Abstract
In the era of neuroendoscopic surgery, an endoscopic transorbital approach via the superior eyelid crease has been proposed as a viable way to access the orbital apex and anterior and middle cranial fossa. In addition to the visualization of cranio-orbital tumors, a surgical corridor through this approach allows excellent visualization of the lateral cavernous sinus while avoiding the need for brain retraction. In addition, this approach allows a more direct and shorter anteromedial surgical access to Meckel’s cave without violation of the temporalis muscle. This surgical technique could facilitate minimally invasive surgery for skull base tumors involving the orbital apex. In this chapter, we describe the step by step endoscopic transorbital approach to the orbital apex.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The classic approaches to the orbital apex have involved a requirement for lateral or medial orbitotomy according to the circumferential extension of the lesion around the optic nerve or craniotomy, eventually combined with zygomatic osteotomy for larger lesions [1, 2]. However, transcranial approaches to the orbital apex are laborious and time-consuming with a high risk of temporalis muscle atrophy and operative morbidity associated with brain retraction and damage of critical neurovascular structures en route to the target lesion [3]. In the past few years, alternative approaches, including endoscopic approaches, have been suggested for the treatment of orbital apex lesions with the goal of decreasing the overall invasiveness of the procedure, tailoring the corridor, and, ultimately, achieving better visual and cosmetic outcomes [2]. In the era of neuroendoscopic surgery, an endoscopic transorbital approach (ETOA) has emerged as a recent technique of skull base surgery, enabling a superolateral corridor to the orbit as well as anterior and middle cranial fossae, although the idea itself is no longer novel.
Orbital apex lesions, including orbital tumors, remain a surgical challenge, because they often require an extensive fronto-temporo-orbital zygomatic approach and a multidisciplinary team approach to provide the best outcomes [3]. They mainly include cavernous hemangioma, meningioma, schwannoma, neurofibroma, and fibrous tumors, which have different characteristics and different imaging findings on CT or MRI [4]. The main goal of surgery for orbital apex lesions is to preserve or restore the vision by maximum safe resection of the lesions, with minimal morbidity. In this regard, selection of the optimal approach tailored to each orbital apex lesion is of utmost importance in order to obtain the best possible functional and cosmetic outcomes. For orbital apex lesions, various techniques have been described, including transcranial approaches (such as pterional and supraorbital), lateral orbitotomies, transconjuctival, transantral, and transnasal transethmoidal approaches [2].
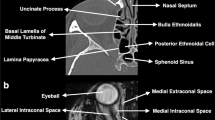
We have recently proposed a neuro-topographic four-zone model with the epicenter around the optic nerve on the coronal plane using endoscopic transorbital and endoscopic endonasal approaches, which successfully provided minimally invasive 360° circumferential access to the entire orbit with acceptable morbidity (Fig. 24.1) [5]. Although open transcranial approaches have classically been performed for access to the lesions located in zones I and II, our study demonstrated that ETOA resulted in clinical outcomes comparable to those achieved with conventional transcranial approaches with less morbidity. It provides a more direct and shorter operative corridor to the lesions located superiorly and laterally to the optic nerve via the superior eyelid crease incision. This access-related advantage is clinically associated with a shorter operation time, excellent cosmesis, reduced postoperative pain, a shorter hospital stay, a faster return to normal activities, and lower risks of CSF leak and wound infection [5,6,7].
Illustration showing the four-zone model with its epicenter around the optic nerve (gray circle) on the coronal plane, consisting of zones I and II (temporal side) and zones III and IV (nasal side). (Adapted with permission from Jeon et al. [5])
We have previously demonstrated the feasibility of ETOA for paramedian skull base tumors, such as trigeminal schwannomas, spheno-orbital meningiomas, and other cranio-orbital tumors [5,6,7,8,9]. In this chapter, we will review the ETOA via the superior eyelid approach for the orbital apex lesions (Fig. 24.2).
3D reconstruction showing the surgical corridor to the orbital apex using ETOA. (Adapted with permission from Jeon et al. [5])
2 Procedure
2.1 Patient Position
All patients have preoperative imaging for intraoperative image guidance with computed tomography and magnetic resonance imaging. ETOA is performed by a multidisciplinary team, consisting of a neurosurgeon and oculoplastic surgeon. After induction of general anesthesia with endotracheal intubation, the patient is placed in a supine position with the head in a neutral or slightly flexed position in a Mayfield three-point fixation, and intraoperative multiplanar MRI–CT fusion neuronavigation is registered. Standard surgical preparation is then performed with 5% povidone-iodine solution on the periocular skin and eyelid margins. One should keep in mind that chlorhexidine is contraindicated on the face due to the potential risk of corneal damage. When draping the patient, both eyes should be left exposed so that the symmetry of the globe positions can be assessed intraoperatively. A lubricated corneal protector is placed over the ipsilateral eye to avoid corneal damage.
