Abstract
Sleep disruption and circadian misalignment are associated with an increased risk for infectious and inflammatory pathologies, including cardiometabolic, neoplastic, autoimmune, and neurodegenerative diseases. Sleep and circadian rhythms are closely involved in the regulation of the immune system. Impairments of sleep quantity, quality, and timing, as well as circadian misalignment, result in derangements of innate and adaptive immune responses leading to a chronic inflammatory state and a decrease of the immune defense and reaction to threats (infection or injury). The immune system potentially plays an important mechanistic role in the relation between sleep disruption and circadian misalignment, and adverse health effects. By regulating the immune system, sleep- and circadian-centered intervention may beneficially impact overall health and on the prevention—and treatment—of infections and chronic diseases, especially in the modern lifestyles characterized by a multiplicity of social and environmental pressures on sleep and circadian rhythms, and in times of infectious disease outbreaks, such as COVID-19, where an effective immunity is of utmost importance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
There is a time for many words, and there is also a time for sleep. Homer. The Odyssey.
Since antiquity, sleep has been recognized as essential for overall health. Sleep is an active physiological process necessary for life, being involved in the regulation of physical, mental, and emotional health.
Key components in the regulation and maintenance of the sleep/wake cycle are the endogenous 24-h body clock/circadian system that drives wakefulness throughout the day and sleep during the night; and the wake-dependent homeostatic drive that generates a sleep pressure during the day, which is dissipated during sleep. However, an exogenous drive that is the product of our societal temporal structure (e.g., school and work times) forces an exogenous sleep/wake pattern influencing sleep opportunities and thereby sleep duration, quality, and efficiency, potentially resulting in sleep and circadian rhythm disruption (Zielinski et al. 2016).
On this background, patterns of sleep quantity, quality, and timing are influenced by health status, cultural, social, psychological, behavioral, and environmental factors (Grandner 2017). Expert consensus recommendations suggest that adults should sleep a minimum of 7 h per night on a regular night to promote optimal health (Consensus Conference et al. 2015a, b). Apart from sleep/circadian disorders however many factors, including jet lag syndrome, social jet lag, work schedule, growing frequency of technology use, higher rates of obesity and diabetes, inadequate nutrition, and other lifestyle factor changes occurring in the twenty-first century as well as the COVID-19 pandemic are increasingly and often negatively impacting on sleep quantity, quality, and timing, so that sleep problems have constantly arisen in the general population and special groups of vulnerable people, including children/adolescents or workers. Population-based studies reported that sleep duration among adults has changed over the past few decades and, in particular, the prevalence of adults sleeping less than 6 h per night has stably increased over a long period (Gilmour et al. 2013; Ford et al. 2015a, b; Zomers et al. 2017). This phenomenon is also occurring among children and adolescents (Matricciani et al. 2012), so 58% and 73% of middle and high school-aged adolescents, respectively, do not meet the American Academy of Sleep Medicine sleep duration recommendations (Wheaton et al. 2018). A parallel increasing prevalence of insomnia has been documented in several countries (Ford et al. 2015a, b).
In addition to excessive daytime sleepiness, fatigue, tiredness, depressed mood, poor daytime functioning, and impaired cognitive and safety-related performance, inadequate sleep is associated with an increased risk of adverse health outcomes, including weight gain, obesity, type 2 diabetes mellitus, hypertension, cardiovascular (CV) and neurodegenerative diseases, mental diseases, cancer as well as all-cause mortality (Vgontzas et al. 2009, 2013; Irwin 2015; Smagula et al. 2016; Cappuccio and Miller 2017).
Another integral part of human physiology and behavior tightly regulating sleep is the circadian rhythms, which are orchestrated by a central “master” clock, i.e., the suprachiasmatic nucleus (SCN) located in the anterior hypothalamus, that coordinates alignment between circadian clocks in other brain regions and peripheral tissues with external synchronizing agents (the environment and behaviors). Indeed, circadian rhythms are modulated by endogenous (genetic, physiological) as well as environmental (light) and behavioral (activity, feeding) factors. Various behaviors and physiological functions of the body show circadian rhythms, such as the sleep–wake cycle and food intake, blood pressure, blood lipids, coagulation/fibrinolysis system, heart rate, body temperature, locomotor activity, hormone levels, cell metabolism and proliferation (Sulli et al. 2018). The advantages of internal clocks are to enable individuals to anticipate, rather than react to, daily recurring events and align their physiology and behavior to the changing environment.
The “molecular circadian clock” refers to genes that maintain autoregulatory feedback loops in which oscillating outputs regulate their expression (circadian locomotor output cycles kaput [CLOCK], brain and muscle ARNT-like [BMAL], period [PER], REV-ERB/nuclear receptor subfamily 1, group D [NR1D], and cryptochrome [CRY]). The formation, trafficking, and degradation of different clock protein complexes throughout this transcriptional cycle establish the intrinsic 24 h period of the cellular clock. Furthermore, at the cellular level, molecular clock components generate circadian fluctuation in basic cellular functions, e.g., gene expression, protein translation, and intracellular signaling, which are all involved in fundamental processes, including cell cycle regulation, nutrient sensing/utilization, metabolism, stress response, redox regulation, detoxification, and cell defense (immunity and inflammation) in a tissue-specific manner (Mure et al. 2018). Circadian disruption may occur as a result of a misalignment between external factors (such as the natural light/dark cycle, social and work requirements, and behaviors such as sleep and meal timing) with the master circadian clock as well as with endogenous circadian clocks in other tissues. Misalignments can occur among two (or more) rhythms which may be both internal (central vs. peripheral rhythms), or one may be internal and the other external (e.g., central vs. light/dark or peripheral vs. feeding/fasting). Social jet lag, jet lag syndrome, shift work, and inappropriately timed light exposure (evening or night) are common causes of circadian (external-internal) misalignments in modern society.
Circadian disruption has important public health implications due to its prevalence in modern society as well as its association with serious safety- and performance-related issues (Mitler et al. 1988) and adverse health outcomes (Boivin et al. 2022). Subjects with a nondaytime working schedule (shift work), exposure to light pollution, social jet lag, or evening chronotype are at increased risk for circadian disruption. Circadian disruption in humans is associated with broad and significant consequences for mental and physical health, increasing the risk for the development of cancer (Sulli et al. 2019), neurodegenerative, psychiatric (Abbott et al. 2020), cardiometabolic (Scheer et al. 2009), and immune disorders (Fishbein et al. 2021). Importantly, changes in circadian function are often accompanied by sleep–wake disturbances. Therefore, increasing scientific efforts have been devoted to understanding the health consequences of sleep disruption and circadian misalignment, and to translating this science to directly impact human health.
The immune system functions to preserve the integrity of the body by sensing physiological disturbances (microbes or tissue injury) and reinstating homeostasis via both inflammatory immune responses and processes of tissue repair and physical barrier regulation. In many chronic diseases, a deregulated and/or exacerbated immune response shifts from repair/regulation towards immune-driven unresolved inflammatory responses (Hand et al. 2016). Several anatomic and molecular mechanisms, including neurons, glial cells, leukocytes, nerve fibers, soluble mediators, cellular receptors, the blood–brain barrier (BBB), the neuroendocrine hypothalamus-pituitary-adrenal (HPA) axis, and the autonomic nervous system, participate in orchestrating the bidirectional brain-immune crosstalk that fine tunes the immune response and its relationship with sleep and the circadian systems (Dantzer 2018). In this contest, the circadian system and sleep have emerged as important intertwined regulators of the immune system. It follows that sleep disruption and circadian misalignment may result in deregulated immune responses and pro-inflammatory responses, that contribute to an increase in the risk for the onset and/or worsening of infections as well as inflammation-related chronic diseases, including cancer, cardiometabolic and neurodegenerative diseases (Labrecque and Cermakian 2015; Scheiermann et al. 2018; Garbarino et al. 2021).
This Chapter focuses on the regulation of the immune system by sleep and the circadian rhythm, and the consequences for the immune function of sleep disruption and circadian misalignment.
1.2 Immune Regulation by Sleep
The sleep–immune interaction hypothesis was first suggested by pioneering studies attempting to identify substances involved in sleep regulation. The hypothesis of humoral regulation of sleep dates back to the early 1900s and posited that substances accumulated during waking could trigger subsequent sleep. Accordingly, experimental studies found that injection of cerebrospinal fluid from sleep-deprived dogs into rested recipient dogs caused the recipient dogs to fall into a narcosis-like sleep state (Ishimori 1909; Legendre and Piéron 1913). Subsequent animal studies replicated this result, pointing to the existence of hypnotoxins mediating sleep induction (endogenous factor(s) S, where S stands for sleep), and led to the identification of several sleep-promoting factors.
Among these hypnotoxins, muramyl peptide, a component of bacterial cell wall able of activating the immune system and inducing the release of immune regulators, such as cytokines, was recognized as the first molecular link between the immune system and sleep (Krueger et al. 1982). Other microbial-derived factors such as the endotoxin lipopolysaccharide (LPS), as well as mediators of inflammation, such as cytokines (interleukin [IL]-1, tumor necrosis factor [TNF]-α), prostaglandins (PG), uridine, growth factors, were found to regulate sleep (Zielinski and Krueger 2011). These factors, through the BBB or via afferent nerve fibers, establish a signaling network with other brain factors involved in sleep regulation, such as neurotransmitters (acetylcholine, dopamine, serotonin, norepinephrine, histamine), neuropeptides (orexin), nucleosides (adenosine), the hormone melatonin as well as the hypothalamus-pituitary (HPA) axis.
