Abstract
Environmental pollution from petroleum products for energy generation is of grave concern nowadays. Oxygenated alternative fuels like biodiesels, alcohols, etc., have gained much popularity for internal combustion (IC) engines. Due to their inherent oxygen content, these oxygenated alternative fuels possess lower carbon-to-hydrogen (C/H) ratio for same heating value. Supporting the road map towards decarbonization of mobility, there has been a recent uptick in curiosity about the possibility of alcohols in compression ignition (CI) engines. Biodiesel contains ~10% inherent oxygen (m/m), and alcohols may contains up to 50% oxygen (m/m), affecting the CI engine's combustion, performance, and emission. An overview of CI engines fuelled with oxygenated fuels (biodiesel and alcohol blends) on combustion characteristics, engine performance, and exhaust emissions are presented in this study. Biodiesel and alcohol fuels (methanol, ethanol, and butanol) have been compared to evaluate the impact of different blend ratios, oxygen mass fraction content based on in-cylinder combustion pressure trace, heat release rate, and engine performance as brake thermal efficiency and carbon dioxide (CO2) emission. Up to 25% lowered CO2 emission was recorded for oxygenated fuel compared to diesel, with significantly lower particulate emission.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Oxygenated alternative fuels
- Alcohols
- Waste cooking oil (WCO) based biodiesel
- Compression ignition (CI) engines
1 Introduction
Global population is likely to reach 9 billion by the year 2040, resulting in a 25% increase in energy demand (Ghadikolaei et al. 2018). Increased energy demand and a growing global urge to minimize carbon emissions impact the usage of conventional fuels and encourage the investigation and implementation of alternative fuels for existing engines (Sahu et al. 2021a, 2022a). Sustainable fuel production processes and utilization have drawn attention to environmental protection by reducing overall carbon dioxide (CO2) emissions (Ianniello et al. 2021). Biofuels are produced from renewable and sustainable resources, which have proven their use in compression ignition (CI) engines and have been promising in recent years. In this context, biofuels (such as biodiesels, lower and higher alcohols, and their blends with conventional fuels) are extensively explored, showing exciting results regarding emissions reduction and increased efficiency compared with conventional fuels (Ianniello et al. 2021; Shamun et al. 2020; Luca et al. 2022; Sahu et al. 2022c). Innovative technologies for powertrain systems improved the overall engine efficiency and lowered emissions (Belgiorno et al. 2020; Di Blasio et al. 2019). Improved injector nozzle and fuel injection systems (Sequino et al. 2018; Beatrice et al. 2017; Di Blasio et al. 2019), in-cylinder air motion, and EGR systems (Dimitrakopoulos et al. 2019) are being implemented to meet stringent emission regulations and engine performance requirements. Different injection strategies (Sahu et al. 2022d) as well as advanced combustion modes (dual fuel, partially premixed combustion, etc.) (Belgiorno et al. 2019; Dimitrakopoulos et al. 2017; Sahu et al. 2021b) combined with alternative fuels could be an enabler towards clean combustion and reduce the overall CO2 impact of the transportation sector (Sahu et al. 2022d). It has been observed that oxygenated alternative fuel blends in dual fuel mode lead to the air-fuel mixture in crevice volume showing higher hydrocarbon (HC) emissions (Fraioli et al. 2017; Monsalve-Serrano et al. 2020). Other studies have also reported this behaviour for heavy-duty and light-duty engines (Heuser et al. 2016; Pedrozo et al. 2018). These observations show that HC emissions are higher at lower engine loads and have a similar emission profile to conventional fuel at higher engine loads. Engine operating parameters such as maximum in-cylinder pressure and compression ratio (CR) can affect the air-to-fuel mass trapped in the crevice volume, primarily affecting the HC emission slip in dual fuel combustion (Di Blasio et al. 2015, 2017). Biodiesel-diesel-ethanol (BDE; 78%, 17%, and 5% v/v, diesel, biodiesel, and ethanol, respectively) blends were studied by Krishna et al. (2019) and a marginal decrease in efficiency with reduced carbon monoxide (CO) and comparable CO2 emissions for BDE has been reported. Saravanan et al. (2020) have tested exhaust gas recirculation (EGR) on ethanol-diesel blends in CI engines and reported that an increase in EGR reduces the efficiency, reduces peak pressure (Pmax) and heat release rate (HRR) compared to ethanol-diesel fuelled conditions. This is due to a drop in thermal efficiency caused by replacing a large quantity of combustion air with recirculated exhaust gases. Verma et al. (2020) have tested hybrid fuel (ethanol-methanol-diesel-microalgae blends) in a CI engine and observed that the presence of spirulina microalgae shortens the ignition delay while increasing efficiency and decreasing the exhaust gas temperature. Another study shows that EGR is an effective technique to reduce the oxides of nitrogen (NOx) associated with alcohol fuel where Duan et al. (2021) have observed an advancement in injection timing which advances the HRR and HRRmax decreases with increasing the EGR ratio. Li et al. (2019) reported that utilization of isopropanol-butanol-ethanol blend with diesel (IBE30) reduces HRRmax value and NOx emissions. Higher molecular weight alcohols (butanol, pentanol, hexanol, or octanol) blends with Ultra-low sulfur diesel (ULSD) were tested and investigated by Rajesh Kumar et al. (2016). They observed that butanol served promising with the lowest emission levels of smoke, NOx, and CO; however, it showed longer ignition delay, higher Pmax and HRRmax, and shortest combustion duration among tested alcohols. Neat methanol was tested for varying intake air temperature, resulting in longer ignition delay and lowering the Pmax with decreasing the intake air temperature (Zincir et al. 2019). Shamun et al. (2018) examined NOx and Indicated mean effective pressure (IMEP) in a Partially premixed combustion (PPC) engine with methanol and iso-octane. They compared the charge-cooling impact of methanol and iso-octane using a single injection technique and observed that methanol resulted in lower nitrogen oxide (NOx) emissions. Higher latent heat of methanol vaporization results in lower NOx emission (Shamun et al. 2018). It has been observed that a reduction in the fuel injection rate reduced the NOx and CO2 emissions and increased the smoke and HC emissions (Sayin et al. 2009). According to the research summarised in the literature survey, it can be noted that biodiesel, methanol, ethanol, and butanol have been tested and compared extensively with varying volume fractions under various injection techniques, blends dual fuel modes, and PPC techniques. Researchers found that the lower carbon-to-hydrogen (C/H) ratio and inherent oxygen content may be the reasons for reduced exhaust emissions. In this framework, this study provides a thorough literature review on comparing different oxygenated alternative fuels (biodiesel, methanol, ethanol, and butanol) blended with diesel, keeping almost constant the oxygen fraction of blends. The analysis includes the comparative study of the main combustion analysis parameters and efficiencies. The first part of the chapter discusses the characteristics of oxygenated alternative fuels. The later part of the chapter discusses the combustion and performance behaviours of oxygenated fuels. This discussion emphasized in-cylinder pressure, heat release rate (HRR), ignition delay, and engine efficiencies.
2 Fuel Properties of Oxygenated Alternative Fuels
This section discusses the selected fuel properties of diesel and some of the alternative oxygenated fuels. Table 4.1 reports various fuel properties such as density, kinematic viscosity, heating value, latent heat of vaporization, autoignition temperature, carbon, hydrogen, oxygen content, and cetane number for different alcohols and biodiesels. Alcohols (methanol, ethanol and butanol) show a lower carbon-to-hydrogen ratio (up to 50%) with 50–21% inherent oxygen content (m/m). Primarily, alcohols and biodiesel are being used as oxygenated alternative fuels due to the ease of their production, availability, and renewable nature. Alcohol properties significantly change with increasing molecular weight, which can be observed in Table 4.1. For example, the kinematic viscosity of methanol, ethanol, and butanol are 0.59 mm2/s, 1.2 mm2/s and 2.51 mm2/s, respectively. Similarly, heating values are 20.2, 27.1, and 33.1 MJ/kg for methanol, ethanol, and butanol.
Table 4.1 show that the stoichiometric air-fuel ratios for methanol, ethanol, and butanol are 6.6, 9.0, and 11.1 kg of air/kg of fuel, which are significantly lower than diesel, i.e., 14.49 kg of air/kg of fuel. Lower air requirement for chemically complete combustion increases the chances for a higher degree of complete combustion and reduces unburnt hydrocarbon emission. Alcohols show a lower cetane number, higher autoignition temperature, and higher latent heat of evaporation, increasing the ignition delay and decreasing the autoignition characteristics of the fuel.
3 Combustion and Performance of Oxygenated Fuels
CI engines are more widespread than SI engines in the farming and heavy-duty transportation sectors owing to their higher durability, performance, and fuel economy. Using oxygenated fuels (mainly alcohols and biodiesels) in CI engines might offer a more environmentally friendly and viable alternative to conventional diesel (Sahu and Shukla 2022). Despite having renewable nature and superior fuel properties, specific challenges still need to be investigated for alcohols and biodiesels. Since alcohol has a lower cetane number (CN) value than diesel, the autoignition temperature value is higher, resulting in a longer ignition delay in contrast to diesel. In order to avoid this, it is essential to make modifications to the fuel design, and injection strategy or adding ignition improver additives (Sahu and Shukla 2022). Also, hardware modifications like increasing the compression ratio, increasing the intake temperature, and optimizing the EGR rate are ways to make combustion possible for alcohols. In this section, the combustion and performance of oxygenated fuels are discussed and compared with conventional diesel. Jamrozik et al. have tested butanol diesel fuel for oxygen fractions of 0–14.2% (m/m), corresponding to 60% butanol energy fraction (66% in mass fraction) in diesel. Figure 4.1 shows the carbon-hydrogen and oxygen mass fractions of prepared butanol blends. Higher peak pressure and HRR (Fig. 4.2) were observed for butanol blends (till 30% energy fraction of butanol). Further increase in butanol energy fraction showed reduced peak pressure and reduced HRR with retarded location of Pmax and HRRmax. It is attributable primarily to the rising latent heat of vaporization of the blend, which deteriorates the fuel evaporation process, and the low cetane number of n-butanol, which weakens the autoignition characteristics.
