Abstract
Platinum group metals (PGM) are used as a catalyst in the automotive catalytic converters to curb engine emissions. The modern catalytic converter (three-way) executes oxidation of CO and unburnt HC, and reduction of NO using its large active surfaces containing PGM, which are precious metals with high cost all over the world. Due to the high cost of the PGM, researchers are working on efficient methods for extracting and reusing these valuable metals from catalytic converters. Pyrometallurgy and hydrometallurgy are the most common ways for the extraction of the PGMs among other methods. Alternative to platinum, materials like titanium dioxide and other metal-based oxides can be used for carrying out redox reactions of toxic vehicular emissions. The use of such alternative catalysts can help in reducing the increasing demands and cost of PGMs. This chapter focuses on the possibilities of recycling the PGMs from catalytic converters and also of reducing the ever-increasing requirement of PGMs in the manufacturing of autocatalysts in the catalytic converters. The chapter reports the recent global trends of PGM recycling and its demand for use as autocatalysts, alternative materials of to PGMs in catalytic converters and alternative methods for emission reduction. Further, the engine-related challenges and research on future directions of replacing PGM’s as autocatalysts has been performed; it includes some experimental results of direct decomposition of NOx using non-noble metal catalysts such as Cu-COK12, Cu-Nb2O5, Cu-YZeolite, and Cu-ZSM5. The article should also provide a quicker understanding of research on development of low-cost non-noble metal-based alternative autocatalysts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
The harmful gas emissions from the engine exhausts contributes to air pollution and imposes a great threat to environment [1,2,3,4,5,6,7]; thus, the automotive industries are much inclined to counter the engine emissions. Hence, catalytic converters are applied after the exhaust pipe to reduce the emission levels from the engine exhaust [8,9,10]. These catalytic converters consist of an active surface of platinum group metals (palladium, platinum, and rhodium), also sometimes denoted as PGM. PGMs are preferred due to their catalytic ability for the conversion of the exhaust gases into less harmful oxides [11]. The PGM’s are called the state-of-the-art Industries’ Vitamin because of its exceptional characteristics like resistance toward corrosion, catalytic activity, stability (thermal and electrical), and inertness [12,13,14,15,16]. By employing catalytic converters, the engine exhaust emissions are controlled to be in the permitted range set by the emission controlling body.
The automobiles are used extensively in the modern world; they have now been basic daily need of an individual. But apart from all the advantages automotives have, we are paying a huge cost in the form of environmental damage, as they emit large amount of NOx, CO, unburnt hydrocarbons, Particulate matter emissions (also known as Soot), and other toxic gases. There are many regulatory bodies around the globe who have made laws, norms and emission regulations, standards according to the country’s environmental conditions and extent of air pollution. The catalytic converter serves as leading aid to fulfill such regulations, so their demand has become obvious in today’s world. With the growing demand of catalytic converters, the demand of their main ingredient’s such as PGM has of course increased and they are under extensive research. Carrying the fact of increased demand of PGM in such amounts, has led to beat the supply or yield globally [17,18,19], and consequently making them “precious and costlier metals”. Table 17.1 shows the cost increment of PGMs over consecutive years.
Figure 17.1 depicts the data of Platinum Group Metal’s Demand Vs Supply for 5 consecutive years for various sectors. From the above graphical representation, it can be observed that supply of PGM has been lesser as compared to its demand since these last 5 years. The following Table 17.2 presents the platinum group metals’ percentage of change among total demand and supply for 5 consecutive years.
Data of Platinum Group Metal’s Demand Vs Supply for 5 consecutive years for various sectors [21]
Additionally, the demand of PGMs as autocatalysts in the automobile industries for some countries around the globe is as shown in Fig. 17.2.
Demand of PGMs as autocatalysts around the globe (ROW in the graph refers to the “rest of world”) [21]
Moreover, this study further reviews the difference in usual usage or demand of platinum group metals as compared to its demand in the automobile industry (as autocatalysts), is shown in Fig. 17.3.
Representation of demand of PGMs in all sectors as compared to automotive sector [21]
A comparison of the total gross demand of PGMs with the demand for its use as autocatalysts is given in Fig. 17.3.
Figure 17.3 and Table 17.3 reflects that good percentages of the demand of platinum group metals in automotive industry as autocatalysts with respect to its demand in global market. The platinum metal has proved its dependence in the jewelries and ornaments, so a huge percentage of platinum goes in the global market as compared to its demand as autocatalysts.
The huge requirements of PGMs convey that, if by any possibility we can reduce the demand of platinum group metals in the automotive industry, the global prices will be reduced significantly. Recycling of platinum group metals has been in consideration since a long time, and it has been proved as a good practice in view of scenario of increasing cost and demand of PGMs. Researchers are investigating new materials and techniques as the alternatives to PGMs to reduce exhaust emissions in more efficient ways [22]. However, due to the superior activity and results by the PGMs, the most used metals for the catalytic conversion are platinum, palladium, and rhodium [13, 23,24,25,26].
17.1.1 History of Emission Control Research
Please note that the first paragraph of a section or subsection is not indented. The first paragraph that follows a table, figure, equation, etc., does not have an indent, either.
Subsequent paragraphs, however, are indented.
In 1975, after beginning of emission control research in the US, the investigations directed that palladium and platinum as most suitable oxidation catalyst. While the iridium, rubidium, and osmium showed their oxides are volatile [27]. From 1975 to the 1980s, researchers were working actively to control emissions; the other challenge was to finalize a catalyst to reduce NOx. For the very first time, a two-bed converter came into the picture; in the primary bed, reduction of NOx took place, and in the secondary bed, HC and CO were treated and oxidized. But the main discrepancy in this came when NH3 formed into the primary bed went to the second one to again form NOx into the secondary bed. Moreover, ruthenium (even while readily forming volatile oxides) was also tested and seen as a possible catalyst, and its chemical properties were tested in many different studies [28,29,30,31,32,33,34,35,36], but they were not preferred for further production because they did not serve the purpose as they not only proved to be an inefficient reducing agent for NO, but also showed higher selectivity toward NH3. Additionally, rhodium was taken into consideration for reduction of NOx, as they shown good NOx reducing nature with lesser selectivity toward NH3.
