Abstract
With the rapid development of next-generation manufacturing, communication, and display technologies, smartphone has been widely integrated with multifunctional modules, such as sensor chips and handheld detectors for biochemical detections. Owing to the merits of high computing speed, high-resolution image analysis, and user-friendly human-computer interface, smartphone-based point-of-care testing (POCT) devices for personalized healthcare provide an affordable and accessible way for on-site diagnosis without using sophisticated and expensive instruments. The conventional biochemical analytical instruments are always bulky, not portable, and especially expensive; therefore, further applications are rather limited. The smartphone-based sensors and electronics have been developing rapidly, playing an increasingly important part upon the challenges confronting medical service, food industry, and public safety. This chapter presents smartphone interface and wearable biosensors for on-site analysis and takes our team’s work as examples to elaborate. The sensing mechanism, design principle of smartphone-based portable and wearable sensing system, and implementation of biosensing strategies were discussed. For better insights into the important and valuable smartphone-based portable and wearable sensing devices, several examples were carefully discussed of device designing, application scenarios, analytical targets, and future applications. In the smartphone-based portable system, optical sensing, electrochemical sensing, and photoelectrochemical sensing were introduced with specific exciting and sampling circuit implementation and on-site diagnosis applications. In the smartphone-based wearable system, we summarized the representative applications of the perspiration analysis system, implantable system, ingestible system, and wound monitoring system. Due to the wide-range permeability rate of smartphone in our lives, it can be expected that smartphone interface and wearable biosensors will gradually complement and dominate the existing health management approaches.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
With the growth need of point-of-care testing (POCT) for on-site diagnosis of analytes and pathogens in a variety of clinical conditions, there has been a transformation from the centralized biomedical analysis to easier, cheaper, and more reliable health monitoring at home or anywhere (Liu et al. 2019; Xu et al. 2018; Vashist et al. 2015; Tran et al. 2019; Jung et al. 2015). The conventional analytical instruments are bulky and more expensive and require professional operator, although they are sensitive and more authoritative. The strictly controlled laboratory conditions and time-consuming also limits large-scale household use. Thanks to the advances in electronics, functional material, nanotechnology, and micromachining technology, constructing biosensor on a variety of substrate provides an alternative solution to this problem (Turner 2013; Kuila et al. 2011; Kanchi et al. 2018). From a typical perspective, sensitive element modification, optimization, and signal sampling and analyzing are necessary steps to build biosensor for POCT devices (Kim et al. 2019; Miller et al. 2020; Perumal and Hashim 2014; Kirsch et al. 2013). In such case, how to effectively process signal, transmit data, process data, and display result is a new issue to be addressed. Designing and fabricating such biochemical sensing system to reach the function mentioned above is the key to fulfill the momentous needs.
Smartphones were initially brought to market by IBM and BellSouth in 1993, and the total number of users reaches a staggering 3.9 billion by 2021 (Huang et al. 2018). By now, smartphones are widely equipped with multicore processor, big memory, large storage, large battery, high-resolution camera, universal serial ports, and stable operating system. The wireless data transmission by 4G or 5G cellular net service for telecommunications, as well as Bluetooth, Wi-Fi, and near-field communication (NFC) for local communication, forms a fast connected network (Purohit et al. 2020). The cameras built into the smartphones have an easy access to contribute to the sensing system to achieve optical detection (Severi et al. 2020; Ardalan et al. 2020). In the meantime, more and more peripheral modules are integrated into the smartphones to realize product iteration, where the sensing system could be developed as stand-alone device with less space consumption and cost (Chen et al. 2021; Sun and Hall 2019). As there have been many interesting, unique, and applicable smartphone-based POCT devices, it is necessary to summarize recent advances in designing and developing of such devices.
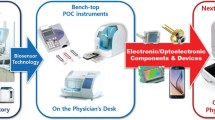
Therefore, this chapter described the smartphone-based biosensors from two aspects, (1) portable system and (2) wearable system (Fig. 13.1), and the content is a selection of classic and recent research advances. In the field of portable system, spectroscopy detection (optical, surface plasmon resonance, and electrochemiluminescence), electrochemistry detection (amperometry, potentiometry, and impedimetric), and photoelectrochemical detection on smartphone were summarized and discussed with device design principles and sensing mechanism. As for the wearable biosensors, epidermal, implantable, ingestible, and integrated wearable devices for drug delivery were introduced with detailed technical circuit designing and fabricating, and their application in combination with smartphone-based devices has been briefly discussed.
13.2 Smartphone-Based Portable Detection System for On-Site Diagnosis
As the high computing speed and camera technology advance rapidly, smartphones have been providing a portable detection method that can be applied in a variety of scenarios. In this section, smartphone-based optical, electrochemical, and photoelectrochemical systems were summarized in the following part.
13.2.1 Smartphone-Based Optical Sensing System
Smartphones are widely used in biosensing system for optical detection with the built-in capabilities of high resolution and image acquisition (McCracken and Yoon 2016; Geng et al. 2017). The first smartphone-based optical sensor was reported by Martinez et al., in which the analysis of glucose and proteins was conducted by a paper-based microfluidic chips with smartphone (Martinez et al. 2008). Compared with the high cost of large laboratory instruments, which cost time and need special operators, smartphone-based platforms for optical sensors have lower costs and more simplified operation requirements (Wang et al. 2016; Zhang and Liu 2016). In this section, smartphone-based spectroscopy system, surface plasmon resonance (SPR) system, and electrochemiluminescence system were summarized with device designs and imaging analysis.
13.2.1.1 Spectroscopy Sensing
The urgent need of good health management is accelerating the development of health monitoring devices combined with smartphone sensing technologies. At the same time, a variety of smartphone-based platforms including smartphone units and integrated circuits for optical analysis of biological samples, such as saliva, urine, and serum were developed (Li et al. 2018a). Compared with the traditional laboratory analytical instruments, the acquired microscopic images can be analyzed based on the smartphone camera and processor with the features of the target components, such as the color, bulk, and brightness (Zhu et al. 2011; Smith et al. 2011). To acquire the image and spectra of micron-scale regions, a microspectroscopy/imaging system consisting of a portable spectrometer as an optical sensor and a compact homemade microscope was proposed by Guang et al. (Chen et al. 2020). In their work, the quantification of protein concentration was demonstrated with the smartphone-based proposed microspectroscopy/imaging system to analyze the spectrometer signals which highlighted the possibility for adopting the smartphone device for building a microspectroscopy/imaging system for point-of-care testing. With the development of the detection technology, colorimetric and fluorescence biosensors are gradually applied based on microscopic imaging. A colorimetric paper-based analytical device with high sensitivity was reported by Huy et al. for the detection of tetracycline by applying the green fluorescent carbon nitride (g-C3N4) nanoparticles using a smartphone for imaging (Huy et al. 2020). However, the sensitivity of smartphone-based colorimetric sensing system still needs to be improved. To address this, nanomaterial-enabled colorimetric detection integrated in the smartphone-based point-of-care platform was developed by Xia et al. with high sensitivity and selectivity for the detection of the avian influenza virus (Xia et al. 2019). The modification of nanomaterials and the smartphone-based imaging system with giant data acquiring and processing ability improved the limit of detection, which reached down to 8 × 103 EID50/mL.
