Abstract
CO2 capture, utilization, and storage (CCUS) is a beneficial measure for controlling carbon emissions and reducing greenhouse gases. At present, most of the studies on CO2 storage describe the mechanism, calculation of storage volume, calculation of cap breakthrough pressure and CO2 migration law at the macroscopic level, but there are few studies on the CO2 transport law in the storage process at the microscopic pore scale. This paper establishes numerical formulas for CO2 migration and interface characterization at the pore scale based on numerical simulation methods. Using a two-dimensional model of porous media, a pressure-driven approach is used to simulate the microscopic migration characteristics of CO2 during the storage process of salt water layers. The CO2 migration law is explained, and the influence of pressure and wettability on the migration law is analyzed. Research has shown that during the injection process, CO2 will migrate along the mainstream toward the outlet. Meanwhile, due to the density difference between the two phases, CO2 will migrate upward until it reaches the top of the model. At the end of injection, residual water is mainly distributed at the edges and corners of the model. During the suction process, residual CO2 gas will be trapped at the top and upper right corner of the model under hydraulic and boundary effects. The increase in injection pressure will reduce the full displacement time and increase the residual gas burial rate. The weakening of wall hydrophilicity will shorten the displacement process time, increase the suction process time, and gradually reduce the maximum burial rate. This study characterizes the flow pattern of two phases during CO2 storage in the salt water layer from a microscopic perspective, which has reference significance for further understanding the CO2 storage mechanism in mines.
Copyright 2023, IFEDC Organizing Committee.
This paper was prepared for presentation at the 2023 International Field Exploration and Development Conference in Wuhan, China, 20–22 September 2023.
This paper was selected for presentation by the IFEDC Committee following review of information contained in an abstract submitted by the author(s). Contents of the paper, as presented, have not been reviewed by the IFEDC Technical Team and are subject to correction by the author(s). The material does not necessarily reflect any position of the IFEDC Technical Committee its members. Papers presented at the Conference are subject to publication review by Professional Team of IFEDC Technical Committee. Electronic reproduction, distribution, or storage of any part of this paper for commercial purposes without the written consent of IFEDC Organizing Committee is prohibited. Permission to reproduce in print is restricted to an abstract of not more than 300 words; illustrations may not be copied. The abstract must contain conspicuous acknowledgment of IFEDC. Contact email: paper@ifedc.org.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
With the acceleration of industrialization, the world is facing challenges in addressing climate change and high greenhouse gas emissions. The signing of the Paris Agreement on December 12, 2015, meant that the signatories reached a consensus to cut greenhouse gas emissions and achieve a temperature rise of no more than 2 ℃ [1]. Current measures to effectively reduce CO2 emissions include improving resource efficiency; using low-carbon or carbon-free energy; and adopting CO2 capture, utilization and storage (CCUS) technologies. CO2 capture, utilization, and storage (CCUS) is the process of capturing industrial emissions of CO2 for water, oil, gas, and mining applications while achieving carbon sequestration. CCUS technology is still considered the main way to reduce carbon emissions [2,3,4]. CO2 storage is mainly achieved by injecting CO2 into geological structures (such as deep saltwater layers, abandoned oil and gas reservoirs, and unexplainable coal seams) combined with cap rock traps. There are four main mechanisms of CO2 storage in the formation: structural storage, residual storage, dissolution storage, and mineral storage. After CO2 injection, it is prevented from being transported upward mainly by high capillary forces of the cap, partially dissolved in water by the solubility of CO2 in salt water, disconnected by capillary forces of the pore structure and retained in the pores, and stored in the formation by chemical reactions to generate new substances [5, 6]. Capillary forces are positively correlated with interfacial tension, which is the phase behavior of the fluid and may greatly affect the effectiveness of structural and residual storage. Salt water layer CO2 storage is a method of injecting CO2 into deep salt water layers and permanently storing it through the above mechanisms. It is also currently the most promising CO2 storage method [7, 8]. The study of the migration law of CO2 and salty water two-phase fluids in the pore space is important for understanding the effect of structural and residual storage.
