Abstract
Polymeric micelles (PM) developed in solution by self-assembling of amphiphilic polymers and represents an advanced tool to overcome several issues related to drug delivery like low aqueous solubility drug that may lead to low drug permeability across biological barriers. PM have several benefits, such as their tiny size, high solubility, ease of sterilizing, and controlled release of pharmaceuticals. Since the integrated medicine may be released rapidly in vivo, the physical stability of this carrier is a crucial concern. To effectively develop micelles that can carry drugs to their sites of action, the significant effort still must be done in understanding how PM interact with plasmatic and cellular components. This chapter describes the advantages and challenges of PM for the delivery of proteins, oral uptake of PM, types of polymers utilized in the administration of micellar drugs, and PM for multiple functionality and protein delivery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Although protein medicines offer a lot of potential as targeted therapeutics, their use is hampered by issues including instability, a brief half-life, and unfavourable immune reactions. As a result, ways of effectively increasing the availability and activation of proteins in specific tissues may be found in protein delivery approaches based on stimuli-responsive nanocarriers. Thus, substantial research is being done on PM that may encapsulate proteins (Tao et al. 2020). The core-shell structure of PM for developing nanoscale drug delivery device was created via the self-assembling of amphiphilic block copolymers after dispersion in the aqueous phase. Amphiphilic di-block-copolymers such as polystyrene and poly(ethylene glycol) (PEG) and triblock-copolymers (poloxamers) are common and frequently used polymeric materials for the development of micelles, but graft (like G-chitosan) and ionic [like PEG-poly(-caprolactone)-g-polyethyleneimine] copolymers are also used (Yadav et al. 2019). As excipients for conventional pharmaceutical formulations, biocompatible polymers have been widely used in pharmaceutical research (Bharate et al. 2016; Cabral et al. 2018). More recently, they have been used in nanomedicines to improve the therapeutic results of strong pharmaceuticals. The development of micelle-based delivery methods as possible human nanomedicines has been suggested for several new block copolymers (Lee et al. 2008; Kim et al. 2004). The delivery of medicinal compounds has been greatly improved because of several important developments in PM. Due to the functions the polymer confers on the formulation, standard PM formulations are anticipated to improve the therapeutic efficacy of the medication encapsulated in a protein. Because PM can release their cargo from the core in a regulated way throughout systemic circulation, their PK profiles for therapeutic drugs differ from those of native substances. PM structural characteristics, such as their hydrophilic shell, aid in preventing opsonization by the complement system and unanticipated drug loss from serum components, both of which usually lead to the fast clearance of medicines from systemic circulation (Owens and Peppas 2006). Due to their many advantageous properties, such as their capability to access the affected area with compromised vasculature, longevity, safety, long term drug release, improved stability (in vivo and in vitro), and capacity to successfully emulsify various poor soluble drugs, they have become very popular. Additionally, by modifying the surface of these micelles with different ligands and cell-penetrating moieties, new activities may be added to enable the targeted delivery and intracellular accumulation of protein-based therapeutics (Jhaveri and Torchilin 2014). PM are the most effective alternative drug carriers when compared to other micellar systems. The advantages of mixed PM include the incorporation of noticeably higher medicine doses, longer blood circulation durations, and thermodynamic stability. The PM core has been designed to have the best durability and drug loading capacity. The length of hydrophobic blocks and the kind of substituents found in the core have the biggest effect on PM capacity to carry medicines. The insoluble medications can be contained in the micellar core via either chemical conjugation or physical trapping.