Fluorescence microscopic imaging improves spatial resolution, specificity, and sensitivity for biosensing, which greatly promotes the bioanalysis of proteins, nucleic acids, and cells (Wei et al. 2013). Compared with conventional fluorescence equipment, the smartphone-based fluorescence sensing is limited by the camera and processor of smartphone itself as the cost is also lowered down. To surmount these shortcomings and realize molecule-level detection for on-site diagnosis, a Forster resonance energy transfer (FRET)-based nanoprobe combined with a smartphone RGB camera was proposed by Severi et al. for detection of nucleic acids (Severi et al. 2020). The nanoprobe response of nucleic acids can be detected in time as low as 10 pM in true color and in red-to-green ratio images using the RGB camera. The FRET-based biosensor can be easily applied using a smartphone coupled to an appropriate optical setup, expanding the method to high sensitivity and low cost for point-of-care analysis. Despite the hardware differences between smartphones, the software and high-speed computing of the smartphone itself can compensate for the shortcomings of this method to some extent (Chen et al. 2021).
13.2.1.2 Surface Plasmon Resonance (SPR) Sensing
SPR is a physical phenomenon which resonates under the excitation of incident light at the interface of materials with different conductivity (Karlsson 2004). When this phenomenon happens on the surface of nanoscale metal structures, such as gold and silver, it is also called local surface plasmon resonance (LSPR) (Zhang et al. 2015a; Bian et al. 2019). Due to its merits of interface effect, label-free, real-time, and eco-friendly, LSPR has attracted extensive attention. At the same time, with the rise of mobile health (mHealth) concepts, smartphones have gradually developed into an effective platform for LSPR-based biochemical sensing detection (Li et al. 2018b). Seemesh et al. reported a template-free plasmonic material of Ag-Soret colloids (Ag-SCs) on the surface plasmon-coupled emission (SPCE) platform to realize more than 100-fold enhanced fluorescence which enabled real-time monitoring of glutathione (GSH) (Bhaskar et al. 2020a). The proposed biosensing system was more sensitive and selective, while had lower cost for on-site detection of biomarkers. With the further researches in SCs from various nanomaterials and nanocomposites, the smartphone-based analytical and photonic devices for the next generation will help the system reach to a higher level. Meanwhile, a lateral flow plasmonic biosensor consisting of gold-viral biomineralized nanoclusters (AuVCs) for on-site GSH determination was developed by Pang et al. (2020). The sensing system reached a limit of detection (LoD) of 9.80 μM with the linear in the range of 25–500 μM. At the same time, Seemesh et al. also demonstrated a label-free smartphone-based SPCE biosensor for the detecting spermidine with Au-decorated SiO2 nanoparticles (Bhaskar et al. 2020b). The smartphone platform can capture the multifold hotspots that were rendered by the nanohybrids assisted in augmented electromagnetic (EM)-field intensity, which made it promising for portable and economical biosensing system in such area.
13.2.1.3 Electrochemiluminescence (ECL) Sensing
ECL is widely used in bioanalysis for point-of-care diagnosis, which combines the merits of chemiluminescence (CL) analysis without background light noise and easy control of reactions by applying a voltage to the electrode (Cao et al. 2020; Li et al. 2019a; Qi and Zhang 2020). As an analytical technique, due to its versatility, it can have a simpler optical setup than photoluminescence (PL), better time, and space control than CL. Zhu et al. proposed a handheld device capable of ECL analysis, which used smartphone as optical signal reader for the discrimination of 3-nitrotyrosine (3-NT) (Zhu et al. 2021). The device consisted of printed circuit board for ECL stimulation and a flexible antenna was designed coupled with an NFC for data transmission. In the design of smartphone-based ECL system, the high-definition cameras and programmed software in smartphones can capture luminous images (Rahn et al. 2020). Therefore, the integration of smartphone and ECL is a preferable method for optical analysis as it meets the need of on-site biosensing for point-of-care diagnosis with simplified system and powerful capabilities (Nie et al. 2019).
A smartphone-based ECL device was designed for the biochemical sensing of nicotine and trinitrotoluene (TNT) with the on-site fingerprint mapping (Fig. 13.2a) (Li et al. 2019b). The electrochemical excitation for ECL and optical analysis were integrated in a handheld smartphone-based system with a specially designed device. The bioanalysis of typical enhanced luminescence for nicotine and quenched luminescence for TNT was demonstrated for proof of concept of the smartphone-based ECL sensing system. In addition, this system could also conduct multimode imaging analysis on smartphone for fingerprint biochemical mapping, such as color, gray, and RGB extraction (Fig. 13.2b). At last, in situ analysis of exogenous substances on human hands, such as nicotine and TNT, was realized through the smartphone-based ECL device where the fingerprint’s morphology and grain were clearly exhibited on the optical images (Fig. 13.2c). The fingerprints are widely used for unlocking terminal equipment such as smartphones and laptops. Thus, the combination of fingerprint analysis and smartphone-based sensing platform will contribute to the development of personal health monitoring as well as public safety. In addition, the proposed sensing platform can also provide additional information of diet habit, drug intake, and personal medicine. The excellent performances of smartphone-based ECL system can be further applied in various fields of biochemical detection and imaging analysis.
Smartphone-based ECL system for fingerprint mapping and biochemical analysis. (a) Schematic of the smartphone-based ECL system with the universal serial bus-based electrochemical excitation, the camera-based imaging analysis, and the user interface. (b) The concept of reaction cell and electrodes (platinum and ITO). (c) In situ analysis of nicotine on fingerprints. Reproduced from Li et al. (2019b). Copyright 2019 Elsevier
13.2.2 Smartphone-Based Electrochemical System
Nowadays, the electrochemical biosensors which take advantage of the specificity of biological element, such as enzyme, antibody, and cell, are widely researched and partially commercialized (Maduraiveeran et al. 2018; Cesewski and Johnson 2020). However, traditional lab-based analysis methods always require complicated instrumentation like electrochemical workstation and stringent operating environment. With multiple functional modules, smartphone has been significantly improving the portability of electrochemical detection (Zhang and Liu 2016). To reach a full combination of smartphone and electrochemical methods, an external miniaturized potentiostat applying electrochemical excitation on the surface of the electrode is needed. In addition, the integrated circuits for data sampling, amplifying, and communicating always require individual designs. Besides, the electrochemical electrode is another essential part for the bioanalysis. With the advances in additive and subtraction manufacturing, various kinds of electrode can be fabricated on soft and hard substrate such as paper, polyethylene terephthalate (PET), and polyimide (PI) through the screen-printing technology, laser-based manufactory, etc. Recently, multifold electrochemical sensing methods have been integrated with smartphones, including amperometry (Ji et al. 2017, 2020), potentiometry (Xu et al. 2019; Yao et al. 2019), and impedimetry (Zhang et al. 2016; Rosati et al. 2019). These detection systems extend the potential of electrochemical biosensor for applying in real-time biochemical analysis and on-site diagnosis.
