Abstract
Nanoparticles are of great interest in material science due to their characteristics, such as high surface area, biocompatibility, and unique optical properties. Within the search for more ecological and efficient pathways to synthesize nanoparticles, there is a special interest in the synthesis of bacterial biofilms. Biofilms are self-organized communities of homogeneous or heterogeneous microorganisms that grow on a surface. Naturally, bacteria organize themselves by forming biofilms as a survival mechanism, which is why they are found in different ecosystems. Synthesis of nanoparticles assisted by biofilms is exceptionally a particular methodology in which it is possible to produce nanoparticles with high purity, selectivity, and uniformity, taking advantage of in vivo synthesis. Some of the recent findings in the synthesis of nanoparticles by using biofilms have been focused on the synthesis, mechanism, and stabilization, in which the stabilization represents one of the most critical factors. From a biological point of view, the use of nanoparticles synthetized by using biofilm represents an innovator and direct practical application especially in drug delivery and biocatalysis. In this chapter, a general description of nanoparticles and their different synthesis routes are made, as well as basic concepts of biofilms and their formation processes, to finally present in detail the method of synthesis of nanoparticles into biofilms and their applications. In this order, the first part of the chapter will focus on concepts and methodologies to synthetize nanoparticles, followed by a general description of biofilms. In the third part, an introduction about synthesis of nanoparticles assisted by biofilms will be present. Finally, general applications (agriculture, medicine, and catalysis) will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Nanoparticles Synthesis
Nanotechnology refers to the manipulation of matter at the nanoscale, i.e., at dimensions around 10−9 m. Solids with at least one dimension between 1 and 100 nm are called nanomaterials (Laurent et al. 2010). At this scale, solids might show different properties regarding their macroscopic analogous. Thus, the interest in developing synthesis routes for obtaining nanomaterials has gained attention during the last decades (Li et al. 2013; Shkodenko et al. 2020). In that regard, several experimental protocols, at the laboratory and even pilot scale, have been proposed.
Depending on the composition, nanomaterials are classified as organic or inorganic, and the synthesis route strongly depends on this parameter. Among the most used inorganic nanomaterials, transition metal-based particles are the most used. Metals such as iron, zinc, and copper (Shkodenko et al. 2020) are the most widely used due to their versatile properties and application. Taking advantage of the redox properties of transition metals, common synthesis routes are based on them.
1.1 Chemical Routes
For instance, nowadays it is quite common to favor the reduction, nucleation, and growth of transition metal-based nanoparticles using a proper reducing agent. Briefly, a metal source is solubilized on a specific solvent, commonly water, and then the called reducing agent is added, which produces the reduction of the oxidized metal to a reduced state (or combination of them) as shown in Reaction (6.1) (Lu et al. 1997).
where M stands for the oxidized metal atom, n for the initial oxidation state, and x is the modification on this parameter based on the reducing capacity of the reducing agent. Regarding the subproducts, they depend on the nature of the reducing agent. This simple approach has been widely used in the literature (Lu et al. 1997; Dong et al. 2009; Mercado and Weiss 2018). For instance, for obtaining zero-valent iron nanoparticles, sodium borohydride (NaHB4) has been reported following Reaction (6.2).
In this case, the subproducts are 3H2BO3− + 12H+ + 6H2. More importantly, the reduction from Fe3+ to Fe0 is due to the relatively high standard reduction potential of BH4−, varying from −1.24 V vs SHE (Standard Hydrogen Electrode) at pH 14 to −0.48 V vs SHE at pH 0 (Lu et al. 1997), when compared to the −0.036 V to the Fe3+/Fe0 pair. Thus, selecting the proper reducing agent to promote the formation of zero-valent transition metal nanoparticles depends on the standard reduction potential of the reducing agent and both the initial and final oxidation state of the transition metal. Nevertheless, zero-valent iron nanoparticles are susceptible to oxidation in an air atmosphere. Thus, to obtain air-stable zero-valent iron nanoparticles, the use of an additional functionalization protocol is required. In this way, dicarboxylic acids such as succinic acid have been proven to chelate the surface of the material enhancing their oxidation stability (Mercado and Weiss 2018).
Another example of the previous protocol route is the well-known production of zero-valent gold or silver nanoparticles using the citrate anion as a reducing agent (Turkevich and Copper 1951; Dong et al. 2009). Due to their relatively higher standard reduction potential, Au3+/Au0 (1.52 V vs SHE) and Ag+/Ag0 (0.80 V vs SHE) do not require strong reducing agents such as NaBH4 and citrate anion could be used instead. Moreover, compared to the Fe/NaBH4 system, the Au or Ag/citrate systems do not require the use of an additional surface stabilizer because the oxidized organic subproducts which can be adsorbed on the surface and act as a stabilizer. For instance, the common route to obtain gold nanoparticles is by using AuCl4- (AuCl4−/Au0 1.002 V vs SHE) reduced by citrate which results in the obtention of nanospheres with a mean diameter of 12 nm (De Freitas et al. 2018).
Moving toward a circular economy concept, the use of agro-industrial wastes as feedstock or additives for the synthesis of transition metal oxide nanoparticles has gained interest in the academic community due to its relative simplicity. The reducing agents are typically substituted by an extract from the organic wastes, and the principle of the synthesis is the one previously mentioned. For instance, zero-valent silver nanoparticles have been obtained, instead of using the citrate protocol, avocado seed extracts were suitable as a reducing and stabilization agents. The involved reactive pathway is represented in Fig. 6.1. Immediately after adding the avocado seed extract in contact with the silver ions, the suspension turns brownish-black, which indicates the start of the reduction of the Ag ions. In this case, the nuclei formation of the nanoparticle is given by the interaction of metal ions with organic molecules from avocado extract; mainly hydroxylated molecules, polyphenolic compounds, and organic acids such as perseitol (C7H16O7) or pyrocatechol (C6H6O2), many of which have enough redox potential to complex and reduce Ag+ to Ag0 (Figueroa et al. 2018).