At present, research on CO2 storage in salt water mainly relies on physical experimental methods and numerical simulation methods [9,10,11]. The research direction includes macroscopic mechanism description, storage capacity calculation, cap rock breakthrough pressure calculation, CO2 migration law, and other aspects. There is relatively little research on the migration law and mechanism at the pore scale. Sun et al. [12] established models of different river lithofacies combinations based on numerical simulation methods to study the impact of heterogeneous river structures on CO2 storage. The study noted that low permeability phases can hinder the vertical migration of CO2 plumes. Baban et al. [13] used in situ NMR T1-T2 2D images to visualize fluid configuration in pore networks. The study found that in CO2 wet samples, CO2 is mainly residual in mesopores and micropores, while in water wet rocks, CO2 is mainly residual in macrospores. Ren et al. [14] studied the migration law of CO2 injection into a heterogeneous model through numerical simulation, and the study showed that the migration of CO2 in a heterogeneous deep aquifer exhibits a significant fingering phenomenon. Afanasyev et al. [15] studied the injection of supercritical CO2 into an inclined aquifer and proposed a simple relation to estimate the maximum gas migration distance in the upward dip direction. The above research indicates that CO2 will migrate in the upward direction after being injected into the salt water layer, and due to its low viscosity, there is a fingering phenomenon, which is influenced by reservoir heterogeneity. The injection process is mainly influenced by residual gas burial and dissolution mechanisms. However, the aforementioned studies have all conducted simulation studies at the macro scale, and there is a lack of research on the CO2 migration patterns in saltwater layers at the pore scale. At the macroscopic scale, the migration process of CO2 can only reflect the flow direction of the CO2 front, and cannot characterize the migration and capture mechanism of CO2 in pores.
To address the above research questions, this paper establishes a numerical model for CO2 migration. Based on a two-dimensional model of porous media, a pressure-driven approach is used to simulate the microscopic migration characteristics of CO2 during the storage process of salt water layers at the pore scale. The CO2 migration law is explained, and the influence of pressure and wettability on the migration law is analyzed. This study is a guide to further understanding the migration law of CO2 in the pore space at the mine site and understanding the mechanism of structural storage and residual gas storage.
2 Numerical Simulation Methods
2.1 Numerical Model Establishment
CO2 forms a two-phase interface with saltwater after entering the pore space, and it is transported upward under the effect of gravitational divergence. To simulate the microscopic seepage law of CO2 in saltwater, the following assumptions need to be made:
-
(1) Considering only the migration law of CO2 in pores, without considering the chemical reactions between CO2, water, and rock, the rock does not undergo deformation during fluid seepage;
-
(2) CO2 and salt water are assumed to be Newtonian fluid and incompressible fluid;
-
(3) It is assumed that the values of interfacial tension and contact angle do not change during the two-phase displacement.
The continuity equation is used to control the mass balance of an incompressible fluid, as follows [16]:
where ρ is the fluid density, kgm−3; u is the fluid velocity, ms−1.
The Navier‒Stokes equation is used to control the momentum balance of the two-phase fluid, as follows:
where t equals the time, s; p is the pressure, Pa; I represents the identity matrix; μ denotes the fluid viscosity, Pas; Fst represents the surface tension acting at the interface between CO2 and salt water, Pam−1; and g is the gravity vector, ms−2.
The level set method is used to describe the fluid interface and fluid convection. The interface between CO2 and saltwater is determined by tracing the contour of the level set function ϕ = 0.5. ϕ is 0 in salt water and 1 in CO2. In the transition layer near the interface, the ϕ value transitions smoothly from 0 to 1. The interface moves at fluid velocity u. The equations controlling the transfer and reinitialization of ϕ are as follows [17]:
where ϕ is the level set function, dimensionless; γ is the reinitialization parameter, ms−1; and ε is the interface thickness control parameter, m.