13.2.2.1 Amperometry Sensing
Integrated with smartphone technique, the amperometry-based biosensing is commonly applied in numerous detection fields (Zhang and Liu 2016; Garcia et al. 2018). As the excitation potential applied, the current is related to the oxidation or reduction of the electrical mediating species during the amperometry, which is proportional to substrate concentration. For smartphone-based amperometry system, these extraordinary methods, such as chronoamperometry (CA), cyclic voltammetry (CV), square wave voltammetry (SWV), and differential pulse voltammetry (DPV), could effectively improve the sensitivity of the biosensor system. Due to the good simplicity and excellent adaptability, it became the first reported of smartphone-based biosensor application (Zhang and Liu 2016; Lillehoj et al. 2013). Among various detecting techniques, CV and CA with more practicability for the quantitation of analytes attract much attention. Recently, a smartphone-based CV system was developed by Ji et al. with simplified electrode modification to perform electrochemical detections (Ji et al. 2017). The detecting system consisted of electrochemical modified electrodes, the designed detector, and the smartphone with software. Compared with the standard analytical equipment, the proposed CV-based system had lower cost and smaller size and could perform electrochemical test in real time. To further apply the bioanalysis ability of smartphones, an electrochemical device based on the paper fabrication was proposed by Caratelli et al. for the detection of butyrylcholinesterase (BChE) activity in whole blood (Caratelli et al. 2020). The development of an analytical method for easy self-testing of BChE inhibitor activity in whole blood on a paper-based lab-on-a-chip with lower cost and user-friendly operation made a good contribution to personalized administration of drugs along with Alzheimer’s disease.
In addition to cyclic sweeps, pulsed potential excitation such as SWV, DPV, and differential pulse amperometry (DPA) is also a widely used electrochemical method, which could be employed for biochemical sensing, such as nucleic acids, peptides, and heavy metal ions (Ji et al. 2018, 2020; Shi et al. 2020). For example, a rapid detection system combined with a smartphone-based electrochemical detector was constructed for monitoring of levodopa, which can be applied for Parkinson’s disease prevention and treatment, while the conventional treatment is to supplement levodopa so as it could overcome blood-brain barrier and synthetic dopamine (Ji et al. 2019). The system contained these main parts: a disposable screen-printing electrode, a handheld detecting circuit, and a smartphone with programmed software (Fig. 13.3a). The detecting circuit was powered by lithium ion battery, and the electrochemical excitation can be applied to measure the subsequent current change on the surface of electrode. Finally, the potential and current information were transferred to smartphone via Bluetooth module. This proposed system exhibited good performance in the detection of levodopa from 0 μM to 200 μM and with a limit of detection as low as 0.5 μM in human serum, in which the modified sensor could also distinguish levodopa from representative interference (Fig. 13.3b). To implement the smartphone in the nucleic acid detection, Low et al. demonstrated a detector using smartphone for electrochemical sensing of circulating microRNA-21 (miR-21) biomarker in saliva as a proof of concept (Shin Low et al. 2020). The electrochemical detecting system consisted of commercial screen-printing electrode, which was modified with reduced graphene oxide/gold nanoparticles (rGO/Au), a detector, and a smartphone with programmed Android software (Fig. 13.3c). The salivary miR-21 was detected as a proof of concept with concentration level of 1 pM to 100 μM with correlation coefficient of 0.99 (Fig. 13.3d). Furthermore, with the huge demand for virus testing and family practice, this smartphone-based sensing system is capable to provide timely, low-cost, rapid on-site diagnosis, which satisfied the testing needs and reduced the squeeze of medical resources.
Smartphone-based electrochemical system. (a) Smartphone-based DPA system (the Bluetooth module, controller unit, digital analog converter, potentiostat module, electrode socket, and power management module are in the red box from 1 to 6). (b) The diagram of DPA measurement using the system for levodopa detection at different concentrations. Reproduced from Ji et al. (2019). Copyright 2019, Elsevier. (c) Schematic diagram of smartphone-based DPV system. (d) Corresponding peak current change of the smartphone-based sensing system and standard analytical equipment in miR-21 gradients. Reproduced from Shin Low et al. (2020). Copyright 2020, Elsevier
13.2.2.2 Potentiometry Sensing
The potentiometry is easier to implement in smartphone-based electrochemical sensing system but as important as amperometry, which is widely used to determine the potential change or biochemical activity (Zhang and Liu 2016; Yao et al. 2019). The mechanism of potentiometry is to detect the cumulative charge resulting in potential change in the dielectric layer, which is commonly applied in the detection of electrolyte ion and other biomarkers (Zhang and Liu 2016; Zhang et al. 2015b, c). Therefore, ion-selective membranes are usually modified on the electrode surface for the trace detection using potentiometry (Dimeski et al. 2010). For example, a wearable and battery-free epidermal electrochemical system was reported by Xu et al. with NFC technology and screen-printing electrode. The wearable detection system got rid of the rigid batteries, and it can adhere to the skin surface and detects electrolyte ions in sweat (Xu et al. 2019). The potentiometry can be applied for molecular imprinting or enzyme-based biosensing in the same way. Yao et al. proposed an enzymatic aptasensor using freestanding graphene paper for wirelessly detecting kanamycin (Yao et al. 2019). A nuclease was used to assist to amplify, and it realized an ultrasensitive biosensing platform with the smartphone recording real-time data. This work paved the way for implementing of paper-based material and enzyme-based biosensing, which provides a broader application for smartphone-based potentiometry sensing.
13.2.2.3 Impedimetric Sensing
To measure the impedance of an electrode system, the electrochemical impedance spectroscopy (EIS) is set with the scanning from low frequency to high frequency, which was usually from 0.1 Hz to 1 MHz. The EIS could exhibit the change of electrochemical properties, such as electrode capacitance changes and the resistance caused by electron transfer (Chang and Park 2010). Through a sinusoidal voltage applied, the ratio of alternating voltage and current change at different frequency was measured, where the corresponding electrochemical signal detection can be realized. Due to the high resolution of frequency, smartphone-based impedimetric sensing systems are widely used in the detection of small molecules, proteins, nucleic acid, and gases. The first reported application of smartphone-based impedimetric system was developed by Broeders et al. in 2013 (Broeders et al. 2013). Later, Zhang et al. reported a smartphone-based wirelessly controlled biosensing system developed with electrochemical impedance spectroscopy for detecting proteins along with the data transferring via Bluetooth (Zhang et al. 2016). In addition to the solid/liquid phase biosensing, the volatile organic compounds (VOCs) are usually measured by impedance. For example, a smartphone-based impedance system was developed for monitoring VOCs in expiratory gas by Liu et al., which provided a platform for early diagnosis for pulmonary biomarkers (Liu et al. 2017). Recently, an electronic nose with small size and low cost was developed by Arroyo et al. with a mobile programmed terminal for classification services (Arroyo et al. 2020). Based on frequency resolution, impedance analysis methods can make a unique contribution to material detection especially in selectivity.