12.2 Polymeric Micelles (PM) as Therapeutic Carriers for Protein Delivery
The use of proteins and peptides as PM targeting ligands is also quite common. The creation of transferrin-targeted nanocarriers is made possible by the transferrin receptor (TfR), which is overexpressed in many malignancies (Singh 1999). Transferrin (Tf), an endogenous ligand for PM, or antibodies against TfR may be used to modify them (Torchilin 2006). Sawant et al. developed micelle of transferrin modified PEG-PE (polyethylene glycol-phosphatidylethanolamine) to deliver a CDK inhibitor “R457.” The drug targeting efficacy and cell toxicity were evaluated in vitro and in vivo using A2780 ovarian cancer cell lines (ovarian carcinomas). Antitumour activity was improved as compared to free drug (Sawant et al. 2013). Water is distributed in PM in an anisotropic manner, with the amount of water decreasing as it moves from the surface to the hydrophobic centre. Thus, the polarity of the medication determines where it will be located within micelles: hydrophobic APIs (active pharmaceutical ingredients) will locate within core (lipophilic) or on the surface depending upon drug polarity and hydrophilicity. Generally, hydrophilic candidates are lodged within centre or surface of the micellar structure, whereas lipophilic drug candidate settled within lipophilic core of micelle (Rangel-Yagui et al. 2005). Most of the time, unimers and polyion complex micelles are used to chemically conjugate hydrophilic molecules, or they use electrostatic interactions to load hydrophilic molecules. For instance, RNA is often loaded into amphiphilic block copolymers by the insertion of polycations and then employed for RNA condensation. Amphiphile micellar solutions are an efficient method of delivering medications to their targets. Water-insoluble medicines are easily soluble in the hydrophobic environment of the micelle core and loaded for distribution to the necessary sites. Targeted protein drug delivery systems are created to ensure minimal drug loss and degradation, avoid negative side effects, boost the bioavailability of protein pharmaceuticals, and raise the concentration of medications in the desired zone of interest. Insoluble polymers (synesthetic and natural), soluble polymers, liposomes, micelles, microparticles, cell ghosts, cells, and amphiphilic polymers are just a few of the numerous drug carriers that are often used (Yokoyama 1998).
12.3 Advantages and Challenges of Polymeric Micelles (PM) for the Delivery of Proteins
Due to the potential of PM to boost the solubility and stability of aquaphobic medications and their in vivo therapeutic efficacy, which is equivalent to or better than that of the free drug, PM have received scientific attention in recent years. PM may be made to be big enough to prevent early clearance owing to fast glomerular filtration, which prolongs circulation time, by regulating their size. The particle size is kept tiny enough to safely pass through the smallest veins at the same time. The properties of the PM can improve the cellular localization of the drug loaded micelles and provide a different pathway for endosomal internalization. Because the drug delivery is selective and targeted to tissues, these properties aid PM in having a better mean residence time (MRT), which may lead to a lowered dose, higher bioavailability, as well as a potential reduction in the risk of nonspecific organ toxicity. To improve medication delivery to particular sites and penetrate tumours more effectively, PM have been found to have a higher therapeutic index. Thus, PM may be useful for enhancing biodistribution while posing little threat of accumulation and persistent toxicity in the body. While research on PM has advanced significantly as excellent nanocarriers for pharmaceuticals, particularly hydrophilic medications, their development has been hampered by issues with poor stability and limited drug loading. Nevertheless, due to the chemical adaptability provided by amphiphilic block copolymers, PM may be designed to circumvent these difficulties. Modifying the micellar core is one method for raising the drug loading capacity and micellar stability, and it has been studied in several studies as one method for improving the loading efficiency of PM. Wan et al. loaded paclitaxel and cisplatin into micelles made of amphiphilic copolymers for the purpose of targeting ovarian and breast cancers, resulting in a noticeably higher loading efficiency (Wan et al. 2019). In addition to the numerous uses for PM, there are several difficulties that need to be overcome before they may be considered as viable drug carriers. These include enhancing drug loading effectiveness even further, stabilizing blood aerosol injection, and facilitating transport across cell membranes.
They physically entrap sparingly soluble medications, improve their bioavailability, and transport them to the intended site of action at concentrations that are greater than its intrinsic water solubility. The addition of micelles also improves the drug’s stability. Additionally, compared to free pharmaceuticals, there are less negative side effects due to reduced interaction of the medication with inactivating species, such as enzymes found in bodily fluids (Torchilin 2001). Micellar delivery systems’ tiny size (10–30 nm) and narrow size distribution are by far their most distinctive characteristics, setting them apart from other particulate drug carriers (Florence and Hussain 2001). Nonionic surfactant-based micelles are often employed as drug carrier for controlled drug delivery (Bardelmeijer et al. 2002). Since most organizations established their individual micelle system made from distinct hydrophilic-lipophilic combinations because of the high degree of activity, this field now has a significant lot of diversity. Figure 12.1 depicts the schematic representation of PM.