The global density and dynamic viscosity are determined by the following equations:
where \(\rho_{{\text{W}}}\) is the salt-water density, kgm−3; \(\rho_{{{\text{CO}}_{2} }}\) is the CO2 density, kgm−3; \(\mu_{{\text{W}}}\) is the salt-water viscosity, Pas; and \(\mu_{{{\text{CO}}_{2} }}\) is the CO2 viscosity, Pas.
Based on the level set function, the surface tension is calculated by the following equation:
where σ is the surface tension coefficient, Nm−1; δ is the Dirac δ function, dimensionless, with a nonzero value only at the fluid interface; and κ is the curvature, dimensionless.
The controlling equation for the wetted wall conditions is as follows:
2.2 Two-Dimensional Model Establishment
The porous media model size is 9353 nm×8550 nm, the conventional media diameter is 800 nm, and 11 media are randomly selected to expand the diameter by 10% and marked in purple.
The spacing between the conventional medium is 100 nm, and the spacing between the conventional medium and the expanded medium is 60 nm. The lower left side of the model is the inlet, and the lower right side is the outlet, as shown in Fig. 1.
2.3 Material Parameters
The simulated environment temperature is 323.15 K, pressure is 19 MPa, salt water density is 1094.45 kgm−3, and viscosity is 0.7047 mPas. The density and viscosity parameters of CO2 at different pressure conditions are shown in Table 1.
2.4 Calculation Parameter Setting
The reference pressure level pref in the laminar physical field is 1.9×107 Pa, and the reference temperature Tref and the model input temperature T are 323.15 K. The inlet relative pressure p0 is 1×106 Pa, and the outlet relative pressure p0 is 0 Pa.
Reinitialize parameters in the level set physical field γ 1 ms−1, interface thickness control parameter ε set to the default value ls. ep_ default。
In the level set physical field, the reinitialization parameter γ is 1 ms−1, and the interface thickness control parameter ε is the default value ls-ep_default. In the initial value, the level set variable within the pore domain of the model is specified as fluid 1 (salt water), and the injected phase at the inlet is fluid 2 (CO2).
The boundary condition of the wetting wall is the default Navier slip, and the gas phase contact angle is 120°. The surface tension coefficient of the two phases is 35 mNm−1.
2.5 Grid Division Parameters
The mesh type is triangle, the number of mesh vertices is 3711, the number of triangles is 5154, the number of edge cells is 2464, the number of vertex cells is 489, the number of cells is 5154, the average cell mass is 0.7573, and the mesh area is 2.256×107 nm2.
3 Results and Discussion
3.1 Microscopic Migration Law of CO2 Storage in the Salt Water Layer
Storage of CO2 in the saltwater layer includes the process of displacement and suction. The displacement process is the first stage, in which CO2 displaces salt water. The inhalation process is the second stage, in which the salt water displaces the CO2. The displacement process ends when the gas drive water reaches 98% gas content at the outlet. The suction process continues the displacement process, and the water drive gas ends when the water cut at the outlet reaches 98%. The gas saturation of the model at the end of the displacement process is defined as the maximum burial rate, and the gas saturation of the model at the end of the suction process is defined as the residual gas burial rate.
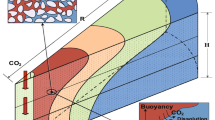
Figure 2 shows the two-phase migration diagram of the displacement process after CO2 injection. Pure red represents salt water, while pure blue represents CO2. When the color is between red and blue, it is the interface where the two phases transition, and the color code is the volume fraction of salt water. The figure shows that when CO2 is injected and pushed toward the right outlet, it will simultaneously migrate toward the top of the model under the effect of the two-phase density difference. After CO2 breaks through the mainstream line at the bottom, it will displace salt water diagonally upward, and the displacement will end at 0.00016 s. The residual water is mainly distributed in the blind end area in the upper left corner and the unaffected area in the upper right corner.