Carbon, hydrogen, and oxygen mass fractions at different diesel-butanol blend ratios (Jamrozik et al. 2021).
In-cylinder pressure and HRR traces at part-load at different diesel-butanol blend ratios (Jamrozik et al. 2021).
Ghadikolaei et al. (2018) investigated CI engines’ combustion, performance, and emissions parameters fueled with five distinct alternative fuel mixtures. They used seven different fuels, diesel (D), waste cooking oil (B), methanol (M), ethanol (E), 2-propanol (Pr), n-butanol (Bu), and n-Pentanol (Pe) labeled as DB, DBM, DBE, DBPr, DBBu, and DBPe. These blends were prepared so that the oxygen content of 5% can be retained in the blend and the calorific value remained constant. They reported that the peak in-cylinder pressure of all tested blends shows a 1% higher Pmax than conventional. Peak HRR was elevated by 22.1% for higher alcohols and 14.8% and 5.9% for lower alcohols (DBE and DBM), respectively. Moreover, 5% oxygenated biodiesel showed a lower peak of HRR by 3%. Labeckas et al. (2017a) have studied the variation of ethanol oxygen fraction and biodiesel oxygen fraction for five different oxygen fractions (0, 0.91, 1.81, 2.71, 3.61 and 4.52%). They observed a slightly higher ignition delay for ethanol blends than biodiesel blends when oxygen fraction (m/m) was maintained at 3.61%. However, ethanol showed 1.7 CAD higher ignition delay for 2.71% oxygen at λ = 1.2 (Fig. 4.3) at 2500 rpm of engine speed.
Ignition delay for ethanol and biodiesel oxygen fraction (Labeckas et al. 2017a).
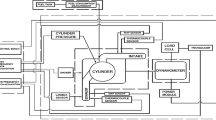
Nour et al. (2019) tested 10% pentanol/octanol with ethanol—diesel blend (10:10:80) and reduces the peak in-cylinder pressure compared to diesel. Diffusion phase of HRR is enhanced with a diminished premixed phase. Break specific fuel consumption (BSFC) was observed to be higher for pentanol substitution and lower for octanol substitution. Both pentanol and octanol report better stability for blends of ethanol-diesel with significantly lower NOx and CO2 emissions. Guedes et al. (Mendes Guedes et al. 2018) have suggested advancing the fuel injection timing by 1 CAD for an increase of every 5% ethanol in the blends of diesel-biodiesel-ethanol, which resulted in a maximum increase of 9% in peak pressure. Prakash et al. (2018) examined the performance of ternary mixtures of bioethanol, diesel, and castor oil. 30% bioethanol demonstrated a longer ignition delay, leading to higher fuel collection and a rapid increase in in-cylinder pressure and HRR. Shamun et al. (2018) tested diesel, biodiesel, and ethanol in a light-duty diesel engine; they underlined the fact that greater oxygen content in the fuel leads to complete combustion and elevates the temperature of the combustion process. Ignition delay was increased when ethanol was present, which resulted in a greater amount of fuel being consumed during the premixed phase of combustion and an increase in the creation of thermal NOx. Pradelle et al. (2019) experimented with a Euro 3 CI engine and reported that the decreased density and heating value of ethanol caused a rise in specific fuel consumption of around 2% for every 5% increase in that anhydrous ethanol was included in the blend. Ethanol possesses a higher latent heat of vaporization, heat capacity at constant pressure, and a lower cetane number than conventional fuel causes an increase in the ignition delay. Despite these limits, useful thermal energy increased with ethanol content. This improved the engine’s efficiency, especially at low loads. Shrivastava et al. (2021) investigated that the ternary blend (Biodiesel 20%—Ethanol 10%-Diesel 70%) delivered superior performance than the conventional diesel. Their study shows a 2% reduction in break thermal efficiency (BTE), a 3% rise in BSFC, a 3% increase in EGT, a 0.86% increase in CO2, a 0.02% reduction in CO, an 8% reduction in NOx, and 12 ppm reduction in HC compared to baseline diesel (Shrivastava et al. 2021). Authors of this chapter too performed an experiment to understand the effect of oxygen variation for different alcohol fuels, and an investigation was performed for 2.5% and 5% oxygenated blends in a twin-cylinder water-cooled CI engine for 1600 rpm at 20 Nm engine loading, schematic of the experimental setup