Nevertheless, non-reductive pathways for obtaining transition metal-oxide-based nanomaterials using agroindustrial wastes have been also reported. Monje et al. have studied the use of Ilex paraguariensis, commonly known as Yerba mate, as both precipitation and dispersion matrix for embedding iron/copper oxide nanomaterials (Monje et al. 2022) as shown in Fig. 6.2. Similar to the case of dicarboxylic acid-coated zero-valent iron nanoparticles, the organic matter acts as a stabilization agent. In this case, the organic compounds on the extract do not completely reduce the metallic ions and the final state corresponds to mixed oxides. They suggest that nanoparticle nucleation and growth depend on the formation of monomers of iron-polyphenol complexes and thus pH synthesis is a crucial parameter to control the final size.
1.2 Microorganisms-Based Route
A relatively new approach to nanoparticle synthesis is through biological processes taking advantage of extracellular redox reactions (similar to those discussed above) in microorganisms. This approach is of interest due to the decrease in the use of harmful reagents and its low cost. Moreover, at the end of the synthesis, the obtained materials are coated with biogenic capping agents due to the interaction with the biomass (Tanzil et al. 2016; Prasad et al. 2016; Srivastava et al. 2021; Eftekhari et al. 2023; Kisimba et al. 2023) which avoids its oxidation (vide supra). Among all microorganisms, bacteria and their biofilms have gained attention as nanomaterials synthesis platforms due to their inherent metal resistance and rapid extracellular metal reduction (Ramanathan et al. 2013). From the kinetic aspect of nanoparticle production, biofilms are superior to their bacteria due to their higher metal resistance properties (Tanzil et al. 2016).
As for the chemical route, the nanomaterial synthesis (Reaction (6.1)) requires the use of metal ions and a reducing agent. This latter reagent is provided indirectly by the bacteria. Thus, the production mechanism varies among the used bacteria according to their reducing metabolites secreted (or even in their intracellular space). Nevertheless, some parameters have been identified to control the size of the nanoparticles, such as the pH, incubation time, composition of the growth medium, and metal concentration (Klaus et al. 1999). In fact, the metal concentration is a key parameter to control. Metal ions might be consumed by the microorganism for biochemical reaction such as oxygenic photosynthesis, nitrogen fixation, and hydrogel assimilation (Klaus et al. 1999). Thus, to avoid high metal concentration, it is necessary that the bacteria adopt a resistive state such as complexation, precipitation, or reduction (Tanzil et al. 2016). This last case is responsible for the production of metal-based nanoparticles. The required electrons are provided by the oxidation of reduced organic matter through electron transfer system mediated by multihaeme cytochromes, which prevail in the membrane (Tanzil et al. 2016).
2 Biofilms
Biofilms are self-organized communities of homogeneous or heterogeneous microorganisms that grow on a surface. Naturally, bacteria organize themselves by forming biofilms as a survival mechanism, which is why they are found in different ecosystems, but it was not until 1970 that Nils Hoiby linked chronic infections with bacterial biofilms (Vestby et al. 2020). Currently, it is known that approximately 65% of bacterial infections involve the formation of a biofilm; the strains mainly found in humans are Staphylococcus aureus and epidermidis, Acinetobacter, Bacteroides fragilis, Klebsiella pneumonia, Pseudomonas aeruginosa, and Escherichia coli (Joshi and Litake 2013; Khan et al. 2017). Biofilms can form on different surfaces, such as living tissues, polymeric devices, pipes, or aquatic systems (natural or synthetic) (Schiebel et al. 2020). The developing environment of the biofilm will largely determine the composition and the growth rate.
Biofilms are formed by the adhesion of planktonic bacteria to a surface, acquiring a characteristic structure in which they are covered with an extracellular matrix that provides protection. That matrix is generally formed by a mixture of proteins, mainly rigid such as fibrin, polysaccharides, and polynucleotides. The exact composition of the biofilm matrix depends on the strains that make it up and the developing medium. The extracellular matrix constitutes between 50 and 90% of the biofilm and is usually made up mostly of neutral or anionic polysaccharides that allow the association of cations that provide strength to the biofilm structure (Karygianni et al. 2020). This physical barrier provides the pathogen resistance against antibiotics and host defenses. Some of the mechanisms that have been studied to explain this resistance are: (1) slow and incomplete penetration of antimicrobial agents since the matrix neutralizes and dilutes them, decreasing their effect; (2) decreases the metabolic rate of bacteria, which allows them to survive in conditions of nutrient deficiency; and (3) gene changes that lead to the formation of persistent cells, which occurs due to the slow rate of growth that allows variations in the bacterial phenotype (Percival et al. 2015; Sonawane et al. 2022).
The formation of biofilms is a process that involves three parameters: the substrate, the medium, and the microorganism. Regarding the substrate, the properties of the surface, such as surface energy, polarity, charge, and morphology, are determinants for bacterial fixation since it is the surface that predisposes to the adhesion of external agents by providing binding sites. Some studies found that rough surfaces increase microbial colonization, as were polar, high surface tension, and charged surfaces (De-la-Pinta et al. 2019; Zheng et al. 2021). According to Belfort et al., nonpolar surfaces facilitate the conformational reorientation of proteins since they exert a destabilizing effect, which allows greater interaction with the surface and favors the creation of a conditioning layer that promotes the microorganism adhesion (Nakanishi et al. 2001).
The medium is a factor of great importance for the development of biofilms since it determines the composition of the conditioning film and the stabilization of the microcolonies. When a surface comes into contact with an aqueous medium, it is initially coated with components found in it, such as proteins, polymers, or salts, creating a conditioning film that will affect the shape, speed, and intensity of microorganism attachment. In addition, the physical-chemical conditions of the medium, such as pH, ionic strength, temperature, and the concentration and type of nutrients, are essential for the development of the biofilm. Cowan et al. demonstrated in in vitro tests that the increase in the concentration of nutrients in the medium increases the number of attached microorganisms (Cowan et al. 1991). Additionally, the hydrodynamics of the medium also plays an important role, and the flow velocity limits sedimentation and association of bacteria cells with the surface (Donlan 2002).
