Abstract
All over the world, different types of influenza viruses are responsible for seasonal pandemics annually. The influenza virus is responsible for 0.3–0.5 million deaths every year. Influenza A, B, and C are the three main types of influenza virus. Influenza A virus is the most infectious one among different types as it has the ability to change genetic shift and transfer from animal to human. Various synthetic drugs are prescribed for the treatment of influenza. But these drugs have their own drawbacks like the production of resistance and side effects. Hence remedies for influenza A can be obtained from nature. Different studies are carried over different plant extracts in the discovery of effective anti-influenza treatments. Various phytochemicals are being screened for effective and safe anti-influenza treatment. This chapter includes an overview of different plants and phytochemicals screened for anti-influenza A activity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
18.1 Influenza A Virus Infections: Introduction and Epidemiology
Viral infections have prevailed throughout the world among humans as well as animal populations and will remain one of the foremost causes of mortality and morbidity. Some of the examples of these viruses are Human Immunodeficiency Virus (HIV), Influenza virus, Ebola virus, SARS, and Coronavirus. There are about 219 virus species that are capable to infect humans. In 1901 yellow fever virus was first to be discovered and three to four new species are still being discovered every year [55]. The influenza virus is one of the viruses which is accountable for more than three million new cases every year and 0.3–0.5 million deaths every year [13, 36]. The influenza virus causes human respiratory infection and has a high morbidity and mortality rate. Influenza viruses can be classified in subspecies like A, B, and C virus. At a times there can be several influenza viruses circulating in humans, which cause seasonal flu having mild symptoms.
Some of the biggest and most dangerous disease outbreaks of humankind are caused by the influenza virus. Spanish flu pandemic originated in 1918 and responsible for killing more than 60 million people all over the world; followed by more pandemics in 1957, 1968, 1977, and 2009 killing millions more. Still, in a year around 0.4 million people probably die because of recurring flu [1, 26, 48].
Influenza is defined as “a contagious respiratory illness caused by influenza viruses that infect the nose, throat, and lungs. It can cause mild to severe illness, and at times can lead to death” by the Centers for Disease Control and Prevention of the USA [18].
Sneezing or coughing by an infected person blows out fine droplets into the air. Inhalation of these droplets by a healthy person is enough to cause infection. The average incubation period is about 48 h. Initially, cells of the upper respiratory tract are infected followed by lower parts of the respiratory tract. Various symptoms observed in an infected person rages from fever, sore throat, cough, rhinitis, muscle aches, tiredness, headache, vomiting, and diarrhea [33].
18.2 Classification and Etiology of Influenza Virus
The influenza virus is from the Orthomyxoviridae family. This family contains viruses having segmented single sense single-strand RNA inside the envelope. Influenza A, Influenza B, and Influenza C are the types of influenza virus. Among these four only two are clinically relevant to humans [3]. World Health Organization (WHO) laid certain norms regarding the nomenclature of the influenza virus in 1980. These norms include factors such as [32] (Fig. 18.1);
-
Virus type (either A, B, C).
-
The country or geographic place where the virus is first secluded.
-
Strain.
-
Isolation year.
-
Proteinous antigenic structure present on the virus indicated by letter and number like H1 to H6 and N1 to N9.
Among these types, Influenza A is the most hazardous one and it can infect a variety of hosts like swine, horses, domestic as well as wild birds, fowl, and dogs [38]. Influenza A viruses contain multiple-segmented genomes, which indicates that the genome is separated in different sections which are joined together. These genome segments rearrange to produce a new combination, producing a new virus subtype [28]. Each year, seasonal outbreaks of influenza viruses occur because of different subtypes of influenza A viruses. Sometimes these subtypes can cause huge pandemic outbreaks like the 2009 H1N1 pandemic influenza, also called swine flu. Human, avian, and swine influenza viruses’ genomic parts are combined in this subtype [45]. The most challenging thing about the influenza virus is its ability to cause a genetic shift, which causes constant evolution and production of new subtypes (Table 18.1).
18.3 Epidemiology
Wild birds and domestic birds act as a reservoir of Influenza A virus in which all HA and NA types occur [52, 53]. Avian plague, also called bird flu occurs in chicken due to highly infectious subtypes H5 and H7. This virus can replicate very fast inside the bird body and may have a maximum death rate. Type A/H3N2 and A/H1N1 are observed to be cocirculated in humans since 1977. Viruses of subtype H1N2 were first observed in 1977. Then in 2001 and 2002 outbreak was observed in some countries [16]. In each winter periodic influenza epidemics can arise in both the southern and northern hemispheres. Due to Influenza, it is estimated that, per year, approximately 500,000 deaths occur worldwide [11] (Fig. 18.2).