Animal studies have consistently reported a role for the cytokines IL-1 and TNF-α and the prostaglandin PGD2 in the physiologic, homeostatic nonrapid eye movement (NREM) sleep regulation in a dose- and time-of-day-dependent manner, so that the inhibition of the biological action of these substances resulted in a decrease of spontaneous NREM sleep, whereas administration of these substances enhanced NREM sleep amount and intensity and suppressed REM sleep (Opp 2005; Urade and Hayaishi 2011). Anti-inflammatory cytokines, including IL-4, IL-10, and IL-13, have been found to inhibit NREM sleep in animal models (Kubota et al. 2000). In humans, the circulating levels of IL-1, IL-6, TNF-α, and PGD2 are highest during sleep and, the available evidence, though indirect, converge to suggest the involvement of these immune mediators in the physiologic regulation of sleep (Besedovsky et al. 2019).
Accordingly, infection or inflammatory diseases induce immune activation and associated altered cytokine concentrations and profiles, which are transmitted to the central nervous system inducing adaptive and energy-saving responses, including sleep (Besedovsky et al. 2019). Acute mild immune activation enhances NREM sleep and suppresses REM sleep, and the increase in NREM sleep was a favorable prognostic factor for rabbits during infectious diseases (Toth et al. 1993). Contrarily, severe immune response with an upsurge of cytokine levels causes sleep disturbance with suppression of both NREM and REM sleep (Toth et al. 1993; Mullington et al. 2000; Sharpley et al. 2016). Chronic infectious, such as HIV infection (Chaponda et al. 2018), and inflammatory diseases, such as inflammatory bowel disease or rheumatoid arthritis (Ranjbaran et al. 2007; Uemura et al. 2016), are associated with sleep disturbances.
Evidence of the immune-supportive effect of sleep that may favor host defense is provided by vaccination studies. In humans, compared with nocturnal wakefulness, sleep after vaccination boosted both the memory phase and the effector phase of the immune response, underscoring the adjuvant-like effect of sleep on the immunological function (Lange et al. 2011). Similarly, habitual (and hence chronic) short sleep duration (less than 6 h) compared with longer sleep duration decreased the long-term clinical protection after vaccination against hepatitis B (Prather et al. 2012).
In exerting these effects, sleep may benefit the immune response through different mechanisms. Sleep influences the T helper (Th) phenotype and the cytokine balance between Th1 and Th2 cells thus determining the types of the effector mechanisms of the immune response. Th1 polarization state is typical of immune response to intracellular viral and bacterial challenges and is characterized by increased release of IFN-γ, IL-2, and TNF-α. It supports various cell-mediated responses, including macrophage activation, phagocytosis, the killing of intracellular microbes, and antigen presentation (Zhu and Zhu 2020). Th2 immunity is characterized by the expression of IL-4, IL-5, IL-10, and IL-13, and mediates humoral defense by stimulating mast cells, eosinophils, and B cells (with the production of IgG2,4 and IgE) against extracellular pathogens (Zhu and Zhu 2020).
The balance of Th1/Th2 immunity is critically involved in antimicrobial and anti-tumor immune responses. Th2 overactivity is found in some forms of allergic responses, and increases the susceptibility to infection (Moser et al. 2018), as well as to tumor development and progression, by limiting cytotoxic T lymphocytes proliferation and modulating other inflammatory cell types (Disis 2010). In contrast, Th1 immunity supports cytotoxic lymphocytes with the potential of elimination or control of tumor cell growth; indeed, a Th1 adaptive immune response may be associated with improved survival or prognosis (Disis 2010; Lee et al. 2019).
Furthermore, sleep is associated with a reduction in circulating immune cells that most likely accumulate into lymphatic tissues thus increasing the probability to encounter antigens (immunological synapse) and trigger the immune response. An effective adaptive immune response to an immunological challenge may be facilitated by specific immune-active hormones associated with slow wave sleep (SWS)-rich early sleep, which is characterized by minimum concentrations of immunosuppressive hormones, such as cortisol, and high levels of immune-stimulating hormones such as growth hormone (GH), prolactin, and aldosterone, which support pro-inflammatory cytokine production and Th1 cell-mediated immunity (Besedovsky et al. 2019). Therefore, through pro-inflammatory hormones and cytokines night-time sleep facilitates the onset of adaptive immune responses, while during daytime activity, anti-inflammatory signals, hormones, and cytokines support immediate reactions to biological and other environmental challenges.
1.3 Sleep Disruption and Immune Consequences
In agreement with the sleep–immunity relationship, several lines of evidence from experimental and epidemiological studies converge on the significant effects of sleep disruption on immune function and related clinical outcomes.
Early animal studies found that sleep loss, besides being lethal after several weeks, was associated with dysfunction of host defense (Everson and Toth 2000; Everson et al. 2008, 2014) thus suggesting the importance of sleep for the immune system. More pertinently, the effect of sleep on immune function has emerged in studies in which immune parameters, including circulating levels of cytokines and cell adhesion molecules, leukocyte counts, and activity, were measured under the manipulation of sleep duration compared with undisturbed sleep.
Collectively most human and animal findings report on the supportive effect of sleep—and the detrimental effect of disturbed sleep—on several immune regulators. Indeed, compared with regular nocturnal sleep, acute and mostly sustained sleep loss has been found: (a) to alter circulating leukocyte counts with studies reporting increased numbers of total leukocytes and specific cell subsets mainly neutrophils, monocytes, B cells, decreased circulating natural killer (NK) cells, and changes in circulating CD4+ T cells (Born et al. 1997; Dimitrov et al. 2007; van Leeuwen et al. 2009; Lasselin et al. 2015; Said et al. 2019); (b) to alter the diurnal rhythm of circulating leukocytes, resulting in higher levels during the night and at awakening and a flattening of the rhythm (Born et al. 1997; Lasselin et al. 2015); (c) to increase the plasma levels of pro-inflammatory cytokines such as IL-1, IL-6, CRP, and, less consistently, TNF-α, MCP-1, and a homeostatic increase in endogenous inhibitors such as IL-1 receptor antagonist (IL-1ra) and TNF receptor I and II in an attempt to limit the increased cytokine levels and activity (Shearer et al. 2001; Hu et al. 2003; Vgontzas et al. 2004; van Leeuwen et al. 2009); (d) to transiently decrease the cytotoxic activity of NK cells, the proliferation capacity of lymphocytes (Irwin et al. 1994), and the phagocytic activity of neutrophils, important against infection (Said et al. 2019); (e) to enhance circulating levels of endothelial adhesion molecules such as intercellular adhesion molecule (ICAM)-1 and E-selectin, suggesting endothelial activation and enhanced risk for vascular dysfunction (Sauvet et al. 2010); (f) to reduce the stimulated production of IL-2 and IL-12, which normally support the adaptive immune response (Dimitrov et al. 2007; Axelsson et al. 2013); (g) to reduce the levels of Mac-1 positive lymphocytes suggesting reduced migratory capacity of immune cells (Redwine et al. 2004). Compared to undisturbed sleep which is predominantly characterized by a Th1 response (mainly during early sleep), experimental sleep deprivation leads to a shift from a Th1 pattern towards a Th2 pattern in humans (Dimitrov et al. 2004; Axelsson et al. 2013). Elderly people (Ginaldi et al. 1999), alcoholic subjects (Redwine et al. 2003) as well as insomnia patients (Sakami et al. 2002), all characterized by disturbed sleep, show a cytokine shift towards Th2.
At the molecular level, findings demonstrate that a single night of partial sleep deprivation (4 h of sleep) (Irwin et al. 2006, 2015) or chronic partial sleep deprivation (4 h of sleep for five nights) (van Leeuwen et al. 2009) in healthy adults led to increased protein production and mRNA levels of inflammatory cytokines (IL-6, IL-1β, TNF-α, IL-17). Accordingly, prominent genome-wide gene expression changes have been found in response to acute (Irwin et al. 2006) or chronic (Aho et al. 2013; Moller-Levet et al. 2013) partial sleep deprivation in human circulating monocytes, so most of the genes and associated biological pathways upregulated after sleep loss compared with unrestricted sleep were related to immune and inflammatory processes (leukocyte activation and differentiation, cytokine positive regulation, innate and adaptive immunity, TLRs signaling), as well as to oxidative stress, response to stress, apoptosis, collectively indicating activation of the immune system. Interestingly, genes associated with B cell activation and Th2 cell differentiation were upregulated, whereas those associated with Th1 cell differentiation were downregulated (Aho et al. 2013), suggesting that the Th2 immune response driven by sleep deprivation, as observed in many studies, is regulated at the level of gene expression. In contrast, biological processes associated with genes downregulated following sleep deprivation compared with unrestricted sleep included chromatic organization and modification, gene expression, cellular macromolecule metabolism (Moller-Levet et al. 2013), cholesterol/lipid metabolism and transport, as well as NK cell function thus contributing to the reduced immune response against pathogens (Aho et al. 2013). The same expression profile of several genes identified in the experimental sleep deprivation was observed in a cohort of subjects with self-report of insufficient sleep (Aho et al. 2013), highlighting the physiological relevance of the experimental results at the population level in real-life conditions.
The pro-inflammatory transcriptomic response observed after sleep deprivation mainly involves the activation of the pro-inflammatory NF-κB family of transcription factors (Irwin et al. 2006, 2008; Aho et al. 2013). NF-κB mediates the expression of genes (e.g., cytokines, chemokines, growth factors, receptors/transporters, enzymes, adhesion molecules) involved in the activation of inflammation, adaptive and innate immunity, proliferation, and apoptosis, and is recognized as a promising therapeutic target in inflammatory diseases (Madonna and De Caterina 2012).