Finally, the composition of the surface of the microorganism also affects the rate and intensity of binding to the substrate. In general, bacteria have a highly hydrophobic and negatively charged surface, given to a large extent by the fimbriae or flagellar appendages, which are the first to adhere to the substrate and facilitate the approach of the microorganism for its subsequent fixation. In addition to fimbriae, recent studies have shown that some proteins, mycolic acid, lipopolysaccharide, and other flagella also affect the adhesion process (Srinivasan et al. 2021).
Within a biofilm, there may be more than one bacterial strain, which usually enter into a competition that ends in the strain elimination or coexistence. Mixed biofilms are syntrophic communities, in which several microorganisms coexist, giving them high resistance. It is estimated that between 60 and 80% of biofilm infections correspond to mixed biofilms (Rao et al. 2020). Cooperation between mixed species can also improve adhesion or produce cross-feeding increasing community growth. Yuan et al. reported that biofilms formed by Lactococcus lactis and Pseudomonas fluorescens have an approximately 20,000 and 100-fold increase in adhesiveness when compared to their pure strains, respectively (Yuan et al. 2020).
The reason why the mixed biofilms present greater resistance is still a field of study, among the proposed speculations is the three-dimensional structuring that allows the protection of the weakest microbial strains and the development of denser, thicker, and more adaptable extracellular matrices. The extracellular matrix thickness largely determines the ability of the biofilm to block harmful substances and maintain good bacterial development (Rao et al. 2020).
2.1 Life Cycle of a Biofilm
The study of biofilms is recent, even though bacteria have been studied for several centuries. The first reports of the observation of these structures date back to 1684 when Anthony van Leeuwenhoek observed this behavior in bacteria attached to teeth. However, it was until 1970 that the adhesion of bacteria to pacemaker leads was observed and associated with persistent infections, awakening medical interest in this type of bacterial conformation (Bjarnsholt 2013). Since then, different models have been proposed to try to understand how the formation of biofilms occurs and what is the cause of their particular characteristics. Currently, the biofilm life cycle is made up of three stages: adhesion, growth, and separation. Figure 6.3 shows the process.
The adhesion process is the first phase (I) of biofilm development; this involves the conditioning of the surface and the accumulation and union of a small population of microorganisms. When a medical device is put in contact with tissues, the surface is covered with proteins and other molecules from the medium, which when adhered form a conditioning film that allows a more friendly interaction between the material and microorganisms. Thus, when the bacteria find a conditioned solid surface, they proceed to form a union via appendages (fibrins, flagella) or by secretion of proteins that facilitate adhesion. This union is reversible and requires interaction time for the microorganisms to cement themselves to the substrate and begin to form aggregates that drive the irreversible union of the bacterium to the surface. At this stage, the gene expression of the flagellum is not distinguished, and the bacteria begin to segregate the base substances that will form the extracellular matrix of the biofilm. The second phase (II) corresponds to the growth or maturation of the microorganism; once the bacterium adheres to the surface, it initiates cell division forming clusters of several layers of cells, which form microcolonies with a protective extracellular barrier, which will depend on the type of bacteria and the conditions in which it grows. In the final stage (III), the separation or dispersion of the biofilm occurs; at this point the bacteria are released from the surface, either individually or by conglomerates that can colonize the surface again, closing the cycle, and causing the propagation of bacterial infection. When biofilms dissociate, their structure becomes unstable, and the cells inside them acquire some mobility, which allows separation through biofilm erosion (Palmer et al. 2007; Francolini and Donelli 2010; Sonawane et al. 2022).
The biofilm formation model was developed based on in vitro studies of P. aeruginosa, which has practical and observable management, and initially included five steps; however, said model did not describe the behavior of complex systems such as industrial, natural, and clinical environments, nor did it find the great diversity of biofilm structures that can be formed, so the model was extended leaving the current model that encompasses the three basic events described above, this model gives a general idea of the behavior of this type of structure, allowing its expansion and adaptation to different environments and behavior (Sauer et al. 2022).
2.2 Beneficial Uses of Biofilms
Although biofilms are normally associated with the development of infections and damage to health and industry, they can be used for the benefit of society. Among the beneficial applications of this type of structure are antibacterial potential, fermentation capacity, biofertilizer, filtration, anticorrosive effect, and bioremediation (Qureshi et al. 2005; Todhanakasem 2013). All these characteristics can be exploited mainly by the agricultural and food industry. Some beneficial microorganisms can be expressed as biofilms, which improves their application. For example, in the food and agricultural industry, the production of probiotics in the form of biofilms from different strains of Lactobacillus has shown technological advantages the manufacture, since biofilms avoid the contamination and deterioration of the strain, as well as improve the characteristics of the final product and also have greater resistance to changes in temperature, pH, and mechanical wear (Jones and Versalovic 2009). Probiotics are microorganisms that improve human health by helping to treat and prevent gastrointestinal diseases, so their production and use are highly desired. Another use of biofilm in the food industry is for the manufacture of fermented products (dairy, meat, and pickles) to maintain the quality of the product as aromas and textures. Thus, to produce Ragusano cheese, a vat with a biofilm of fermentative microflora is used; the milk is added to the vat where fermentation and curing of the cheese can take place. In addition, in the traditional formation of cheese, the formation of biofilms on wooden utensils improves the production of lactic acid (Ünal Turhan et al. 2019).