12.4 Oral Uptake of Polymeric Micelles (PM)
PM have been tested for oral administration for a variety of therapeutic goals, including increasing apparent drug solubility in GI fluids and facilitating absorption, penetrating pathological GI tract regions for locoregional treatment, carrying the drug directly to the bloodstream, minimizing pre-systemic losses, and targeting the drug to precise tissues or cells in the body after oral absorption. PM have been tested for oral administration for a variety of therapeutic goals, including increasing apparent drug solubility in GI fluids and facilitating absorption, penetrating pathological GI tract regions for locoregional treatment, carrying the drug directly to the bloodstream, minimizing pre-systemic losses, and targeting the drug to specific tissue or cells in the body after oral absorption (Simões et al. 2015).
12.5 Types of Polymers Utilized in the Administration of Micellar Drugs
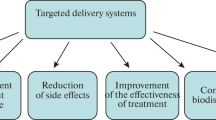
Graft copolymers, amphiphilic block copolymers, and triblock copolymers are frequently used to create PM. According to its compatibility with the integrated medication, stability, drug release profile, and toxicity, the block polymer of the hydrophobic core is selected. One can modify the medication release from these block copolymers. Micelles can also be further altered by having the shell cross-linked, having the PM surfaces functionalized, and linking ligands (aptamers and antibodies) to the surface for active targeting. Multigraft copolymers are generally comprised of minimum three homopolymers joined by a common branch. A graft polymer possessed multiple polymer chain acting as both the side grafted polymer and the backbone of the polymer. They gain from the transplant as well as the advantages of the backbone (Kulthe et al. 2012). A hyperbranched polymer with a significant number of functional end group belongs to another class of polymers. Because they have so many end groups, they may readily have their characteristics changed, making them an ideal choice for drug release under certain responsive stimuli such as pH, electrolytic strength, and temperature. Several varieties of manufactured polyester amide and poly(urea-urethane) hyperbranched amphiphilic polymers can be developed and altered to be utilized as a carrier for hydrophobic medicines, such as anticancer therapy (Gao and Yan 2004). Since each polymer has a special benefit, choosing the right polymer is crucial for extending the circulation period and ensuring a regulated release of the medicine. Since they have all been given FDA approval for biomedical uses in humans, polymers including PLGA [poly(lactic-co-glycolic acid], PCL [poly(-caprolactone)], and PLA [poly(lactic acid)] are frequently utilized to form the lipophilic core (Cagel et al. 2017). Figure 12.2 describes the drug loaded PM with several targeting functions.
12.6 Stimuli Sensitive Micelles for Delivery of Protein
When exposed to high temperatures, thermo-responsive PM alter structurally; this characteristic may be used to direct the deposition of protein drugs precisely where it is desired. Now, scientists utilized the concept of thermoresponsive polymer for the drug release. Therefore, a drug can be allowed to release loaded payload due to temperature triggered transition in the thermosensitive polymer based micelle above or below critical solution temperature (CST) (Rijcken et al. 2007). Above the LCST (low CST), these thermosensitive polymers solubilize, releasing the medication from the carrier. Therefore, in this form of micellar system, LCST is the most important factor. Another external stimulus that has been employed to cause the release of drugs from stimuli-sensitive multifunctional PM is a magnetic field. A thermo-sensitive star-block copolymer was used in one study to create magnetothermally responsive drug loaded micelles by fusing the concepts of temperature-triggered drug release with the application of a magnetic field (Ji et al. 2014). An external magnetic stimulation was used to locate the micelles, and after that, the temperature rose, causing the thermoresponsive micelles to release the medication. To obtain desired release of payloads, PM may be designed to react to a variety of stimuli (intrinsic or extrinsic) of various sources (chemical, physical, and biological sources) (Cheng et al. 2013). The therapeutic payloads of PM that are “environmentally sensitive” or “smart” can be released by altering their structural composition in response to the stimuli. As a result of the reaction, micelles may degrade or become unstable, isomerize, polymerize, or aggregate supramolecular (Fleige et al. 2012) (Table 12.1). Figure 12.3 depicts the multifunctional PM for oral delivery of drugs.
