakash 2020). Over the last two decades, biosensors have emerged as a crucial tool for the analysis of food, and researchers are working on many analytical strategies and technologies for the easy, fast, reliable, sensitive, and economic detection of contaminants, toxins, pesticides, carcinogens, and foodborne pathogens.
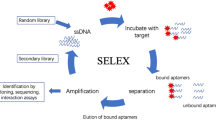
Most common biorecognition elements for the development of biosensors are antibodies, enzymes, molecularly imprinted polymers (MIP), and aptamers. Morales and Halpern (2018) have very well explained different types of biorecognition molecules that have been used for the development of biosensors and how to select the biorecognition element for a biosensor. Aptamers are short single-stranded oligonucleotides that are identified by an iterative process called SELEX (selective evolution of ligand by exponential enrichment). They are known as chemical antibodies, but are easier to produce without any batch difference. Chemical modifications of aptamers are also relatively easy, and therefore, various types of distinct tags can be used for sensing applications (Groff et al. 2015; Kalyani et al. 2021a). In addition to this, they can be extended at both 3′ and 5′ ends for tagging and binding or to incorporate enzymatic activity (Setlem et al. 2020). Aptamers are being used in many biosensors as they have many advantages such as high affinity, small size, no batch variation, easy chemical modifications, and faster production (Fig. 6.1). They have been exploited in conjugation with various detection techniques, viz., fluorescence, SERS, electrochemical, lateral flow assays, and quartz crystal microbalance (QCM), to fabricate biosensors (Dhiman et al. 2017; Arroyo-Currás et al. 2020; Kalyani et al. 2021a; Bayramoglu et al. 2022).
Over the last two decades, there has been exponential growth in the development of optical biosensors as they provide various advantages over other detection techniques. They have been applied for simple, cheap, rapid, and label-free sensing of several chemical and biological molecules in real time. Optical biosensors exploit the interaction of optical field with a biorecognition element that could be an antibody, enzyme, receptor, MIP, or aptamer. They were implemented for clinical diagnosis, food industries, environmental sensing, and medicine. The most commonly used aptamer-based optical methods for food safety applications are colorimetric assays, fluorescence spectroscopy, surface plasmon resonance (SPR), surface-enhanced Raman spectroscopy (SERS), and photonic crystals (Chen and Wang 2020; Asghari et al. 2021; Kaur et al. 2022). The principle, mechanism, advantages, and limitations of each of these techniques are explained with examples in further sections.
6.2 Colorimetric Sensors
It is one of the most widely employed biosensing techniques where target detection can be achieved via observing a change in the color of reaction mixture. The color change is visible to the naked eyes and can be easily quantified using a spectrophotometer (Kalyani et al. 2021b). Aptamer-based colorimetric biosensors employ target-specific aptamers, selected often using SELEX method, and involve conjugation of these aptamers with various nanostructures or enzymes for producing color changes visible to naked eyes. Gold nanoparticles (AuNPs) are most commonly used nanostructures in conjugation with target-specific aptamers where binding of target and aptamer results in salt-induced aggregation of AuNPs and color change. In a study, a highly specific ssDNA aptamer for oxytetracycline in conjugation with gold nanoparticles has been designed where binding of aptamer and target causes release of AuNPs followed by salt-induced aggregation, thereby inducing color change from red to purple (Kim et al. 2010b). Similarly, AuNP-based colorimetric sensors have been devised for detection of mercuric ions (Li et al. 2009), ochratoxin A (Yang et al. 2022), and bisphenol A (Zhang et al. 2016).
In addition to using AuNPs, other novel mechanisms have also been employed for colorimetric assays. In a study a structure-switchable aptasensor has been devised for the detection of aflatoxin B1. Here an aflatoxin B1-specific aptamer and two split halves of DNAzymes having complementary sequence are made to hybridize, resulting in the formation of functional G-quadruplex structure capable of carrying out color change reactions (Fig. 6.2). In the presence of aflatoxin B1, aptamer-DNAzyme complex undergoes structural changes causing release of halves of DNAzymes rendering it incapable of catalyzing color change reaction, thus causing decrease in colorimetric signal (Seok et al. 2015). In the absence of target, the peroxidase-mimicking split DNAzyme is in right configuration and converts light green ABTS to dark green-colored radical anion. When target is present, the DNAzyme remains in split form and no reaction occurs. Table 6.1 includes the list of the colorimetric sensors developed for food safety.
Colorimetric detection of aflatoxin B1 using structure-switching aptasensor. Reused with permission from Seok et al. (2015). Elsevier © 2015
6.3 Fluorescence Sensors
Another type of widely used optical sensor is fluorescence biosensor and used to quantify a number of pollutants and contaminants like heavy metals, antibiotics, food allergens, and toxins. A typical fluorescence-based aptasensor involves the use of a fluorophore and a quencher. Fluorescence-based aptasensors are divided into two groups: (1) “turn-on” fluorescence, i.e., increase in fluorescence can be observed when target molecule binds with aptamer, and (2) “turn-off” fluorescence, where binding leads to decrease in fluorescence (Nsibande and Forbes 2016; Kiruba Daniel et al. 2019; Kalyani et al. 2020). In turn-on fluorescence biosensors, initially fluorophore compound is positioned very close to quencher, and in the presence of target molecule, aptamer-target binding leads to change in its conformation, thus separating the fluorophore from quencher, and increase in fluorescence can be observed. For instance, graphene oxide (GO) sheets have been used to quench the fluorescence of quantum dots in the absence of target molecule (Lu et al. 2015). For the detection of lead (II), the aptamer-QD conjugates are bound to GO sheets, and the resulting energy transfer between QDs and GO leads to quenching. In the presence of lead ions, aptamer-Pb2+ binding causes conformational change in aptamer causing the release of aptamer-QD-Pb2+ complex from GO sheets, and restoration of fluorescence can be observed (Li et al. 2013). Similarly, using turn-on fluorescence-based technique, CdTe quantum dots-aptamer-GO complex was employed for the detection of aflatoxin B1 (AFB1).