On the other hand, an application related with the bioremediation is still more interesting. Bioremediation deals with the use of microorganisms to treat contaminants, transforming them into less toxic substances. Biofilms have been used for the bioremediation of recalcitrant substances, due to their high biomass, biofilms can immobilize the compounds; in addition, this structure is more resistant to toxic environments (Singh et al. 2006). In agriculture, the formation of biofilms on different parts of the plants can produce symbiotic effects and bring biological control to the crop; among the bacteria that fulfill this function are Bacillus subtilis and Pseudomonas that on the root gives the plant robustness and protection. Rhizobium in the root of the legumes produces symbiosis, and Gluconacetobacter diazotrophicus benefits sugar cane (Rudrappa et al. 2008). Biofilms have also been used for the development of bioreactors, which are used in a wide range of industrial applications. By the way, the formation of biofilms in reactors has shown an increase in productivity, providing advantages such as a higher amount of biomass and greater efficiency in the process. These types of reactors have been used for water treatment and the manufacture of biofuels, enzymes, and fatty acids. One of the best-known processes that use this type of bioreactor is the manufacture of vinegar, which uses Acetobacter biofilms on pieces of wood to speed up the production process (Zottola and Sasahara 1994).
3 Nanoparticles Synthesis by Using Biofilms
There are a lot of strategies that can be adequate to synthetize nanoparticles or to improve their dispersion for several catalytic applications. Among these, biofilms are a good option to control the synthesis (biosynthesis) of nanoparticles. The use of these materials present different and relevant advantages which some of them are related with the low precursor concentrations that can be used during the synthesis, the evident large surface area and besides the inert atmosphere which can produce a resistance of different agents (highly charged) in where evidently can decrease the effective synthesis of the nanoparticles. In general, biofilms minimize the risk of contamination by oxidation suggesting a good way to synthetize and incorporate metallic particles into biofilms (Tanzil et al. 2016).
In this way, the synthesis of nanoparticles using biological pathways (by means bacteria and biofilms) has gained more attention because of the application of green chemical routes which effectively reduce the contamination charge. Effective supporting or adhesion of nanoparticles in the matrix of the biofilms can represent a challenge because sometimes can help to reduce its low biocompatibility. In this way, synthetisizing nanoparticles in the wall or inside of the biofilm is of interest. Besides, biofilms have been used to produce nanoparticles, however, the mechanistic details of its formation are not well understood.
Some of the recent findings in the synthesis of nanoparticles by using biofilms have been focused on the synthesis, mechanism, and stabilization, in which the last represents one of the most critical factors. From a biological point of view, the use of nanoparticles synthetized by using biofilm represents an innovate and direct practical application especially in drug delivery and biocatalysis. Although negative effects of biofilms have been reported, their use in many applications including nanotechnology-based drug delivery is still of interest (Dos Santos Ramos et al. 2018). The use of nanoparticles such as liposomes, microemulsions, polymeric, and metallic nanoparticles could help to control the evident negative effect that can represent the microbial biofilms for a specific application (Thambirajoo et al. 2021). To solve this, the use of nanoparticles as a carrier is widely used to avoid any damage and increase biological activities in comparison with biofilms (Thambirajoo et al. 2021). The modification of biofilms with the use of nanoparticles could avoid the adhesion of bacteria or other phenomena in the surface and within the biological behavior of the cell. In addition, nanoparticles could cause an increasing in the resistance to different vectors coming from antimicrobial agents and helping in the multidrug-resistance strains.
For example, recently the synthesis has been described in a single step of extracellular silver nanoparticles through an in-situ bio-reduction of aqueous solution of silver nitrate using Psidium guayaba L. Nanoparticles were obtained by the reduction pathway starting from AgNO3 solution and periodically monitored by using UV-vis (Gupta et al. 2014). Besides, they were well characterized by TEM obtaining a size particle distribution at around 59.39 nm. In comparison, significant Au nanoparticles were synthetized using marine actinomycete of Nocardiopsis sp. GRG1 (KT235640) as biological stabilizing and reducing agent. In this case, these nanoparticles were evaluated as a potential antibiofilm (up to 91% against biofilm-forming MR-CoNS) (Rajivgandhi et al. 2019). Comparatively, ZnO nanoparticles were synthesized using green chemical synthesis with sodium hydroxide as a reducing agent (Velsankar et al. 2022). These nanoparticles were deposited into a biofilm which was tested as a matrix in biomedical field. The biofilm activity confirmed the antibacterial properties of green-ZnO nanoparticles on both Gram-negative (S. typhi) and Gram-positive (B. subtilis and S. aureus) bacteria by films previously disintegrated. As verification of the potential activity of these nanoparticles, the antioxidant, ant-diabetic, and anti-inflammatory activities were tested showing relevant results.
Following the reported literature, a recent study was focused on the effect of molecular weight starting from chitosan depolymerization products on the synthesis and production of silver nanoparticles (AgNPs) as well as chitosan/AgNPs blend films (Affes et al. 2020). In comparison with the films constituted by only chitosan, the properties of most of them were enhanced when the mixture of chitosan/AgNPs was tested. Then, the light barrier, opacity, elongation at break, as well as bioactivities were improved, thus suggesting that films could be used as novel alternative food packaging applications (Chausali et al. 2022).
Among a lot of applications with nanoparticles by the use of biofilms, an antibacterial study was performed to see whether the effect of polymeric PolymP-n Active nanoparticles would present a significant contribution by using an in vitro subgingival biofilm model (Sánchez et al. 2019). Interestingly, the in vitro investigation demonstrated that the coating surface with both nanoparticles and ions from metallic sources could have an impact on the biofilm formation reducing in some ways the vitality and weakness. In other terms, decreases the contact between matrix and colonizer and being more susceptible to detachment.
Comparatively, ultra-small nanoparticles (USNPs) coming from noble metals could represent a great potential in different applications. This fact is related with their high surface areas and high reactivity, increasing its potential interest in biology. Several pathways have been reported, however, the electrochemical route has been reported recently to obtain USNPs (Au, Pd, Pt) using single bacterium strain of Shewanella loihica PV-4; comparatively, these USNPs had a size range between 2 and 7 nm and showed to be active as potential catalysts for dye degradation (Ahmed et al. 2018).
The mechanism of the interaction of the nanoparticles in systems involving the drug delivery on biofilm formation process is divided into different stages involving, among other, the adherence of microbial cells, the reversible adhesion, irreversible adhesion, maturation, and finally the detachment of cells (Martin et al. 2015).