Eugene Houndry, a French-based mechanical engineer is the inventor of catalytic converter [37,38,39]. However, it was further developed by Carl D. Keith and John J. Mooney in the year 1973, where it was produced for the very first time. After 5 years of its production, the three-way catalyst was also studies for indispensable reduction of NOx [40,41,42,43,44,45]. The platinum group metals are considered as a group of highly valued and rarely available transition metals that consists of platinum, palladium, rhodium, iridium, osmium, ruthenium, (all are periodic table’s d-block elements). They are all white silvery metals that are unreactive, having almost the same chemical and physical characteristics, and are also found at the same place together in mineral deposits. There is a nice history behind using Pt, Pd, and Rh as autocatalysts [46]. Due to difficulties in availability and the high costs of noble metals, in the mid-1970s the research was focused on non-noble metal-based catalysts. But after some extent of the research, it was found that non-noble metals with their oxides, for example, cobalt oxide, nickel oxides, manganese oxide, copper oxide, and chromium oxide, etc., proved to have less durability, less abatement activity toward automotive emissions [22, 27, 46,47,48]. So, the research was moved forward on the catalysts with noble metals due to their exceptional capabilities of higher temperature stability and lesser likeliness to react or interact with the material of support. Pardiwala et al., in their study for the USA and Japan, stated that the catalytic converter has the ability to abate the lethal emissions in forms of CO2, H2O, O2, and N2, and it has become a compulsory for every vehicle [38]. Researches also stated the same for Indian government has also made catalytic converters obligatory, and strict regulations are there for harmful emission prevention [39,40,41]. In 1970s, Japan and the United States of America are the countries, which made catalytic converter to be mandatory, and further, this rule was also implemented in Asia, Europe, and Australia after 10 years; additionally, later on other countries such as India, Brazil, and Mexico took 10 more years to make it mandatory for all vehicles [49].
In the same scenario for the manufacturers, platinum group metal-containing catalytic converter production became a must requirement. The position of catalytic converter as shown in Fig. 17.4, is on rear side of vehicle, inside the exhaust pipe, so that all kind of exhaust gases produced by engine combustion can pass through it and they can be dealt in the Platinum group metal-containing catalytic converter [37]. Additionally, researchers have also worked on the placement of catalysts concerning the engine and explored interesting behaviors in the reduction of emissions. For example, with the increase in distance of placement, the non-methane hydrocarbon emissions increased [50]. However, they also used another factor of palladium loading in their research and showed higher palladium loading results in lesser non-methane hydrocarbon emissions.
17.1.2 Requirement of PGMs in the Catalytic Converters
PGMs are coated on the monolith substrate inside the catalytic converter so as to deal with lethal exhaust emissions; the coated surface remains active and redox reactions take place on it [5]. The catalytic converters can be further studied as two-way (where HC and CO get oxidized to H2O and CO2) and three-way catalytic converters (including function of converting HC and CO), it also reduces nitrogen oxides into O2 and N2 [2]. Researchers studied some potential candidates similar to PGMs such as Cr2O3, TiO2, NiO, CeO2, Fe2O3, and ZrO2. But in 1980s, PGM was established well and found efficient in their role in the catalytic converters [2].
17.1.3 The Different Aspects of Platinum Group Metals
As discussed in the previous sections, the exhaust emissions undergo redox reactions which is the primary goal of a catalytic converter, contributing to reduce the lethal emissions [40]. The palladium metal and the platinum metal are mostly used as oxidizing agent for unburnt hydrocarbons and carbon monoxide to convert them into H2O and CO2, respectively, as shown in the following reactions.
However, rhodium is deployed to break NO into the N2 and also steam reforming [51, 52]. Figure 17.5 shows a typical three-way catalytic converter.
Typical three-way catalytic converter [53]
The alumina washcoat which is porous in structure helps for distribution of platinum, palladium, and rhodium on the substrate’s surface. The washcoat is made up of γ-Al2O3. It provides a greater contact area for the interaction of exhaust gases and active phases and a greater heating stability. In some cases, it may happen that if temperature rises, the surface area of γ-Al2O3 may get decreased; for this reason, metal oxides like CeO2, La2O3, BaO, and ZrO2 are used as stabilizing agents [54]. Furthermore, to assist the distribution of PGM on the substrate, a traditional additive (mixture of ZrO2 with CeO2) is used; this combination also helps in oxidation reactions and promotes catalytic activity [55].
17.1.4 Availability of PGMs
The maximum supply of PGM in the world market is accomplished by South Africa. Following Tables 17.4, 17.5, and 17.6 represents the supply chain of platinum, palladium, and rhodium separately from some regions around the globe.
The data in Tables 17.4, 17.5, and 17.6 clearly specifies that PGMs are immensely being used as a autocatalysts; there is a very high demand. The purpose of these tables is to give an idea that there is a need of PGM recycling and reuse and focus that recycling should be done as much as possible.
As shown in the Sect. 17.1, the current research inculcates the numbers of demand and supply of PGM metals and focuses on the need of recycling of such precious noble metals. There is ample amount of research already available on the topic of PGM recycling and reuse, the current research reviews the developments made in the methods of recycling, reuse, and alternative catalysts tested in place of PGMs. The current study also presents experimental results of catalytic activity of alternative catalysts such as Cu-COK12, Cu- Nb2O5, Cu-YZeolite, and Cu-ZSM5 using DeNOx (Direct decomposition of NOx) technology.
17.2 Current Scenario of the Use and Recycling of PGMs from Catalytic Converters
As discussed in the earlier sections that the demand of PGM are higher than supply, hence the idea of recycling the PGMs would be great. Figure 17.6 shows the trends of demand volume and PGMs recycling volume being performed in recent times.
Trends of demand volume and PGMs recycling volume, being performed in recent times [21]
Figure 17.6 shows that the amount of PGMs obtained from the recycling of autocatalysts is very small as compared to their total demand in the autocatalysts industry. This low fraction of the extraction of PGMs from the autocatalysts adds more to its demand and supply gap. This percentage needs to be increased so that the maximum possible share of demand as autocatalysts gets fulfilled by recycling the old autocatalysts from discarded automobiles.
17.3 Methods of PGM Recycling
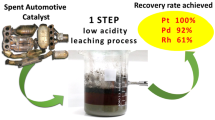
The recycling of PGMs in catalytic converters has been of particular interest to many researchers [10, 56,57,58,59,60,61]. It is considered that the recycling of autocatalysts from the catalytic converter takes less effort than to purify or separate them as compared to the ones taken out directly from mineral ores. Additionally, it is also a fact that the content of PGM is higher when recycled from spent autocatalysts as compared to the ones extracted directly from the earth [51]. Figure 17.7 gives a flowchart of the steps involved in common techniques of extraction of platinum group metals from the catalytic converters.
17.3.1 HydroMetallurgy
Hydrometallurgy is the most basic and common metallurgical process of PGM extraction from the autocatalysts. In this process, the catalytic carrier is first immersed into a solution of chlorides, nitrates, aqua regia, etc. Consequently, PGMs get converted into their respective chlorides (MCl6-2). The obtained solution is then concentrated and separated by electrolytic decomposition of the metal chloride complexes.
Dissolving PGMs in common acids is not easy, as they show high chemical inertness. Hence, aqua regia solution (firmly acidic) is availed to dissolve PGMs. The presence of nitric acid helps as a reducing agent as the reducing potentials for the formation of the chloride complexes of the PGMs is quite high. About 90% of the PGMs used in a car’s catalytic converter get extracted by this process. However, processing takes considerable time due to slow phenomenon of PGMs dissolution in solution of aqua regia. Also, a lot of liquid waste gets created in this process which may be hazardous due to the presence of strong metals [67].