In turn-off sensors, the presence of target molecule brings fluorophore and quencher in close proximity, thus reducing the fluorescence signal, which was otherwise powerful in the absence of target (Akki and Werth 2018). There are other mechanisms which are based on photoinduced electron transfer (PIET) mechanism between fluorescent molecules and guanine residues. For instance, for the rapid detection of ochratoxin A (OTA), carboxyfluorescein (FAM)-labeled aptamer, when hybridized to its complementary strand containing guanine residue at its 3″ end, results in the quenching of fluorescence (Fig. 6.3). In the absence of ochratoxin A, the aptamer is bound to complementary strand that leads to fluorescence quenching. In the presence of target, the complementary strand leaves the aptamer resulting in fluorescence. OTA presence causes the binding of OTA with FAM-labeled aptamer, releasing complementary strand and restoration of fluorescence (Zhao et al. 2019). Table 6.2 highlights some of the aptamer based fluorescensce sensors developed for food safety.
Fluorescence-based sensor for the detection of ochratoxin A. (Zhao et al. 2019)
6.4 Surface Plasmon Resonance-Based Sensors
Surface plasmon resonance (SPR)-based sensors rely on the strong oscillation of electromagnetic field at the interface of dielectric medium and nanometal film via p-polarized incident light. At specific incident angle and wavelength of light, this results in a dark band profile of light reflectivity (Prabowo et al. 2018). In SPR, the recognition molecule can be immobilized on chip to capture the target. Various nanostructures have been studied with different SPR properties (Mahato et al. 2018). It provides real-time signals of recognition molecule and target interactions by closely monitoring the change in refractive index. SPR sensors are very sensitive to the external solution refractive index, and the resonant angle and wavelength alter the external solution refractive index. SPR offer advantages of label-free and real-time detection of molecules (Wang et al. 2018). As they are highly sensitive and label-free, they have been extensively used for biological sensing. SPR biosensors have been shown to detect various biomolecules such as virus, proteins, bacteria, and small molecules (Chinowsky et al. 2007; Wang and Zhao 2018; Akgönüllü et al. 2020).
Wang et al. have developed an SPR-based tetrahedron-assisted aptasensor to detect tetracycline (Wang et al. 2018). To reduce steric hindrance between aptamer molecules and improve the accessibility of the aptamer to tetracycline, DNA tetrahedron was used (Fig. 6.4). Using this strategy, aptamer can be oriented in both directions: lateral and vertical, with precisely 6 nm distance. The pyramid structure of the tetrahedron can act as spacer and provide enough space for aptamer to fold properly. It is reported that there was tenfold improvement when DNA tetrahedron is used. In the absence of tetrahedron, the LOD was 0.0183 μg/kg, whereas when aptamer was properly oriented with the help of DNA tetrahedron, the LOD was 0.0069 μg/kg. The specificity of the sensor was tested with close analogues of tetracycline, viz., oxytetracycline and chlortetracycline. For real sample, different types of honey samples were spiked with tetracycline and recovery of 86–114% was observed.
A novel triple-amplification SPR electro-chemiluminescence (ECL) approach was developed by Miao et al. for detecting chloramphenicol (Miao et al. 2016). It is based on horseradish peroxidase enzyme-linked polymer (EV) and single-stranded DNA-binding protein (SSB) attached to gold nanoparticles (EV-Au-SSB) as nanotracer and exonuclease-assisted target recycling. Au nanoparticles in the EV-Au-SSB system efficiently enhance the intensity of ECL of nanocrystals by −1.35 V using SPR technique.
6.5 Surface-Enhanced Raman Scattering (SERS) Sensors
In recent years, SERS has been used for its unique molecular sensitivity and spectral resolution [18] in biomolecular analysis (Lee et al. 2015; Hanif et al. 2017), food industry (Deneva et al. 2019; Muhammad et al. 2020), antibiotics response (Moritz et al. 2010; Kumar et al. 2020), and detection of water pollutants. SERS is an enhancement in the Raman signal from molecules adsorbed on the surface of the SERS substrate. However, this phenomenon can be explained by two general mechanism that are accepted but have distinct factors: electromagnetic enhancement mechanism and chemical enhancement mechanism (Stiles et al. 2008; Ding et al. 2017). The mechanism of electromagnetic factor is mainly based on electric field enhancement caused by LSPR (localized surface plasmon resonance), while chemical enhancement mechanism is based on the charge transfer state between the SERS substrate and molecule absorbed on the nanoparticles (chemisorbed molecules) (Stiles et al. 2008). Therefore, the production of such substrates or platforms is very important, and it’s still a huge task to improve the specificity and sensitivity of Raman signals in complex environments like biological samples.