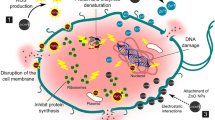
4 General Applications (Fig. 6.4)
4.1 Nanomedicine
4.1.1 Oral Treatments
Nanoparticles have significant promise for addressing the challenges of oral biofilm drug delivery. The chemical flexibility and relative ease of nanoparticle preparation enable the development of unique biofilm treatments. Nanoparticles can either be directly bactericidal or be designed to enhance drug aqueous solubility and transport into bacterial cells. Antibiofilm nanoparticles can be developed from metals or metal oxides, synthetic or natural polymers, or hybrids therein. Furthermore, by modifying chemical compositions, size, surface charge, and other properties, nanoparticles provide great flexibility to ensure robust biofilm targeting and retention through biofilm matrix interactions, thereby enhancing substantivity and antibiofilm efficacy. Nanoparticles’ high surface-area-to-volume ratios enable robust drug or drug combination loading that may result in synergistic antibiofilm efficacy. Data suggest that nanoparticles can also lower the potential for bacterial resistance and protect conventional drugs from pH and/or enzymatic degradation in the harsh biofilm microenvironments (Wang et al. 2016; Benoit et al. 2019).
4.1.2 Drug Delivery Systems
Biofilms can help nanoparticles to improve the aqueous solubility of drugs. Through fine adjustments to chemical compositions such as size, surface charge, and other properties, biofilms together with nanoparticles can provide flexibility for transport, retention, and deliver drugs (Wang et al. 2016). For example, depending on the shape and morphology of nanoparticle, can penetrate the bacterial biofilm, making resistant bacteria more sensitive to breaking the film, thus improving the penetration of antibacterial agents (Shah et al. 2022), in general shape, size, and morphology are the most important parameters for determining the property of nanoparticles (Velsankar et al. 2022).
4.1.3 Anti-Functional Activities
In the recent years, the synthesis of metal oxide nanoparticles has attained much interest in a scientific community. Among them, the ZnO nanostructures are most preferable and attractable due to its cost-effective and easily accessible dynamic nature in emerging bio-sensing, photocatalytic, optical, and electronic device applications (Yadav et al. 2019; Velsankar et al. 2022; Bhuyan et al. 2015).
ZnO nanostructures are nontoxic and a biocompatible material and are used in drug delivery, pharmaceutical, and biomedical fields, for example, some uses are antioxidant, anti-inflammatory, and antidiabetic activities (Kavitha et al. 2023).
The antioxidant activity has been ascribed to a variety of mechanisms, including transition metal ion catalyst binding, chain initiation prevention, hydrogen abstraction prevention, radical scavenging activity, breakdown of peroxides, and reductive capacity. The antioxidants play a significant role in all the living organisms due to the interaction of biomolecules with molecular oxygen. The different chemical entities that consist of unpaired electrons are known as free radicals. These are highly unstable in nature. They extract electrons from neighboring molecules to attain stability and causing damage to them. They are highly reactive, causing severe damage to short-lived chemical species, which results in degradation of biomolecules. These free radicals are essential to human body for detoxification, energy supply, chemical signaling, and immune function, and they are produced continuously in our human body. The antioxidant activity of any studied material is mostly owing to their redox property, which can help in the absorption and neutralization of free radicals, as well as quenching of singlet and triplet oxygen (Rehana et al. 2017; Rajakumar et al. 2018; Velsankar et al. 2022).
4.1.4 Antibacterial Systems
Silver nanoparticles have become the focus of intensive research due to their applications in different areas such as antimicrobials and biomaterial production. Recently nanoparticles have been successfully used for the delivery of therapeutic agents in chronic disease diagnostics, to reduce bacterial infections in skin and burn wounds, to prevent bacterial colonization on medical devices, and in food and clothing industries as an antimicrobial agent (Gupta et al. 2014; Prasad et al. 2020, Inamuddin et al. 2021).
It must be considered that to deliver the antimicrobial agents to the infected area, the dense physical obstacle presented by biofilms must be overcome. Antimicrobial agents administered systemically are not able to effectively reach the infected areas. In addition to the presence of biofilms, in infected wounds, the necrotic tissue covering the wound bed is another issue to consider in the treatment process. Antimicrobial agents delivered from conventional topical dosage forms have been found to display poor penetration due to this obstruction, leading to low concentrations of the antimicrobial agents in the infected area. One way to improve these systems is by developing films from chitosan laden with silver NPs as a delivery system, this allows for improved treatment of chronic wounds with the presence of bacterial biofilms (Permana et al. 2021; Prasad et al. 2020).
Other uses of AgNPs in biomedical applications are antiviral, antifungal, antiparasitic, and antifouling activity (Almatroudi 2020).
4.2 Agriculture
In agriculture, applications of nanomaterials include efficient, slow-releasing plant growth, and protection products such as fertilizers, pesticides, seed cover treatments, improved pathogen detection systems, and improved delivery systems (Prasad et al. 2014, 2017a, b; Bhattacharyya et al. 2016). One potential benefit of nanoparticles is their use for control of pathogenic microorganisms that pose a major threat to crop productivity (Ismail et al. 2017; Gupta et al. 2018; Prasad et al. 2017c). Biological control of pathogens is an effective alternative that also provides the benefit of soil sustainability (Osherov et al. 2023). Biocontrol agents are self-replicating and therefore limit the need for repeated application. The problem of resistance to these agents of disease control is also not encountered. The immobilization of nanoparticles in polymeric matrices helps to improve biocompatibility, control release, and reduce toxicity levels (Bhatia et al. 2021).