Figure 3 shows the two-phase migration diagram during the suction process of salt water, with the same color representation as Fig. 2. As seen from the figure, the salt water suction approximates a fan shape advancing forward and will displace the CO2 when it reaches the outlet and will combine with the boundary to close part of the CO2 when it reaches the top of the model. When the salt water converges with the residual water in the upper right corner of the model, it truncates the continuous gas phase and displaces the lower gas toward the outlet under the action of gravity and capillary force, and the upper gas eventually accumulates mostly in the upper right corner of the model under hydraulic and porous media trapping. The suction process ended at 0.00027 s.
3.2 Effect of Different Factors on Microscopic Migration Law
Effect of Injection Pressure on Microscopic Migration Law.
To study the effect of injection pressure on microscopic migration laws, injection pressures of 19.8, 20, 20.3, and 20.5 MPa are selected to study the seepage characteristics of the displacement and suction processes.
Figure 4 shows the gas saturation diagram of the entire process under different injection pressure conditions. The figure shows that the gas saturation in the first stage of the displacement process first rapidly increases, and then the increase amplitude slows down. After the second stage of the suction process begins, the gas saturation first rapidly decreases and then gradually flattens out in the region. An increase in injection pressure will reduce the entire displacement time and increase the residual gas burial rate. At injection pressures of 19.8, 20, 20.3, and 20.5 MPa, the maximum burial rates are 77.84%, 93.42%, 87.85%, and 89.77%, respectively, and the residual gas burial rates are 7.88%, 3.04%, 4.24%, and 4.29%, respectively. As the injection pressure increases, the maximum burial of CO2 at the end of displacement first increases, then decreases, and then increases in a fluctuating manner. When the pressure is low, increasing the pressure will increase the swept area of CO2; When the pressure is high, increasing the pressure will cause the formation of the mainstream channel earlier, exacerbate gas channeling, and reduce the affected area. As the injection pressure increases, the residual gas burial rate first decreases and then increases. When the suction pressure is low, increasing the pressure will increase the injection time and reduce the residual gas burial rate; When the pressure is high, increasing the pressure will shorten the time of the suction process, reduce the gas discharge volume, and increase the residual gas volume.
Effect of Wettability on Microscopic Migration Law.
To study the effect of wettability on microscopic migration laws, water phase contact angles of 45, 60, 75, and 90° are selected to study the seepage characteristics of displacement and suction processes.
Figure 5 shows the gas saturation diagram of the entire process under different water phase contact angles. It can be seen from the figure that as the water phase contact angle increases, the displacement process time decreases and the suction process time increases. An increase in the contact angle of the water phase will gradually reduce the maximum burial rate, and the residual gas burial will first increase and then decrease. The maximum burial rates are 91.51%, 87.85%, 85.3%, and 85.2% at water phase contact angles of 45, 60, 75, and 90°, respectively, and the residual gas burial rates are 3.15%, 4.24%, 4.31%, and 4.15%, respectively. An increase in the contact angle of the water phase will reduce the hydrophilicity of the wall, making it easier for CO2 to flow out to the outlet during the displacement process, gradually reducing the maximum burial rate. An increase in the contact angle of the water phase will reduce the capillary force, and the residual gas burial rate will increase at 45°. The increase in the water phase contact angle increases the suction process time, more gas is expelled, and the residual gas burial rate tends to decrease at 90°.
4 Conclusion
The following conclusions are obtained from the simulation of the displacement process and the suction process of CO2 storage in salt water:
-
(1) During the displacement process, when CO2 is injected and pushed toward the right outlet, it will simultaneously migrate toward the top of the model under the effect of the two-phase density difference. The residual water is mainly distributed in the blind end area in the upper left corner and the unaffected area in the upper right corner.
-
(2) During the suction process, the salty water is sucked in and pushed forward in an approximate fan shape, and when it reaches the top of the model, it will cut off the continuous phase of CO2 with the residual water and combine with the boundary to trap part of the CO2 so that the CO2 is trapped at the edge and angle.
-
(3) The increase in injection pressure will reduce the full process displacement time and increase the residual gas burial rate. The residual gas burial rate decreases and then increases with increasing injection pressure.