The specificity and sensitivity factor of aptamer-based SERS assays is due to the specific interaction of sample target molecules with the aptamer. There are generally two types of aptamer-based SERS tests: with Raman-labeled molecule (label-based) and without Raman-labeled molecule (label-free). In the label-free strategy, the aptamer can interact with the target molecule and induce conformal changes that alter the aptamer’s Raman signal. On the other hand, in Raman labeling method, externally sensitive Raman molecules attached to the detected target help with the measurement. In both the cases, specificity is a main key characteristic of aptamer-based detection, which depends on the target molecule interaction and the capturing DNA sequences of aptamer, and sensitivity depends on the Raman signal generated by various mechanisms of amplification, including the SERS effect, which can be significantly improved.
Since the first application of aptamers in SERS (Barhoumi et al. 2008a, 2008b; Kim et al. 2010a), many SERS biosensors based on diversified aptamers have been developed. It is expected for these sensors to be sensitive and targeted to facilitate quick diagnosis in various fields and applications. Wang et al. review the detection of various substances using aptamer-based SERS sensors (Wang et al. 2019). Similarly, the free use of aptamer labels in Raman spectroscopy is also discussed by Scatena et al. (2019). Table 6.3 lists the SERS based sensors developed for food safety.
An interesting strategy for using SERS-aptamer-based sensors is to combine them with catalytic reactions (Li et al. 2018a; Sun et al. 2019a; Yang et al. 2020). The fundamental idea is to replace the detection target molecule, which can be more easily detected or enhanced by a catalytic reaction. When encountering biological enzymes, the combination of catalytic amplification and SERS becomes especially important. Basically, the reaction product is enhanced by the introduction of the molecule or the catalytic enhancement of the catalytic activity of the process. The design of target-specific enzymatic reactions is very useful for the development of aptamer-SERS biosensors, especially when enzymes start acting on DNA/RNA.
Fang et al. reported a dual mode of aptamer-attached and enzyme-assisted SERS module for the detection of chloramphenicol (CAP) antibiotic trace at very low section limit of 15 fM (Fang et al. 2019). In this study, a special aptamer was designed against CAP (anti-CAP) which can recognize the alteration in the conformation of the analyte, which initiates the de-hybridization of the target aptamer’s DNA (Fig. 6.5a). The addition of a DNA probe labeled with the reporter gene and the exonuclease (III) increases the SERS signal when hybridized with the captured DNA due to the creation of high number of surplus DNA molecules during the amplification loop.
(a) Chloramphenicol (CAP), the target molecule recognition process induces a structural change in biorecognition aptamer that is further enhanced by multiple rounds of DNA hybridization with Exo III. (b) An aptamer-functionalized sensor for mercuric ions detection uses GO to assist the HAuCl4 reduction reaction. Adapted from reference Fang et al. (2019)
In addition, the catalytic reaction approach can facilitate the generation of SERS-active nanoparticles that can enhance the SERS signal. Li et al. presented redox-GO reactions for the synthesis of enhanced SERS nanoparticles (Fig. 6.5b) that can amplify the SERS signaling reporter molecules for indirect identification of mercury ions (Li et al. 2018b). The aptamer binds to the target Hg2+ specifically in their presence which leads to the inhibition of their further adsorption onto the GO surface. Due to this process, catalysis reaction of citrate and HAuCl4 took place on GO surface that leads to production of AuNPOs that can be detected via SERS.
The sensitive detection of contaminants in food requires pretreatment to inhibit interference from nontarget molecules. Using gold nanoparticle conjugated with Raman tag 4-(mercaptomethyl) benzonitrile aptamers as Raman probes and AgNPs tagged cDNA-as signal enhancers, a new SERS-based aptameric sensor has been developed to minimize interference from nontarget species. When food samples contain acetamiprid, the target aptamer complex prevents the appearance of MMBN-AuNPs-aptamer-cDNA-AgNPs@Si, and the intensity of the Raman signal MMBN in Au-AgNPs@Si decreases. The real sample testing of the sensor was done to sense acetamiprid in apple juice with a detection limit of only 6.8 nm, which is expected to be used to detect traces of pesticides in complex food matrices.
6.6 Photonic Crystal-Based Sensors
Photonic crystals can be defined as the highly ordered nanostructures with varying dielectric constant and periodic scale of visible light wavelengths. Photonic crystals are nanomaterials and dielectrics having optical sensing features. Their nanostructures affect the movement of photons via periodic spatial modulation of refractive index. The repeated array of refractive index and Bragg reflections on photonic crystals lattice structure lead to impeded propagation of light and culminate in the formation of photonic bandgap (Fathi et al. 2021). Analogous to flow of electrons in semiconductors, photons move in photonic crystals, and the hindrance in the propagation of photons in all directions led to full photonic bandgap. A pseudogap prohibits photon propagation in only some directions and results in incomplete photonic bandgap. The capability of photonic crystals to alter the spectral position at certain frequencies and the presence of photonic bandgap makes them really interesting and useful material for application in biosensors. Notably, there is no absorption of light in the process, and the photonic bandgap corresponds to the reflection of light via periodic arrays. In the simplest way, the optical sensing can be performed by monitoring the photonic crystal reflectivity shift or transmission spectra (Zhang et al. 2008). Photonic crystals such as liquid crystals, inverse opals, and fibers have been widely exploited for biosensing applications. Photonic crystal-incorporated biosensors have been used to detect a number of molecules like proteins, nucleic acids, pathogens, viruses, and cancer (Shafiee et al. 2014; Panda and Puspa Devi 2020).