General pattern of influenza annual season [44]
Slight is identified about influenza’s tropical epidemiology, but it is considered that influenza can arise around the year. Apart from seasonal dependency, infections can also occur around the year in temporarily limited areas. It is estimated that during the annual outbreaks of influenza around 10–15% of the population is affected. The majority of deaths associated with Influenza are over 60 years old [49, 60]. In the past century, three big pandemics strike the human race, in 1918 Spanish flu (H1N1) is responsible for nearly 40 million deaths, in 1957 Asian influenza (H2N2) caused the death of 1–2 million population and 1968 Hong Kong flu (H2N3) caused the death of 0.75–1 million population [11].
18.4 Treatment/Management of Influenza Virus Disease
The influenza virus is self-limiting and symptoms are mild in most individuals. If infections are mild, then there is no need for antiviral treatment. Antiviral treatment is necessary during outbreaks. Several medications belonging to the neuraminidase inhibitor family-like Oseltamivir, Zanamivir, and Peramivir are used in the treatment of Influenza A as well as B. Other medications like Amantadine and Rimantadine are effective versus type A, however, not against type B. Along with this vaccination is highly recommended before the seasonal outbreak [21, 23, 57]. These drugs have their own drawbacks like side effects, specific activity. Remedies over Influenza A can be obtained from nature. Nature is acting as a reservoir of medicines since human civilization. Various plants are found to be active against the Influenza A virus. Alternative therapy against Influenza A virus is necessary for several reasons like resistance to already present drugs, the emergence of new virus types, unpredictable availability of the vaccine, and the cost of available drugs.
18.5 Medicinal Plants Used in the Treatment of Influenza A Virus Infections
Plants across the world have been explored for Anti-Influenza activity.
-
(a)
Ribes nigrum L. [10, 25]: This plant is also recognized as black currant or cassis. It is a woody shrub from the family Grossulariaceae. Crude fruit and leave extract of this plant is found to be active against Influenza A as well as B. Crude fruit extract was rich in anthocyanins like delphinidin, peonidin, and cyanidin along with organic acids like ascorbic acid and citric acid. The concentration of plant fruit extract to inhibit plaque formation by 50% (Also termed as IC50) for Influenza virus A (IVA) was found to be 3.2 μg/mL. The dose of 10 μg/mL directly inactivates 99.9% IVA at a pH of 2.8 [25]. Plant extract is found to have broad antiviral activity towards IVA. One of the studies indicates that extract mainly acts by suppressing the late stage of growth of the virus in the cell [25] while another study indicates that the early stage of the infection process is blocked [10]. Experimental results indicate that plant constituents inhibit viral internalization but do not interfere with cellular activity [10]. The mechanism may involve direct antiviral effects of the extract by a combination of constituents of an extract with haemagglutinin on the viral envelope. In vivo studies also indicated antiviral activity of leave extract in mice [10].
-
(b)
Cistus incanus P. [9]: This plant is a hybrid between Cistus albidus and Cistus crispus. It is a shrub with pink to red flowers belonging to the family Cistaceae. CYSTUS052 is the extract obtained from this plant and high in polyphenolic content (more than 26%) and other constituents like gallocatechin, gallic acid, catechin, and epicatechin [9]. The Anti-Influenza effect of CYSTUS052 was studied using Madine Darby Canine Kidney (MDCK) cell line. These cell lines were infected with A/Puerto-Rico/8/34 (H1N1) (PR8), the extremely infective avian influenza virus (HPAIV), A/FPV/Bratislava/79 (H7N7) (FPV) along with a human isolate of the HPAIV of the H5N1 subtype (A/Thailand/1(KAN-1)/2004 (H5N1)). Dose-dependent reduction of progeny virus was showed by the extract. At a concentration of 50 μg/mL CYSTUS052 showed a maximum reduction in progeny virus. Cell morphology and viability was not affected by CYSTUS052 and does not undesirably affect cellular production and metabolism. The mechanism of action of extract indicates that it may hinder the virus itself and avoid infection by a reduction in virus uptake by the cell. Cells pretreated with CYSTUS052 showed the inability of the virus to bind with RBC which indicates that components of the extract are able to interact directly with viral Hemagglutinin and block binding of the virus to cellular receptors. Also, IVA does not show resistance easily to CYSTUS052.