-
(4) When the contact angle of the water phase increases, the displacement process time is shortened, and the suction process time is increased. An increase in the contact angle of the water phase will gradually reduce the maximum burial rate, and the residual gas burial will first increase and then decrease.
References
Zhang, K., Lau, H., Chen, Z.: CO2 enhanced gas recovery and sequestration as CO2 hydrate in shallow gas fields in Alberta, Canada. J. Nat. Gas Sci. Eng. 103, 104654 (2022)
Cui, Y., Bai, J., Liao, S., et al.: Suggestions on the development of environmental monitoring technology of CO2 geological storage and leakage under the background of China’s “Double-Carbon” strategy. Atmosphere 14(1), 51 (2022)
Chen, Z., Zhou, Y., Li, H.: A review of phase behavior mechanisms of CO2 EOR and storage in subsurface formations. Ind. Eng. Chem. Res. 61(29), 10298–10318 (2022)
Zhang, K., Lau, H.: Regional opportunities for CO2 capture and storage in Southeast Asia. Int. J. Greenhouse Gas Control 116, 103628 (2022)
Chenrai, P., et al.: A preliminary assessment of geological CO2 storage in the Khorat Plateau, Thailand. Front. Energy Res. 10, 1164 (2022)
Ren, D., Wang, X., Kou, Z., et al.: Feasibility evaluation of CO2 EOR and storage in tight oil reservoirs: a demonstration project in the ordos basin. Fuel 331, 125652 (2023)
Peter, A., Yang, D., Eshiet, K., et al.: A review of the studies on CO2–brine–rock interaction in geological storage process. Geosciences 12(4), 168 (2022)
Jung, S.: Expansion of geological CO2 storage capacity in a closed aquifer by simultaneous brine production with CO2 injection. Sustainability 15(4), 3499 (2023)
Wang, G., et al.: Integrated numerical simulation of CO2 flooding、heat recovery and storage in high temperature reservoir. Pet. Sci. Technol. 41(12), 1250–1271 (2022)
Qi, S., et al.: Experimental investigation on the CO2 effective distance and CO2-EOR storage for tight oil reservoir. Energy Fuels 37(1), 339–349 (2022)
Li, Z., et al.: Investigation of CO2 storage and EOR of alternating N2 and CO2 injection using experiments and numerical simulation. Fuel 340, 127517 (2023)
Sun, X., et al.: Effects of fluvial sedimentary heterogeneity on CO2 geological storage: Integrating storage capacity, injectivity, distribution and CO2 phases. J. Hydrol. 617, 128936 (2023)
Baban, A., et al.: Effect of organic acids on CO2 trapping in carbonate geological formations: pore-scale observations using NMR. Energy Fuels 37(4), 2983–2992 (2023)
Ren, J., et al.: Characterization method and application of heterogeneous reservoir based on different data quantity. Lithosphere 2022, 8267559 (2022)
Afanasyev, A., Vedeneeva, E., Grechko, S.: Scaling analysis for a 3-D CO2 plume in a sloping aquifer at a late stage of injection. J. Nat. Gas Sci. Eng. 106, 104740 (2022)
Eiswirth, R., et al.: Experimental and numerical investigation of a free rising droplet. Chem. Eng. Process.-Process Intensificat. 50(7), 718–727 (2011)
Hysing, S.: Mixed element FEM level set method for numerical simulation of immiscible fluids. J. Comput. Phys. 231(6), 2449–2465 (2012)
Acknowledgments
The project is supported by National Natural Science Foundation (Number 51974343).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Li, J., Cui, Cz. (2024). Research on the Microscopic Migration Law of CO2 in Salt Water Storage Based on Pressure Driving. In: Lin, J. (eds) Proceedings of the International Field Exploration and Development Conference 2023. IFEDC 2023. Springer Series in Geomechanics and Geoengineering. Springer, Singapore. https://doi.org/10.1007/978-981-97-0268-8_42
Download citation
DOI: https://doi.org/10.1007/978-981-97-0268-8_42
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-97-0267-1
Online ISBN: 978-981-97-0268-8
eBook Packages: EngineeringEngineering (R0)