Sleep disruption is also associated with oxidative stress, which represents an imbalance in the production and/or detoxification of free radicals such as reactive oxygen species (ROS). Oxygen-derived free radicals are generated during oxidative metabolism and energy production processes and normally serve, at very low physiologic concentrations, as important second messengers in many intracellular signaling pathways for maintaining cell homeostasis and survival in response to stress (Liguori et al. 2018). At higher levels which are not counteracted by the antioxidant defenses of the cell, ROS can cause damage to cells and tissues resulting in cell senescence and injury, unbalanced local/systemic inflammation, metabolic dysfunction as well as immune derangements. Pertinently, ROS are essential, especially at low levels for a wide range of immune functions, including anti-viral, anti-bacterial, and anti-tumor responses through, for example, killing of pathogens, regulation of T cell activation, expansion and effector function, and induction of balanced inflammatory reaction (Sena and Chandel 2012). However, excessive uncompensated ROS production can lead to aberrant immune responses and unbalanced inflammatory reactions, including apoptosis and functional suppression of T cells with following reduced anti-tumor function and chronic activation of pro-inflammatory signaling pathways including NF-κB (Chen et al. 2016). Oxidative stress is now recognized to play a central role in the pathophysiology of many different disorders with immune components, mostly neurodegenerative, cardiovascular, and metabolic diseases as well as cancer (Liguori et al. 2018), which are also disease conditions triggered or exacerbated by sleep disturbance. Accordingly, most animal studies found an increase in oxidative stress markers and/or a decrease in endogenous antioxidants and antioxidant enzymes in brain regions and peripheral tissues (liver, heart, plasma, etc.) after sleep disturbance, while recovery sleep restored the antioxidant/oxidant balance (Villafuerte et al. 2015). Recent human findings agree with animal results, as shown in night workers with chronic sleep loss (Teixeira et al. 2019), and in young adults subjected to acute (overnight) sleep deprivation (Trivedi et al. 2017). Therefore, sleep shows an antioxidant function, responsible for eliminating ROS produced during wakefulness, and contrarily sleep curtailment may exert negative health effects by causing oxidative stress.
A breakdown of host defense against microorganisms has been found in animals subjected to insufficient sleep, as shown by the increased mortality after septic insult in sleep-deprived mice compared with control mice (Friese et al. 2009), or by systemic invasion by opportunistic microorganisms leading to increased morbidity and lethal septicemia in sleep deprived-rats (Everson and Toth 2000). Patients with sleep disorders exhibited a 1.23-fold greater risk of herpes zoster than did the comparison cohort, after adjustment for potential covariates (Chung et al. 2016). Accordingly, increased susceptibility to respiratory infections has been reported in sleep-deprived human subjects, as those with habitual short sleep (≤5 h) compared with 7–8 h sleep, in cross-sectional and prospective studies (Patel et al. 2012; Prather and Leung 2016), and after an experimental viral challenge (Cohen et al. 2009; Prather et al. 2015). Similarly, compared with long sleep duration (around 7 h), short sleep duration (around 6 h) is associated with an increased risk of common illnesses, including cold, flu, gastroenteritis, and other common infectious diseases, in adolescents (Orzech et al. 2014).
In sum, most of these immune responses to sleep loss are suggestive of a systemic low-grade pro-inflammatory reaction. Following experimental findings, population-based studies in adult and younger individuals found that habitual short sleep duration (generally <5 or 6 h) is directly and independently associated with elevated circulating pro-inflammatory markers, such as acute phase proteins (CRP, IL-6), cytokines (TNF-α, IFN-γ, IL-1, etc.), adhesion molecules, white blood cell counts (Miller et al. 2009; Patel et al. 2009; Ferrie et al. 2013; Perez de Heredia et al. 2014; Bakour et al. 2017; Richardson and Churilla 2017). Furthermore, reduced NK cell activity (Fondell et al. 2011) and a decline in naive T cells (Carroll et al. 2017) were also found to be associated with habitual short sleep. A shortening of leukocyte telomere length, which is a marker of cellular senescence and inflammation damage, was also shown to be associated with shorter sleep duration in adults and children (Jackowska et al. 2012; James et al. 2017). Systemic low-grade inflammation has been shown to predict the risk of disease and mortality (Li et al. 2017); thus, it is suggested to mediate the increased risk of morbidity and mortality associated with sleep disruption (Hall et al. 2015; Smagula et al. 2016).
1.4 Circadian Clock Regulation of the Immune System
The circadian system encompasses a central clock representing a master circadian pacemaker located in the SCN of the hypothalamus, and peripheral circadian clocks are distributed in different cells or tissues outside the brain and functioning autonomously and flexibly (Patke et al. 2020). The central clock mainly responds to the light/dark cycle, so that the ambient light (one of the strongest zeitgebers, i.e., external time givers) is transmitted to the hypothalamus through the retinal ganglion cells leading to glutamate release at the nerve terminals and an increase in the SCN neuronal activity (i.e., wake pressure).
The central and peripheral clocks interact through neural, endocrine pathways and body temperature, to produce daily rhythms in sleep, physical activity, and nutrient metabolism via self-sustained near-24 h and alternating activation-repression cycles of core clock transcriptional and translational regulators (Clock, Bmal1, Npas2, Crys, Pers, Rors, and Rev-erbs) (Sulli et al. 2018). The peripheral clocks also respond to other synchronizers such as meal times and humoral factors, and body temperature. Several metabolites and proteins interact with the core clock components to influence their function and modulate specific outputs of the circadian system. Whole-genome transcriptomic studies have revealed circadian variation (i.e., with a ~24-h periodicity) in gene expression, and that almost every gene including those encoding drug targets show diurnal rhythmic expression in a tissue-specific manner and a bimodal distribution (with peaks predominantly occurring during the biological night and day) thus suggesting a marked temporal segmentation of biological processes and functions and contributing to the circadian rhythmicity of basic cellular functions, including metabolism, immune function, and tissue repair (Mure et al. 2018; Sulli et al. 2018; Christou et al. 2019).
Both the innate and adaptive arms of the immune response are under circadian control, which is instrumental to gate the immune functions, such as immune cell trafficking, production of cytokines, or host–pathogen interaction, and to increase organismal fitness, while minimizing metabolic costs of immune activation or collateral tissue damage due to uncontrolled immune response. Both circulating immune cell counts and inflammatory cytokine levels show variations during the sleep/wake cycle dependent on both sleep- and circadian rhythm-associated processes (Scheiermann et al. 2013): indeed, human natural killer (NK) cells and neutrophils peak at midday and show minimum levels during the night; while monocytes, B, and T cells peak during the first half of the night and have minimum levels during the day (Born et al. 1997). Similarly, blood levels of pro-inflammatory cytokines show a peak during early nocturnal sleep (Lange et al. 2010).
The rhythmic oscillation of immune function allows an appropriate magnitude of immune response and ensures the resolution of injury without progressing towards chronic inflammation. It follows that different aspects of innate immunity (e.g., production of cytokines and chemokines, expression of Toll-like receptors [TLRs], antimicrobial peptides, phagocytosis, secretion of complement and coagulation factors, barrier functions) are temporally gated (with nadir and peaks) to distinct phases of the circadian cycle preventing their synchronous activation and limiting the duration of the inflammatory response (Gibbs et al. 2012; Bellet et al. 2013). The exit of differentiated immune cells from the bone marrow and trafficking of innate and adaptive immune cells also display a circadian rhythm (Mendez-Ferrer et al. 2008; Druzd et al. 2017). The rhythmic cellular clock-based expression of TLRs as well as the temporal gating of T cell activation and proliferation can maximize the immune response at a specific time window, and contribute to the time-of-the-day dependence of immunization after vaccination (Silver et al. 2012). Accordingly, studies found that morning vaccination resulted in higher viral-specific antibody responses compared with afternoon vaccination (Long et al. 2016).
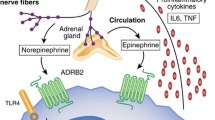
The rhythmic outputs of the immune function can be generated by both extrinsic (e.g., central clock) or intrinsic (cell-autonomous circadian clock) entrainment cues (Man et al. 2016). For example, studies found that the central clock-regulated rhythmic output of the sympathetic nervous system or glucocorticoids is the dominant entrainment cue for the recruitment of hematopoietic stem cells, innate immune cells, as well as adaptive immune cells, respectively. On the other hand, diurnal oscillations in the abundance of inflammatory monocytes are under the control of Bmal1 thus gating the host’s vulnerability to infection and associated tissue damage (Man et al. 2016). Furthermore, the cellular circadian clock establishes the rhythmic oscillations in the expression of basal and inducible inflammatory genes such as cytokine and chemokines, which involves among others the regulation of the transcription factor NF-κB, the major transcriptional activator of inflammation. Bmal1 has been shown to modulate monocyte trafficking, immune response and NF-κB signaling (Man et al. 2016) as well as to regulate metabolic utilization in peripheral tissue (Peek et al. 2017) and to maintain the BBB function (Nakazato et al. 2017). Bmal1 knockout caused neuroinflammation, redox imbalance, and neurodegeneration (Musiek et al. 2013). Similarly, REV-ERB-α and REV-ERB-β, and CRY contribute to rhythmically repress inflammatory gene expression by acting on NF-𝜅B signaling (Narasimamurthy et al. 2012; Man et al. 2016).
Diurnal oscillations have also been observed in the relative abundance and composition of gut microbial communities, which translate to rhythmic production of microbial metabolites, in turn, able to influence host circadian activity as well as immune cell functions (Thaiss et al. 2016). Genetic ablation of molecular clock components, circadian disruption due to jet lag, sleep disruption, or high-fat diet may lead to alterations of the gut microbiota ecosystem (dysbiosis), which subsequently impairs host metabolism and immune function (Murakami and Tognini 2019).