17.3.2 PyroMetallurgy
It is second most used common way for the extraction of PGMs from the catalytic converters. In this method, the catalyst carrier is first ground to break up its surface. It is then melted with additional metal collectors, forming PGMs alloys and metal collector, together with the slag. Further, the metal alloys are purified to yield pure PGMs, and the slag is removed simultaneously.
For the choice of collector metal, several properties like melting point, mutual solubility, and chemical properties between PGMs and collectors have to be considered thoroughly. Mostly copper, nickel, lead, and iron are considered good collector. This technique has many advantages like a lesser investment, low melting temperature, a simple refining process, and simple operation. However, it has a disadvantage of low extraction of rhodium and also by the formation of lead oxide, as it is a toxic waste added into the environment [13].
17.3.3 Pressure Cyanidation
The use of pressure cyanidation is a potential way of extracting PGMs from the automotive catalytic converters. In this method, the spent catalyst is first pre-treated with pressure alkaline leaching. The metal concentrate so obtained is then once again treated with two stages of pressure cyanide leaching. It is then followed by the zinc cementation process giving out concentrates of the respective precious metals. And ultimately, the PGMs are separated by electrolytic decomposition of the metal concentrates [3]. Although it can be categorized under the hydro-metallurgical process, the pressure cyanidation process is, however, more complex and advanced. Firstly, the selective dissolution of base metals by the acidic leaching process was done with elevated pressure conditions, keeping nearly all the precious metals in residue of iron. However, its results were not very satisfactory, partly due to the uncertainty of extraction of PGM, high reagent consumption, lesser rhodium recoveries, and severe pollution, etc. The organization of the United States Bureau of Mines consequently executed investigations on convalescing the platinum group metals by cyanide leaching at high temperatures. The recoveries reported were not much high and also the cyanide exhaustion was quite high in the process of leaching. Thus, a conclusion was made that new pertinent pre-treatment methods are much needed. Chen et al. [6] suggested a new method of pre-treatment, where followed by the 2 stages of leaching of pressure cyanide, the automotive catalysts are pre-treated with pressure alkaline leaching. This leads to a very high number of recoveries of platinum, palladium, and rhodium [3].
17.3.4 Industries Involved in Recycling
The art of recycling catalytic converters has now become well known and gained the focus of industrialists globally. Some used catalytic converters are shown in Fig. 17.8. The processes discussed above in Sect. 17.3 are employed to extract PGMs from them and use them to make fresh catalytic converters.
Some used catalytic converters before process of PGM extraction [59]
Different companies have now begun works to recycle the old, discarded autocatalysts used in automobile vehicles to extract these metals back. Currently, most of the companies that have been involved for production of PGMs are also actively growing a facility for the recycling of catalytic converters to extract back the used PGMs. Some companies are listed in Table 17.7.
Among the above-listed companies, the Umicore Autocatalysts recycling is the most established name in the entire world that works to extract back the PGMs from the used autocatalysts with its plants in Germany, Brazil, the U.S.A., Belgium, etc. Moreover, researchers have studied to substitute PGMs with different noble and non-noble metal catalysts as discussed in the next section.
17.4 Alternatives of Platinum Group Metals as Autocatalysts in Catalytic Converters
some potential catalysts, to replicate PGMs in the catalytic converters. Following Table 17.8 shows the work of researchers since many years for the development of reliable and low-cost solution for exhaust gas treatment. Selected PGM alternatives are discussed in this section.
17.5 Alternative Methods/Techniques
17.5.1 Selective Catalytic Reduction (SCR)
The selective catalytic reduction can control the nitrogen oxides emissions, and the technology involves a reducing agent coupled with catalyst which needs to be injected in the exhaust gas flow stream [100]. Anhydrous ammonia, liquid ammonia, and urea can be used as the reagent for the reduction of NOx. The use of liquid ammonia is preferred over anhydrous ammonia, as it is safer to store and is not toxic [101]. The initially manufactured SCR catalyst system was comprised of TiO2 anatase containing active components (mostly V2O5 & WO3). However, toxicity of vanadium and need of catalyst activity at elevated temperatures gave an idea to focus on another type of research, for example, the highly active metal zeolites [102].
Nowadays, catalysts are generally metal-based zeolites like iron, copper, chromium, etc. The NO2 is generated at just the center of metals on these metal exchanged zeolites, whereas the selective catalytic reduction reaction occurs inside zeolite lattice. On these metal-exchanged zeolites, the NO2 is produced at the metal centers, while the SCR reaction itself takes place within the zeolite framework. Because the NO2 is destroyed as soon as it is produced in the SCR method, it doesn’t appear as gas-phase NO2 [103].
The main reactions for this process are:
17.5.2 NOx Traps
NOx traps or absorbers are applied to reduce the NOx emissions from the automotive exhausts. Various metal-based Zeolites were employed as adsorbents in this study, and their unique characteristics in a variety of applications such as ion exchange, adsorbents, and catalysts have piqued the interest of automotive industry makers [104, 105].
Das et al. [106] developed iron-exchanged X-zeolite and investigated it in real-time exhaust of SI engine. For NOx, the conversion efficiency of 55.8% was achieved, and the conversion of 57.4% was reported for CO. A numerical mathematical equation model for assessing the actions of a catalytic converter incorporating the Fe-X catalyst was also created [107].
17.5.3 DeNOx (Direct Decomposition of NOx)
The direct decomposition of NOx has been in research since a long time due to its easy applicability to break NO into nitrogen and oxygen with the help of catalysts. The direct decomposition of NOx method do not employ a reducing agent and deals in temperature less than 1000 °C while decomposing the NO, hence known to me thermodynamically favorable method [108]. The method includes conversion of NO to Nitrogen and Oxygen (as shown in reaction below) at the catalyst’s surface, with N desorbing as N2 and O remaining strongly attached at surface of catalyst.
The dissolution and absorbance of NO occur efficiently on surface of transition metal. The direct decomposition of NOx has widely been studies for PGM metals. There's also an article there in 1920s that stated that at a temperature of approximately 800 °C, DeNOx progressed on the surface of platinum metal [109], and though research that time in this area slowed due to lack in activity of catalyst and necessary catalysts just weren’t readily available to recompense platinum metal. Also in early 1990s, the studies shown that Cu-ZSM-5, Ag/Co3O4, Pd/MgAl2O4 and perovskite-type oxides shown good activity for decomposition of nitrogen oxides, so researchers focused on the rare earth metals and the compounds of the oxides of pyrochlore [110,111,112,113,114,115].