12.7 Polymeric Micelles (PM) for Multiple Functionality and Protein Delivery
At present, multifunctional PM have been the focus of substantial research for localized delivery drug and nucleic acid such as RNA. Over the past several years, various amphiphilic block copolymers were synthesized and developed for delivery of siRNA in micellar construct. These fundamental building blocks are regularly enhanced to get the most out of them by adding certain ligands or ethically sound building blocks or linkages. A fundamental goal of all siRNA delivery systems, including micelles, is to stop siRNA from degrading from the point at which it enters the bloodstream to the point at which it travels via the endocytic route for intracellular trafficking and escapes the endosome. In their paper, several authors utilized multifunctional micelles for delivery either drug or nucleic acid, or co-administration of drug and NA by allowing self-assembling of RNA with block copolymer(s) (Christie et al. 2012) (Table 12.2). Figure 12.4 shows the schematic representation of the phenomena regulating in vivo drug delivery, in terms of both rate and location, from PM.
12.8 Co-delivery of Drugs and siRNA Using Multifunctional Micelles
The siRNA therapy based on PM has shown remarkable promise and is presently the focus of intensive study. However, it is extremely possible that tumours will have genetic changes, which might lower the usefulness of siRNA as a solitary agent in the therapy of malignancies (Liu et al. 2013). Traditional anticancer medications also have issues with off-target effect and MDR which is mainly responsible for substantial impairment in cancer treatment. Due to the nonspecific character of these inhibitors, there has been relatively little clinical success in the creation of compounds that block the action of drug transporter proteins like P-glycoprotein (Pg-p), which is produced by the MDR1-gene, to sensitize tumour cells to anticancer drugs (Shukla et al. 2008). In these circumstances, it would be more advantageous to treat cancer using RNAi to directly reduce Pg-p expression rather than only its function by suppressing the expression of MDR genes after conventional chemotherapy (Wu et al. 2003). Studies show that pre-treating cancer cells with siRNAs prior to treating them with conventional anticancer medications can greatly increase the cells’ sensitivity to the drug and increase the effectiveness of therapy (Spankuch et al. 2007; Macdiarmid et al. 2009). To have the greatest effect in vivo, however, siRNA and medicine must be given to the same tumour cell at the same time following systemic distribution. For the best potential collaboration, they ought to also be distributed optimally among cells (Sun et al. 2011). In this chapter, we examine many examples of such PM, which include siRNA and medications inside of a single nanocarrier. Multifunctional PEO-b-PCL block copolymer micelles having functional modifications on both blocks. These micelles may carry out a number of functions, including pH-triggered drug release in endosomes, siRNA and DOX co-delivery, facilitated cell membrane translocation, passive and active targeting, and siRNA and DOX administration. These micelles may carry out a range of functions, such as pH-triggered drug release in endosomes, siRNA and DOX co-delivery, simpler cell membrane translocation, passive and active targeting, and siRNA and DOX administration. Using a pH-sensitive hydrazone linkage, the PCL core of the micelles may conjugate short polyamines [spermine (SP)] to chemically combine MDR1 siRNA and DOX, as well as fluorescent imaging probes to monitor micelles in vitro and in vivo. Two ligands were added to the virus-like shell of these micelles: a cell-penetrating TAT-peptide to aid in intracellular absorption and an active targeting ligand, RGD4C specific for integrin (ɑvꞵ3) receptors (Table 12.3).