1.5 Immune Responses Under Circadian Misalignment
One potential common feature of the negative health consequences of circadian disruption may be the dysregulation of the immune system. The loss of immune regulation due to circadian disruption may increase the susceptibility to tissue damage in response to infection or other challenges. Several studies assessed the perturbation of circadian systems by environmental and/or genetic manipulations in animal models. Animal studies found that global, brain, or peripheral knockout of clock genes alters this circadian fluctuation and leads to an exacerbated inflammatory response to infection or other pathogenic stimuli, oxidative stress, and age-related phenotypes thus revealing a direct role for clock genes in suppressing chronic inflammation and ensuring its timely resolution (Nguyen et al. 2013; Scheiermann et al. 2013) Notably, cytokines, including TNF-α, IL-1β, and endotoxin, as observed in inflammation and infection, inhibit the expression of core clock genes and clock-controlled genes, resulting in reduced locomotor activity and prolonged rest time (Cavadini et al. 2007), and leading to the loss of basal oscillatory rhythm and to a reprogramming of the temporal relationships between gene expression, metabolites and leukocyte trafficking (Haspel et al. 2014). Modulation of clock molecules leads to increased replication of herpes, influenza, respiratory syncytial virus, parainfluenza type 3, and hepatitis C virus (Majumdar et al. 2017; Zhuang et al. 2019) pointing to an important role of the circadian clock in virus infection. Herpes viruses target molecular clock components of the host, which in turn affects the viral replication rate (Edgar et al. 2016).
A study in mice investigating the environmental perturbation of circadian rhythms shows that experimentally induced circadian disruption schedule (four consecutive weekly 6 h phase-advances of the light-dark) increased endotoxemic shock and mortality compared to unshifted control mice in response to the immune challenge LPS (Castanon-Cervantes et al. 2010). This result was associated with a heightened inflammation in shifted animals as exemplified by higher levels of pro-inflammatory cytokines and activation of peritoneal macrophages in response to LPS treatment. Rhythms in the expression of clock genes in the central clock, liver, thymus, and peritoneal macrophages were also altered and/or inhibited after chronic jet lag, while no sleep loss or stress was documented (Castanon-Cervantes et al. 2010). Furthermore, another study investigating the chronic effects of circadian misalignment in mice revealed that the long-term nonadjustive shifted condition of the light-dark cycle, simulating the chronic jet lag, induced chronic inflammation and accelerated immune senescence in association with a reduced survival rate (Inokawa et al. 2020). Therefore, the mouse model system of exposure to long-term nonadjustive shifted light conditions may mirror the pathophysiology of chronic circadian rhythm disruption in humans.
In humans, the most common forms—and causes—of circadian misalignment due to human behaviors are chronic jet lag, when traveling across several time zones; social jet lag, which corresponds to the time difference between routine sleep cycles during the work/school week and free time on weekends, involving a discrepancy social clock and individual’s circadian rhythm; and shift work, an atypical working schedule where individuals experience an unnatural routine of activity during the dark phase and sleep during the light phase of the day so that work and sleep occur at times that conflict with the circadian rhythm. These are conditions associated with sleep disruption and psychosocial stress, and adversely affect the immune system due to circadian misalignment. Human studies have found that centrally controlled rhythms as well as peripheral clocks are disturbed in such conditions (Koshy et al. 2019). In night shift workers, light exposure at night has a large impact on the resetting of peripheral circadian clocks (Cuesta et al. 2017).
Compared with the non-shift workers, the shift work schedule was associated with a decline in innate immune response, e.g., NK cell activity (Okamoto et al. 2008; Nagai et al. 2011), and an increase in the number of circulating leukocytes (Lu et al. 2016; Wirth et al. 2017), a lower CD4/CD8 ratio, cytokines and systemic endotoxemia (Atwater et al. 2021), besides to shifted and desynchronized cytokine release by immune cells (Cuesta et al. 2016). A 4-day simulated night shift work protocol in healthy subjects changed the circadian regulation of the human transcriptome and mostly affected biological processes related to the adverse health effects associated with night shift work, notably the natural killer cell-mediated immune response and inflammatory pathways (Kervezee et al. 2018). A recent cross-sectional study showed that shift work, particularly night work, was associated with a 1.85-fold increased risk of COVID-19 infection (Fatima et al. 2021). In a case-control study on healthcare workers, people with sleep problems had greater odds of COVID-19 (Kim et al. 2021). Individuals with social jet lag were also significantly associated with a higher risk of COVID-19 infection (2.07-fold) (Coelho et al. 2022). A recent study on the vaccination response in people with circadian disruption, i.e., shift workers, found that compared with day workers shift workers had altered sleep architecture, with a lower slow wave sleep and REM duration, higher levels of cytokines and a weaker specific leukocyte-mediated immune response to vaccination against meningococcal C meningitis (Ruiz et al. 2020). Research concerning the impact of sleep disruption and circadian misalignment in shift workers on the immune response to COVID-19 vaccination is currently ongoing (Lammers-van der Holst et al. 2022).
A relationship between sleep and the circadian system has emerged in an investigation carried out in humans where sleep deprivation (5.70 h compared with 8.50 h sleep per 24 h, for 1 week) led in the blood transcriptome to a significant reduction in rhythmic transcripts (from 8.8% to 6.9%) and affected many genes associated with sleep homeostasis, oxidative stress, metabolism, inflammatory and stress responses, chromatin organization and modification (Moller-Levet et al. 2013). Among the genes whose rhythmic expression was altered by sleep loss, there were several genes classified as circadian, including classic clock genes, such as Bmal1, PERs, CRY, neuronal PAS domain-containing protein (NPAS), REV-ERB-α, REV-ERB-β, with a significant reduction in the number of rhythmic transcripts, in the circadian amplitude and the width of the period of expression. On the other hand, another set of genes, including those associated with RNA metabolic processes, became circadian following sleep loss (Moller-Levet et al. 2013).
These data are following previous animal studies (Barclay et al. 2012) and provide potential molecular mechanisms whereby sleep loss can lead to circadian disruption, and cause negative health consequences. Furthermore, while many transcripts in the human blood transcriptome have a circadian expression profile when sleep occurred in phase with the central circadian rhythm (as indexed by the melatonin rhythm), when sleep and associated locomotor, feeding, and metabolic rhythms are phase-shifted compared with the circadian clock (mistimed sleep or forced desynchrony), the majority of circadian transcripts became arrhythmic (reduction in the number of circadian transcripts from 6.4% to 1% and changes in the overall time course of expression of 34% of transcripts) (Archer et al. 2014). In addition, results from a mathematical modeling analysis, which separated the relative contribution of sleep and circadian rhythmicity on the temporal gene expression profile, suggests that circadian-driven transcripts are mainly associated with cellular metabolic and homeostatic processes, whereas sleep-driven transcripts are linked with the regulation of transcription and protein synthesis (Archer et al. 2014).
Overall, these data underscore the important role of sleep and circadian rhythmicity in the regulation of tissue gene expression and functions (Maret et al. 2007; Mongrain et al. 2010; Pellegrino et al. 2012; Anafi et al. 2013; Perrin et al. 2018), and indicate that a common feature is a 24-h organization of molecular processes, including the immune function, in humans (Fig. 1.1). They also suggest that appropriate sleep duration, quality, and timing significantly contribute to the overall temporal organization of the human transcriptome, and desynchrony of sleep and centrally driven circadian rhythms, as occurs in shift work and jet lag, may lead to disruption of rhythmicity in physiology and endocrinology.
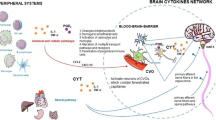
Regulation of the immune system by sleep and the circadian system. Immune system functions, including cell proliferation, differentiation, trafficking, activity, and cytokine production, are regulated by both homeostatic and circadian drives of sleep. The circadian clocks, with the cell-autonomous transcriptional–translational feedback loop mechanism (depicted on the right inset), include the central clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus, which is entrained primarily by environmental cues from light. This SCN coordinates 24-h rhythms in physiology and behavior through neural and hormonal signals that synchronize peripheral clocks in peripheral organs or tissues, including the immune cells, through the expression of clock-controlled genes (CCGs). Peripheral clocks can also be synchronized by behaviors, including eating and physical activity. (Illustrations are adapted from Servier Medical Art (http://smart.servier.com/))
1.6 Conclusion and Perspectives
Epidemiological data suggest that increasing numbers of individuals are becoming sleep disturbed and circadian misaligned, and the accumulation of these disturbances over years may induce safety-related problems and have gradual cumulative adverse effects on health, increasing the risk of chronic diseases and the susceptibility to pathogens and pollution (Fig. 1.2). Sleep and the circadian rhythm exert immune-supportive and regulatory functions, and impairments of the immune inflammatory system are plausible mechanisms mediating the negative health effects of sleep and circadian disruption. Evidence-based molecular and clinical studies have provided a framework to understand the link between these disturbances and inflammatory immune diseases, positing a causal role for the immune derangements in the development and/or exacerbation of inflammatory and infectious diseases. Sleep deprivation superimposed on preexisting sleep disorders, as occurred in abstinent alcohol-dependent subjects, more strongly increases susceptibility to inflammation compared with subjects without sleep problems (Irwin et al. 2004). Similarly, sleep deprivation can worsen the risk profile in individuals with hypertension, in which even half a night of sleep deprivation elevates blood pressure the next day (Lusardi et al. 1999).