17.6 Future Scope and DeNOx Results Using Non-noble Metal Catalysts
The use of emission control techniques is however depends on particular characteristics associated with different engines. Certain modifications in the discussed techniques might improve the control of the engine exhaust emissions. For example, for lean-burn Spark Ignition (SI) and Compression Ignition (CI) engines, the three-way catalyst system is not effective for reducing NOx emission. This is because the reducing catalyst is used up in reducing the high level of O2 in diesel exhaust gases. To overcome this problem, other techniques such as Selective Catalytic Reduction (SCR), in which ammonia is used as a reductant and metal oxides as an oxidizer [101], are applied. Also, NOx traps or NOx adsorbers are applied, in which different metal-based zeolites are used as an adsorbent [104].
Authors have conducted a study for direct decomposition of NOx using alternative catalyst to PGMs. The experimental tests were based on the development of non-noble metal-based catalysts in order to provide a low-cost solution. The catalysts Cu-COK12, Cu- Nb2O5, Cu-YZeolite, and Cu-ZSM5 were prepared by the standard wet impregnation method, the supports ZSM-5, Y-Zeolite and Nb2O5 were obtained from Zeolyst International, the Netherlands, and COK12 support was prepared in the laboratory of CSIR-Indian Institute of Petroleum, Dehradun, India. Furthermore, the reactivity tests of prepared catalysts toward NO decomposition were performed with the help of a quartz glass fixed bed reactor setup. This experimental setup at the laboratory of EATA, AFLAD, and CSIR-Indian Institute of Petroleum consists of quartz reactor, thermocouples, mass flow controllers (MFCs), furnace, gas shut ON/OFF valves, gas regulators, and temperature control units. Helium and nitrogen gases were simultaneously used as purging agents to remove the impurities in the gas lines and fixed bed reactor. The prepared catalyst (of amount 250 mg) was placed on the quartz wool (fixed bed) and NO gas was made to pass through the fixed bed reactor; this fixed bed reactor was placed in a furnace to maintain the temperature of the reaction from 200 to 600 ℃. For this case, the flow rate of NO was maintained (using mass flow controllers) at 100 ml/min. Similarly, for each catalysts, the tests were performed at a NO flow rate of 100 ml/min, and temperature of furnace was varied from 200 to 600 ℃. The reacted NO gas coming out of quartz fixed bed reactor was then taken into DANI Master Gas Chromatography machine, equipped with the thermal conductivity detectors, and the output was seen in the form of voltage signals with the help of Clarity software attached with the DANI Master GC. The interpreted results are hence plotted for the percentage conversion versus the temperature as shown in Figure 17.9.
Figure 17.9 shows the catalytic activity of Cu-COK12, Cu- Nb2O5, Cu-YZeolite, and Cu-ZSM5 at the NO flow rate of 100 ml/min. The reactivity of Cu-COK12 and Cu-ZSM5 remains approximately 40% at lower temperatures and falls down with subsequently higher temperatures; but, the reactivity of Cu-YZeolite and Cu-Nb2O5 remains low. Moreover, in order to enhance the catalytic activity and to accomplish the aim to get comparable reactivity with platinum group metals, the further investigations are planned to vary the flow rates of NO and the non-noble metal catalyst weight. In the future, it is planned to calculate the dependence of NO decomposition activity of catalysts in terms of space velocities. This study can be extended for developing the diesel oxidation catalysts for the abatement of HC, CO, and particulate matter emissions.
17.7 Conclusions
Platinum, palladium, and rhodium, have powerful catalytic abilities. Additionally, platinum metals are naturally beautiful, which elevates their value as jewelery in many cultures. The automotive sector is anticipated to have a substantial impact on future PGM demand, while important Asian nations like China are anticipated to have significant rise in jewelery market for platinum. Along with the expansion of the world economy, there is also anticipated growth in the demand for platinum metals for industrial uses. These metals, which are utilized in electronic components and automotive catalytic converters, are increasingly being recycled because of recent increases in PGM costs. PGM recycling often begins with scrap refiners, which gather scrap materials such used catalytic converters and electronic components that contain PGMs to recover the precious metals. To further improve the quality of the recycled material, other refiners often purchase the recovered PGM material. Technological developments that rely on the catalytic capabilities of PGMs, such as fuel cells, become more extensively employed across the globe, the market for recycled platinum metals is anticipated to rise over the upcoming years. The study highlights the following conclusions:
-
The high demands of the PGMs emphasize the need to recycle the catalytic converters for the chemical extraction of these precious metals used as autocatalysts.
-
The gap between total gross supply and total gross demand of PGMs is around 30%; this is what it makes them precious.
-
Only about 32% of the demand for PGMs as an autocatalysts gets fulfilled by recycling from used catalytic converters. This percentage should be increased with a target to achieve above 90% recycling.
-
The use of PGMs in catalytic converters can be reduced by replacing them with other materials and techniques like nickel oxide, titanium dioxide, CeO2 composite catalysts, Cu/Cr Oxide Catalysts, zeolites, selective catalytic reduction, NOx traps, etc.
-
A direct decomposition of engine exhaust is possible using alternative catalyst to PGMs with comparable reactivity with platinum group metals.
-
The current research showed an experimental investigation on NO decomposition activity using Cu-based non-noble metal-based catalysts through DeNOx technology. The maximum reactivity achieved was for Cu-COK12 and Cu-ZSM5, it remains approximately 40% at lower temperatures.
In the future, the plan will be to find ways to increase the reactivity of the catalysts, so that we can add to the research of alternative catalysis. Using non-noble metal catalysts instead of PGMs would be a great breakthrough in this area of research.