shown in Fig. 4.4. The engine was fitted with a piezoelectric pressure sensor for measuring in-cylinder pressure. Engine was operated at desired operating load for a minimum of 15 minutes to reach the steady state condition. Upon reaching the steady state, combustion data were taken for 120 thermodynamic consecutive cycles for a given blend of fuel. Performance and emissions data were measured multiple times, and average data were used for analysis. Methanol, ethanol, and butanol were used for preparing the oxygenated blend, named as M2.5, E2.5, and B2.5 for 2.5% oxygen m/m fraction and M5, E5, and B5 for 5% oxygen m/m fraction. It contains 5.2% methanol, 7.4% ethanol, and 11.7% butanol respectively for a 2.5% oxygenated blend and 10.3% methanol, 14.8% ethanol, and 23.3% butanol respectively for a 5% oxygenated blend. Figure 4.5 shows the measured in-cylinder pressure and calculated HRR for tested fuels at 20 Nm loading conditions. For 2.5% oxygenated case (Fig. 4.5a), pressure and HRR pattern were similar with slightly higher peak pressure for M2.5, HRR curve was observed to shift away from top dead center (TDC) for all three tested fuels, E2.5 resulted in 2 CAD retarded location of HRRmax compared to diesel. Figure 4.5b shows the pressure and HRR pattern for 5% oxygenated fuel. M5 showed the advanced location of peak pressure; however, E5 and B5 showed the retarded location of peak pressure than diesel. E5 and B5 also showed a higher ignition delay and higher HRRmax and compared to diesel, the location of HRRmax was also retarded by ~3 CAD.
Figure 4.6 shows the value of combustion phasing (CA05, CA50, and CA90 correspond to total heat release of 5, 50, and 90%), combustion duration, and BTE for 2.5 and 5% oxygenated blends at 20 Nm engine loading condition for 1600 rpm. It was noted that all three fuel CA05 locations were advances for 5% oxygenated fuel than 2.5% oxygenated fuel. A similar pattern was also observed for CA50 and CA90 locations. Early CA05 and CA90 of oxygenated fuel resulted in a lower combustion duration (lowered by ~2–3 CAD) for both 2.5 and 5% oxygenated fuel compared to baseline diesel. BTE was observed comparable for both 2.5 and 5% oxygenated fuel, with maximum efficiency of 22% was achieved for M5 and a minimum efficiency of 18.9% was achieved for E2.5 observed for the tested condition (Fig. 4.6).
Labeckas et al. (2017b) reported that BTE is higher after 2.71% oxygen fraction; further, it is intensified with a higher oxygen fraction for ethanol-oxygen mass content (shown in Fig. 4.7) for higher engine rpm of 2000 and 2500 rpm. Biodiesel oxygen mass fraction showed a similar comparable efficiency for 2500 rpm; moreover, it is a slightly increasing trend with an increase in oxygen fraction for 2000 rpm. Compared to both cases, ethanol oxygen fraction is more beneficial than biodiesel oxygen fraction as it showed a relatively higher BTE for tested conditions. Variations of BTE are shown in Fig. 4.8 for the experiment performed by Ghadikolaei et al. (2018) for different fuel blends with a 5% oxygen mass fraction. Figure 4.8 shows that the highest BTE of the tested fuels was observed at 199.5 Nm, resulted in the best engine performance. Low load operations caused lower combustion temperatures, which possibly resulted to incomplete combustion. At high loads, fuel/air ratio is richer, and combustion temperature is higher; however, there is insufficient time for mixing, leading to incomplete combustion and a decrease in BTE. Five loads show that the BTE of DBM (3.5%) and DBBu (1.5%) is higher than that of diesel fuel, while the BTE of other blended fuels is comparable [Ghadikolaei et al. (2018)]. Lower fuel viscosity, improved fuel atomization, and higher oxygen content etc. Improved BTE by enhancing the combustion process, which transforms fuel chemical energy into useful engine work. DBM showed the highest BTE of all the evaluated fuels. Among the investigated alcohols, methanol indicated the shortest chain and lowest molecular weight, which promotes better combustion and the highest BTE (Fig. 4.8). Methanol showed the lowest boiling point, which lowered heat losses and raises BTE.
BTE for an ethanol-oxygen mass fraction (left) and biodiesel oxygen mass fraction (right) (Labeckas et al. 2017b).