Conditions of sleep disruption (e.g., sleep deprivation) and misaligned sleep–wake cycles alter immune processes and are associated with or increase the risk for many diseases (right). (Illustrations are adapted from Servier Medical Art (http://smart.servier.com/))
A practical implication of these observations is that immune homeostasis may be a crucial target for intervention against diseases associated with sleep and circadian clock disruption. Currently, limited evidence exists in this context. Treatment of insomnia has been demonstrated to reverse humoral and cell inflammatory activation, as well as to reduce diabetic and cardiovascular risk, as assessed by a multisystem biological risk based on eight biomarkers (Carroll et al. 2015). Randomized controlled trials assessing the effect of the treatment of sleep and circadian disturbances on inflammatory immune dysfunction and/or health outcomes are needed to provide a cause–effect relationship. Knowledge of inflammatory and immunological signatures in response to sleep curtailment, mostly through omic-based approaches, may inform not only on the underlying molecular links but also contribute to refining risk profiles to be used for developing biomarkers of disturbed sleep and sleep disturbance-related health outcomes. Recent metabolomics (Weljie et al. 2015) and transcriptomic (Laing et al. 2019) studies hold promise in biomarker discovery, not only confirming the activation of pathways related to immune, inflammatory, and cell stress responses following sleep disruption but also identifying, along these pathways, potential blood biomarkers and associated prediction model for sleep debt status with practical applications (e.g., diagnosis of sleep disorders, risk stratification for health outcomes and safety driving, evaluation of therapeutic interventions on sleep) (Bragazzi et al. 2019; Laing et al. 2019).
These efforts may converge towards a new ground fostering interaction between sleep research, chronobiology, and the medical community to translate scientific knowledge into lifestyle recommendations and clinical practice, and to prevent and/or treat the negative health consequences of sleep disruption and circadian misalignment. Particular attention deserves sleep management in the hospital environment, where sleep disturbances often occur and in patients with acute or chronic illness and hence particularly vulnerable may benefit from good sleep in terms of immunity boosting and recovery of health.
These actions might also foster health literacy and empowerment of individuals to actively better manage their health and well-being throughout their life course utilizing lifestyle, nutritional and behavioral habits including sleep hygiene and circadian lifestyle that include timing of sleep, activity, nutrition, and lighting (Garbarino and Scoditti 2020; Scoditti et al. 2022). Of course, behavioral, lifestyle, or pharmacological approaches to improve sleep beneficially impact one key component of the circadian rhythm and may indirectly benefit other aspects of the daily rhythms thereby leading to a better quality of life. Further knowledge in the circadian system and its interaction with immunity may advance strategies to prevent or treat several chronic diseases, establishing how to maintain a regular circadian rhythm in sleep–wake, feeding–fasting, or light–dark cycles; how to optimize the timing of drug treatment; and how to directly target a circadian clock component (clock-targeting pharmacotherapy) for treating inflammatory disorders (Sulli et al. 2018).
Conclusively, in the perspective of staying healthy in this rapidly changing world, the sleep/circadian clock–immunity relationship raises relevant clinical implications for promoting health. During the COVID-19 pandemic, these issues have become particularly important because of sleep disturbances often reported in association with the heavy social, work, and lifestyle changes imposed to contain the virus spreading. Moreover, healthcare workers, which are subjected to night shifts, irregular sleep–wake schedules, changes in daily routine, and circadian rhythm problems, have been found to have more severe insomnia and a greater risk of coronavirus infection (Kim et al. 2021). Pending further research, sleep and circadian-based intervention may reduce the susceptibility to SARS-CoV-2 infection and the severity of COVID-19, and improve immune response to COVID-19 vaccination (Meira et al. 2020).
References
Abbott SM, Malkani RG, Zee PC (2020) Circadian disruption and human health: a bidirectional relationship. Eur J Neurosci 51:567–583. https://doi.org/10.1111/ejn.14298
Aho V, Ollila HM, Rantanen V, Kronholm E, Surakka I, Van Leeuwen WM, Lehto M, Matikainen S, Ripatti S, Harma M, Sallinen M, Salomaa V, Jauhiainen M, Alenius H, Paunio T, Porkka-Heiskanen T (2013) Partial sleep restriction activates immune response-related gene expression pathways: experimental and epidemiological studies in humans. PLoS One 8:e77184. https://doi.org/10.1371/journal.pone.0077184
Anafi RC, Pellegrino R, Shockley KR, Romer M, Tufik S, Pack AI (2013) Sleep is not just for the brain: transcriptional responses to sleep in peripheral tissues. BMC Genomics 14:362. https://doi.org/10.1186/1471-2164-14-362
Archer SN, Laing EE, Moller-Levet CS, Van Der Veen DR, Bucca G, Lazar AS, Santhi N, Slak A, Kabiljo R, Von Schantz M, Smith CP, Dijk DJ (2014) Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci U S A 111:E682–E691. https://doi.org/10.1073/pnas.1316335111
Atwater AQ, Immergluck LC, Davidson AJ, Castanon-Cervantes O (2021) Shift work predicts increases in lipopolysaccharide-binding protein, interleukin-10, and leukocyte counts in a cross-sectional study of healthy volunteers carrying low-grade systemic inflammation. Int J Environ Res Public Health 18(24):13158. https://doi.org/10.3390/ijerph182413158
Axelsson J, Rehman JU, Akerstedt T, Ekman R, Miller GE, Hoglund CO, Lekander M (2013) Effects of sustained sleep restriction on mitogen-stimulated cytokines, chemokines and T helper 1/T helper 2 balance in humans. PLoS One 8:e82291. https://doi.org/10.1371/journal.pone.0082291
Bakour C, Schwartz S, O’Rourke K, Wang W, Sappenfield W, Couluris M, Chen H (2017) Sleep duration trajectories and systemic inflammation in young adults: results from the National Longitudinal Study of Adolescent to Adult Health (Add Health). Sleep 40(11):zsx156. https://doi.org/10.1093/sleep/zsx156
Barclay JL, Husse J, Bode B, Naujokat N, Meyer-Kovac J, Schmid SM, Lehnert H, Oster H (2012) Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS One 7:e37150. https://doi.org/10.1371/journal.pone.0037150
Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, Edwards RA, Sahar S, Dandekar S, Baldi P, George MD, Raffatellu M, Sassone-Corsi P (2013) Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci U S A 110:9897–9902. https://doi.org/10.1073/pnas.1120636110
Besedovsky L, Lange T, Haack M (2019) The sleep-immune crosstalk in health and disease. Physiol Rev 99:1325–1380. https://doi.org/10.1152/physrev.00010.2018
Boivin DB, Boudreau P, Kosmadopoulos A (2022) Disturbance of the circadian system in shift work and its health impact. J Biol Rhythm 37:3–28. https://doi.org/10.1177/07487304211064218
Born J, Lange T, Hansen K, Molle M, Fehm HL (1997) Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol 158:4454–4464
Bragazzi NL, Guglielmi O, Garbarino S (2019) SleepOMICS: how big data can revolutionize sleep science. Int J Environ Res Public Health 16(2):291. https://doi.org/10.3390/ijerph16020291
Cappuccio FP, Miller MA (2017) Sleep and cardio-metabolic disease. Curr Cardiol Rep 19:110. https://doi.org/10.1007/s11886-017-0916-0
Carroll JE, Seeman TE, Olmstead R, Melendez G, Sadakane R, Bootzin R, Nicassio P, Irwin MR (2015) Improved sleep quality in older adults with insomnia reduces biomarkers of disease risk: pilot results from a randomized controlled comparative efficacy trial. Psychoneuroendocrinology 55:184–192. https://doi.org/10.1016/j.psyneuen.2015.02.010
Carroll JE, Irwin MR, Levine M, Seeman TE, Absher D, Assimes T, Horvath S (2017) Epigenetic aging and immune senescence in women with insomnia symptoms: findings from the Women’s Health Initiative Study. Biol Psychiatry 81:136–144. https://doi.org/10.1016/j.biopsych.2016.07.008
Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ (2010) Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol 185:5796–5805. https://doi.org/10.4049/jimmunol.1001026
Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A (2007) TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci U S A 104:12843–12848. https://doi.org/10.1073/pnas.0701466104
Chaponda M, Aldhouse N, Kroes M, Wild L, Robinson C, Smith A (2018) Systematic review of the prevalence of psychiatric illness and sleep disturbance as co-morbidities of HIV infection in the UK. Int J STD AIDS 29:704–713. https://doi.org/10.1177/0956462417750708
Chen X, Song M, Zhang B, Zhang Y (2016) Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxidative Med Cell Longev 2016:1580967. https://doi.org/10.1155/2016/1580967
Christou S, Wehrens SMT, Isherwood C, Moller-Levet CS, Wu H, Revell VL, Bucca G, Skene DJ, Laing EE, Archer SN, Johnston JD (2019) Circadian regulation in human white adipose tissue revealed by transcriptome and metabolic network analysis. Sci Rep 9:2641. https://doi.org/10.1038/s41598-019-39668-3