Abbreviations
- ATR-FTIR:
-
Attenuated total reflectance-Fourier transform infrared
- BET:
-
Brunauer–Emmett–Teller
- CHN:
-
Carbon Hydrogen Nitrogen analysis
- CI:
-
Compression ignition
- CO:
-
Carbon monoxide
- DeNOx:
-
Direct decomposition of NOx
- DPF:
-
Diesel particulate filter
- FSP:
-
Flame spray pyrolysis
- FTIR:
-
Fourier Transform Infrared Spectroscopy
- H2-TPR:
-
Hydrogen-temperature programmed reduction
- HC:
-
Hydrocarbons
- HRTEM:
-
High-Resolution Transmission Electron Microscopy
- ICP-OES:
-
Inductively coupled plasma—optical emission spectrometry
- LRS:
-
Laser Raman spectroscopy
- MFC:
-
Mass flow controller
- N2O:
-
Nitrous oxide
- NDIR:
-
Nondispersive infrared sensor
- NH3-TPD:
-
Ammonia-Temperature programmed desorption
- NO:
-
Nitrogen oxide
- NO2:
-
Nitrogen dioxide
- NO-TPO:
-
Nitrogen oxide- Temperature programmed oxidation
- NOx:
-
Nitrogen oxide gases
- PGM:
-
Platinum group metals
- PM:
-
Particulate matter
- PXRD:
-
Powder X-ray diffraction
- ROW:
-
Rest of World
- SA:
-
Surface Area
- SCR:
-
Selective catalytic reduction
- SEM:
-
Scanning electron microscope
- SEM-EDX:
-
Energy-dispersive X-ray spectroscopy
- SI:
-
Spark ignition
- TEM:
-
Transmission electron microscopy
- TGA:
-
Thermogravimetric analysis
- TGA-DTA:
-
Thermal gravimetric analysis -Differential thermal analysis
- TPD:
-
Temperature programmed desorption
- TPO:
-
Temperature programmed oxidation
- TPR:
-
Temperature programmed reduction
- UHC:
-
Unburnt hydrocarbons
- USA:
-
United States of America
- UV vis DRS:
-
UV–vis diffuse reflectance spectroscopy
- XPS:
-
X-ray photoelectron spectroscopy
- XRD:
-
X-ray diffraction
References
Ambroziak, T., Gołębiowski, P., Pyza, D., Jacyna-Gołda, I., Merkisz-Guranowska, A.: Identification and analysis of parameters for the areas of the highest harmful exhaust emissions in the model EMITRANSYS. J. KONES 20(3), 9–20 (2013)
Agarwal, A.K.: Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 33(3), 233–271 (2007)
Gritsuk, I., Volkov, V., Mateichyk, V., Gutarevych, Y., Tsiuman, M., Goridko, N.: The evaluation of vehicle fuel consumption and harmful emission using the heating system in a driving cycle. SAE Int. J. Fuels Lubr. 10(1), 236–248 (2017)
Thiyagarajan, S., Geo, V.E., Ashok, B., Nanthagopal, K., Vallinayagam, R., Saravanan, C.G., Kumaran, P.: NOx emission reduction using permanent/electromagnet-based fuel reforming system in a compression ignition engine fueled with pine oil. Clean Technol. Environ. Policy 21(4), 815–825 (2019)
Watson, A.Y., Bates, R.R., Kennedy, D.: Assessment of human exposure to air pollution: methods, measurements, and models. In: Air Pollution, The Automobile, and Public Health. National Academies Press (US) (1988)
Chen, J., Huang, K.: A new technique for extraction of platinum group metals by pressure cyanidation. Hydrometallurgy 82(3–4), 164–171 (2006)
Reşitoğlu, İA., Altinişik, K., Keskin, A.: The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 17(1), 15–27 (2015)
Abitha, J.: Prevention of exhaust from gasoline and diesel engines. J. Adv. Mech. Eng. Sci. 2(3), 21–28 (2016)
Gao, J., Tian, G., Sorniotti, A., Karci, A.E., Di Palo, R.: Review of thermal management of catalytic converters to decrease engine emissions during cold start and warm up. Appl. Therm. Eng. 147, 177–187 (2019)
Saternus, M., Fornalczyk, A.: Possible ways of refining Precious Group Metals (PGM) obtained from recycling of the used auto catalytic converters. Metalurgija 52(2), 267–270 (2013)
Farrauto, R.J., Heck, R.M.: Catalytic converters: state of the art and perspectives. Catal. Today 51(3–4), 351–360 (1999)
Panda, R., Jha, M.K., Pathak, D.D.: March. Commercial processes for the extraction of platinum group metals (PGMs). In: TMS Annual Meeting & Exhibition, pp. 119–130. Springer, Cham (2018)
Dong, H., Zhao, J., Chen, J., Wu, Y., Li, B.: Recovery of platinum group metals from spent catalysts: a review. Int. J. Miner. Process. 145, 108–113 (2015)
Jha, M.K., Lee, J.C., Kim, M.S., Jeong, J., Kim, B.S., Kumar, V.: Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: a review. Hydrometallurgy 133, 23–32 (2013)
Mpinga, C.N., Eksteen, J.J., Aldrich, C., Dyer, L.: Direct leach approaches to Platinum Group Metal (PGM) ores and concentrates: a review. Miner. Eng. 78, 93–113 (2015)
Afolabi, A.S., Nkobane, M.P., Abdulkareem, A.S.: Development of PGMs and chrome extraction circuit from UG-2 ore. In: Proceedings of the World Congress on Engineering, vol. 3 (2012)
Leman, A.M., Jajuli, A., Feriyanto, D., Rahman, F., Zakaria, S.: Advanced catalytic converter in gasoline engine emission control: a review. In: MATEC Web of Conferences, vol. 87, p. 02020. EDP Sciences (2017)
Renner, H., Schlamp, G., Kleinwächter, I., Drost, E., Lüschow, H.M., Tews, P., Panster, P., Diehl, M., Lang, J., Kreuzer, T., Knödler, A.: Platinum group metals and compounds. Ullmann’s Encyclopedia of Industrial Chemistry, pp. 1–73 (2000)
Kondo, S., Takeyama, A., Okura, T.: A study on the forecasts of supply and Demand of Platinum Group Metals. J.-Min. Mater. Process. Inst. Jpn. 122(8), 386 (2006)
Loferski, P.J.: Platinum-Group Metals Statistics and Information. US Geological Survey, February. http://minerals.usgs.gov/minerals/pubs/commodity/platinum (2015). Last accessed 13 Apr 2015
Matthey, J.: PGM market report, November 2016 (2019). www.platinum.mathey.com
Kummer, J.T.: Catalysts for automobile emission control. Prog. Energy Combust. Sci. 6(2), 177–199 (1980)
Matsuda, S., Kato, A.: Titanium oxide based catalysts-a review. Appl. Catal. 8(2), 149–165 (1983)
Angelidis, T.N.: Development of a laboratory scale hydrometallurgical procedure for the recovery of Pt and Rh from spent automotive catalysts. Top. Catal. 16(1), 419–423 (2001)
Atkinson, G.B., Kuczynski, R.J., Desmond, D.P.: US Department of the Interior. Cyanide leaching method for recovering platinum group metals from a catalytic converter catalyst. U.S. Patent No. 5,160,711 (1992)