13.2.3 Smartphone-Based Photoelectrochemical (PEC) System
Based on the photoexcitation of photoelectric compound under exogenous excitation, PEC analysis is an emerging analytical method with potential application for on-site diagnosis recently (Zhao et al. 2017; Zhou and Tang 2020). The exogenous excitation facilitates the separation of surface charges in photoelectric compound and to stimulate the electrochemical reaction. As the excitation source and electrochemical current/potential response are separated, the PEC-based analysis system has lower background interference and improved sensitivity (Zhang et al. 2017, 2019). Conventional PEC analysis depends on the large excitation source and standard measuring equipment, such as high-power xenon lamp and electrochemical workstation, which hinders PEC analysis for portable and on-site rapid testing. Recently, the rapid development of integrated electronics, the processing, and fabrication of miniaturized devices also provides opportunities for the application of PEC analysis method (Zhang and Liu 2016). At the same time, combining terminal equipment such as smartphones with the designed analytical devices can achieve lower cost and faster testing.
Combined with the advantages of smartphone and PEC analysis, a portable and universal biosensing platform was constructed for rapid on-site diagnosis (Fig. 13.4a) (Zhang et al. 2021). The portable system consisted of the electrodes, detecting circuit board, and a smartphone. The miniaturized detecting circuit board and battery provided photoexcitation; then, the photocurrent was measured by the sampling module. Smartphone was used as commander and data display via Bluetooth control. The graphitic g-C3N4 was used as photoelectric compound, and the gold nanoparticles (AuNPs) were applied to immobilize matrix metalloproteinase-2 (MMP-2) for specific cleavage peptide. The small light-emitting diode (LED) excited g-C3N4 to generate photocurrent, and the hydrolyzed and cleaved process of MMP-2 for peptide was evaluated by photocurrent (Fig. 13.4b). The photocurrent varies linearly with the concentration of MMP-2 ranging from 1 pg/mL to 100 ng/mL (Fig. 13.4c). This system reached a limit of detection buffer and 0.55 pg/mL in artificial serum. These results verified the effectiveness of the portable smartphone-based PEC device, which provided a potential solution for miniaturized and portable on-site diagnosis.
Smartphone-based PEC system. (a) Illustration of the smartphone-based PEC detection system, which is composed of a reaction tank, the electrodes, detecting circuit board, and a smartphone. (b) The electron-hole transfer mechanism in the proposed PEC system. (c) Photocurrent response of MMP-2 gradients. Reproduced from Zhang et al. (2021). Copyright 2021, Elsevier
13.3 Smartphone-Based Wearable System for On-Site Diagnosis
Over the past few decades, with the rapid development of microelectronics technology and materials science, wearable electronics have achieved tremendous development (Gao et al. 2016; Nyein et al. 2016). Here, smartphone-based biosensors for epidermal application, implantable application, ingestible application, and integrated wearable drug delivery system were summarized in the following section.
13.3.1 Epidermal Biosensor System
The epidermis covers most of the human body, which is the largest organ capable of protection, absorption, sensation, metabolism, and immunity. The biological fluids in epidermis, such as sweat and interstitial fluid, contain plenty of health information, which can be analyzed in real time by epidermal biosensors (Menon 2002). In the above example, smartphone-based electrochemical sensors and biosensors are mainly employed to measure small molecules and ions in sweat, such as lactic acid, glucose, sodium ion, potassium ion, hydrogen ion, calcium ion, chloride ion, etc. These sensors can measure trace molecules or ions accurately, and they exhibited outstanding sensing performance. However, much biochemical analysis has not been able to establish a clear correlation with body condition or specific diseases. For instance, the correlation of sweat glucose and serum glucose has not yet been studied clearly, which is still under research stage (Bandodkar et al. 2016). The concentration of sodium and potassium ions is related to the state of exercise metabolism. However, there is no clear research to explain its mechanism (Corrie et al. 2015). Chloride ions in sweat have been used to indicate cystic fibrosis (CF), but this disease is relatively rare (Bariya et al. 2018). However, with the giant health information in sweat, developing a wearable device to monitor biomarkers is important for preclinical diagnosis of specific diseases (Nimse et al. 2016). To find a solution, Gao et al. reported a fully integrated wearable and flexible sweat analysis device in 2016 (Gao et al. 2016). The devices executing embedded programs can run various electrochemical detection methods with flexible and bendable circuit. The development of microelectronics technology enables miniaturized circuit systems to be designed in the size of a single wristband. Later, Xu et al. reported a battery-free and flexible detection patch with wireless communication based on NFC technology for the more stretchable and comfortable sweat analysis (Xia et al. 2019; Xu et al. 2019).
Detection of sweat substances like ions, glucose, and lactic acid only reflects electrolyte loss and exercise health. However, the hormones and sterols might contain more health information in body regulation. In regard to this consideration, a flexible skin-attached detection device that could monitor the concentration of cortisol in sweat was developed (Fig. 13.5a) (Cheng et al. 2021a). Secreted by the adrenal cortex, cortisol was an adrenal cortex hormone, whose concentration in serum and sweat indicates the mental state of the human (Katsu and Baker 2021). Cortisol concentration in sweat was associated with clinical psychiatric disorders such as anxiety, depression, and post-traumatic stress disorder (PTSD). Detecting cortisol in sweat could aid in the diagnosis and treatment of these diseases. Based on the flexible substrate, the proposed detection device contained a signal processing module and NFC module, which can be bent and folded to fit the surface of the human skin closely without causing discomfort (Fig. 13.5b). The detection device adopted DPV as the detection technology, which could be applied to measure the target substance quickly in real time. The detection device was compared in experiments with a commercial large-scale electrochemical workstation, in which the testing results showed similar performance. The device took advantage of electrochemical immunoassay technology with the detection range that could reach 0 nM to 500 nM for cortisol (Fig. 13.5c). Further, the device also tested sweat samples of the volunteers in vivo. During 7 days, the volunteers’ sweat was collected in the morning and evening for cortisol analysis. These data reflected the circadian rhythm of cortisol content in subjects over 7 days (Fig. 13.5d). The detection system proposed by Cheng et al. established an integrated, small, flexible detection platform for the sweat cortisol monitoring, which had potential value for the health management of human mental state.
Smartphone-based epidermal biosensor system. (a) Using a smartphone to read data from a sweat cortisol analysis device. (b) Photograph of the flexible and wearable analysis system. (c) Cortisol detection with DPV from 0 nM to 500 nM. (d) Sweat cortisol detecting results during 7 days. Reproduced from Cheng et al. (2021a). Copyright 2021, Elsevier
13.3.2 Implantable Biosensor System
The urgent need for continuous monitoring of body condition with the purpose for solving the problem to the routine requirement of hospitalization and supervision of the patients drives integrated biosensing system field growing fast. Hence, much research aims to explore and develop skin-integrated and implantable medical devices (Bobrowski and Schuhmann 2018; Ashammakhi et al. 2021; Oh et al. 2021). In these devices, the common monitoring signals are heart electrocardiogram (ECG), blood pressure, pulse rate, blood glucose level, and respiration efficacy. Except for physiological or biochemical sensing, external excitation for tissues is also a research hotspot for implanted devices. With the rapid development in biocompatible material, miniaturized design, wireless communication, and power supply technology, the electrochemical detection device is converted from rigid and wired designs to wireless, battery-free, and flexible system, which makes in vivo, in situ, and real-time physiological data monitoring available (Boutry et al. 2018).