BTE for 5% oxygen mass fraction for different alternative fuels (diesel (D), waste cooking oil biodiesel (B), methanol (M), ethanol (E), 2-propanol (Pr), n-butanol (Bu), and n-pentanol (Pe)) (Ghadikolaei et al. 2018).
Jamrozik et al. (2021) reported that DB40 (40% butanol energy fraction in diesel) showed maximum efficiency of 41.3% (Fig. 4.9) with the lowest energy consumption among the tested butanol-diesel blends which is approximately 11% higher than diesel. Higher efficiency is achieved possibly due to the acceleration of chemical oxidation with the higher butanol fraction, however, a further increase in butanol fraction shifted the HRR curve away from TDC resulting in lower useful work and lower efficiency.
Indicated thermal efficiency (ITE) and indicated specific energy consumption (ISEC) for diesel-butanol blends (Jamrozik et al. 2021).
Nour et al. (2019) demonstrated that pentanol (10%) increased the BTE by 3% at lower loads for ethanol-diesel blends (10:10:80). Adding octanol to diesel raises BTE by 5% at low and mid loads, whereas at higher loads, diesel is 1% more efficient. The ternary blends’ oxygen concentration improved combustion and boosted BTE, especially with octanol.
Overall, it can be observed that using oxygenated fuel in a limited fraction improves the combustion and enhances the performance characteristics of the engine, however, a much higher fraction also led to retard the peak pressure and offered higher ignition delay. Considering conventional CI engines up to 15% of alcohols can be utilized with diesel, a further higher fraction requires alterations in engine hardware and fuel injection systems.
4 Exhaust Emissions Characteristics of Oxygenated Fuels
Locally peak in-cylinder temperature locations and a high degree of pyrolysis lead to NOx and PM emissions in CI engines. It has been reported that alcohols are beneficial in reducing PM emissions in diesel combustion drastically with almost no/slight change in NOx emissions. Jamrozik et al. (2021) tested butanol blends with oxygen percentages of 2.7–14.2% (shown in Fig. 4.1, mentioned as DB10 to DB60). They reported NOx is an increasing trend from DB10 to DB40; moreover, DB60 shows significantly lower NOx (Fig. 10a) compared to DB50 and similar to diesel D100. This is mainly due to the higher amount of alcohol present in DB50 and DB60 blends, resulting in higher latent heat of vaporization, and reducing the peak cylinder temperature lowers the NOx formation. CO and CO2 emissions are in decreasing trend with an increase in butanol fraction; CO shows a sharp decline (Fig. 10b) with an increase in oxygen fraction due to improving combustion quality and lower C/H ratio of overall blends with an increase in butanol.
NOx, THC, CO, and CO2 emissions for different butanol-diesel blends (Jamrozik et al. 2021).
Kumar et al. (2022) tested the particulate matter (PM) in diesel engines fueled with ethanol-diesel blends and reported that 5–10% of ethanol with diesel fuel reduced significant particulate emissions. 50% reduction in PM was observed using ethanol compared to diesel fuel. This may be due to the use of ethanol fuel with diesel improving the combustion and increasing the combustion temperature which resulted in a reduction in soot formation. Smoke results shown in Fig. 4.11 were performed by authors, which show the emission characteristics of diesel engine fueled with different alcohols with the same oxygen fraction (2.5 and 5% inherent oxygen). Butanol blends with 5% oxygen mass fraction showed a higher reduction compared to diesel and 2.5% butanol oxygenated fuel blend. It was observed that E5 and B5 resulted in significantly higher HRRmax and lower combustion duration (Figs. 4.5 and 4.6), which showed that a higher degree of complete combustion resulted in lower smoke emissions.
5 Summary
A comparative study was performed to study the combustion and performance characteristics of oxygenated alternative fuels in CI engines. In addition to this, an engine experiment was also performed with the same oxygen concentration for different fuels. Butanol blends resulted in a promising way compared to mineral diesel and lower molecular alcohol blends. Biodiesel and light alcohols have a high oxygen content that is chemically inherited in their molecular structure helps to boost combustion efficiency. Apart from that, the following conclusions can be drawn:
-
Alcohols (methanol, ethanol, and butanol) with the same oxygen mass fractions showed different results due to different volume fractions of alcohol, ethanol, and butanol. Ethanol and butanol showed higher HRR than methanol blends (keeping the oxygen mass fraction same). Higher volume fraction of ethanol/butanol is required to make the same oxygen mass fraction.
-
Methanol blends showed improved efficiency due to the shortest chain and lowest molecular weight among investigated alcohols, leading to better combustion and higher thermal efficiency.
-
In general, higher alcohol fraction addition showed increased in-cylinder pressure and higher HRR, which may be damped using ignition improver additives, or higher alcohols such as the use of pentanol or octanol.