Photonic crystal structures comprised of spatially organized periodic dielectric material that uniquely interacts with light, which results in high-efficiency reflection at specific wavelengths. Naturally, there are many photonic crystal-type nanostructures that exist (Vukusic and Sambles 2003). The most common examples are peacock, and Morpho rhetenor butterfly (Kinoshita et al. 2002; Zi et al. 2003). Apart from this, sea mouse, opals, and Eupholus magnificus also have geometrical patterns on the surface like photonic crystals through which light illuminates and reflects (McPhedran et al. 2003; Marlow et al. 2009; Pouya et al. 2011). On the basis of their geometry, the periodicity can be iterated in one, two, and three directions that implies the different dielectric constants. Thus, the fabrication of the photonic crystals can be done in one-dimensional (1D), two-dimensional (2D), or three-dimensional (3D) orientation (Fig. 6.6). Also, different types of materials have been used for their fabrication such as glass, polymer, silicon, colloids, and silk (Colvin 2001; Edrington et al. 2001; Jamois et al. 2003; Meseguer 2005; Freeman et al. 2008; González-Urbina et al. 2011; Kim et al. 2012; Han et al. 2012; MacLeod and Rosei 2013; Diao et al. 2013). Various top-down (electron beam lithography, thin film deposition, nanoimprint lithography, and electrochemical etching) and bottom-up (self-assembly) approaches have been utilized for the fabrication of photonic structures (López 2003; Kouba et al. 2006). Photonic crystals can be fabricated via various economic fabrication methods like colloidal self-assembly, mold-based replica printing, and hydrogels (Choi and Cunningham 2007; Yan et al. 2011; Fenzl et al. 2013). The advantages of photonic crystal-based biosensors over other techniques are cost-efficient fabrication and shorter assay time. Table 6.4 constitutes the faad safety sensors reported using photonic crystals.
Various examples of photonic crystals in nature constituting different colors: (a) one-dimensional neck feathers of domestic pigeons; (b) 1D, wings of Morpho butterflies; (c) two-dimensional, barbules of male peacocks; (d) 2D, iridescent setae from polychaete worms; (e) three-dimensional green spots in the wings of Parides sesostris butterfly; (f) 3D – Lamprocyphus augustus beetle (Chiappini et al. 2020)
6.7 Future Perspective and Conclusion
Optical biosensors are common analytical tools that can be utilized for point-of-care applications. Their small size enables their use in high-throughput applications to test wide array of samples in different conditions. Many optical biosensors are integrated with nanoparticles to enhance sensitivity or specificity. While developing an optical sensor for real-time detection, crucial factors to consider are simplicity, selectivity, sensitivity, robustness, and ease of use. All the discussed techniques have the potential to be practically employed in POC applications for food safety. Various optical sensors have been reported for food safety to detect various contaminants like toxins, pathogens, pesticides, and heavy metals. However, many sensors are in development stage and require substantial efforts before applying in real applications. The major challenge in the field of optical sensing for food safety is to develop the point-of-care sensors that are accessible and commercially available for resource-limited settings or remote areas so that illnesses or harmful effects due to adulterated and contaminated food products can be prevented.
References
Akgönüllü S, Yavuz H, Denizli A (2020) SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 219:121219
Akhtar MH, Hussain KK, Gurudatt NG, Chandra P, Shim Y-B (2018) Ultrasensitive dual probe immunosensor for the monitoring of nicotine induced-brain derived neurotrophic factor released from cancer cells. Biosens Bioelectron 116:108
Akki SU, Werth CJ (2018) Critical review: DNA Aptasensors, are they ready for monitoring organic pollutants in natural and treated water sources? Environ Sci Technol 52:8989–9007
Arduini F, Cinti S, Scognamiglio V, Moscone D (2016) Nanomaterials in electrochemical biosensors for pesticide detection: advances and challenges in food analysis. Microchim Acta 183:2063–2083
Arroyo-Currás N, Dauphin-Ducharme P, Scida K, Chávez JL (2020) From the beaker to the body: translational challenges for electrochemical, aptamer-based sensors. Anal Methods 12:1288–1310
Asghari A, Wang C, Yoo KM et al (2021) Fast, accurate, point-of-care COVID-19 pandemic diagnosis enabled through advanced lab-on-chip optical biosensors: opportunities and challenges. Appl Phys Rev 8:031313
Barhoumi A, Zhang D, Halas NJ (2008a) Correlation of molecular orientation and packing density in a dsDNA self-assembled monolayer observable with surface-enhanced Raman spectroscopy. J Am Chem Soc 130:17