Chung WS, Lin HH, Cheng NC (2016) The incidence and risk of Herpes Zoster in patients with sleep disorders: a population-based cohort study. Medicine (Baltimore) 95:e2195. https://doi.org/10.1097/MD.0000000000002195
Coelho J, Micoulaud-Franchi JA, Wiet AS, Nguyen D, Taillard J, Philip P (2022) Circadian misalignment is associated with Covid-19 infection. Sleep Med 93:71–74. https://doi.org/10.1016/j.sleep.2022.03.015
Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB (2009) Sleep habits and susceptibility to the common cold. Arch Intern Med 169:62–67. https://doi.org/10.1001/archinternmed.2008.505
Consensus Conference Panel, Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E (2015a) Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med 11:931–952. https://doi.org/10.5664/jcsm.4950
Consensus Conference Panel, Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E, Non-Participating Observers, Twery M, Croft JB, Maher E, American Academy of Sleep Medicine Staff, Barrett JA, Thomas SM, Heald JL (2015b) Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med 11:591–592. https://doi.org/10.5664/jcsm.4758
Cuesta M, Boudreau P, Dubeau-Laramee G, Cermakian N, Boivin DB (2016) Simulated night shift disrupts circadian rhythms of immune functions in humans. J Immunol 196:2466–2475. https://doi.org/10.4049/jimmunol.1502422
Cuesta M, Boudreau P, Cermakian N, Boivin DB (2017) Rapid resetting of human peripheral clocks by phototherapy during simulated night shift work. Sci Rep 7:16310. https://doi.org/10.1038/s41598-017-16429-8
Dantzer R (2018) Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev 98:477–504. https://doi.org/10.1152/physrev.00039.2016
Dimitrov S, Lange T, Tieken S, Fehm HL, Born J (2004) Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun 18:341–348. https://doi.org/10.1016/j.bbi.2003.08.004
Dimitrov S, Lange T, Nohroudi K, Born J (2007) Number and function of circulating human antigen presenting cells regulated by sleep. Sleep 30:401–411. https://doi.org/10.1093/sleep/30.4.401
Disis ML (2010) Immune regulation of cancer. J Clin Oncol 28:4531–4538. https://doi.org/10.1200/JCO.2009.27.2146
Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, Uhl O, Yao L, Sander LE, Chen CS, Kraus K, De Juan A, Hergenhan SM, Ehlers M, Koletzko B, Haas R, Solbach W, Oster H, Scheiermann C (2017) Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 46:120–132. https://doi.org/10.1016/j.immuni.2016.12.011
Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O’Neill JS, Reddy AB (2016) Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci U S A 113:10085–10090. https://doi.org/10.1073/pnas.1601895113
Everson CA, Toth LA (2000) Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol 278:R905–R916. https://doi.org/10.1152/ajpregu.2000.278.4.R905
Everson CA, Thalacker CD, Hogg N (2008) Phagocyte migration and cellular stress induced in liver, lung, and intestine during sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol 295:R2067–R2074. https://doi.org/10.1152/ajpregu.90623.2008
Everson CA, Henchen CJ, Szabo A, Hogg N (2014) Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep 37:1929–1940. https://doi.org/10.5665/sleep.4244
Fatima Y, Bucks RS, Mamun AA, Skinner I, Rosenzweig I, Leschziner G, Skinner TC (2021) Shift work is associated with increased risk of COVID-19: findings from the UK Biobank cohort. J Sleep Res 30:e13326. https://doi.org/10.1111/jsr.13326
Ferrie JE, Kivimaki M, Akbaraly TN, Singh-Manoux A, Miller MA, Gimeno D, Kumari M, Davey Smith G, Shipley MJ (2013) Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II Study. Am J Epidemiol 178:956–961. https://doi.org/10.1093/aje/kwt072
Fishbein AB, Knutson KL, Zee PC (2021) Circadian disruption and human health. J Clin Invest 131(19):e14828. https://doi.org/10.1172/JCI148286
Fondell E, Axelsson J, Franck K, Ploner A, Lekander M, Balter K, Gaines H (2011) Short natural sleep is associated with higher T cell and lower NK cell activities. Brain Behav Immun 25:1367–1375. https://doi.org/10.1016/j.bbi.2011.04.004
Ford ES, Cunningham TJ, Croft JB (2015a) Trends in self-reported sleep duration among US adults from 1985 to 2012. Sleep 38:829–832. https://doi.org/10.5665/sleep.4684
Ford ES, Cunningham TJ, Giles WH, Croft JB (2015b) Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med 16:372–378. https://doi.org/10.1016/j.sleep.2014.12.008
Friese RS, Bruns B, Sinton CM (2009) Sleep deprivation after septic insult increases mortality independent of age. J Trauma 66:50–54. https://doi.org/10.1097/TA.0b013e318190c3a1
Garbarino S, Scoditti E (2020) On the role of sleep hygiene in health management during COVID-19 pandemic. Sleep Med 77:74. https://doi.org/10.1016/j.sleep.2020.11.036
Garbarino S, Lanteri P, Bragazzi NL, Magnavita N, Scoditti E (2021) Role of sleep deprivation in immune-related disease risk and outcomes. Commun Biol 4:1304. https://doi.org/10.1038/s42003-021-02825-4
Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS (2012) The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A 109:582–587. https://doi.org/10.1073/pnas.1106750109
Gilmour H, Stranges S, Kaplan M, Feeny D, Mcfarland B, Huguet N, Bernier J (2013) Longitudinal trajectories of sleep duration in the general population. Health Rep 24:14–20
Ginaldi L, De Martinis M, D’Ostilio A, Marini L, Loreto MF, Corsi MP, Quaglino D (1999) The immune system in the elderly: I. Specific humoral immunity. Immunol Res 20:101–108. https://doi.org/10.1007/BF02786466
Grandner MA (2017) Sleep, health, and society. Sleep Med Clin 12:1–22. https://doi.org/10.1016/j.jsmc.2016.10.012
Hall MH, Smagula SF, Boudreau RM, Ayonayon HN, Goldman SE, Harris TB, Naydeck BL, Rubin SM, Samuelsson L, Satterfield S, Stone KL, Visser M, Newman AB (2015) Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: the Health, Aging and Body Composition Study. Sleep 38:189–195. https://doi.org/10.5665/sleep.4394
Hand TW, Vujkovic-Cvijin I, Ridaura VK, Belkaid Y (2016) Linking the microbiota, chronic disease, and the immune system. Trends Endocrinol Metab 27:831–843. https://doi.org/10.1016/j.tem.2016.08.003
Haspel JA, Chettimada S, Shaik RS, Chu JH, Raby BA, Cernadas M, Carey V, Process V, Hunninghake GM, Ifedigbo E, Lederer JA, Englert J, Pelton A, Coronata A, Fredenburgh LE, Choi AM (2014) Circadian rhythm reprogramming during lung inflammation. Nat Commun 5:4753. https://doi.org/10.1038/ncomms5753
Hu J, Chen Z, Gorczynski CP, Gorczynski LY, Kai Y, Lee L, Manuel J, Gorczynski RM (2003) Sleep-deprived mice show altered cytokine production manifest by perturbations in serum IL-1ra, TNFa, and IL-6 levels. Brain Behav Immun 17:498–504. https://doi.org/10.1016/j.bbi.2003.03.001
Inokawa H, Umemura Y, Shimba A, Kawakami E, Koike N, Tsuchiya Y, Ohashi M, Minami Y, Cui G, Asahi T, Ono R, Sasawaki Y, Konishi E, Yoo SH, Chen Z, Teramukai S, Ikuta K, Yagita K (2020) Chronic circadian misalignment accelerates immune senescence and abbreviates lifespan in mice. Sci Rep 10:2569. https://doi.org/10.1038/s41598-020-59541-y
Irwin MR (2015) Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol 66:143–172. https://doi.org/10.1146/annurev-psych-010213-115205
Irwin M, Mascovich A, Gillin JC, Willoughby R, Pike J, Smith TL (1994) Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom Med 56:493–498. https://doi.org/10.1097/00006842-199411000-00004
Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C (2004) Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun 18:349–360. https://doi.org/10.1016/j.bbi.2004.02.001
Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S (2006) Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med 166:1756–1762. https://doi.org/10.1001/archinte.166.16.1756
Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S (2008) Sleep loss activates cellular inflammatory signaling. Biol Psychiatry 64:538–540. https://doi.org/10.1016/j.biopsych.2008.05.004
Irwin MR, Witarama T, Caudill M, Olmstead R, Breen EC (2015) Sleep loss activates cellular inflammation and signal transducer and activator of transcription (STAT) family proteins in humans. Brain Behav Immun 47:86–92. https://doi.org/10.1016/j.bbi.2014.09.017
Ishimori K (1909) True causes of sleep: a hypnogenic substance as evidenced in the brain of sleep-deprived animals. Tokyo Igakki Zasshi 23:429–457
Jackowska M, Hamer M, Carvalho LA, Erusalimsky JD, Butcher L, Steptoe A (2012) Short sleep duration is associated with shorter telomere length in healthy men: findings from the Whitehall II cohort study. PLoS One 7:e47292. https://doi.org/10.1371/journal.pone.0047292
James S, Mclanahan S, Brooks-Gunn J, Mitchell C, Schneper L, Wagner B, Notterman DA (2017) Sleep duration and telomere length in children. J Pediatr 187:247–252.e241. https://doi.org/10.1016/j.jpeds.2017.05.014
Kervezee L, Cuesta M, Cermakian N, Boivin DB (2018) Simulated night shift work induces circadian misalignment of the human peripheral blood mononuclear cell transcriptome. Proc Natl Acad Sci U S A 115:5540–5545. https://doi.org/10.1073/pnas.1720719115
Kim H, Hegde S, Lafiura C, Raghavan M, Luong E, Cheng S, Rebholz CM, Seidelmann SB (2021) COVID-19 illness in relation to sleep and burnout. BMJ Nutr Prev Health 4:132–139. https://doi.org/10.1136/bmjnph-2021-000228
Koshy A, Cuesta M, Boudreau P, Cermakian N, Boivin DB (2019) Disruption of central and peripheral circadian clocks in police officers working at night. FASEB J 33:6789–6800. https://doi.org/10.1096/fj.201801889R