Smart devices such as smartphones with NFC function are suitable for implantable applications, because they could be designed to be smaller and thinner without the requirement of battery, along with data transmitting outside of body wirelessly. Based on this, an implantable, wireless, and battery-free electrochemical system was proposed (Fig. 13.6a) (Xu et al. 2021a). It could perform detection of the glucose concentration in the peritoneum while a patient had peritoneal cancer. The electrochemical detection system was mainly composed of a flexible circuit patch and an integrated platinum nanotree microelectrode. The circuit patch consisted of a potentiostat for electrochemical excitation and NFC module. The developed system was capable of fast detection of glucose concentration, wireless data transmission, and power supply (Fig. 13.6b). The modification of highly sensitive platinum nanostructure and Nafion on implantable electrode enhanced the performance for in vitro and in vivo test in rat peritoneal cavity. In addition, results from the biochemical analyzer with the implantable and wireless patient ascites monitoring system were compared. The reliability of monitoring system was demonstrated in Fig. 13.6c. The implantable electrochemical system for detecting peritoneal glucose owned the characteristics of less invasiveness and fast feedback and provided a hassle-free method for the monitoring of peritoneal cancer.
Smartphone-based implantable glucose sensors. (a) The explosive view of the battery-free and implantable electrochemical system. (b) Image of microelectrode and the flexible circuit patch. (c) The variation of response currents after glucose injections. Reproduced from Xu et al. (2021a). Copyright 2021 Elsevier
13.3.3 Ingestible Biosensor System
The rapid development of microelectronics and materials science has promoted the emergence of small-sized, low-power consumption and ingestible biomedical electronic devices (Abramson et al. 2021). The ingestible electronic devices have the feature of small size which is enough to be swallowed into the gastrointestinal (GI) tract without anesthesia. They are not available in previous semi-invasive devices. Hence, the development of ingestible devices has attracted much attention in recent few years. However, designing competent ingestible biomedical electronic devices faces many challenges. For instance, the volume of the device needs to be small enough, and shape of the device must conform to the characteristics of the GI tract. These allow it to smoothly pass through the narrow parts of the GI tract without causing additional damage such as perforation, intestinal obstruction, ulcers, etc.
Based on wireless communication and electrochemical sensing method, a capsule-based ingestible sensing system was developed with IrOx modified for monitoring the pH of the GI tract in real time (Fig. 13.7a) (Cheng et al. 2021b). The capsule system uses IrOx-deposited screen-printed electrodes (SPE) featuring high sensitivity as the pH sensor. The capsule detection circuit is designed with rigid-flexible composited printed circuit board (RFPCB) technology and can be loaded with the pH sensor (Fig. 13.7b). Capsule structure was fabricated by 3D-printed technology. The capsule detection circuit can be bent and folded into a small volume and assembled into the capsule structure. Polydimethylsiloxane (PDMS) was covered on the outer surface of the capsule structure to improve its biocompatibility and ensure its reliability. The data and control command of the capsule detection circuit is wirelessly transmitted by the Bluetooth module to the external receiving device, where the external receiving device could be smartphone, personal computer (PC), or programmed auxiliary circuit. At last, the capsule system was applied to monitoring the pH value of artificial digestive juice as an in vitro simulation. For in vivo evaluation, the pH value of the GI tract of canine was detected. The results of the detection process were compared with commercial pH sensors, which exhibited excellent performance (Fig. 13.7c). The proposed method could be used to evaluate the performance of the capsule system for monitoring or drug releasing and even laid the groundwork for further clinical trials.
Smartphone-based wireless capsule sensing device for GI pH detection. (a) The view of smartphone-based ingestible capsule. (b) The capsule detection circuit designed by rigid-flexible composite printed circuit board (RFPCB) technology consists of four flexible substrates and five rigid substrates. Among them, the flexible substrate only plays the role of connection, and the five rigid substrates are respectively placed: lithium ion battery and Bluetooth module, download interface and power circuit, microcontroller (MCU) and digital-to-analog conversion (DAC) module, potentiostat and analog-to-digital conversion (ADC) module, and electrical interface of pH sensor. (c) The results of real-time detection of pH in the GI tract of beagle dogs by capsule system. Reproduced from Cheng et al. (2021b). Copyright 2021, Elsevier
13.3.4 Integrated Wearable Drug Delivery System
The acute illnesses on skin are usually treated by hypodermic injection or oral administration of drugs, while the chronic diseases require an integrated wearable drug delivery system for long-term treatment and protection (Mirvakili and Langer 2021). The destruction of the intact skin or subcutaneous tissue by sharps, burns, and blows usually causes chronic wounds, which increases the pain and long-term stress for patients. Wounds could be infected by pathogens if without proper care where the infection may cause slow healing and further pain to the patient (Liang et al. 2021; Farahani and Shafiee 2021). Chronic wound and a series of complications such as tissue damage, organ failure, and septicemia could be caused by severe wound infections. Hence, biochemical monitoring and medical treatment of wounds are vital for wound recovery. In recent years, with the development of intelligent materials and structures that can respond to various external stimuli, wound treatment can be realized through various kinds of wearable smart wound dressings (Tang et al. 2021; Tu et al. 2021). Thus, constructing a biocompatible and wearable wound diagnosis and treatment device to monitor wound status by integrated biosensor and provide treatment by smart wound dressing is quite important.
To address this, a fully integrated, battery-free, and wireless smart wound dressing was proposed, which had the functions of wound infection monitoring and on-demand drug delivery (Fig. 13.8a) (Xu et al. 2021b). The proposed wound dressing established a closed-loop monitoring and drug delivery platform, in which the system showed its electrical controllability and stability. Through miniaturized circuits and integrated smartphone application, the smart wound dressings with integrated NFC modules can realize wireless energy harvesting, data transmission, on-site signal processing, and on-demand drug delivery control (Fig. 13.8b). To assess wound condition, the sensors of the dressing synchronously detected three indicators including wound temperature, pH value, and uric acid. In the meantime, through electronically controlled antibiotic delivery, the drug delivery electrodes in the dressing could provide on-demand treatment for wound infections. In situ animal studies showed that the integrated wearable drug delivery system can effectively promote wound healing, which fully validated to be effective in the wound treatment (Fig. 13.8c, d). Utilizing the advantages of NFC technology and flexible electronics, the battery-free and integrated design of sensing and treatment provides a promising solution for the development of a closed-loop biomedical system integrating monitoring, diagnosis, and therapy in single device.