-
The incorporation of alcohol into diesel fuel led to a considerable decrease in CO and smoke emissions.
The present study indicated that alcohols are promising alternative fuels in terms of PM reduction. Alcohols (especially butanol) indicated significantly higher efficiency compared to diesel due to better combustion. Overall, utilization of these alcohol fuels is also beneficial in terms of total CO2 reduction in the environment as it is considered as green alternative fuel produced by first-generation or second-generation vegetation/agro products.
Abbreviations
- ABE:
-
Acetone-butanol-ethanol
- BDE:
-
Biodiesel-diesel-ethanol
- BTE:
-
Brake thermal efficiency
- CA05:
-
5% of total burn fraction
- CA50:
-
50% of total burn fraction
- CA90:
-
90% of total burn fraction
- CAD:
-
Crank angle degree
- CI:
-
Compression ignition
- C/H:
-
Carbon to hydrogen ratio
- CO:
-
Carbon monoxide
- CO2:
-
Carbon dioxide
- EGR:
-
Exhaust gas recirculation
- HC:
-
Hydrocarbon
- HRR:
-
Heat release rate
- IBE:
-
Isopropanol-butanol-ethanol
- IC:
-
Internal combustion
- ISEC:
-
Indicated specific energy consumption
- IMEP:
-
Indicated mean effective pressure
- ITE:
-
Indicated thermal efficiency
- LTC:
-
Low-temperature combustion
- NOx:
-
Nitrogen oxides
- PPC:
-
Partially premixed combustion
- SI:
-
Spark ignition
- TDC:
-
Top dead center
- ULSD:
-
Ultra-low sulfur diesel
References
Alemahdi N, Tuner M (2020) The effect of 2-ethyl-hexyl nitrate on HCCI combustion properties to compensate ethanol addition to gasoline. Fuel 270:117569
Beatrice C, Belgiorno G, Di Blasio G, Mancaruso E, Sequino L, Vaglieco BM (2017) Analysis of a prototype high-pressure “Hollow Cone Spray” diesel injector performance in optical and metal research engines. SAE Technical Paper 2017-24-0073
Belgiorno G, Blasio GD, Beatrice C (2019) Advances of the natural gas/diesel RCCI concept application for light-duty engines: comprehensive analysis of the influence of the design and calibration parameters on performance and emissions. In: Natural gas engines. Springer, pp 251–266
Belgiorno G, Boscolo A, Dileo G, Numidi F, Pesce FC, Vassallo A et al (2020) Experimental study of additive-manufacturing-enabled innovative diesel combustion bowl features for achieving ultra-low emissions and high efficiency. SAE Int J Adv Curr Pract Mob 3(2020–37–0003):672–684
Di Blasio G, Belgiorno G, Beatrice C, Fraioli V, Migliaccio M (2015) Experimental evaluation of compression ratio influence on the performance of a dual-fuel methane-diesel light-duty engine. SAE Int J Engines 8(5):2253–2267
Di Blasio G, Belgiorno G, Beatrice C (2017) Parametric analysis of compression ratio variation effects on thermodynamic, gaseous pollutant and particle emissions of a dual-fuel CH 4-diesel light duty engine. SAE Technical Paper 2017-01-0764
Di Blasio G, Beatrice C, Ianniello R, Pesce FC, Vassallo A, Belgiorno G et al (2019a) Balancing hydraulic flow and fuel injection parameters for low-emission and high-efficiency automotive diesel engines. SAE Int J Adv Curr Pract Mob 2(2019-24-0111):638–652
Di Blasio G, Vassallo A, Pesce FC, Beatrice C, Belgiorno G, Avolio G (2019b) The key role of advanced, flexible fuel injection systems to match the future CO2 targets in an ultra-light mid-size diesel engine. SAE Int J Engines 12(2):129–144
Di Luca G, Pipicelli M, Ianniello R, Belgiorno G, Di Blasio G (2022) Alcohol fuels in spark ignition engines. In: Di Blasio G, Agarwal AK, Belgiorno G, Shukla PC (eds) Application of clean fuels in combustion engines. Springer Nature Singapore, Singapore, pp 33–54
Dimitrakopoulos N, Belgiorno G, Tuner M, Tunestal P, Di Blasio G, Beatrice C (2017) PPC operation with low ron gasoline fuel. A study on load range on a euro 6 light duty diesel engine. Jpn Soc Mech Eng C308
Dimitrakopoulos N, Belgiorno G, Tunér M, Tunestål P, Di Blasio G (2019) Effect of EGR routing on efficiency and emissions of a PPC engine. Appl Therm Eng 152:742–750
Duan X, Xu Z, Sun X, Deng B, Liu J (2021) Effects of injection timing and EGR on combustion and emissions characteristics of the diesel engine fuelled with acetone–butanol–ethanol/diesel blend fuels. Energy 231:121069
Fraioli V, Beatrice C, Di Blasio G, Belgiorno G, Migliaccio M (2017) Multidimensional simulations of combustion in methane-diesel dual-fuel light-duty engines. SAE Technical Paper 2017-01-0568