Yakoumis, I., Moschovi, A.M., Giannopoulou, I., Panias, D.: Real life experimental determination of platinum group metals content in automotive catalytic converters. In: IOP Conference Series: Materials Science and Engineering, vol. 329, no. 1, p. 012009. IOP Publishing (2018)
Kummer, J.T.: Use of noble metals in automobile exhaust catalysts. J. Phys. Chem. 90(20), 4747–4752 (1986)
Shelef, M., Gandhi, H.S.: The reduction of nitric oxide in automobile emissions. Platin. Met. Rev. 18(1), 2–14 (1974)
Gandhi, H.S., Stepien, H.K., Shelef, M.: Optimization of ruthenium-containing, stabilized, nitric oxide reduction catalysts. Mater. Res. Bull. 10(8), 837–845 (1975)
Gandhi, H.S., Stepien, H.K., Shelef, M.: Paper No. 750177. Society of Automotive Engineers (1975)
Voorhoeve, R.J.H., Remeika, J.P., Trimble, L.E.: Perovskites containing ruthenium as catalysts for nitric oxide reduction. Mater. Res. Bull. 9(10), 1393–1404 (1974)
Yao, H.C., Japar, S., Shelef, M.: Surface interactions in the system RhAl2O3. J. Catal. 50(3), 407–418 (1977)
McCabe, R.W., Usmen, R.K., Ober, K., Gandhi, H.S.: The effect of alumina phase-structure on the dispersion of rhodium/alumina catalysts. J. Catal. 151(2), 385–393 (1995)
Wong, C., McCabe, R.W.: Effects of high-temperature oxidation and reduction on the structure and activity of RhAl2O3 and RhSiO2 catalysts. J. Catal. 119(1), 47–64 (1989)
Chen, J.G., Colaianni, M.L., Chen, P., Yates, J.T., Fisher, G.B.: Thermal behavior of a rhodium/alumina model catalyst: disappearance of surface rhodium upon heating. J. Phys. Chem. 94(12), 5059–5062 (1990)
Beck, D.D., Capehart, T.W., Wong, C., Belton, D.N.: XAFS characterization of Rh/Al2O3 after treatment in high-temperature oxidizing environments. J. Catal. 144(1), 311–324 (1993)
Pundir, B.P.: Engine Emissions: Pollutant Formation and Advances in Control Technology. Alpha Science International, Limited (2007)
Pardiwala, J.M., Patel, F., Patel, S.: Review paper on catalytic converter for automotive exhaust emission. In: International Conference on Current Trends in Technology, pp. 382–481. NUiCONE (2011)
Bera, P., Hegde, M.S.: Recent advances in auto exhaust catalysis. J. Indian Inst. Sci. 90(2), 299–325 (2010)
Heck, R.M., Farrauto, R.J.: Automobile exhaust catalysts. Appl. Catal. A 221(1–2), 443–457 (2001)
Shelef, M., McCabe, R.W.: Twenty-five years after introduction of automotive catalysts: what next? Catal. Today 62(1), 35–50 (2000)
Traa, Y., Burger, B., Weitkamp, J.: Zeolite-based materials for the selective catalytic reduction of NOx with hydrocarbons. Microporous Mesoporous Mater. 30(1), 3–41 (1999)
Nishita, Y., Mizuki, J., Tanka, H., Uenishi, M., Kimura, M.: Self regeneration of palladium-perovskite catalysts in modern automobile. J. Phys. Chem. Solids 66, 274–282 (2005)
He, H.X.D.H., Dai, H.X., Au, C.T.: An investigation on the utilization of perovskite-type oxides La1−xSrxMO3 (M = Co0.77Bi0. 20Pd0. 03) as three-way catalysts. Appl. Catal. B Environ. 33(1), 65–80 (2001)
Taylor, K.C.: Why rhodium in three way catalysts? Catal. Rev.-Sci. Eng. 35(4), 457–481 (1993)
Gandhi, H.S., Graham, G., McCabe, R.: Automotive exhaust catalysis. J. Catal. 216(1–2), 433–442 (2003)
Shelef, M., Otto, K., Otto, N.C.: Poisoning of automotive catalysts. In: Advances in Catalysis, vol. 27, pp. 311–365. Academic Press (1979)
Heck, R.M., Farrauto, R.J.: Breakthrough catalytic technologies: the future. Catal. Air Pollut. Control. Commer. Technol. 71–112 (1995)
Fornalczyk, A., Saternus, M.: Removal of platinum group metals from the used auto catalytic converter. Metalurgija 48(2), 133–136 (2009)
Zammit, M., Wuttke, J., Ravindran, P., Aaltonen, S.: The effects of catalytic converter location and palladium loading on tailpipe emissions. No. 2012-01-1247. SAE Technical Paper (2012)
Trinh, H.B., Lee, J.C., Suh, Y.J., Lee, J.: A review on the recycling processes of spent auto-catalysts: towards the development of sustainable metallurgy. Waste Manage. 114, 148–165 (2020)
Hickey, N., Boscarato, I., Kaspar, J.: Air pollution from mobile sources: formation and effects and abatement strategies. In: Current Environmental Issues and Challenges, pp. 15–43. Springer, Dordrecht (2014)
Arcoumanis, C. (ed.): Internal Combustion Engines. Elsevier (2012)
Kašpar, J., Fornasiero, P., Hickey, N.: Automotive catalytic converters: current status and some perspectives. Catal. Today 77(4), 419–449 (2003)
Ozawa, M.: Role of cerium–zirconium mixed oxides as catalysts for car pollution: a short review. J. Alloys Compd. 275, 886–890 (1998)
Kirichenko, A.S., Seregin, A.N., Volkov, A.I.: Developing a technology for recycling automotive exhaust-gas catalysts. Metallurgist 58(3), 250–255 (2014)
Hagelüken, B.C.: Recycling the platinum group metals: a European perspective. Platin. Met. Rev. 56(1), 29–35 (2012)
Rumpold, R., Antrekowitsch, J.: September. Recycling of platinum group metals from automotive catalysts by an acidic leaching process. In: Proceedings of the 5th International Platinum Conference, Sun City, South Africa, The Southern African Institute of Mining and Metallurgy, pp. 695–713 (2012)
Tekper, I., Lewballah, J.K., Quaisie, J.K., Adzabe, F.J.K., Osei, E.Y., Asamoah, E., Baidoo, P., Danquah, A.: An Investigation into the Comparisons of Exhaust Emissions Through Catalytic Converters Installed on a Kia Sportage Lx (Exhaust System) (2020)
Benson, M., Bennett, C.R., Harry, J.E., Patel, M.K., Cross, M.: The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour. Conserv. Recycl. 31(1), 1–7 (2000)
Kizilaslan, E., Aktaş, S., Şeşen, M.K.: Towards environmentally safe recovery of platinum from scrap automotive catalytic converters. Turk. J. Eng. Environ. Sci. 33(2), 83–90 (2010)