12.9 Conclusion
PM appear to be a perfect carrier for poorly water-soluble drugs due to their benefits, such as tiny size, high solubility, ease of sterilizing, and controlled release of drugs. Since the integrated medicine may be released rapidly in vivo, the physical stability of this carrier is a crucial concern. To effectively develop micelles that can carry drugs to their sites of action, significant effort still must be done in understanding how PM interact with plasmatic and cellular components. PM have made significant strides in recent years in the delivery of a wide range of payloads, including traditional anticancer medications and biological macromolecules including antibodies, siRNA, DNA, and oligonucleotides. Chemically modified structure of various block copolymers generated micelles which enabled the formation of complex micelles that integrate numerous modalities inside a single carrier. Purposely, multifunctional micelles were fabricated for targeted delivery to the infected sites (by surface functionalization with ligands), alteration caused for imaging contrast in disease diagnosis, and responsive drug delivery using magnetic field, pH, electrolyte strength and temperature provided externally or internally (tumour microenvironment). These imposed physical characteristics innate in polymer control drug release from micelle due to deformation or transition in construct cargoes or drug effectiveness at the site of action (tumour lesion, lysozymes, and enzymatic degradation). The fact that they may be tweaked and altered to meet demands gives them a clear edge over conventional drug delivery methods. Although appearing straightforward at first glance, it is obvious that PM constitute a far more complicated system than what is initially believed. Clinical studies for many PM formulations have begun after achieving some promising preclinical results, but only a small number of them have obtained regulatory permission for usage in humans. Numerous obstacles have made it difficult for them to follow the regulatory road. Clinically significant PM formulations serve as a supply path for insoluble small molecules, and due to reason that the existing PM formulation systems must develop further to act as efficient carriers. A detailed analysis of drug encapsulation and the successive drug release profile in systemic circulation will reveal how novel PM systems for human usage could be developed in the future.
References
Bardelmeijer HA, Ouwehand M, Malingré MM, Schellens JH, Beijnen JH, van Tellingen O (2002) Entrapment by Cremophor EL decreases the absorption of paclitaxel from the gut. Cancer Chemother Pharmacol 49(2):119–125
Bharate SS, Bharate SB, Bajaj A (2016) Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: a comprehensive review. J Excip Food Chem 1:1131
Cabral H, Miyata K, Osada K, Kataoka K (2018) Block copolymer micelles in nanomedicine applications. Chem Rev 118:6844–6892
Cagel M, Tesan FC, Bernabeu E et al (2017) Polymeric mixed micelles as nanomedicines: achievements and perspectives. Eur J Pharma Biopharm 113:211–228
Cheng R, Meng F, Deng C, Klok HA, Zhong Z (2013) Dual and multistimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 34:3647–3657. https://doi.org/10.1016/j.biomaterials.2013.01.084
Chitkara D, Mittal A, Behrman SW, Kumar N, Mahato RI (2013) Self-assembling, amphiphilic polymer-gemcitabine conjugate shows enhanced antitumor efficacy against human pancreatic adenocarcinoma. Bioconjug Chem 24:1161–1173. https://doi.org/10.1021/bc400032x
Choi SW, Lee SH, Mok H, Park TG (2010) Multifunctional siRNA delivery system: polyelectrolyte complex micelles of six-arm PEG conjugate of siRNA and cell penetrating peptide with crosslinked fusogenic peptide. Biotechnol Prog 26:57–63. https://doi.org/10.1002/btpr.310