Smartphone-based integrated wearable drug delivery system. (a) Schematic diagram of the smart dressing, which could monitor temperature, pH, and uric acid simultaneously when attached to the wound and provide feedback treatment through the electronically controlled release of drug molecules according to the monitoring results. (b) Side view of the wearable drug delivery patch. (c) Experiments in rats to assess the effect of dressings on wound healing. (d) Image of relative wound area over time during wound healing. Reproduced from Xu et al. (2021b). Copyright 2021, Wiley
13.4 Conclusion and Future Perspective
In summary, this chapter focuses on the smartphone interface and wearable biosensors for on-site diagnosis. According to the way of application, the development of biosensor technology on smartphone can be divided into two parts: portable and wearable system. As for smartphones portable biosensors are concerned, the optical, electrochemical, and PEC-based sensing system were summarized. Among them, smartphone-based optical biosensors have been making good use of the integrated camera imaging and optical intensity measurements. These designs are characterized by simplicity, high miniaturization, and multifunction. However, the complex operation and quantitation of optical biosensors were not as accurate as electrochemical methods. The electrochemical biosensors rely on the interface or external device to excite redox reactions and to sample signal with wireless communication via Bluetooth, radio frequency (RF) antenna, etc. Among them, the application of smartphone-based PEC system for the on-site diagnosis could be expanded to more fields especially in nucleic acid detection. As for the wearable biosensors, trends of flexible electronics in building next-generation biosensors for in situ detection have emerged. The wearable biosensors take advantage of smartphone to achieve wireless communication and power supply for integrated and miniaturized sensing system. The implantable and ingestible biosensors have attracted more researchers for the capability to capture more direct biochemical information. With the integrated wearable system, the transdermal drug delivery to human body would greatly reduce the pain of hypodermic injection, increase the comfort and adherence of patients, and finally enhance the efficiency of drug administration.
With the revolutionary invention of smartphone, the evolution of electronics technology has led to decreasing the size but increasing the diverse functions. These devices can also integrate various sensors and biosensors, making them portable and wearable with high-resolution image processing and fast computing ability. As mentioned with above examples, the smartphone-based devices in portable sensing system could be designed to the size of the palm of hands, and the wearable system could be miniaturized to a microneedle size or electronic tattoo. Researches on wearable sensing devices have been widely developed on smartphones for biomedical applications. But in most application, smartphone serves only as a control and display analysis device. As the materials and electronics developed, customizing sensor, special function hardware, and integrated smartphones will finally meet the growing need for personalization of health management. In addition, due to the complexity of ergonomic design and the obstacles to energy supply, these devices still face enormous commercialization challenges. In the meantime, plenty of clinical trials should be carried out, and collected samples can be used to prove the availability, biocompatibility, and the anti-interference properties of these biosensors previous to the next generation of wearable sensors entering in commercial application. By then, the whole smartphone-based sensing system can be closely connected with people’s healthcare for sustaining physiological monitoring.
References
Abramson A, Frederiksen MR, Vegge A, Jensen B, Poulsen M, Mouridsen B et al (2021) Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nat Biotechnol 40:103–109
Ardalan S, Hosseinifard M, Vosough M, Golmohammadi H (2020) Towards smart personalized perspiration analysis: an IoT-integrated cellulose-based microfluidic wearable patch for smartphone fluorimetric multi-sensing of sweat biomarkers. Biosens Bioelectron 168:112450
Arroyo P, Meléndez F, Suárez JI, Herrero JL, Rodríguez S, Lozano J (2020) Electronic nose with digital gas sensors connected via Bluetooth to a smartphone for air quality measurements. Sensors 20:786
Ashammakhi N, Hernandez AL, Unluturk BD, Quintero SA, Barros NR, Hoque Apu E et al (2021) Biodegradable implantable sensors: materials design, fabrication, and applications. Adv Funct Mater 31:2104149
Bandodkar AJ, Jeerapan I, Wang J (2016) Wearable chemical sensors: present challenges and future prospects. ACS Sensors 1:464–482
Bariya M, Nyein HYY, Javey A (2018) Wearable sweat sensors. Nat Electron 1:160–171
Bhaskar S, Moronshing M, Srinivasan V, Badiya PK, Subramaniam C, Ramamurthy SS (2020a) Silver Soret nanoparticles for femtomolar sensing of glutathione in a surface plasmon-coupled emission platform. ACS Appl Nano Mater 3:4329–4341
Bhaskar S, Kowshik NCSS, Chandran SP, Ramamurthy SS (2020b) Femtomolar detection of spermidine using au decorated SiO2 nanohybrid on plasmon-coupled extended cavity nanointerface: a smartphone-based fluorescence dequenching approach. Langmuir 36:2865–2876
Bian J, Xing X, Zhou S, Man Z, Lu Z, Zhang W (2019) Patterned plasmonic gradient for high-precision biosensing using a smartphone reader. Nanoscale 11:12471–12476
Bobrowski T, Schuhmann W (2018) Long-term implantable glucose biosensors. Curr Opin Electroche 10:112–119
Boutry CM, Kaizawa Y, Schroeder BC, Chortos A, Legrand A, Wang Z et al (2018) A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat Electron 1:314–321
Broeders J, Croux D, Peeters M, Beyens T, Duchateau S, Cleij TJ et al (2013) Mobile application for impedance-based biomimetic sensor readout. IEEE Sensors J 13:2659–2665
Cao Z, Shu Y, Qin H, Su B, Peng X (2020) Quantum dots with highly efficient, stable, and multicolor electrochemiluminescence. ACS Cent Sci 6:1129–1137
Caratelli V, Ciampaglia A, Guiducci J, Sancesario G, Moscone D, Arduini F (2020) Precision medicine in Alzheimer’s disease: an origami paper-based electrochemical device for cholinesterase inhibitors. Biosens Bioelectron 165:112411
Cesewski E, Johnson BN (2020) Electrochemical biosensors for pathogen detection. Biosens Bioelectron 159:112214
Chang BY, Park SM (2010) Electrochemical impedance spectroscopy. Annu Rev Anal Chem 3:207–229
Chen G, Chai HH, Fu JJ, Yu L, Fang C (2020) A smartphone-supported portable micro-spectroscopy/imaging system to characterize morphology and spectra of samples at the microscale, anal. Methods 12:4166–4171