Barhoumi A, Zhang D, Tam F, Halas NJ (2008b) Surface-enhanced Raman spectroscopy of DNA. J Am Chem Soc 130:5523–5529
Bayraç C, Eyidoğan F, Avni Öktem H (2017) DNA aptamer-based colorimetric detection platform for Salmonella Enteritidis. Biosens Bioelectron 98:22–28
Bayramoglu G, Kilic M, Yakup Arica M (2022) Selective isolation and sensitive detection of lysozyme using aptamer based magnetic adsorbent and a new quartz crystal microbalance system. Food Chem 382:132353
Birader K, Kumar P, Tammineni Y et al (2021) Colorimetric aptasensor for on-site detection of oxytetracycline antibiotic in milk. Food Chem 356:129659
Caglayan MO (2020) Aptamer-based ellipsometric sensor for ultrasensitive determination of aminoglycoside group antibiotics from dairy products. J Sci Food Agric 100:3386–3393
Chandra P, Das D, Abdelwahab AA (2010) Gold nanoparticles in molecular diagnostics and therapeutics. Dig J Nanomater Biostruc 5:363
Chandra P, Prakash R (2020) Nanobiomaterial engineering. Springer, Singapore. https://doi.org/10.1007/978-981-32-9840-8
Chen C, Wang J (2020) Optical biosensors: an exhaustive and comprehensive review. Analyst 145:1605–1628
Chiappini A, Tran LTN, Trejo-García PM et al (2020) Photonic crystal stimuli-responsive chromatic sensors: a short review. Micromachines (Basel) 11:290
Chinowsky TM, Soelberg SD, Baker P et al (2007) Portable 24-analyte surface plasmon resonance instruments for rapid, versatile biodetection. Biosens Bioelectron 22:2268–2275
Choi CJ, Cunningham BT (2007) A 96-well microplate incorporating a replica molded microfluidic network integrated with photonic crystal biosensors for high throughput kinetic biomolecular interaction analysis. Lab Chip 7:550–556
Choi JR, Yong KW, Choi JY, Cowie AC (2019) Emerging point-of-care technologies for food safety analysis. Sensors (Basel) 19:817
Colvin VL (2001) From opals to optics: colloidal photonic crystals. MRS Bull 26:637–641
Dang X, Zhang X, Zhao H (2019) Signal amplified photoelectrochemical sensing platform with g-C3N4/inverse opal photonic crystal WO3 heterojunction electrode. J Electroanal Chem 840:101–108
Deneva V, Bakardzhiyski I, Bambalov K (2019) Using Raman spectroscopy as a fast tool to classify and analyze Bulgarian winesa feasibility study. Molecules 25:170. https://doi.org/10.3390/molecules25010170
Dhiman A, Kalra P, Bansal V et al (2017) Aptamer-based point-of-care diagnostic platforms. Sens Actuators B Chem 246:535–553
Diao YY, Liu XY, Toh GW et al (2013) Multiple structural coloring of silk-fibroin photonic crystals and humidity-responsive color sensing. Adv Funct Mater 23:5373–5380
Ding S-Y, You E-M, Tian Z-Q, Moskovits M (2017) Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem Soc Rev 46:4042
Écija-Arenas Á, Kirchner EM, Hirsch T, Fernández-Romero JM (2021) Development of an aptamer-based SPR-biosensor for the determination of kanamycin residues in foods. Anal Chim Acta 1169:338631
Edrington AC, Urbas AM, DeRege P et al (2001) Polymer-based photonic crystals. Adv Mater 13:421–425
Fang Q, Li Y, Miao X et al (2019) Sensitive detection of antibiotics using aptamer conformation cooperated enzyme-assisted SERS technology. Analyst 144:3649–3658
Fathi F, Rashidi MR, Pakchin PS et al (2021) Photonic crystal based biosensors: Emerging inverse opals for biomarker detection. Talanta 221:121615
Fenzl C, Wilhelm S, Hirsch T, Wolfbeis OS (2013) Optical sensing of the ionic strength using photonic crystals in a hydrogel matrix. ACS Appl Mater Interfaces 5:173–178
Freeman D, Grillet C, Lee MW et al (2008) Chalcogenide glass photonic crystals. Photonics Nanostruct 6:3–11
González-Urbina L, Baert K, Kolaric B et al (2011) Linear and nonlinear optical properties of colloidal photonic crystals. Chem Rev 112:2268–2285
Groff K, Brown J, Clippinger AJ (2015) Modern affinity reagents: recombinant antibodies and aptamers. Biotechnol Adv 33:1787–1798
Guo X, Wen F, Qiao Q et al (2019) A novel graphene oxide-based aptasensor for amplified fluorescent detection of aflatoxin M1 in milk powder. Sensors 19:1–9
Ha NR, Jung IP, La IJ, Jung HS, Yoon MY (2017) Ultra-sensitive detection of kanamycin for food safety using a reduced graphene oxide-based fluorescent aptasensor. Sci Rep 7(1):40305
Han MG, Shin CG, Jeon SJ et al (2012) Full color tunable photonic crystal from crystalline colloidal arrays with an engineered photonic stop-band. Adv Mater 24:6438–6444
Hanif S, Liu H, Chen M et al (2017) Organic cyanide decorated SERS active nanopipettes for quantitative detection of hemeproteins and Fe3+ in single cells. Anal Chem 89:2522–2530
Jamois C, Wehrspohn RB, Andreani LC et al (2003) Silicon-based two-dimensional photonic crystal waveguides. Photonics Nanostruct 1:1–13