2.2 Surgical Technique
After infiltration of a local anesthetic with a mixture of 2% lidocaine with 1:100,000 epinephrine and 0.5% bupivacaine, a skin marking is made with a No. 15 Bard-Parker scalpel blade at the lateral half of the eyelid crease line for patients with an eyelid crease and at the same length of supraciliary line for patients without an eyelid crease (Fig. 24.3a). The line is extended approximately 1 cm laterally over the lateral orbital rim. The skin and orbicularis muscle flap are raised and extended superolaterally until the lateral orbital rim is reached, with the utmost care taken to avoid encroaching on the levator muscle and aponeurosis. Lateral orbital rim is not usually removed for orbital tumors. The periosteum is cut. A hand-held malleable retractor is gently set in place in an infero-medial fashion, and a rigid 0° endoscope is then introduced into the operative field. A subperiosteal dissection is undertaken in a lateral-to-inferomedial direction until the margins of the superior and inferior orbital fissures are identified (Fig. 24.3b). The cranio-orbital foramen (also known as Hirtl’s foramen), through which the recurrent meningeal artery or meningolacrimal branch of the middle meningeal artery traverses, is a good anatomical landmark during the subperiosteal dissection. Being located 1–2 cm anterior to the superior orbital fissure, it is present in 50–60% of patients [10, 11]. After interrupting the recurrent meningeal branch with monopolar cauterization, further dissection to the edges of superior and inferior orbital fissures is made (Fig. 24.3c).
Key steps approaching the orbital apex during endoscopic transorbital approach. (a) Superior eyelid crease incision after placement of a lubricated corneal protector over the ipsilateral eye. (b) Subperiosteal dissection on the superolateral orbital rim. (c) Coagulation of the recurrent meningeal artery at the cranio-orbital foramen. (d) Exposure of the superior orbital fissure. (e) Drilling of the greater sphenoid wing after placement of a silastic sheet along the periorbita. (f) Exposure of the orbital apex, meningo-orbital band, frontal dura, and temporal dura
The superior orbital fissure is the next important landmark to identify prior to drilling the greater sphenoid wing and orbital roof (Fig. 24.3d). When dealing with exposing the margin of the IOF, it is essential to keep the subperiosteal dissection toward the inferomedial side. High-speed drilling of the sphenoid bone using a coarse diamond burr is carefully performed from lateral to medial, protecting the periorbita with a Silastic sheet (Fig. 24.3e). Minimal retraction force is applied with slight displacement of the orbital contents in an inferomedial direction while performing drilling as well as dissection. Given that the greater sphenoid wing has a wide triangular shape, drilling of the lateral orbital wall in the same direction also provides sufficient working space. Once the greater wing of the sphenoid bone is drilled until the dura mater covering the temporal pole comes into view, the hand-held malleable retractor is removed. Subsequently, natural retraction with the surgical instruments is intermittently carried out, resulting in minimal retraction force.
Above all, extreme care should be taken to minimize the retraction-induced pressure on the globe, which may induce the oculo-cardiac reflex, while dissecting the periorbita or drilling the greater sphenoid wing. In addition, an elevated intraocular pressure due to globe retraction during ETOA might lower the eye perfusion pressure, potentially leading to perioperative visual loss. We recommend the instruments be withdrawn from the operative corridor every 15 min while checking the pupils. If necessary, additional osteotomy of the lateral orbital rim using chiseling, so-called an extended ETOA, may be helpful in selected cases to avoid excessive retraction of the globe and to gain adequate working space.
Under guidance of neuronavigation, the periorbital overlying the lesion or the tumor capsule is appropriately opened, and the tumor is carefully removed. Microsurgical technique is used throughout tumor removal.
For the orbital apex lesions extending into Meckel’s cave or cavernous sinus, an interdural dissection plane should be obtained. At this phase, the key anatomical landmark is the meningo-orbital band (MOB) (also known as the fronto-orbital-temporal polar dura), tethering the frontotemporal basal dura to the periorbita, which leads directly to the interdural space of the cavernous sinus without interrupting the intradural space (Fig. 24.3f). A slight incision at the MOB and a peel-off technique is used to gently dissect the interdural layers between the outer membrane of the cavernous sinus and the dura propria. This avascular dissection plane introduces the route to Meckel’s cave and, most importantly, avoids profuse bleeding from the cavernous sinus. The intersection of the dura allows for entry into the cavernous sinus with direct visualization of the V1 and V2 space. Such an approach, through the anteromedial triangle, enables a Hakuba approach along the lateral border of the outer membrane of the cavernous sinus in an anteroposterior direction. The tumor is then removed by applying the same microsurgical techniques as for the conventional transcranial approach.