Ghadikolaei MA, Cheung CS, Yung K-F (2018) Study of combustion, performance and emissions of diesel engine fueled with diesel/biodiesel/alcohol blends having the same oxygen concentration. Energy 157:258–269
Heuser B, Kremer F, Pischinger S, Rohs H, Holderbaum B, Körfer T (2016) An experimental investigation of dual-fuel combustion in a light duty diesel engine by in-cylinder blending of ethanol and diesel. SAE Int J Engines 9(1):11–25
Ianniello R, Belgiorno G, Di Luca G, Beatrice C, Di Blasio G (2021) Ethanol in dual-fuel and blend fueling modes for advanced combustion in compression ignition engines. In: Alcohol as an alternative fuel for internal combustion engines. Springer, pp 5–27
Jamrozik A, Tutak W, Grab-Rogaliński K (2021) Combustion stability, performance and emission characteristics of a CI engine fueled with diesel/n-butanol blends. Energies 14(10)
Krishna SM, Abdul Salam P, Tongroon M, Chollacoop N (2019) Performance and emission assessment of optimally blended biodiesel-diesel-ethanol in diesel engine generator. Appl Therm Eng 155:525–533
Kumar A, Sahu TK, Shukla PC (2022) Design and fabrication of a partial flow dilution tunnel for particulate mass sampling in an ethanol blended CI engine, 1st edn, vol 1042. IOP Publishing, p 012006
Labeckas G, Slavinskas S, Kanapkienė I (2017a) The individual effects of cetane number, oxygen content or fuel properties on the ignition delay, combustion characteristics, and cyclic variation of a turbocharged CRDI diesel engine—Part 1. Energy Convers Manag 148:1003–1027
Labeckas G, Slavinskas S, Kanapkienė I (2017b) The individual effects of cetane number, oxygen content or fuel properties on performance efficiency, exhaust smoke and emissions of a turbocharged CRDI diesel engine—Part 2. Energy Convers Manag 149:442–466
Li G, Lee TH, Liu Z, Lee CF, Zhang C (2019) Effects of injection strategies on combustion and emission characteristics of a common-rail diesel engine fueled with isopropanol-butanol-ethanol and diesel blends. Renew Energy 130:677–686
Mendes Guedes AD, Leal Braga S, Pradelle F (2018) Performance and combustion characteristics of a compression ignition engine running on diesel-biodiesel-ethanol (DBE) blends—Part 2: optimization of injection timing. Fuel 225:174–183
Monsalve-Serrano J, Belgiorno G, Di Blasio G, Guzmán-Mendoza M (2020) 1D simulation and experimental analysis on the effects of the injection parameters in methane–diesel dual-fuel combustion. Energies 13(14)
Nour M, Attia AMA, Nada SA (2019) Improvement of CI engine combustion and performance running on ternary blends of higher alcohol (pentanol and octanol)/hydrous ethanol/diesel. Fuel 251:10–22
Pedrozo VB, May I, Guan W, Zhao H (2018) High efficiency ethanol-diesel dual-fuel combustion: A comparison against conventional diesel combustion from low to full engine load. Fuel 230:440–451
Pipicelli M, Luca GD, Ianniello R, Gimelli A, Beatrice C (2022) Alcohol fuels in compression ignition engines. In: Application of clean fuels in combustion engines. Springer, pp 9–31
Pradelle F, Leal Braga S, Fonseca de Aguiar Martins AR, Turkovics F, Nohra Chaar Pradelle R (2019) Performance and combustion characteristics of a compression ignition engine running on diesel-biodiesel-ethanol (DBE) blends—Potential as diesel fuel substitute on an Euro III engine. Renew Energy 136:586–598
Prakash T, Geo VE, Martin LJ, Nagalingam B (2018) Effect of ternary blends of bio-ethanol, diesel and castor oil on performance, emission and combustion in a CI engine. Renew Energy 122:301–309
Rajesh Kumar B, Saravanan S, Rana D, Nagendran A (2016) A comparative analysis on combustion and emissions of some next generation higher-alcohol/diesel blends in a direct-injection diesel engine. Energy Convers Manag 119:246–256
Sahu TK, Sarkar S, Shukla PC (2021a) Combustion investigation of waste cooking oil (WCO) with varying compression ratio in a single cylinder CI engine. Fuel 283:119262
Sahu TK, Kshatri R, Shukla PC (2021b) Impact of ethanol on combustion, performance, and emission characteristics of diesel engine. In: Alcohol as an alternative fuel for internal combustion engines. Springer, pp 251–266
Sahu TK, Shukla PC (2022a) Effect of Inherent oxygen mass fraction of alcohol blends with diesel on combustion and emission parameters. Environ Prog Sustain Energy e14030
Sahu TK, Shukla PC (2022b) Combustion and emission characteristics of butanol-diesel blend (B15) doped with diethyl ether, diglyme and ethyl diglyme in a CRDI diesel engine. SAE Technical Paper 2022-01-1073.