Krueger JM, Pappenheimer JR, Karnovsky ML (1982) The composition of sleep-promoting factor isolated from human urine. J Biol Chem 257:1664–1669
Kubota T, Fang J, Kushikata T, Krueger JM (2000) Interleukin-13 and transforming growth factor-beta1 inhibit spontaneous sleep in rabbits. Am J Physiol Regul Integr Comp Physiol 279:R786–R792. https://doi.org/10.1152/ajpregu.2000.279.3.R786
Labrecque N, Cermakian N (2015) Circadian clocks in the immune system. J Biol Rhythm 30:277–290. https://doi.org/10.1177/0748730415577723
Laing EE, Moller-Levet CS, Dijk DJ, Archer SN (2019) Identifying and validating blood mRNA biomarkers for acute and chronic insufficient sleep in humans: a machine learning approach. Sleep 42(1):zsy186. https://doi.org/10.1093/sleep/zsy186
Lammers-Van Der Holst HM, Lammers GJ, Van Der Horst GTJ, Chaves I, De Vries RD, Geurtsvankessel CH, Koch B, Van Der Kuy HM (2022) Understanding the association between sleep, shift work and COVID-19 vaccine immune response efficacy: protocol of the S-CORE study. J Sleep Res 31:e13496. https://doi.org/10.1111/jsr.13496
Lange T, Dimitrov S, Born J (2010) Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci 1193:48–59. https://doi.org/10.1111/j.1749-6632.2009.05300.x
Lange T, Dimitrov S, Bollinger T, Diekelmann S, Born J (2011) Sleep after vaccination boosts immunological memory. J Immunol 187:283–290. https://doi.org/10.4049/jimmunol.1100015
Lasselin J, Rehman JU, Akerstedt T, Lekander M, Axelsson J (2015) Effect of long-term sleep restriction and subsequent recovery sleep on the diurnal rhythms of white blood cell subpopulations. Brain Behav Immun 47:93–99. https://doi.org/10.1016/j.bbi.2014.10.004
Lee HL, Jang JW, Lee SW, Yoo SH, Kwon JH, Nam SW, Bae SH, Choi JY, Han NI, Yoon SK (2019) Inflammatory cytokines and change of Th1/Th2 balance as prognostic indicators for hepatocellular carcinoma in patients treated with transarterial chemoembolization. Sci Rep 9:3260. https://doi.org/10.1038/s41598-019-40078-8
Legendre R, Piéron H (1913) Recherches sur le besoin de sommeil consécutif à une veille prolongée. Z Physiol 14:235–262
Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, Wan Q, He R, Wang Z (2017) Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: a meta-analysis. Atherosclerosis 259:75–82. https://doi.org/10.1016/j.atherosclerosis.2017.02.003
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772. https://doi.org/10.2147/CIA.S158513
Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, Phillips AC (2016) Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine 34:2679–2685. https://doi.org/10.1016/j.vaccine.2016.04.032
Lu LF, Wang CP, Tsai IT, Hung WC, Yu TH, Wu CC, Hsu CC, Lu YC, Chung FM, Jean MC (2016) Relationship between shift work and peripheral total and differential leukocyte counts in Chinese steel workers. J Occup Health 58:81–88. https://doi.org/10.1539/joh.15-0137-OA
Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R (1999) Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens 12:63–68. https://doi.org/10.1016/s0895-7061(98)00200-3
Madonna R, De Caterina R (2012) Relevance of new drug discovery to reduce NF-kappaB activation in cardiovascular disease. Vasc Pharmacol 57:41–47. https://doi.org/10.1016/j.vph.2012.02.005
Majumdar T, Dhar J, Patel S, Kondratov R, Barik S (2017) Circadian transcription factor BMAL1 regulates innate immunity against select RNA viruses. Innate Immun 23:147–154. https://doi.org/10.1177/1753425916681075
Man K, Loudon A, Chawla A (2016) Immunity around the clock. Science 354:999–1003. https://doi.org/10.1126/science.aah4966
Maret S, Dorsaz S, Gurcel L, Pradervand S, Petit B, Pfister C, Hagenbuchle O, O’Hara BF, Franken P, Tafti M (2007) Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci U S A 104:20090–20095. https://doi.org/10.1073/pnas.0710131104
Matricciani L, Olds T, Petkov J (2012) In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev 16:203–211. https://doi.org/10.1016/j.smrv.2011.03.005
Meira ECM, Miyazawa M, Gozal D (2020) Putative contributions of circadian clock and sleep in the context of SARS-CoV-2 infection. Eur Respir J 55(6):2001023. https://doi.org/10.1183/13993003.01023-2020
Mendez-Ferrer S, Lucas D, Battista M, Frenette PS (2008) Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452:442–447. https://doi.org/10.1038/nature06685
Miller MA, Kandala NB, Kivimaki M, Kumari M, Brunner EJ, Lowe GD, Marmot MG, Cappuccio FP (2009) Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep 32:857–864
Mitler MM, Carskadon MA, Czeisler CA, Dement WC, Dinges DF, Graeber RC (1988) Catastrophes, sleep, and public policy: consensus report. Sleep 11:100–109. https://doi.org/10.1093/sleep/11.1.100
Moller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, Lo JC, Santhi N, Von Schantz M, Smith CP, Dijk DJ (2013) Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A 110:E1132–E1141. https://doi.org/10.1073/pnas.1217154110
Mongrain V, Hernandez SA, Pradervand S, Dorsaz S, Curie T, Hagiwara G, Gip P, Heller HC, Franken P (2010) Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep 33:1147–1157. https://doi.org/10.1093/sleep/33.9.1147
Moser EK, Field NS, Oliver PM (2018) Aberrant Th2 inflammation drives dysfunction of alveolar macrophages and susceptibility to bacterial pneumonia. Cell Mol Immunol 15:480–492. https://doi.org/10.1038/cmi.2016.69
Mullington J, Korth C, Hermann DM, Orth A, Galanos C, Holsboer F, Pollmacher T (2000) Dose-dependent effects of endotoxin on human sleep. Am J Physiol Regul Integr Comp Physiol 278:R947–R955. https://doi.org/10.1152/ajpregu.2000.278.4.R947
Murakami M, Tognini P (2019) The circadian clock as an essential molecular link between host physiology and microorganisms. Front Cell Infect Microbiol 9:469. https://doi.org/10.3389/fcimb.2019.00469
Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, Panda S (2018) Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359(6381):eaao0318. https://doi.org/10.1126/science.aao0318
Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Giasson BI, Weaver DR, Holtzman DM, Fitzgerald GA (2013) Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 123:5389–5400. https://doi.org/10.1172/JCI70317
Nagai M, Morikawa Y, Kitaoka K, Nakamura K, Sakurai M, Nishijo M, Hamazaki Y, Maruzeni S, Nakagawa H (2011) Effects of fatigue on immune function in nurses performing shift work. J Occup Health 53:312–319. https://doi.org/10.1539/joh.10-0072-oa
Nakazato R, Kawabe K, Yamada D, Ikeno S, Mieda M, Shimba S, Hinoi E, Yoneda Y, Takarada T (2017) Disruption of Bmal1 impairs blood-brain barrier integrity via pericyte dysfunction. J Neurosci 37:10052–10062. https://doi.org/10.1523/JNEUROSCI.3639-16.2017
Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM (2012) Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A 109:12662–12667. https://doi.org/10.1073/pnas.1209965109
Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A (2013) Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341:1483–1488. https://doi.org/10.1126/science.1240636
Okamoto H, Tsunoda T, Teruya K, Takeda N, Uemura T, Matsui T, Fukazawa S, Ichikawa K, Takemae R, Tsuchida K, Takashima Y (2008) An occupational health study of emergency physicians in Japan: health assessment by immune variables (CD4, CD8, CD56, and NK cell activity) at the beginning of work. J Occup Health 50:136–146. https://doi.org/10.1539/joh.l6084
Opp MR (2005) Cytokines and sleep. Sleep Med Rev 9:355–364. https://doi.org/10.1016/j.smrv.2005.01.002
Orzech KM, Acebo C, Seifer R, Barker D, Carskadon MA (2014) Sleep patterns are associated with common illness in adolescents. J Sleep Res 23:133–142. https://doi.org/10.1111/jsr.12096
Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S (2009) Sleep duration and biomarkers of inflammation. Sleep 32:200–204. https://doi.org/10.1093/sleep/32.2.200
Patel SR, Malhotra A, Gao X, Hu FB, Neuman MI, Fawzi WW (2012) A prospective study of sleep duration and pneumonia risk in women. Sleep 35:97–101. https://doi.org/10.5665/sleep.1594
Patke A, Young MW, Axelrod S (2020) Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21:67–84. https://doi.org/10.1038/s41580-019-0179-2
Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, Mcnulty MR, Ramsey KM, Bass J (2017) Circadian clock interaction with HIF1alpha mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab 25:86–92. https://doi.org/10.1016/j.cmet.2016.09.010
Pellegrino R, Sunaga DY, Guindalini C, Martins RC, Mazzotti DR, Wei Z, Daye ZJ, Andersen ML, Tufik S (2012) Whole blood genome-wide gene expression profile in males after prolonged wakefulness and sleep recovery. Physiol Genomics 44:1003–1012. https://doi.org/10.1152/physiolgenomics.00058.2012
Perez de Heredia F, Garaulet M, Gomez-Martinez S, Diaz LE, Warnberg J, Androutsos O, Michels N, Breidenassel C, Cuenca-Garcia M, Huybrechts I, Gottrand F, Ferrari M, Santaliestra-Pasias AM, Kafatos A, Molnar D, Sjostrom M, Widhalm K, Moreno LA, Marcos A, HELENA Study Group (2014) Self-reported sleep duration, white blood cell counts and cytokine profiles in European adolescents: the HELENA study. Sleep Med 15:1251–1258. https://doi.org/10.1016/j.sleep.2014.04.010
Perrin L, Loizides-Mangold U, Chanon S, Gobet C, Hulo N, Isenegger L, Weger BD, Migliavacca E, Charpagne A, Betts JA, Walhin JP, Templeman I, Stokes K, Thompson D, Tsintzas K, Robert M, Howald C, Riezman H, Feige JN, Karagounis LG, Johnston JD, Dermitzakis ET, Gachon F, Lefai E, Dibner C (2018) Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. Elife 7:e34114. https://doi.org/10.7554/eLife.34114