Christie RJ, Matsumoto Y, Miyata K, Nomoto T, Fukushima S, Osada K et al (2012) Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano 6:5174–5189. https://doi.org/10.1021/nn300942b
Desale SS, Cohen SM, Zhao Y, Kabanov AV, Bronich TK (2013) Biodegradable hybrid polymer micelles for combination drug therapy in ovarian cancer. J Control Release 171:339–348. https://doi.org/10.1016/j.jconrel.2013.04.026
Elsabahy M, Wazen N, Bayó-Puxan N, Deleavey G, Servant M, Damha MJ et al (2009) Delivery of nucleic acids through the controlled disassembly of multifunctional nanocomplexes. Adv Funct Mater 19:3862–3867. https://doi.org/10.1002/adfm.200901139
Fleige E, Quadir MA, Haag R (2012) Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: concepts and applications. Adv Drug Deliv Rev 64:866–884. https://doi.org/10.1016/j.addr.2012.01.020
Florence AT, Hussain N (2001) Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv Drug Deliv Rev 50:S69–S89
Gao C, Yan D (2004) Hyperbranched polymers: from synthesis to applications. Prog Polym Sci 29(3):183–275
Glover AL, Bennett JB, Pritchett JS, Nikles SM, Nikles DE, Nikles JA et al (2013) Magnetic heating of iron oxide nanoparticles and magnetic micelles for cancer therapy. IEEE Trans Magn 49:231–235. https://doi.org/10.1109/TMAG.2012.2222359
Guo J, Hong H, Chen G, Shi S, Zheng Q, Zhang Y et al (2013) Image guided and tumor-targeted drug delivery with radiolabeled unimolecular micelles. Biomaterials 34:8323–8332. https://doi.org/10.1016/j.biomaterials.2013.07.085
Hoang B, Ekdawi SN, Reilly RM, Allen C (2013) Active targeting of block copolymer micelles with trastuzumab fab fragments and nuclear localization signal leads to increased tumor uptake and nuclear localization in HER2-overexpressing xenografts. Mol Pharm 10:4229–4241. https://doi.org/10.1021/mp400315p
Howell M, Mallela J, Wang C, Ravi S, Dixit S, Garapati U et al (2013) Manganese-loaded lipid-micellar theranostics for simultaneous drug and gene delivery to lungs. J Control Release 167:210–218. https://doi.org/10.1016/j.jconrel.2013.01.029
Husseini GA, Velluto D, Kherbeck L, Pitt WG, Hubbell JA, Christensen DA (2013) Investigating the acoustic release of doxorubicin from targeted micelles. Colloids Surf B Biointerfaces 101:153–155. https://doi.org/10.1016/j.colsurfb.2012.05.025
Jhaveri AM, Torchilin VP (2014) Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol 5:77
Ji G, Yang J, Chen J (2014) Preparation of novel curcumin-loaded multifunctional nanodroplets for combining ultrasonic development and targeted chemotherapy. Int J Pharm 466(1–2):314–320
Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, Kim NK, Bang YJ (2004) Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res 10:3708–3716
Kim DH, Vitol EA, Liu J, Balasubramanian S, Gosztola DJ, Cohen EE et al (2013) Stimuli-responsive magnetic nanomicelles as multifunctional heat and cargo delivery vehicles. Langmuir 29:7425–7432. https://doi.org/10.1021/la3044158
Kulthe SS, Choudhari YM, Inamdar NN, Mourya V (2012) Polymeric micelles: authoritative aspects for drug delivery. Des Monomer Polym 15(5):465–521
Kumar S, Allard J-F, Morris D, Dory YL, Lepage M, Zhao Y (2012) Near-infrared light sensitive polypeptide block copolymer micelles for drug delivery. J Mater Chem 22:7252–7257. https://doi.org/10.1039/c2jm16380b
Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, Rha SY, Lee MY, Ro J (2008) Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat 108:241–250
Lee SH, Mok H, Lee Y, Park TG (2011) Self-assembled siRNA-PLGA conjugate micelles for gene silencing. J Control Release 152:152–158. https://doi.org/10.1016/j.jconrel.2010.12.007
Lee I, Park M, Kim Y, Hwang O, Khang G, Lee D (2013) Ketal containing amphiphilic block copolymer micelles as pH-sensitive drug carriers. Int J Pharm 448:259–266. https://doi.org/10.1016/j.ijpharm.2013.03.017