Hoffmann, J.E.: Recovery of platinum-group metals from gabbroic rocks metals from auto catalysts. JOM 40(6), 40–44 (1988)
Kim, W., Kim, B., Choi, D., Oki, T., Kim, S.: Selective recovery of catalyst layer from supporting matrix of ceramic-honeycomb-type automobile catalyst. J. Hazard. Mater. 183(1–3), 29–34 (2010)
Sasaki, H., Maeda, M.: Zn-vapor pretreatment for acid leaching of platinum group metals from automotive catalytic converters. Hydrometallurgy 147, 59–67 (2014)
Liu, G., Tokumaru, A., Owada, S.: Separation of PGMs bearing alumina phase from cordierite in spent automobile catalyst by thermal shock. Resour. Process. 60(1), 28–35 (2013)
Trinh, H.B., Lee, J.C., Srivastava, R.R., Kim, S.: Total recycling of all the components from spent auto-catalyst by NaOH roasting-assisted hydrometallurgical route. J. Hazard. Mater. 379, 120772 (2019)
Paiva, A.P., Piedras, F.V., Rodrigues, P.G., Nogueira, C.A.: Hydrometallurgical recovery of platinum-group metals from spent auto-catalysts–focus on leaching and solvent extraction. Sep. Purif. Technol. 286, 120474 (2022)
Schippers, A., Hedrich, S., Vasters, J., Drobe, M., Sand, W., Willscher, S.: Biomining: metal recovery from ores with microorganisms. Geobiotechnology I, 1–47 (2013)
Yong, P., Rowson, N.A., Farr, J.P.G., Harris, L.R., Macaskie, L.E.: A novel electro biotechnology for the recovery of precious metals from spent automotive catalysts. Environ. Technol. 24(3), 289–297 (2003)
Kriek, R.J.: Leaching of selected PGMs: a thermodynamic and electrochemical study employing less aggressive lixiviants. Master’s thesis (2008). University of Cape Town
Wang, S., Chen, A., Zhang, Z., Peng, J.: Leaching of palladium and rhodium from spent automobile catalysts by microwave roasting. Environ. Prog. Sustain. Energy 33(3), 913–917 (2014)
Tyson, D.R., Bautista, R.G.: Leaching kinetics of platinum and palladium from spent automotive catalysts. Sep. Sci. Technol. 22(2–3), 1149–1167 (1987)
Lanaridi, O., Platzer, S., Nischkauer, W., Betanzos, J.H., Iturbe, A.U., Del Rio Gaztelurrutia, C., Sanchez-Cupido, L., Siriwardana, A., Schnürch, M., Limbeck, A., Konegger, T.: Benign recovery of platinum group metals from spent automotive catalysts using choline-based deep eutectic solvents. Green Chem. Lett. Rev. 15(2), 404–414 (2022)
Harjanto, S., Cao, Y., Shibayama, A., Naitoh, I., Nanami, T., Kasahara, K., Okumura, Y., Liu, K., Fujita, T.: Leaching of Pt, Pd and Rh from automotive catalyst residue in various chloride based solutions. Mater. Trans. 47(1), 129–135 (2006)
Puvvada, G.V.K., Sridhar, R., Lakshmanan, V.I.: Chloride metallurgy: PGM recovery and titanium dioxide production. JOM 55(8), 38–41 (2003)
Karim, S., Ting, Y.P.: Recycling pathways for platinum group metals from spent automotive catalyst: a review on conventional approaches and bio-processes. Resour. Conserv. Recycl. 170, 105588 (2021)
Meng, X., Han, K.N.: Recovery of platinum and palladium from spent automobile catalytic converters by leaching with solutions containing halogen salts, ammonium and oxidants (No. CONF-951105-). Minerals, Metals and Materials Society, Warrendale, PA (United States) (1995)
Bollinger, M.A., Vannice, M.A.: A kinetic and DRIFTS study of low-temperature carbon monoxide oxidation over Au—TiO2 catalysts. Appl. Catal. B 8(4), 417–443 (1996)
Harada, S., Ohnishi, T., Ogura, M.: Two-step catalytic system using pulsatile heating to achieve NO decomposition in the presence of water vapor. Chem. Lett. 45(11), 1283–1284 (2016)
Iwamoto, M., Furukawa, H., Mine, Y., Uemura, F., Mikuriya, S.I., Kagawa, S.: Copper (II) ion-exchanged ZSM-5 zeolites as highly active catalysts for direct and continuous decomposition of nitrogen monoxide. J. Chem. Soc. Chem. Commun. 16, 1272–1273 (1986)
Ziolek, M., Sobczak, I., Decyk, P., Nowak, I.: NO adsorption and decomposition on Cu-containing mesoporous molecular sieves-comparison with CuZSM-5, vol. 125. In: Studies in Surface Science and Catalysis, pp. 633–640. Elsevier (1999)
Hasna, A.M.: Reduction of NOx gases using copper zeolite catalyst, vol. 1. In: Proceedings of the World Congress on Engineering (2009)
An, H., McGinn, P.J.: Catalytic behavior of potassium containing compounds for diesel soot combustion. Appl. Catal. B 62(1–2), 46–56 (2006)
Teraoka, Y., Fukuda, H., Kagawa, S.: Catalytic activity of perovskite-type oxides for the direct decomposition of nitrogen monoxide. Chem. Lett. 19(1), 1–4 (1990)
Querini, C.A., Cornaglia, L.M., Ulla, M.A., Miro, E.E.: Catalytic combustion of diesel soot on Co, K/MgO catalysts. Effect of the potassium loading on activity and stability. Appl. Catal. B: Environ. 20(3), 165–177 (1999)
Liu, S., Obuchi, A., Uchisawa, J., Nanba, T., Kushiyama, S.: An exploratory study of diesel soot oxidation with NO2 and O2 on supported metal oxide catalysts. Appl. Catal. B 37(4), 309–319 (2002)
Mul, G., Kapteijn, F., Moulijn, J.A.: Catalytic oxidation of model soot by metal chlorides. Appl. Catal. B 12(1), 33–47 (1997)
Tikhomirov, K., Kröcher, O., Elsener, M., Wokaun, A.: MnOx-CeO2 mixed oxides for the low-temperature oxidation of diesel soot. Appl. Catal. B 64(1–2), 72–78 (2006)
Shangguan, W.F., Teraoka, Y., Kagawa, S.: Promotion effect of potassium on the catalytic property of CuFe2O4 for the simultaneous removal of NOx and diesel soot particulate. Appl. Catal. B 16(2), 149–154 (1998)
Teraoka, Y., Kanada, K., Kagawa, S.: Synthesis of La K Mn O perovskite-type oxides and their catalytic property for simultaneous removal of NOx and diesel soot particulates. Appl. Catal. B 34(1), 73–78 (2001)
Mul, G., Neeft, J.P., Kapteijn, F., Makkee, M., Moulijn, J.A.: Soot oxidation catalyzed by a Cu/K/Mo/Cl catalyst: evaluation of the chemistry and performance of the catalyst. Appl. Catal. B 6(4), 339–352 (1995)
Wu, X., Liang, Q., Weng, D., Lu, Z.: The catalytic activity of CuO–CeO2 mixed oxides for diesel soot oxidation with a NO/O2 mixture. Catal. Commun. 8(12), 2110–2114 (2007)