Sahu TK, Sahu VK, Mondal A, Shukla PC, Gupta S, Sarkar S (2022a) Investigation of sugar extraction capability from rice paddy straw for potential use of bioethanol production towards energy security. Energy Sour Part A: Recovery Utiliz Environ Effects 44(1):272–286
Sahu TK, Shukla PC, Belgiorno G, Maurya RK (2022b) Alcohols as alternative fuels in compression ignition engines for sustainable transportation: a review. Energy Sour Part A: Recovery Utiliz Environ Effects 44(4):8736–8759
Sahu TK, Kshatri R, Kumar A, Shukla PC (2022c) Combustion stability investigation of ethanol blends (E05, E10) in a twin-cylinder ci engine. SAE Technical Paper 2022-01-0521
Sahu VK, Sahu TK, Shukla PC (2022d) Fuel injection strategies for alcohol utilization in combustion engines. In: Application of clean fuels in combustion engines. Springer, pp 55–69
Saravanan P, Kumar NM, Ettappan M, Dhanagopal R, Vishnupriyan J (2020) Effect of exhaust gas re-circulation on performance, emission and combustion characteristics of ethanol-fueled diesel engine. Case Stud Thermal Eng 20:100643
Sayin C, Ilhan M, Canakci M, Gumus M (2009) Effect of injection timing on the exhaust emissions of a diesel engine using diesel–methanol blends. Renew Energy 34(5):1261–1269
Sequino L, Belgiorno G, Di Blasio G, Mancaruso E, Beatrice C, Vaglieco BM (2018) Assessment of the new features of a prototype high-pressure “Hollow Cone Spray” diesel injector by means of engine performance characterization and spray visualization. SAE Technical Paper 2018-01-1697
Shamun S, Belgiorno G, Di Blasio G, Beatrice C, Tunér M, Tunestål P (2018) Performance and emissions of diesel-biodiesel-ethanol blends in a light duty compression ignition engine. Appl Therm Eng 145:444–452
Shamun S, Belgiorno G, Di Blasio G (2020) Engine parameters assessment for alcohols fuels application in compression ignition engines. In: Singh AP, Sharma YC, Mustafi NN, Agarwal AK (eds) Alternative fuels and their utilization strategies in internal combustion engines. Springer Singapore, Singapore, pp 125–139
Shamun S, Zincir B, Shukla P, Garcia Valladolid P, Verhelst S, Tunér M (2018) Quantification and analysis of the charge cooling effect of methanol in a compression ignition engine utilizing PPC strategy, vol 51982. American Society of Mechanical Engineers, p V001T02A7
Shrivastava K, Thipse SS, Patil ID (2021) Optimization of diesel engine performance and emission parameters of Karanja biodiesel-ethanol-diesel blends at optimized operating conditions. Fuel 293:120451
Verhelst S, Turner JWG, Sileghem L, Vancoillie J (2019) Methanol as a fuel for internal combustion engines. Prog Energy Combust Sci 70:43–88
Verma TN, Nashine P, Chaurasiya PK, Rajak U, Afzal A, Kumar S et al (2020) The effect of ethanol-methanol-diesel-microalgae blends on performance, combustion and emissions of a direct injection diesel engine. Sustain Energy Technol Assess 42:100851
Zhang ZH, Tsang KS, Cheung CS, Chan TL, Yao CD (2011) Effect of fumigation methanol and ethanol on the gaseous and particulate emissions of a direct-injection diesel engine. Atmos Environ 45(11):2001–2008
Zincir B, Shukla P, Shamun S, Tuner M, Deniz C, Johansson B (2019) Investigation of effects of intake temperature on low load limitations of methanol partially premixed combustion. Energy Fuels 33(6):5695–5709
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sahu, T.K., Shukla, P.C. (2023). Combustion and Emission Characteristics of Oxygenated Alternative Fuels in Compression Ignition Engines. In: Shukla, P.C., Belgiorno, G., Blasio, G.D., Agarwal, A.K. (eds) Renewable Fuels for Sustainable Mobility. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-99-1392-3_4
Download citation
DOI: https://doi.org/10.1007/978-981-99-1392-3_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-1391-6
Online ISBN: 978-981-99-1392-3
eBook Packages: EnergyEnergy (R0)