5 Summary
In this contribution, we have described most of the basic concepts to understand different synthetic pathways for the synthesis of nanoparticles and also their stabilization in matrices as biofilms. Synthesis of nanoparticles assisted by biofilms is currently an exceptional methodology emerging from another because of the low environmental impact. Recent advances in the use of biofilms as biological carrier for the delivery and synthesis of particles at the nano size have been described focused on the mechanism and stabilization, highlighted the most critical factors. On the other hand, description about its chemical flexibility and relative introduction in biological applications were introduced using examples such as oral treatments, agriculture, antibacterial, and drug delivery systems. In general, nanoparticles as a carrier are widely used to avoid any damage and increasing biological activities in comparison with biofilms which open new synthetic methodologies to apply in materials science. In the same way, modification of biofilms with the use of nanoparticles could avoid the adhesion of bacteria or other phenomena in the surface and within the biological behavior of the cell giving more resistance against diseases, aggregations, and among others. In general, nanoparticles synthetized by using biofilms can generate an increase in the substrate interactions toward the typical properties of these films such as surface energy, polarity, charge, and morphology. The critical factors are determinants for bacterial fixation since it is the surface that predisposes to the adhesion of external agents by providing binding sites. In summary, nanoparticles within the use of biofilms are an emerging topic with critical and potential applications in connection with biology, chemistry, and material science.
References
Affes S, Maalej H, Aranaz I et al (2020) Controlled size green synthesis of bioactive silver nanoparticles assisted by chitosan and its derivatives and their application in biofilm preparation. Carbohydr Polym 236:116063. https://doi.org/10.1016/j.carbpol.2020.116063
Ahmed E, Kalathil S, Shi L et al (2018) Synthesis of ultra-small platinum, palladium and gold nanoparticles by Shewanella loihica PV-4 electrochemically active biofilms and their enhanced catalytic activities. J Saudi Chem Soc 22:919–929. https://doi.org/10.1016/j.jscs.2018.02.002
Almatroudi A (2020) Silver nanoparticles: synthesis, characterisation and biomedical applications. Open Life Sci 15:819–839. https://doi.org/10.1515/biol-2020-0094
Benoit DSW, Sims KR, Fraser D (2019) Nanoparticles for oral biofilm treatments. ACS Nano 13:4869–4875. https://doi.org/10.1021/acsnano.9b02816
Bhattacharyya A, Duraisamy P, Govindarajan M, Buhroo AA, Prasad R (2016) Nano-biofungicides: emerging trend in insect pest control. In: Prasad R (ed) Advances and applications through fungal nanobiotechnology. Springer International Publishing Switzerland, pp 307–319
Bhatia R, Gulati D, Sethi G (2021) Biofilms and nanoparticles: applications in agriculture. Folia Microbiol 66:159–170. https://doi.org/10.1007/s12223-021-00851-7
Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32:55–61
Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. APMIS Suppl 136:1–51. https://doi.org/10.1111/apm.12099
Chausali N, Jyoti Saxena J, Prasad R (2022) Recent trends in nanotechnology applications of bio-based packaging. J Agric Food Res. https://doi.org/10.1016/j.jafr.2021.100257
Cowan MM, Warren TM, Fletcher M (1991) Mixed-species colonization of solid surfaces in laboratory biofilms. Biofouling 3:23–34. https://doi.org/10.1080/08927019109378159
De Freitas LF, Varca GHC, Batista JGDS, Lugão AB (2018) An overview of the synthesis of gold nanoparticles using radiation technologies. Nano 8:939. https://doi.org/10.3390/nano8110939
De-la-Pinta I, Cobos M, Ibarretxe J et al (2019) Effect of biomaterials hydrophobicity and roughness on biofilm development. J Mater Sci Mater Med 30:77. https://doi.org/10.1007/s10856-019-6281-3
Dong X, Ji X, Wu H et al (2009) Shape control of silver nanoparticles by stepwise citrate reduction. J Phys Chem C 113:6573–6576. https://doi.org/10.1021/jp900775b
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. https://doi.org/10.3201/eid0809.020063
Dos Santos Ramos MA, Da Silva PB, Spósito L et al (2018) Nanotechnology-based drug delivery systems for control of microbial biofilms: a review. Int J Nanomedicine 13:1179–1213. https://doi.org/10.2147/IJN.S146195
Eftekhari A, Khalilov R, Kavetskyy T, Keskin C, Prasad R, Rosic GL (2023) Biological/chemical-based metallic nanoparticles synthesis, characterization, and environmental applications. Front Chem 11:1191659. https://doi.org/10.3389/fchem.2023.1191659
Figueroa JG, Borrás-Linares I, Lozano-Sánchez J, Segura-Carretero A (2018) Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res Int 105:752–763. https://doi.org/10.1016/j.foodres.2017.11.082
Francolini I, Donelli G (2010) Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol Med Microbiol 59:227–238. https://doi.org/10.1111/j.1574-695X.2010.00665.x
Gupta K, Hazarika SN, Saikia D et al (2014) One step green synthesis and anti-microbial and anti-biofilm properties of Psidium guajava L. leaf extract-mediated silver nanoparticles. Mater Lett 125:67–70. https://doi.org/10.1016/j.matlet.2014.03.134
Gupta N, Upadhyaya CP, Singh A, Abd-Elsalam KA, Prasad R (2018) Applications of silver nanoparticles in plant protection. In: Abd-Elsalam K, Prasad R (eds) Nanobiotechnology applications in plant protection. Springer International Publishing AG, pp 247–266