Chen W, Yao Y, Chen T, Shen W, Tang S, Lee HK (2021) Application of smartphone-based spectroscopy to biosample analysis: a review. Biosens Bioelectron 172:112788
Cheng C, Li X, Xu G, Lu Y, Low SS, Liu G et al (2021a) Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens Bioelectron 172:112782
Cheng C, Wu Y, Li X, An Z, Lu Y, Zhang F et al (2021b) A wireless, ingestible pH sensing capsule system based on iridium oxide for monitoring gastrointestinal health. Sensor Actuat B-Chem 130781
Corrie SR, Coffey JW, Islam J, Markey KA, Kendall MA (2015) Blood, sweat, and tears: developing clinically relevant protein biosensors for integrated body fluid analysis. Analyst 140:4350–4364
Dimeski G, Badrick T, John AS (2010) Ion selective electrodes (ISEs) and interferences-a review. Clin Chim Acta 411:309–317
Farahani M, Shafiee A (2021) Wound healing: from passive to smart dressings. Adv Healthc Mater 10:e2100477
Gao W, Emaminejad S, Nyein HYY, Challa S, Chen K, Peck A et al (2016) Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529:509–514
Garcia PT, Guimarães LN, Dias AA, Ulhoa CJ, Coltro WKT (2018) Amperometric detection of salivary α-amylase on screen-printed carbon electrodes as a simple and inexpensive alternative for point-of-care testing. Sensor Actuat B-Chem 258:342–348
Geng Z, Zhang X, Fan Z, Lv X, Su Y, Chen H (2017) Recent progress in optical biosensors based on smartphone platforms. Sensors 17:2449
Huang X, Xu D, Chen J, Liu J, Li Y, Song J et al (2018) Smartphone-based analytical biosensors. Analyst 143:5339–5351
Huy BT, Nghia NN, Lee Y-I (2020) Highly sensitive colorimetric paper-based analytical device for the determination of tetracycline using green fluorescent carbon nitride nanoparticles. Microchem J 158:105151–105151
Ji D, Liu L, Li S, Chen C, Lu Y, Wu J et al (2017) Smartphone-based cyclic voltammetry system with graphene modified screen printed electrodes for glucose detection. Biosens Bioelectron 98:449–456
Ji D, Liu Z, Liu L, Low SS, Lu Y, Yu X et al (2018) Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes. Biosens Bioelectron 119:55–62
Ji D, Xu N, Liu Z, Shi Z, Low SS, Liu J et al (2019) Smartphone-based differential pulse amperometry system for real-time monitoring of levodopa with carbon nanotubes and gold nanoparticles modified screen-printing electrodes. Biosens Bioelectron 129:216–223
Ji D, Shi Z, Liu Z, Low SS, Zhu J, Zhang T et al (2020) Smartphone-based square wave voltammetry system with screen-printed graphene electrodes for norepinephrine detection. Smart Mater Med 1:1–9
Jung W, Han J, Choi J-W, Ahn CH (2015) Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron Eng 132:46–57
Kanchi S, Sabela MI, Mdluli PS, Inamuddin, Bisetty K (2018) Smartphone based bioanalytical and diagnosis applications: a review. Biosens Bioelectron 102:136–149
Karlsson R (2004) SPR for molecular interaction analysis: a review of emerging application areas. J Mol Recognit 17:151–161
Katsu Y, Baker ME (2021) Subchapter 123D - cortisol. In: Ando H, Ukena K, Nagata S (eds) Handbook of hormones (2nd ed). Academic Press, San Diego, pp 947–949
Kim J, Campbell AS, de Avila BE, Wang J (2019) Wearable biosensors for healthcare monitoring. Nat Biotechnol 37:389–406
Kirsch J, Siltanen C, Zhou Q, Revzin A, Simonian A (2013) Biosensor technology: recent advances in threat agent detection and medicine. Chem Soc Rev 42:8733–8768
Kuila T, Bose S, Khanra P, Mishra AK, Kim NH, Lee JH (2011) Recent advances in graphene-based biosensors. Biosens Bioelectron 26:4637–4648
Li S, Zhang Q, Lu Y, Zhang D, Liu J, Zhu L et al (2018a) Gold nanoparticles on graphene oxide substrate as sensitive nanoprobes for rapid L-cysteine detection through smartphone-based multimode analysis. Chem Select 3:10002–10009
Li S, Liu J, Lu Y, Zhu L, Li C, Hu L et al (2018b) Mutual promotion of electrochemical-localized surface plasmon resonance on nanochip for sensitive sialic acid detection. Biosens Bioelectron 117:32–39
Li S, Zhang D, Liu J, Cheng C, Zhu L, Li C et al (2019a) Electrochemiluminescence on smartphone with silica nanopores membrane modified electrodes for nitroaromatic explosives detection. Biosens Bioelectron 129:284–291
Li S, Lu Y, Liu L, Low SS, Su B, Wu J et al (2019b) Fingerprints mapping and biochemical sensing on smartphone by electrochemiluminescence. Sensor Actuat B-Chem 285:34–41
Liang Y, He J, Guo B (2021) Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 15:12687–12722
Lillehoj PB, Huang MC, Truong N, Ho CM (2013) Rapid electrochemical detection on a mobile phone. Lab Chip 13:2950–2955
Liu L, Zhang D, Zhang Q, Chen X, Xu G, Lu Y et al (2017) Smartphone-based sensing system using ZnO and graphene modified electrodes for VOCs detection. Biosens Bioelectron 93:94–101
Liu J, Geng Z, Fan Z, Liu J, Chen H (2019) Point-of-care testing based on smartphone: the current state-of-the-art (2017-2018). Biosens Bioelectron 132:17–37
Maduraiveeran G, Sasidharan M, Ganesan V (2018) Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens Bioelectron 103:113–129
Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM (2008) Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem 80:3699–3707
McCracken KE, Yoon J-Y (2016) Recent approaches for optical smartphone sensing in resource-limited settings: a brief review. Anal Methods 8:6591–6601
Menon GK (2002) New insights into skin structure: scratching the surface. Adv Drug Deliver Rev 54:S3–S17
Miller BS, Bezinge L, Gliddon HD, Huang D, Dold G, Gray ER et al (2020) Spin-enhanced nanodiamond biosensing for ultrasensitive diagnostics. Nature 587:588–593
Mirvakili SM, Langer R (2021) Wireless on-demand drug delivery. Nat Electron 4:464–477
Nie Y, Liu Y, Zhang Q, Su X, Ma Q (2019) Novel coreactant modifier-based amplified electrochemiluminescence sensing method for point-of-care diagnostics of galactose. Biosens Bioelectron 138:111318–111318
Nimse SB, Sonawane MD, Song KS, Kim T (2016) Biomarker detection technologies and future directions. Analyst 141:740–755
Nyein HY, Gao W, Shahpar Z, Emaminejad S, Challa S, Chen K et al (2016) A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca(2+) and pH. ACS Nano 10:7216–7224
Oh YS, Kim JH, Xie Z, Cho S, Han H, Jeon SW et al (2021) Battery-free, wireless soft sensors for continuous multi-site measurements of pressure and temperature from patients at risk for pressure injuries. Nat Commun 12:5008
Pang HH, Ke YC, Li NS, Chen YT, Huang CY, Wei KC et al (2020) A new lateral flow plasmonic biosensor based on gold-viral biomineralized nanozyme for on-site intracellular glutathione detection to evaluate drug-resistance level. Biosens Bioelectron 165:112325–112325