Jiang Y, Sun DW, Pu H, Wei Q (2019) Ultrasensitive analysis of kanamycin residue in milk by SERS-based Aptasensor. Talanta 197:151–158
Kalyani N, Chatterjee B, Sharma TK (2021a) Aptamer mediated sensing of environmental pollutants utilizing peroxidase mimic activity of nanozymes. In: Nanozymes for environmental engineering. Environmental chemistry for a sustainable world. Springer, Cham, pp 111–143
Kalyani N, Goel S, Jaiswal S (2020) Point-of-care sensors for on-site detection of pesticides. In: Tuteja SK, Arora D, Dilbaghi N, Lichtfouse E (eds) Nanosensors for environmental applications. Springer Nature, Cham, pp 197–224
Kalyani N, Goel S, Jaiswal S (2021b) On-site sensing of pesticides using point-of-care biosensors: a review. Environ Chem Lett 19:345–354
Kaur B, Kumar S, Kaushik BK (2022) Recent advancements in optical biosensors for cancer detection. Biosens Bioelectron 197:113805
Kim NH, Lee J, Moskovits M (2010a) Aptamer-mediated surface-enhanced Raman spectroscopy intensity amplification. Nano Lett 10:4181–4185
Kim S, Mitropoulos AN, Spitzberg JD et al (2012) Silk inverse opals. Nat Photonics 6:818–823
Kim TY, Lim JW, Lim MC et al (2020) Aptamer-based fluorescent assay for simple and sensitive detection of fipronil in liquid eggs. Biotechnol Bioprocess Eng 25:246–254
Kim YS, Kim JH, Kim IA et al (2010b) A novel colorimetric aptasensor using gold nanoparticle for a highly sensitive and specific detection of oxytetracycline. Biosens Bioelectron 26:1644–1649
Kinoshita S, Yoshioka S, Kawagoe K (2002) Mechanisms of structural colour in the Morpho butterfly: cooperation of regularity and irregularity in an iridescent scale. Proc R Soc B Biol Sci 269:1417
Kiruba Daniel SCG, Kumar A, Sivasakthi K, Thakur CS (2019) Handheld, low-cost electronic device for rapid, real-time fluorescence-based detection of Hg2+, using aptamer-templated ZnO quantum dots. Sens Actuators B Chem 290:73–78
Kouba J, Kubenz M, Mai A et al (2006) Fabrication of nanoimprint stamps for photonic crystals. J Phys Conf Ser 34:149
Kumar S, Gopinathan R, Chandra GK et al (2020) Rapid detection of bacterial infection and viability assessment with high specificity and sensitivity using Raman microspectroscopy. Anal Bioanal Chem 412:2505–2516
Lee C, Carney RP, Hazari S et al (2015) 3D plasmonic nanobowl platform for the study of exosomes in solution. Nanoscale 7:9290–9297
Li C, Qin Y, Li D et al (2018a) A highly sensitive enzyme catalytic SERS quantitative analysis method for ethanol with Victoria blue B molecular probe in the stable nanosilver sol substrate. Sens Actuators B Chem 255:3464–3471
Li C, Wang X, Liang A et al (2018b) A simple gold nanoplasmonic SERS method for trace Hg2+ based on aptamer-regulating graphene oxide catalysis. Luminescence 33:1113–1121
Li M, Zhou X, Guo S, Wu N (2013) Detection of lead (II) with a “turn-on” fluorescent biosensor based on energy transfer from CdSe/ZnS quantum dots to graphene oxide. Biosens Bioelectron 43:69–74
Li Q, Liang B, Li W et al (2021) A capillary device made by aptamer-functionalized silica photonic crystal microspheres for the point-of-care detection of Ochratoxin A. Sens Actuators B Chem 330:129367
Li Q, Lu Z, Tan X et al (2017) Ultrasensitive detection of aflatoxin B1 by SERS aptasensor based on exonuclease-assisted recycling amplification. Biosens Bioelectron 97:59–64
Li T, Dong S, Wang E (2009) Label-free colorimetric detection of aqueous mercury ion (Hg2+) using Hg 2+-modulated G-quadruplex-based dnazymes. Anal Chem 81:2144–2149
Liu R, Huang Y, Ma Y et al (2015) Design and synthesis of target-responsive aptamer-cross-linked hydrogel for visual quantitative detection of ochratoxin A. ACS Appl Mater Interfaces 7:6982. https://doi.org/10.1021/acsami.5b01120
López C (2003) Materials aspects of photonic crystals. Adv Mater 15:1679–1704
Lu Z, Chen X, Wang Y, Zheng X, Li CM (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182:571–578
Lu Y, Zhong J, Yao G, Huang Q (2018) A label-free SERS approach to quantitative and selective detection of mercury (II) based on DNA aptamer-modified SiO2@Au core/shell nanoparticles. Sens Actuators B Chem 258:365–372
Lu Z, Chen X, Wang Y et al (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182:571–578
Lu Z, Zhang H, Huang J et al (2022) Gelatinase-responsive photonic crystal membrane for pathogenic bacteria detection and application in vitro health diagnosis. Biosens Bioelectron 202:114013
Luan Y, Lu A, Chen J, Fu H, Xu L (2016) A label-free aptamer-based fluorescent assay for cadmium detection. Appl Sci 6(12):432
MacLeod J, Rosei F (2013) Photonic crystals: sustainable sensors from silk. Nat Mater 12:98–100
Mahato K, Maurya PK, Chandra P (2018) Fundamentals and commercial aspects of nanobiosensors in point-of-care clinical diagnostics. 3 Biotech 8:1–14