Inamuddin, Ahamed MI, Prasad R (2021) Advanced antimicrobial materials and applications. Springer Singapore. (ISBN: 978-981-15-7098-8) https://www.springer.com/gp/book/9789811570971
Ismail M, Prasad R, Ibrahim AIM, Ahmed ISA (2017) Modern prospects of nanotechnology in plant pathology. In: Prasad R, Kumar M, Kumar V (eds) Nanotechnology. Springer Nature Singapore Pte Ltd, pp 305–317
Jones SE, Versalovic J (2009) Probiotic Lactobacillus reuteribiofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol 9:35. https://doi.org/10.1186/1471-2180-9-35
Joshi S, Litake G (2013) Acinetobacter: an emerging pathogenic threat to public health. World J Clin Infect Dis 3(3):25–36. https://doi.org/10.5495/wjcid.v3.i3.25
Karygianni L, Ren Z, Koo H, Thurnheer T (2020) Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol 28:668–681. https://doi.org/10.1016/j.tim.2020.03.016
Kavitha A, Doss A, Pole RPP, Pushpa Rani TPK, Prasad R, Satheesh S (2023) A mini review on plant-mediated zinc oxide nanoparticles and their antibacterial potency. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2023.102654
Khan HA, Baig FK, Mehboob R (2017) Nosocomial infections: epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed 7:478–482. https://doi.org/10.1016/j.apjtb.2017.01.019
Kisimba K, Krishnan A, Faya M, Byanga K, Kasumbwe K, Vijayakumar K, Prasad R (2023) Synthesis of metallic nanoparticles based on green chemistry and their medical biochemical applications: synthesis of metallic nanoparticles. J Renew Mater 11(6):2575–2591. https://doi.org/10.32604/jrm.2023.026159
Klaus T, Joerger R, Olsson E, Granqvist CG (1999) Silver-based crystalline nanoparticles, microbially fabricated. Proc Natl Acad Sci U S A 96:13611–13614. https://doi.org/10.1073/pnas.96.24.13611
Laurent S, Forge D, Port M et al (2010) Erratum: Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications (Chemical Reviews (2008) 108 (2064)). Chem Rev 110:2574. https://doi.org/10.1021/cr900197g
Li Y, Liu H, Song C et al (2013) The dehydration of fructose to 5-hydroxymethylfurfural efficiently catalyzed by acidic ion-exchange resin in ionic liquid. Bioresour Technol 133:347–353. https://doi.org/10.1016/j.biortech.2013.01.038
Lu J, Dreisinger DB, Cooper WC (1997) Cobalt precipitation by reduction with sodium borohydride. Hydrometallurgy 45:305–322. https://doi.org/10.1016/S0304-386X(96)00086-2
Martin C, Low WL, Gupta A et al (2015) Strategies for antimicrobial drug delivery to biofilm. Curr Pharm Des 21:43–66. https://doi.org/10.2174/1381612820666140905123529
Mercado DF, Weiss RG (2018) Polydimethylsiloxane as a matrix for the stabilization and immobilization of zero⇑valent iron nanoparticles. Applications to dehalogenation of environmentally deleterious molecules. J Braz Chem Soc 29:1427–1439. https://doi.org/10.21577/0103-5053.20180006
Monje DS, Ruiz OS, Valencia GC, Mercado DF (2022) Iron oxide nanoparticles embedded in organic microparticles from Yerba Mate useful for remediation of textile wastewater through a photo-Fenton treatment: ilex paraguariensis as a platform of environmental interest – part 1. Environ Sci Pollut Res 29:57127–57146. https://doi.org/10.1007/s11356-022-19744-4
Nakanishi K, Sakiyama T, Imamura K (2001) On the adsorption of proteins on solid surfaces, a common but very complicated phenomenon. J Biosci Bioeng 91:233–244. https://doi.org/10.1016/S1389-1723(01)80127-4
Osherov A, Prasad R, Chrzanowski W, New EJ, Brazaca L, Sadik O, Haynes CL, Maine E (2023) Responsible nanotechnology for a sustainable future. One Earth 6(7):763–766. https://doi.org/10.1016/j.oneear.2023.06.010
Palmer J, Flint S, Brooks J (2007) Bacterial cell attachment, the beginning of a biofilm. J Ind Microbiol Biotechnol 34:577–588. https://doi.org/10.1007/s10295-007-0234-4
Percival SL, Suleman L, Vuotto C, Donelli G (2015) Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol 64:323–334. https://doi.org/10.1099/jmm.0.000032
Permana AD, Anjani QK, Sartini et al (2021) Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater Sci Eng C 120:111786. https://doi.org/10.1016/j.msec.2020.111786
Prasad R, Kumar V, Prasad KS (2014) Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr J Biotechnol 13(6):705–713
Prasad R, Pandey R, Barman I (2016) Engineering tailored nanoparticles with microbes: quo vadis. WIREs Nanomed Nanobiotechnol 8:316–330. https://doi.org/10.1002/wnan.1363
Prasad R, Bhattacharyya A, Nguyen QD (2017a) Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front Microbiol 8:1014. https://doi.org/10.3389/fmicb.2017.01014
Prasad R, Kumar M, Kumar V (2017b) Nanotechnology: an agriculture paradigm. Springer Nature Singapore Pte Ltd. (ISBN: 978-981-10-4573-8)
Prasad R, Gupta N, Kumar M, Kumar V, Wang S, Abd-Elsalam KA (2017c) Nanomaterials act as plant defense mechanism. In: Prasad R, Kumar M, Kumar V (eds) Nanotechnology. Springer Nature Singapore Pte Ltd, pp 253–269
Prasad R, Siddhardha B, Dyavaiah M (2020) Nanostructures for antimicrobial and antibiofilm applications. Springer International Publishing. (ISBN 978-3-030-40336-2) https://www.springer.com/gp/book/9783030403362
Qureshi N, Annous BA, Ezeji TC et al (2005) Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb Cell Factories 4:24. https://doi.org/10.1186/1475-2859-4-24
Rajakumar G, Thiruvengadam M, Mydhili G et al (2018) Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst Eng 41:21–30. https://doi.org/10.1007/s00449-017-1840-9
Rajivgandhi G, Maruthupandy M, Muneeswaran T et al (2019) Biosynthesized silver nanoparticles for inhibition of antibacterial resistance and biofilm formation of methicillin-resistant coagulase negative Staphylococci. Bioorg Chem 89:103008. https://doi.org/10.1016/j.bioorg.2019.103008