Prather AA, Leung CW (2016) Association of insufficient sleep with respiratory infection among adults in the United States. JAMA Intern Med 176:850–852. https://doi.org/10.1001/jamainternmed.2016.0787
Prather AA, Hall M, Fury JM, Ross DC, Muldoon MF, Cohen S, Marsland AL (2012) Sleep and antibody response to hepatitis B vaccination. Sleep 35:1063–1069. https://doi.org/10.5665/sleep.1990
Prather AA, Janicki-Deverts D, Hall MH, Cohen S (2015) Behaviorally assessed sleep and susceptibility to the common cold. Sleep 38:1353–1359. https://doi.org/10.5665/sleep.4968
Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A (2007) The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res 56:51–57. https://doi.org/10.1007/s00011-006-6067-1
Redwine L, Dang J, Hall M, Irwin M (2003) Disordered sleep, nocturnal cytokines, and immunity in alcoholics. Psychosom Med 65:75–85. https://doi.org/10.1097/01.psy.0000038943.33335.d2
Redwine L, Dang J, Irwin M (2004) Cellular adhesion molecule expression, nocturnal sleep, and partial night sleep deprivation. Brain Behav Immun 18:333–340. https://doi.org/10.1016/j.bbi.2004.01.001
Richardson MR, Churilla JR (2017) Sleep duration and C-reactive protein in US adults. South Med J 110:314–317. https://doi.org/10.14423/SMJ.0000000000000632
Ruiz FS, Rosa DS, Zimberg IZ, Dos Santos Quaresma MV, Nunes JO, Apostolico JS, Weckx LY, Souza AR, Narciso FV, Fernandes-Junior SA, Goncalves B, Folkard S, Bittencourt L, Tufik S, Tulio De Mello M (2020) Night shift work and immune response to the meningococcal conjugate vaccine in healthy workers: a proof of concept study. Sleep Med 75:263–275. https://doi.org/10.1016/j.sleep.2020.05.032
Said EA, Al-Abri MA, Al-Saidi I, Al-Balushi MS, Al-Busaidi JZ, Al-Reesi I, Koh CY, Idris MA, Al-Jabri AA, Habbal O (2019) Sleep deprivation alters neutrophil functions and levels of Th1-related chemokines and CD4(+) T cells in the blood. Sleep Breath 23:1331–1339. https://doi.org/10.1007/s11325-019-01851-1
Sakami S, Ishikawa T, Kawakami N, Haratani T, Fukui A, Kobayashi F, Fujita O, Araki S, Kawamura N (2002) Coemergence of insomnia and a shift in the Th1/Th2 balance toward Th2 dominance. Neuroimmunomodulation 10:337–343. https://doi.org/10.1159/000071474
Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Van Beers P, Bourrilhon C, Florence G, Chennaoui M (2010) Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985) 108:68–75. https://doi.org/10.1152/japplphysiol.00851.2009
Scheer FA, Hilton MF, Mantzoros CS, Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 106:4453–4458. https://doi.org/10.1073/pnas.0808180106
Scheiermann C, Kunisaki Y, Frenette PS (2013) Circadian control of the immune system. Nat Rev Immunol 13:190–198. https://doi.org/10.1038/nri3386
Scheiermann C, Gibbs J, Ince L, Loudon A (2018) Clocking in to immunity. Nat Rev Immunol 18:423–437. https://doi.org/10.1038/s41577-018-0008-4
Scoditti E, Tumolo MR, Garbarino S (2022) Mediterranean diet on sleep: a health alliance. Nutrients 14(14):2998. https://doi.org/10.3390/nu14142998
Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48:158–167. https://doi.org/10.1016/j.molcel.2012.09.025
Sharpley AL, Cooper CM, Williams C, Godlewska BR, Cowen PJ (2016) Effects of typhoid vaccine on inflammation and sleep in healthy participants: a double-blind, placebo-controlled, crossover study. Psychopharmacology 233:3429–3435. https://doi.org/10.1007/s00213-016-4381-z
Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF (2001) Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol 107:165–170. https://doi.org/10.1067/mai.2001.112270
Silver AC, Arjona A, Walker WE, Fikrig E (2012) The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 36:251–261. https://doi.org/10.1016/j.immuni.2011.12.017
Smagula SF, Stone KL, Redline S, Ancoli-Israel S, Barrett-Connor E, Lane NE, Orwoll ES, Cauley JA, Osteoporotic Fractures in Men Research Group (2016) Actigraphy- and polysomnography-measured sleep disturbances, inflammation, and mortality among older men. Psychosom Med 78:686–696. https://doi.org/10.1097/PSY.0000000000000312
Sulli G, Manoogian ENC, Taub PR, Panda S (2018) Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci 39:812–827. https://doi.org/10.1016/j.tips.2018.07.003
Sulli G, Lam MTY, Panda S (2019) Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends Cancer 5:475–494. https://doi.org/10.1016/j.trecan.2019.07.002
Teixeira KRC, Dos Santos CP, De Medeiros LA, Mendes JA, Cunha TM, De Angelis K, Penha-Silva N, De Oliveira EP, Crispim CA (2019) Night workers have lower levels of antioxidant defenses and higher levels of oxidative stress damage when compared to day workers. Sci Rep 9:4455. https://doi.org/10.1038/s41598-019-40989-6
Thaiss CA, Levy M, Korem T, Dohnalova L, Shapiro H, Jaitin DA, David E, Winter DR, Gury-Benari M, Tatirovsky E, Tuganbaev T, Federici S, Zmora N, Zeevi D, Dori-Bachash M, Pevsner-Fischer M, Kartvelishvily E, Brandis A, Harmelin A, Shibolet O, Halpern Z, Honda K, Amit I, Segal E, Elinav E (2016) Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 167:1495–1510.e1412. https://doi.org/10.1016/j.cell.2016.11.003
Toth LA, Tolley EA, Krueger JM (1993) Sleep as a prognostic indicator during infectious disease in rabbits. Proc Soc Exp Biol Med 203:179–192. https://doi.org/10.3181/00379727-203-43590
Trivedi MS, Holger D, Bui AT, Craddock TJA, Tartar JL (2017) Short-term sleep deprivation leads to decreased systemic redox metabolites and altered epigenetic status. PLoS One 12:e0181978. https://doi.org/10.1371/journal.pone.0181978
Uemura R, Fujiwara Y, Iwakura N, Shiba M, Watanabe K, Kamata N, Yamagami H, Tanigawa T, Watanabe T, Tominaga K, Arakawa T (2016) Sleep disturbances in Japanese patients with inflammatory bowel disease and their impact on disease flare. Springerplus 5:1792. https://doi.org/10.1186/s40064-016-3408-6
Urade Y, Hayaishi O (2011) Prostaglandin D2 and sleep/wake regulation. Sleep Med Rev 15:411–418. https://doi.org/10.1016/j.smrv.2011.08.003
Van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, Harma M, Porkka-Heiskanen T, Alenius H (2009) Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One 4:e4589. https://doi.org/10.1371/journal.pone.0004589
Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP (2004) Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 89:2119–2126. https://doi.org/10.1210/jc.2003-031562
Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A (2009) Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 32:491–497. https://doi.org/10.1093/sleep/32.4.491
Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO (2013) Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev 17:241–254. https://doi.org/10.1016/j.smrv.2012.09.005
Villafuerte G, Miguel-Puga A, Rodriguez EM, Machado S, Manjarrez E, Arias-Carrion O (2015) Sleep deprivation and oxidative stress in animal models: a systematic review. Oxidative Med Cell Longev 2015:234952. https://doi.org/10.1155/2015/234952
Weljie AM, Meerlo P, Goel N, Sengupta A, Kayser MS, Abel T, Birnbaum MJ, Dinges DF, Sehgal A (2015) Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc Natl Acad Sci U S A 112:2569–2574. https://doi.org/10.1073/pnas.1417432112
Wheaton AG, Jones SE, Cooper AC, Croft JB (2018) Short sleep duration among middle school and high school students - United States, 2015. MMWR Morb Mortal Wkly Rep 67:85–90. https://doi.org/10.15585/mmwr.mm6703a1
Wirth MD, Andrew ME, Burchfiel CM, Burch JB, Fekedulegn D, Hartley TA, Charles LE, Violanti JM (2017) Association of shiftwork and immune cells among police officers from the Buffalo Cardio-Metabolic Occupational Police Stress study. Chronobiol Int 34:721–731. https://doi.org/10.1080/07420528.2017.1316732
Zhu X, Zhu J (2020) CD4 T helper cell subsets and related human immunological disorders. Int J Mol Sci 21(21):8011. https://doi.org/10.3390/ijms21218011
Zhuang X, Magri A, Hill M, Lai AG, Kumar A, Rambhatla SB, Donald CL, Lopez-Clavijo AF, Rudge S, Pinnick K, Chang WH, Wing PC, Brown R, Qin X, Simmonds P, Baumert TF, Ray D, Loudon A, Balfe P, Wakelam M, Butterworth S, Kohl A, Jopling CL, Zitzmann N, Mckeating JA (2019) The circadian clock components BMAL1 and REV-ERBalpha regulate flavivirus replication. Nat Commun 10:377. https://doi.org/10.1038/s41467-019-08299-7
Zielinski MR, Krueger JM (2011) Sleep and innate immunity. Front Biosci (Schol Ed) 3:632–642. https://doi.org/10.2741/s176
Zielinski MR, Mckenna JT, Mccarley RW (2016) Functions and mechanisms of sleep. AIMS Neurosci 3:67–104. https://doi.org/10.3934/Neuroscience.2016.1.67
Zomers ML, Hulsegge G, Van Oostrom SH, Proper KI, Verschuren WMM, Picavet HSJ (2017) Characterizing adult sleep behavior over 20 years-the population-based Doetinchem Cohort Study. Sleep 40(7):zsx085. https://doi.org/10.1093/sleep/zsx085
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Garbarino, S., Bragazzi, N.L., Scoditti, E. (2023). The Effect of Sleep Disruption and Circadian Misalignment on the Immune System. In: BaHammam, A., Pandi-Perumal, S.R., Jahrami, H. (eds) COVID-19 and Sleep: A Global Outlook. Progress in Sleep Research. Springer, Singapore. https://doi.org/10.1007/978-981-99-0240-8_1
Download citation
DOI: https://doi.org/10.1007/978-981-99-0240-8_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0239-2
Online ISBN: 978-981-99-0240-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)