Marlow F, Muldarisnur, Sharifi P et al (2009) Opals: status and prospects. Angew Chem Int Ed 48:6212–6233
McPhedran RC, Nicorovici NA, McKenzie DR et al (2003) Structural colours through photonic crystals. Phys B Condens Matter 338:182–185
Meseguer F (2005) Colloidal crystals as photonic crystals. Colloids Surf 270:1–7
Miao YB, Ren HX, Gan N et al (2016) A triple-amplification SPR electrochemiluminescence assay for chloramphenicol based on polymer enzyme-linked nanotracers and exonuclease-assisted target recycling. Biosens Bioelectron 86:477–483
Morales MA, Halpern JM (2018) Guide to selecting a biorecognition element for biosensors. Bioconjug Chem 29(10):3231–3239
Moritz TJ, Polage CR, Taylor DS et al (2010) Evaluation of Escherichia coli cell response to antibiotic treatment by use of Raman spectroscopy with laser tweezers. J Clin Microbiol 48:4287–4290
Muhammad M, Yao G, Zhong J et al (2020) A facile and label-free SERS approach for inspection of fipronil in chicken eggs using SiO2@Au core/shell nanoparticles. Talanta 207:120324
Nie Y, Teng Y, Li P et al (2018) Label-free aptamer-based sensor for specific detection of malathion residues by surface-enhanced Raman scattering. Spectrochim Acta A Mol Biomol Spectrosc 191:271–276
Nsibande SA, Forbes PBC (2016) Fluorescence detection of pesticides using quantum dot materials – a review. Anal Chim Acta 945:9–22
Panda A, Puspa Devi P (2020) Photonic crystal biosensor for refractive index based cancerous cell detection. Opt Fiber Technol 54:102123
Park JH, Byun JY, Mun H et al (2014) A regeneratable, label-free, localized surface plasmon resonance (LSPR) aptasensor for the detection of ochratoxin A. Biosens Bioelectron 59:321–327
Pouya C, Stavenga DG, Vukusic P et al (2011) Discovery of ordered and quasi-ordered photonic crystal structures in the scales of the beetle Eupholus magnificus. Opt Express 19:11355–11364
Prabowo BA, Purwidyantri A, Liu KC (2018) Surface Plasmon resonance optical sensor: a review on light source technology. Biosensors (Basel) 8:80
Qiao Q, Guo X, Wen F et al (2021) Aptamer-based fluorescence quenching approach for detection of aflatoxin M1 in milk. Front Chem 9:1–8
Rehmat Z, Mohammed WS, Sadiq MB et al (2019) Ochratoxin A detection in coffee by competitive inhibition assay using chitosan-based surface plasmon resonance compact system. Colloids Surf B: Biointerfaces 174:569–574
Scatena E, Baiguera S, Del Gaudio C (2019) Raman spectroscopy and aptamers for a label-free approach: diagnostic and application tools. J Healthc Eng 2019:2815789
Seok Y, Byun JY, Shim WB, Kim MG (2015) A structure-switchable aptasensor for aflatoxin B1 detection based on assembly of an aptamer/split DNAzyme. Anal Chim Acta 886:182–187
Setlem K, Mondal B, Shylaja R, Parida M (2020) Dual aptamer-DNAzyme based colorimetric assay for the detection of AFB1 from food and environmental samples. Anal Biochem 608:113874
Shafiee H, Lidstone EA, Jahangir M et al (2014) Nanostructured optical photonic crystal biosensor for HIV viral load measurement. Sci Rep 4:1–7
Shen H, Bai J, Zhao X et al (2022) Highly ordered, plasmonic enhanced inverse opal photonic crystal for ultrasensitive detection of staphylococcal enterotoxin B. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.1c18386
Shi X, Sun J, Yao Y et al (2020) Novel electrochemical aptasensor with dual signal amplification strategy for detection of acetamiprid. Sci Total Environ 705:135905
Stiles PL, Dieringer JA, Shah NC, Van Duyne RP (2008) Surface-enhanced Raman spectroscopy. Nat Rev Methods Primers 1:87
Sudha P, Lavu R, Mondal B et al (2016) Selection and characterization of Aptamers using a modified whole cell bacterium SELEX for the detection of Salmonella enterica Serovar Typhimurium. ACS Comb Sci. https://doi.org/10.1021/acscombsci.5b00123
Sun D, Xu W, Xu S (2019a) Ultrasensitive Raman sensing of alkaline phosphatase activity in serum based on an enzyme-catalyzed reaction. Anal Methods 11:3501–3505
Sun L, Wu L, Zhao Q (2017) Aptamer based surface plasmon resonance sensor for aflatoxin B1. Microchim Acta 184:2605–2610
Sun Y, Li Z, Huang X et al (2019b) A nitrile-mediated aptasensor for optical anti-interference detection of acetamiprid in apple juice by surface-enhanced Raman scattering. Biosens Bioelectron 145:111672
Vukusic P, Sambles JR (2003) Photonic structures in biology. Nature 424:852–855
Wang H, Huang X, Wen G, Jiang Z (2019) A dual-model SERS and RRS analytical platform for Pb(II) based on Ag-doped carbon dot catalytic amplification and aptamer regulation. Sci Rep 9:1–10
Wang Q, Zhao WM (2018) Optical methods of antibiotic residues detections: a comprehensive review. Sens Actuators B Chem 269:238–256