Ramanathan R, Field MR, O’Mullane AP et al (2013) Aqueous phase synthesis of copper nanoparticles: a link between heavy metal resistance and nanoparticle synthesis ability in bacterial systems. Nanoscale 5:2300–2306. https://doi.org/10.1039/c2nr32887a
Rao Y, Shang W, Yang Y et al (2020) Fighting mixed-species microbial biofilms with cold atmospheric plasma. Front Microbiol 11:1000. https://doi.org/10.3389/fmicb.2020.01000
Rehana D, Mahendiran D, Kumar RS, Rahiman AK (2017) Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed Pharmacother 89:1067–1077. https://doi.org/10.1016/j.biopha.2017.02.101
Rudrappa T, Biedrzycki ML, Bais HP (2008) Causes and consequences of plant-associated biofilms. FEMS Microbiol Ecol 64:153–166. https://doi.org/10.1111/j.1574-6941.2008.00465.x
Sánchez MC, Toledano-Osorio M, Bueno J et al (2019) Antibacterial effects of polymeric PolymP-n active nanoparticles. An in vitro biofilm study. Dent Mater 35:156–168. https://doi.org/10.1016/j.dental.2018.11.015
Sauer K, Stoodley P, Goeres DM et al (2022) The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat Rev Microbiol 20:608–620. https://doi.org/10.1038/s41579-022-00767-0
Schiebel J, Noack J, Rödiger S et al (2020) Analysis of three-dimensional biofilms on different material surfaces. Biomater Sci 8:3500–3510. https://doi.org/10.1039/D0BM00455C
Shah S, Famta P, Bagasariya D et al (2022) Nanotechnology based drug delivery systems: does shape really matter? Int J Pharm 625:122101. https://doi.org/10.1016/j.ijpharm.2022.122101
Shkodenko L, Kassirov I, Koshel E (2020) Metal oxide nanoparticles against bacterial biofilms: perspectives and limitations. Microorganisms 8:1–21. https://doi.org/10.3390/microorganisms8101545
Singh R, Paul D, Jain RK (2006) Biofilms: implications in bioremediation. Trends Microbiol 14:389–397. https://doi.org/10.1016/j.tim.2006.07.001
Sonawane JM, Rai AK, Sharma M, Tripathi M, Prasad R (2022) Microbial biofilms: recent advances and progress in environmental bioremediation. Sci Total Environ 824:153843. https://doi.org/10.1016/j.scitotenv.2022.153843
Srinivasan R, Santhakumari S, Poonguzhali P et al (2021) Bacterial biofilm inhibition: a focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front Microbiol 12:676458. https://doi.org/10.3389/fmicb.2021.676458
Srivastava S, Usmani Z, Atanasov AG, Singh VK, Singh NP, Abdel-Azeem AM, Prasad R, Gupta G, Sharma M, Bhargava A (2021) Biological nanofactories: using living forms for metal nanoparticle synthesis. Mini-Rev Med Chem 21(2):245–265
Tanzil AH, Sultana ST, Saunders SR et al (2016) Biological synthesis of nanoparticles in biofilms. Enzym Microb Technol 95:4–12. https://doi.org/10.1016/j.enzmictec.2016.07.015
Thambirajoo M, Maarof M, Lokanathan Y et al (2021) Potential of nanoparticles integrated with antibacterial properties in preventing biofilm and antibiotic resistance. Antibiotics 10:111338. https://doi.org/10.3390/antibiotics10111338
Todhanakasem T (2013) Microbial biofilm in the industry. Afr J Microbiol Res 7:1625–1634. https://doi.org/10.5897/AJMR12.2146
Turkevich J, Copper PHJ (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75
Ünal Turhan E, Erginkaya Z, Korukluoğlu M, Konuray G (2019) Beneficial biofilm applications in food and agricultural industry. In: Malik A, Erginkaya Z, Erten H (eds) BT—health and safety aspects of food processing technologies. Springer, Cham, pp 445–469
Velsankar K et al (2022) Green inspired synthesis of ZnO nanoparticles and its characterizations with biofilm, antioxidant, anti-inflammatory, and anti-diabetic activities. J Mol Struct 1255:132420. https://doi.org/10.1016/j.molstruc.2022.132420
Vestby LK, Grønseth T, Simm R, Nesse LL (2020) Bacterial biofilm and its role in the pathogenesis of disease. Antibiotica 9:20059. https://doi.org/10.3390/antibiotics9020059
Wang LS, Gupta A, Rotello VM (2016) Nanomaterials for the treatment of bacterial biofilms. ACS Infect Dis 2:3–4. https://doi.org/10.1021/acsinfecdis.5b00116
Yadav LSR, Pratibha S, Manjunath K et al (2019) Green synthesis of AgZnO nanoparticles: structural analysis, hydrogen generation, formylation and biodiesel applications. J Sci Adv Mater Devices 4:425–431. https://doi.org/10.1016/j.jsamd.2019.03.001
Yuan L, Hansen MF, Røder HL et al (2020) Mixed-species biofilms in the food industry: current knowledge and novel control strategies. Crit Rev Food Sci Nutr 60:2277–2293. https://doi.org/10.1080/10408398.2019.1632790
Zheng S, Bawazir M, Dhall A et al (2021) Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front Bioeng Biotechnol 9:643722. https://doi.org/10.3389/fbioe.2021.643722
Zottola EA, Sasahara KC (1994) Microbial biofilms in the food processing industry–should they be a concern? Int J Food Microbiol 23:125–148. https://doi.org/10.1016/0168-1605(94)90047-7
Acknowledgments
J.S.-V. is grateful to Plan Promoción de la Investigación de la UJI (Investigadores postdoctorales-2022). R.P.G. thanks to Plan Invetigación 2022 – Ayudas investigadores tempranos UNED-SANTANDER 2022 (2022VI/TEMP/001). David Valverde thanks UNED (Costa Rica) for the technical support. Lorena Duarte-Peña is grateful to UNAM for its doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Duarte-Peña, L., Fabio-Mercado, D., Valverde, D., Porcar-García, R., Sánchez-Velandia, J.E. (2023). Synthesis of Nanoparticles in Biofilms. In: Maddela, N.R., Rodríguez Díaz, J.M., Branco da Silva Montenegro, M.C., Prasad, R. (eds) Microbial Processes for Synthesizing Nanomaterials . Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-99-2808-8_6
Download citation
DOI: https://doi.org/10.1007/978-981-99-2808-8_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2807-1
Online ISBN: 978-981-99-2808-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)