After complete resection of the lesion, hemostasis is achieved with bipolar forceps and Floseal®. In cases in which the dura mater is opened and/or CSF leakage is evident, skull base reconstruction using a multilayer technique with collagen matrix, autologous fascia, or acellular allogenic dermis is performed. A wedge-shaped piece of polymerized absorbable Medpor can be introduced as a rigid buttress to prevent postoperative enophthalmos when a large bony defect is expected. Finally, the periosteum is sutured with 5-0 absorbable suture, and the skin is closed with 6-0 fast-absorbing plain gut. No lumbar drainage is usually required postoperatively, unless the frontal sinus is breached during surgery.
3 Tips for ETOA to the Orbital Apex
-
A multidisciplinary team with expertise in endoscopic techniques, consisting of a neurosurgeon, orbital surgeon, and otolaryngologist is essential in ETOA.
-
When approaching the orbital apex via the ETOA, one should keep in mind key anatomical landmarks, such as recurrent meningeal artery, superior orbital fissure, and meningo-orbital band.
-
Because long-standing globe retraction may often cause elevation of orbital pressure, resulting in cardiac arrhythmias, frequent monitoring of pupil size, heart rate, and blood pressure, along with electrocardiography, are required, and intermittent release from retraction of the eyeball is recommended. One should be cognizant and cautious of the potential risk of postoperative visual loss due to retraction of the ipsilateral globe during ETOA.
-
As with any novel surgical technique, a concerning drawback is the long learning curve that is required for the maneuverability of the zero-degree and angled endoscopes in a narrow operative field via the transorbital corridor. It is thus important that practice in the cadaver laboratory be made mandatory to gain familiarity with this minimally invasive approach.
References
Paluzzi A, Gardner PA, Fernandez-Miranda JC, et al. “Round-the-Clock” surgical access to the orbit. J Neurol Surg B Skull Base. 2015;76(1):12–24.
Luzzi S, Zoia C, Rampini AD, et al. Lateral transorbital neuroendoscopic approach for intraconal meningioma of the orbital apex: technical nuances and literature review. World Neurosurg. 2019;131:10–7.
Roth J, Fraser JF, Singh A, et al. Surgical approaches to the orbital apex: comparison of endoscopic endonasal and transcranial approaches using a novel 3D endoscope. Orbit. 2011;30(1):43–8.
Mendoza-Santiesteban E, Mendoza-Santiesteban CE, Berazain AR, et al. Diagnosis and surgical treatment of orbital tumors. Semin Ophthalmol. 2010;25(4):123–9.
Jeon C, Hong SD, Woo KI, et al. Use of endoscopic transorbital and endonasal approaches for 360 degrees circumferential access to orbital tumors. J Neurosurg. 2020:1–10.
Jeon C, Hong CK, Woo KI, et al. Endoscopic transorbital surgery for Meckel’s cave and middle cranial fossa tumors: surgical technique and early results. J Neurosurg. 2018:1–10.
Kong DS, Young SM, Hong CK, et al. Clinical and ophthalmological outcome of endoscopic transorbital surgery for cranioorbital tumors. J Neurosurg. 2018;131(3):667–75.
Kong DS, Kim YH, Hong CK. Optimal indications and limitations of endoscopic transorbital superior eyelid surgery for spheno-orbital meningiomas. J Neurosurg. 2020;134(5):1472–9.
Park HH, Hong SD, Kim YH, et al. Endoscopic transorbital and endonasal approach for trigeminal schwannomas: a retrospective multicenter analysis (KOSEN-005). J Neurosurg. 2020;133(2):467–76.
Abed SF, Shams P, Shen S, et al. A cadaveric study of the cranio-orbital foramen and its significance in orbital surgery. Plast Reconstr Surg. 2012;129(2):307e–11e.
Erturk M, Kayalioglu G, Govsa F, et al. The cranio-orbital foramen, the groove on the lateral wall of the human orbit, and the orbital branch of the middle meningeal artery. Clin Anat. 2005;18(1):10–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jeon, C., Kong, DS. (2023). Endoscopic Transorbital Approach for Orbital Apex Lesions. In: POON, T.L., MAK, C., YUEN, H.K.L. (eds) Orbital Apex and Periorbital Skull Base Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-99-2989-4_24
Download citation
DOI: https://doi.org/10.1007/978-981-99-2989-4_24
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2988-7
Online ISBN: 978-981-99-2989-4
eBook Packages: MedicineMedicine (R0)