Li Y, Qian Y, Liu T, Zhang G, Liu S (2012) Light-triggered concomitant enhancement of magnetic resonance imaging contrast performance and drug release rate of functionalized amphiphilic diblock copolymer micelles. Biomacromolecules 13:3877–3886. https://doi.org/10.1021/bm301425j
Li G, Meng Y, Guo L, Zhang T, Liu J (2013a) Formation of thermo-sensitive polyelectrolyte complex micelles from two biocompatible graft copolymers for drug delivery. J Biomed Mater Res A 102(7):2163–2172. https://doi.org/10.1002/jbm.a.34894
Li X, Li H, Yi W, Chen J, Liang B (2013b) Acid-triggered core crosslinked nanomicelles for targeted drug delivery and magnetic resonance imaging in liver cancer cells. Int J Nanomedicine 8:3019–3031. https://doi.org/10.2147/IJN.S45767
Li J, Cheng D, Yin T, Chen W, Lin Y, Chen J et al (2014) Copolymer of poly(ethylene glycol) and poly(l-lysine) grafting polyethylenimine through a reducible disulfide linkage for siRNA delivery. Nanoscale 6:1732–1740. https://doi.org/10.1039/c3nr05024f
Liao C, Sun Q, Liang B, Shen J, Shuai X (2011) Targeting EGFR overexpressing tumor cells using Cetuximab-immunomicelles loaded with doxorubicin and superparamagnetic iron oxide. Eur J Radiol 80:699–705. https://doi.org/10.1016/j.ejrad.2010.08.005
Liu X-Q, Sun C-Y, Yang X-Z, Wang J (2013) Polymeric-micelle-based nanomedicine for siRNA delivery. Part Part Syst Charact 30:211–228. https://doi.org/10.1002/ppsc.201200061
Macdiarmid JA, Amaro-Mugridge NB, Madrid-Weiss J, Sedliarou I, Wetzel S, Kochar K et al (2009) Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat Biotechnol 27:643–651. https://doi.org/10.1038/nbt.1547
Menon S, Thekkayil R, Varghese S, Das S (2011) Photoresponsive soft materials: synthesis and photophysical studies of a stilbene-based diblock copolymer. J Polym Sci A Polym Chem 49:5063–5073. https://doi.org/10.1002/pola.24973
Owens DE 3rd, Peppas NA (2006) Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 307:93–102
Rangel-Yagui C, Pessoa A, Tavares L (2005) Micellar solubilization of drugs. J Pharm Pharm Sci 8:147–165
Rijcken CJ, Snel CJ, Schiffelers RM, Van Nostrum CF, Hennink WE (2007) Hydrolysable core-crosslinked thermosensitive polymeric micelles: synthesis, characterisation and in vivo studies. Biomaterials 28(36):5581–5593
Sawant RR, Jhaveri AM, Koshkaryev A, Zhu L, Qureshi F, Torchilin VP (2013) Targeted transferrin-modified polymeric micelles: enhanced efficacy in vitro and in vivo in ovarian carcinoma. Mol Pharm 11:375–381. https://doi.org/10.1021/mp300633f
Shukla S, Wu CP, Ambudkar SV (2008) Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol 4:205–223. https://doi.org/10.1517/17425255.4.2.205
Simões SM, Figueiras AR, Veiga F, Concheiro A, Alvarez-Lorenzo C (2015) Polymeric micelles for oral drug administration enabling locoregional and systemic treatments. Expert Opin Drug Deliv 12(2):297–318
Singh M (1999) Transferrin as a targeting ligand for liposomes and anticancer drugs. Curr Pharm Des 5:443–451
Spankuch B, Kurunci-Csacsko E, Kaufmann M, Strebhardt K (2007) Rational combinations of siRNAs targeting Plk1 with breast cancer drugs. Oncogene 26:5793–5807. https://doi.org/10.1038/sj.onc.1210355
Sun TM, Du JZ, Yao YD, Mao CQ, Dou S, Huang SY et al (2011) Simultaneous delivery of siRNA and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano 5:1483–1494. https://doi.org/10.1021/nn103349h
Tanaka K, Kanazawa T, Horiuchi S, Ando T, Sugawara K, Takashima Y et al (2013) Cytoplasm-responsive nanocarriers conjugated with a functional cell penetrating peptide for systemic siRNA delivery. Int J Pharm 455:40–47. https://doi.org/10.1016/j.ijpharm.2013.07.069
Tao A, Huang GL, Igarashi K, Hong T, Liao S, Stellacci F, Matsumoto Y, Yamasoba T, Kataoka K, Cabral H (2020) Polymeric micelles loading proteins through concurrent ion complexation and pH-cleavable covalent bonding for in vivo delivery. Macromol Biosci 20(1):1900161