Perumal V, Hashim U (2014) Advances in biosensors: principle, architecture and applications. J Appl Biomed 12:1–15
Purohit B, Kumar A, Mahato K, Chandra P (2020) Smartphone-assisted personalized diagnostic devices and wearable sensors, current opinion in biomedical. Engineering 13:42–50
Qi H, Zhang C (2020) Electrogenerated chemiluminescence biosensing. Anal Chem 92:524–534
Rahn KL, Rhoades TD, Anand RK (2020) Alternating current voltammetry at a bipolar electrode with smartphone luminescence imaging for point-of-need sensing. ChemElectroChem 7:1172–1181
Rosati G, Ravarotto M, Sanavia M, Scaramuzza M, De Toni A, Paccagnella A (2019) Inkjet sensors produced by consumer printers with smartphone impedance readout. Sensing Bio-Sensing Res 26:100308
Severi C, Melnychuk N, Klymchenko AS (2020) Smartphone-assisted detection of nucleic acids by light-harvesting FRET-based nanoprobe. Biosens Bioelectron 168:112515
Shi Z, Lu Y, Chen Z, Cheng C, Xu J, Zhang Q et al (2020) Electrochemical non-enzymatic sensing of glycoside toxins by boronic acid functionalized nano-composites on screen-printed electrode. Sensor Actuat B-Chem 129197
Shin Low S, Pan Y, Ji D, Li Y, Lu Y, He Y et al (2020) Smartphone-based portable electrochemical biosensing system for detection of circulating microRNA-21 in saliva as a proof-of-concept, vol 308. Sensor Actuat B-Chem, p 127718
Smith ZJ, Chu K, Espenson AR, Rahimzadeh M, Gryshuk A, Molinaro M et al (2011) Cell-phone-based platform for biomedical device development and education applications. PLoS One 6:e17150
Sun AC, Hall DA (2019) Point-of-care smartphone-based electrochemical biosensing. Electroanalysis 31:2–16
Tang N, Zheng Y, Cui D, Haick H (2021) Multifunctional dressing for wound diagnosis and rehabilitation, vol 10. Adv Healthc Mater, p e2101292
Tran V, Walkenfort B, König M, Salehi M, Schlücker S (2019) Rapid, quantitative, and ultrasensitive point-of-care testing: a portable SERS reader for lateral flow assays in clinical chemistry. Angew Chem Int Edit 58:442–446
Tu Z, Chen M, Wang M, Shao Z, Jiang X, Wang K et al (2021) Engineering bioactive M2 macrophage-polarized anti-inflammatory, antioxidant, and antibacterial scaffolds for rapid angiogenesis and diabetic wound repair. Adv Funct Mater 31:2100924
Turner AP (2013) Biosensors: sense and sensibility. Chem Soc Rev 42:3184–3196
Vashist SK, Luppa PB, Yeo LY, Ozcan A, Luong JHT (2015) Emerging technologies for next-generation point-of-care testing. Trends Biotechnol 33:692–705
Wang Y, Liu X, Chen P, Tran NT, Zhang J, Chia WS et al (2016) Smartphone spectrometer for colorimetric biosensing. Analyst 141:3233–3238
Wei Q, Qi H, Luo W, Tseng D, Ki SJ, Wan Z et al (2013) Fluorescent imaging of single nanoparticles and viruses on a smart phone. ACS Nano 7:9147–9155
Xia Y, Chen Y, Tang Y, Cheng G, Yu X, He H et al (2019) Smartphone-based point-of-care microfluidic platform fabricated with a ZnO nanorod template for colorimetric virus detection. ACS Sensors 4:3298–3307
Xu D, Huang X, Guo J, Ma X (2018) Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens Bioelectron 110:78–88
Xu G, Cheng C, Liu ZY, Yuan W, Wu XZ, Lu YL et al (2019) Battery-free and wireless epidermal electrochemical system with all-printed stretchable electrode array for multiplexed in situ sweat analysis. Adv Mater Technol 4:1800658
Xu J, Cheng C, Li X, Lu Y, Hu S, Liu G et al (2021a) Implantable platinum nanotree microelectrode with a battery-free electrochemical patch for peritoneal carcinomatosis monitoring. Biosens Bioelectron 185:113265
Xu G, Lu Y, Cheng C, Li X, Xu J, Liu Z et al (2021b) Battery-free and wireless smart wound dressing for wound infection monitoring and electrically controlled on-demand drug delivery. Adv Funct Mater 31:2100852
Yao Y, Jiang C, Ping J (2019) Flexible freestanding graphene paper-based potentiometric enzymatic aptasensor for ultrasensitive wireless detection of kanamycin. Biosens Bioelectron 123:178–184
Zhang D, Liu Q (2016) Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens Bioelectron 75:273–284
Zhang D, Lu Y, Jiang J, Zhang Q, Yao Y, Wang P et al (2015a) Nanoplasmonic biosensor: coupling electrochemistry to localized surface plasmon resonance spectroscopy on nanocup arrays. Biosens Bioelectron 67:237–242
Zhang L, Yang W, Yang Y, Liu H, Gu Z (2015b) Smartphone-based point-of-care testing of salivary α-amylase for personal psychological measurement. Analyst 140:7399–7406
Zhang D, Jiang J, Chen J, Zhang Q, Lu Y, Yao Y et al (2015c) Smartphone-based portable biosensing system using impedance measurement with printed electrodes for 2, 4, 6-trinitrotoluene (TNT) detection. Biosens Bioelectron 70:81–88
Zhang D, Lu Y, Zhang Q, Liu L, Li S, Yao Y et al (2016) Protein detecting with smartphone-controlled electrochemical impedance spectroscopy for point-of-care applications. Sensor Actuat B-Chem 222:994–1002
Zhang N, Zhang L, Ruan YF, Zhao WW, Xu JJ, Chen HY (2017) Quantum-dots-based photoelectrochemical bioanalysis highlighted with recent examples. Biosens Bioelectron 94:207–218
Zhang X, Peng J, Song Y, Chen Y, Lu F, Gao W (2019) Porous hollow carbon nanobubbles@ZnCdS multi-shelled dodecahedral cages with enhanced visible-light harvesting for ultrasensitive photoelectrochemical biosensors. Biosens Bioelectron 133:125–132
Zhang Q, Chen Z, Shi Z, Li Y, An Z, Li X et al (2021) Smartphone-based photoelectrochemical biosensing system with graphitic carbon nitride/gold nanoparticles modified electrodes for matrix metalloproteinase-2 detection. Biosens Bioelectron 193:113572
Zhao WW, Xu JJ, Chen HY (2017) Photoelectrochemical enzymatic biosensors. Biosens Bioelectron 92:294–304
Zhou Q, Tang D (2020) Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. TrAC-Trend Anal Chem 124:115814
Zhu H, Yaglidere O, Su T-W, Tseng D, Ozcan A (2011) Cost-effective and compact wide-field fluorescent imaging on a cell-phone. Lab Chip 11:315–322
Zhu L, Li S, Liu W, Chen J, Yu Q, Zhang Z et al (2021) Real time detection of 3-nitrotyrosine using smartphone-based electrochemiluminescence. Biosens Bioelectron 187:113284
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81971703) and the Zhejiang Provincial Natural Science Foundation of China (Grant No. LZ23C100001).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Liu, G., Lu, Y., Cheng, C., Xu, J., Liu, Q. (2023). Smartphone Interface and Wearable Biosensors for on-Site Diagnosis. In: Purohit, B., Chandra, P. (eds) Surface Engineering and Functional Nanomaterials for Point-of-Care Analytical Devices. Springer, Singapore. https://doi.org/10.1007/978-981-99-3025-8_13
Download citation
DOI: https://doi.org/10.1007/978-981-99-3025-8_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-3024-1
Online ISBN: 978-981-99-3025-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)