Wang S, Dong Y, Liang X (2018) Development of a SPR aptasensor containing oriented aptamer for direct capture and detection of tetracycline in multiple honey samples. Biosens Bioelectron 109:1
Wei W, Nong J, Mei Y et al (2018) Single-layer graphene-coated gold chip for enhanced SPR imaging immunoassay. Sens Actuators B Chem 273:1548–1555
World Health Organization (2022) WHO global strategy for food safety 2022–2030
Wu S, Wang Y, Duan N et al (2015) Colorimetric aptasensor based on enzyme for the detection of vibrio parahemolyticus. J Agric Food Chem 63:7849–7854
Wu W, Zhu Z, Li B et al (2018) A direct determination of AFBs in vinegar by aptamer-based surface plasmon resonance biosensor. Toxicon 146:24–30
Wu Y, Zhan S, Wang L, Zhou P (2014) Selection of a DNA aptamer for cadmium detection based on cationic polymer mediated aggregation of gold nanoparticles. Analyst 139:1550–1561
Wu K, Ma C, Zhao H, Chen M, Deng Z (2019) Sensitive aptamer-based fluorescene assay for ochratoxin A based on RNase H signal amplification. Food Chem 277:273–278
Wu Z (2019) AuNP tetramer-based Aptasensor for SERS sensing of oxytetracycline. Food Anal Methods 12:1121–1127
Wu Z, Xu E, Chughtai MFJ et al (2017) Highly sensitive fluorescence sensing of zearalenone using a novel aptasensor based on upconverting nanoparticles. Food Chem 230:673–680
Xu J, Li Y, Bie J et al (2015) Colorimetric method for determination of bisphenol A based on aptamer-mediated aggregation of positively charged gold nanoparticles. Microchim Acta 182:2131–2138
Xuan H, Ren J, Zhu Y et al (2016) Aptamer-functionalized P(NIPAM-AA) hydrogel fabricated one-dimensional photonic crystals (1DPCs) for colorimetric sensing. RSC Adv 6:36827–36833
Yan Q, Yu J, Cai Z, Zhao XS (2011) Colloidal photonic crystals: fabrication and applications. In: Hierarchically structured porous materials: from nanoscience to catalysis, separation, optics, energy, and life science. Wiley-VCH, Weinheim, pp 531–576
Yan Z, Tian C, Qu X et al (2017) DNA-functionalized photonic crystal microspheres for multiplex detection of toxic metal ions. Colloids Surf B: Biointerfaces 154:142–149
Yang C, Abbas F, Rhouati A et al (2022) Design of a quencher-free fluorescent aptasensor for ochratoxin A detection in red wine based on the guanine-quenching ability. Biosensors (Basel) 12:297
Yang C, Wang Y, Marty JL, Yang X (2011) Aptamer-based colorimetric biosensing of ochratoxin A using unmodified gold nanoparticles indicator. Biosens Bioelectron 26:2724–2727
Yang Y, Li W, Shen P et al (2017) Aptamer fluorescence signal recovery screening for multiplex mycotoxins in cereal samples based on photonic crystal microsphere suspension array. Sens Actuators B Chem 248:351–358
Yang Y, Zhang Z, Wan M et al (2020) Highly sensitive surface-enhanced Raman spectroscopy substrates of Ag@PAN electrospinning nanofibrous membranes for direct detection of bacteria. https://doi.org/10.1021/acsomega.0c02735
Ye B, Rong F, Gu H et al (2013) Bioinspired angle-independent photonic crystal colorimetric sensing. Chem Commun 49:5331–5333
Ye BF, Zhao YJ, Cheng Y et al (2012) Colorimetric photonic hydrogel aptasensor for the screening of heavy metal ions. Nanoscale 4:5998–6003
Yu T, Xu H, Zhao Y et al (2020) Aptamer based high throughput colorimetric biosensor for detection of staphylococcus aureus. Sci Rep 10:1–6
Yue S, Jie X, Wei L et al (2014) Simultaneous detection of ochratoxin A and fumonisin B1 in cereal samples using an aptamer-photonic crystal encoded suspension array. Anal Chem 86:11797–11802
Zhang D, Yang J, Ye J et al (2016) Colorimetric detection of bisphenol A based on unmodified aptamer and cationic polymer aggregated gold nanoparticles. Anal Biochem 499:51–56
Zhang W, Ganesh N, Block ID, Cunningham BT (2008) High sensitivity photonic crystal biosensor incorporating nanorod structures for enhanced surface area. Sens Actuators B Chem 131:279–284
Zhang Y, Wu Q, Sun M, Zhang J, Mo S, Wang J et al (2018) Magnetic-assisted aptamer-based fluorescent assay for allergen detection in food matrix. Sensors Actuators B Chem 263:43–49
Zhao H, Xiang X, Chen M, Ma C (2019) Aptamer-based fluorometric ochratoxin a assay based on photoinduced electron transfer. Toxins (Basel):11–65
Zi J, Yu X, Li Y et al (2003) Coloration strategies in peacock feathers. Proc Natl Acad Sci 100:12576–12578
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Goel, S., Singh, S., Kalyani, N. (2023). Aptamer-Based Optical Sensors for Food Safety. In: Purohit, B., Chandra, P. (eds) Surface Engineering and Functional Nanomaterials for Point-of-Care Analytical Devices. Springer, Singapore. https://doi.org/10.1007/978-981-99-3025-8_6
Download citation
DOI: https://doi.org/10.1007/978-981-99-3025-8_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-3024-1
Online ISBN: 978-981-99-3025-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)