Taylor RM, Sillerud LO (2012) Paclitaxel-loaded iron platinum stealth immunomicelles are potent MRI imaging agents that prevent prostate cancer growth in a PSMA-dependent manner. Int J Nanomedicine 7:4341–4352. https://doi.org/10.2147/IJN.S34381
Torchilin VP (2001) Structure and design of polymeric surfactant-based drug delivery systems. J Control Release 73(2–3):137–172
Torchilin VP (2006) Multifunctional nanocarriers. Adv Drug Deliv Rev 58:1532–1555. https://doi.org/10.1016/j.addr.2006.09.009
Varshosaz J, Sadeghi-Aliabadi H, Ghasemi S, Behdadfar B (2013) Use of magnetic folate-dextran-retinoic acid micelles for dual targeting of doxorubicin in breast cancer. Biomed Res Int 2013:680712. https://doi.org/10.1155/2013/680712
Wan X, Beaudoin JJ, Vinod N, Min Y, Makita N, Bludau H, Jordan R, Wang A, Sokolsky M, Kabanov AV (2019) Co-delivery of paclitaxel and cisplatin in poly (2-oxazoline) polymeric micelles: implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials 192:1–14
Wang H-X, Xiong M-H, Wang Y-C, Zhu J, Wang J (2013) N-acetylgalactosamine functionalized mixed micellar nanoparticles for targeted delivery of siRNA to liver. J Control Release 166:106–114. https://doi.org/10.1016/j.jconrel.2012.12.017
Wang X, Li S, Wan Z, Quan Z, Tan Q (2014) Investigation of thermo-sensitive amphiphilic micelles as drug carriers for chemotherapy in cholangiocarcinoma in vitro and in vivo. Int J Pharm 463:81–88. https://doi.org/10.1016/j.ijpharm.2013.12.046
Wu H, Hait WN, Yang JM (2003) Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res 63:1515–1519
Xiong XB, Uludag H, Lavasanifar A (2010) Virus-mimetic polymeric micelles for targeted siRNA delivery. Biomaterials 31:5886–5893. https://doi.org/10.1016/j.biomaterials.2010.03.075
Yadav HKS, Almokdad AA, Shaluf SIM, Debe MS (2019) Chapter 17—polymerbased nanomaterials for drug-delivery carriers. In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, Thomas S (eds) Nanocarriers for drug delivery. Elsevier, Amsterdam, pp 531–556
Yin T, Wang P, Li J, Zheng R, Zheng B, Cheng D et al (2013) Ultrasound sensitive siRNA-loaded nanobubbles formed by hetero-assembly of polymeric micelles and liposomes and their therapeutic effect in gliomas. Biomaterials 34:4532–4543. https://doi.org/10.1016/j.biomaterials.2013.02.067
Yokoyama M (1998) Novel passive targetable drug delivery with polymeric micelles. Academic, San Diego, p 193
Yu H, Zou Y, Wang Y, Huang X, Huang G, Sumer BD et al (2011) Overcoming endosomal barrier by amphotericin B-loaded dual pH-responsive PDMA-b-PDPA micelleplexes for siRNA delivery. ACS Nano 5:9246–9255. https://doi.org/10.1021/nn203503h
Yu C, Gao C, Lu S, Chen C, Yang J, Di X et al (2013) Facile preparation of pH-sensitive micelles self-assembled from amphiphilic chondroitin sulfate histamine conjugate for triggered intracellular drug release. Colloids Surf B Biointerfaces 115C:331–339. https://doi.org/10.1016/j.colsurfb.2013.12.023
Zhang L, Gong F, Zhang F, Ma J, Zhang P, Shen J (2013) Targeted therapy for human hepatic carcinoma cells using folate-functionalized polymeric micelles loaded with superparamagnetic iron oxide and sorafenib in vitro. Int J Nanomedicine 8:1517–1524. https://doi.org/10.2147/IJN.S43263
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ashique, S., Garg, A., Hussain, A., Bhatt, S., Agrawal, A., Mishra, N. (2023). Polymeric Micelles in the Delivery of Proteins. In: Singh, S.K., Gulati, M., Mutalik, S., Dhanasekaran, M., Dua, K. (eds) Polymeric Micelles: Principles, Perspectives and Practices. Springer, Singapore. https://doi.org/10.1007/978-981-99-0361-0_12
Download citation
DOI: https://doi.org/10.1007/978-981-99-0361-0_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0360-3
Online ISBN: 978-981-99-0361-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)