Zhang, H., Wang, J., Cao, Y., Wang, Y., Gong, M., Chen, Y.: Effect of Y on improving the thermal stability of MnOx-CeO2 catalysts for diesel soot oxidation. Chin. J. Catal. 36(8), 1333–1341 (2015)
Zhenhua, G., Xiuli, S., Hua, W., Kongzhai, L.: Structure and catalytic property of CeO2-ZrO2-Fe2O3 mixed oxide catalysts for diesel soot combustion: effect of preparation method. J. Rare Earths 32(9), 817–823 (2014)
Neyertz, C.A., Banús, E.D., Miró, E.E., Querini, C.A.: Potassium-promoted Ce0.65Zr0.35O2 monolithic catalysts for diesel soot combustion. Chem. Eng. J. 248, 394–405 (2014)
Wagloehner, S., Nitzer-Noski, M., Kureti, S.: Oxidation of soot on manganese oxide catalysts. Chem. Eng. J. 259, 492–504 (2015)
Legutko, P., Jakubek, T., Kaspera, W., Stelmachowski, P., Sojka, Z., Kotarba, A.: Soot oxidation over K-doped manganese and iron spinels—how potassium precursor nature and doping level change the catalyst activity. Catal. Commun. 43, 34–37 (2014)
Legutko, P., Kaspera, W., Stelmachowski, P., Sojka, Z., Kotarba, A.: Boosting the catalytic activity of magnetite in soot oxidation by surface alkali promotion. Catal. Commun. 56, 139–142 (2014)
Venkataswamy, P., Jampaiah, D., Rao, K.N., Reddy, B.M.: Nanostructured Ce0.7Mn0.3O2−δ and Ce0.7Fe0.3O2−δ solid solutions for diesel soot oxidation. Appl. Catal. A 488, 1–10 (2014)
Busca, G., Lietti, L., Ramis, G., Berti, F.: Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: a review. Appl. Catal. B 18(1–2), 1–36 (1998)
Grossale, A., Nova, I., Tronconi, E.: Study of a Fe–zeolite-based system as NH3-SCR catalyst for diesel exhaust aftertreatment. Catal. Today 136(1–2), 18–27 (2008)
Brandenberger, S., Kröcher, O., Tissler, A., Althoff, R.: The state of the art in selective catalytic reduction of NOx by ammonia using metal-exchanged zeolite catalysts. Catal. Rev. 50(4), 492–531 (2008)
Cheung, T., Bhargava, S.K., Hobday, M., Foger, K.: Adsorption of NO on Cu exchanged zeolites, an FTIR study: effects Of Cu levels, NO pressure, and catalyst pretreatment. J. Catal. 158(1), 301–310 (1996)
Hepburn, J.S., Thanasiu, E., Dobson, D.A., Watkins, W.L.: Experimental and modeling investigations of NOx trap performance. SAE Transactions, pp. 1987–2009 (1996)
West, B., Huff, S., Parks, J., Lewis, S., Choi, J.S., Partridge, W., Storey, J.: Assessing reductant chemistry during in-cylinder regeneration of diesel lean NOx traps. SAE Transactions, pp. 1975–1985 (2004)
Das, R.K., Bhattacharyya, S., DuttaGupta, M., Ghosh, B.B.: Theoretical and experimental analysis of iron-exchanged X-zeolite catalyst for SI engine emission control. Exp. Therm. Fluid Sci. 19(4), 214–222 (1999)
Putrasari, Y.: Preparation of nio catalyst on fecral substrate using various techniques at higher oxidation process. Doctoral dissertation, Universiti Tun Hussein Onn Malaysia (2011)
Imanaka, N., Masui, T.: Advances in direct NOx decomposition catalysts. Appl. Catal. A 431, 1–8 (2012)
Green, T.E., Hinshelwood, C.N.: CCXXIV—the catalytic decomposition of nitric oxide at the surface of platinum. J. Chem. Soc. (Resumed) 129, 1709–1713 (1926)
Iwamoto, M., Yahiro, H., Tanda, K., Mizuno, N., Mine, Y., Kagawa, S.: Removal of nitrogen monoxide through a novel catalytic process. 1. Decomposition on excessively copper-ion-exchanged ZSM-5 zeolites. J. Phys. Chem. 95(9), 3727–3730 (1991)
Hamada, H., Kintaichi, Y., Sasaki, M., Ito, T.: Silver-promoted cobalt oxide catalysts for direct decomposition of nitrogen monoxide. Chem. Lett. 19(7), 1069–1070 (1990)
Teraoka, Y., Torigoshi, K.I., Yamaguchi, H., Ikeda, T., Kagawa, S.: Direct decomposition of nitric oxide over stannate pyrochlore oxides: relationship between solid-state chemistry and catalytic activity. J. Mol. Catal. A Chem. 155(1–2), 73–80 (2000)
Ogata, A., Obuchi, A., Mizuno, K., Ohi, A., Aoyama, H., Ohuchi, H.: Enhancement effect of Mg2+ ion on direct nitric oxide decomposition over supported palladium catalysts. Appl. Catal. 65(2), L11–L15 (1990)
Imanaka, N., Masui, T., Masaki, H.: Direct decomposition of nitric oxide over C-type cubic (Gd1–x–yYxBay)2O3–y solid solutions. Adv. Mater. 19(21), 3660–3663 (2007)
Zhang, H.M., Shimizu, Y., Teraoka, Y., Miura, N., Yamazoe, N.: Oxygen sorption and catalytic properties of La1−xSrxCo1−yFeyO3 perovskite-type oxides. J. Catal. 121(2), 432–440 (1990)
Acknowledgements
The work was supervised by Dr. Atul Dhar, School of Engineering, Indian Institute of Technology Mandi, H.P., India. The research was performed with the collaboration of CSIR-Indian Institute of Petroleum, Dehradun, U.K., India, Indian Institute of Technology Mandi, H.P., India, and Centre of Earth Observation Science, School of Applied Sciences, University of Brighton, Brighton, BN24GJ, United Kingdom.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this chapter.
Credit Author Statement
M.K. Shukla: Conceptualization, Methodology, Visualization, Writing- Original draft preparation; Balendra V.S. Chauhan: Investigation, Draft preparation, Writing and Editing; Dr. Thallada Bhaskar: Data curation, Visualization and Supervision; Dr. Atul Dhar: Validation, Supervision, Reviewing and Editing; Dr. Ajitanshu Vedratnam: Visualization, Draft preparation, Editing.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Shukla, M.K., Chauhan, B.V.S., Bhaskar, T., Dhar, A., Vedratnam, A. (2023). Recycling of Platinum Group Metals and Alternative Catalysts for Catalytic Converters. In: Upadhyay, R.K., Sharma, S.K., Kumar, V., Valera, H. (eds) Transportation Systems Technology and Integrated Management. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-99-1517-0_17
Download citation
DOI: https://doi.org/10.1007/978-981-99-1517-0_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-1516-3
Online ISBN: 978-981-99-1517-0
eBook Packages: EngineeringEngineering (R0)