-
(c)
Andrographis paniculate [4]: This plant belongs to the family Acanthaceae. Diterpenoid lactones such as andrographolide, dehydroandrographolide (DAP), and neoandrographolide are major components of the plant. DAP showed strong anti-influenza A virus action towards the A/chicken/Hubei/327/2004 (H5N1), A/duck/Hubei/XN/2007 (H5N1), A/PR/8/34 (H1N1), A/NanChang/08/2010 (H1N1), and A/HuNan/01/2014 (H3N2) in vitro. The mechanism of action of DAP was found to be inhibition of H5N1 replication by a reduction in the construction of viral nucleoprotein (NP) mRNA, NS1 proteins. DAP does not affect the absorption and release of the virus. DAP also efficiently controlled the nuclear transfer of viral ribonucleoprotein (vRNP) complexes which is significant for Anti-IVA activity. The selectivity index of DAP was found to be close to ribavirin in A549 cell line. Also, DAP showed a marked effect against the production of viral progeny.

-
(d)
Curcuma Longa [6, 8]: Curcuma longa is a traditional plant used for various purposes belongs to the family Zingiberaceae and found in south to southeast tropical Asia. Rhizomes of C. longa are used for the extraction of active components and to study antiviral activity. Curcumin rhizome contains diarylheptanoids (class of curcuminoids) like curcumin, demethoxycurcumin, and bisdemethoxycurcumin with different essential oils [14]. Anti-Influenza A study was carried out in the MDCK cell line against Human influenza virus PR8, A/Puerto-Rico/8/34 (H1N1), and avian influenza virus A/chicken/Taiwan/NCHU0507/99 (H6N1). Initially, the cell culture medium was provided with different levels of curcumin. The virus population was determined at 12, 18, 24, and 30 h post-infection. After treatment with curcumin, it was observed that the synthesis of the virus was drastically decreased in a dose-dependent manner. Various viral protein synthesis was affected like haemagglutinin (HA), neuraminidase (NA), and matrix protein. Curcumin showed an antiviral effect in the early stages of viral infection like virus attachment but not against penetration. This can be attributed to the blockade of HA activity by curcumin which was confirmed by HA inhibition assay. Loss of the HA activity suggested that curcumin intrudes the connection between the viral HA protein and its cellular receptor by already occupying the binding position on HA protein or by alteration of the virus envelope.

-
(e)
Ginkgo biloba [14]: This tree is also called as maidenhair and native to china belonging to the family Ginkgoaceae. Extracts from leaves of G. biloba contain flavonoids, namely kaempferol, quercetin, isorhamnetin along with terpene lactones such as bilobalide, ginkgolide A, B, C, and J [30]. The anti-Influenza activity of plant leaf extract was studied by using MDCK cells. Leaf extract was found to be nontoxic to MDCK cells. The extract showed no effect on the multiplication of cells as indicated by plaque assay, however, when MDCK cells were first exposed to leaf extract, anti-influenza activity was markedly enhanced as dose is increased. Plant leaf extract at 5 μg/mL completely inhibited plaque formation. This suggests that plant extract inhibits the virus in the early stages before entering the virus in the cytoplasm of the cell. The mechanism of action of plant extract is based on the inhibition of HA on virus-cell and prevents virus adsorption on the surface of the cell. Leaf extract inhibited different types of influenza viruses like influenza A/PR/8/34 (H1N1), A/Udorn/72 (H3N2), and B/Lee/40.
-
(f)
Psidium guajava Linn [46].: Psidium guajava Linn belongs to the family Myrtaceae with different chemical constituents like tannins, alkaloids, saponins, triterpenoids, glycosides, flavonoids, and phenolic compounds. Different polyphenols like catechin, myricetins, quercetin, gallic and ellagic acids are present in leaf extract [35]. Extract of leaves of P. guajava showed 111 mg of tannin per 100 mL. It was observed that the tea leaf extract of the plant does not show any cytotoxic effect on AX4 cells. Leaf extract showed an inhibitory effect on the growth of different species of resistance seasonal viruses with IC50 values of 0.58 ± 0.14% versus A/Yamaguchi/20/06 and 0.23 ± 0.05% versus A/Kitakyushu/10/06. The mechanism of action of leaf extract involves inhibition of hemagglutinin and Neuraminidase of the Influenza virus at a concentration of 0.1%. This prevents the entry of the viral particle. This overall concludes that leaf extract (tea) has potent anti-influenza activity.
-
(g)
Echinacea purpurea [37]: It is a North American species also called as purple coneflower belongs to the daisy family or Asteraceae family. A standardized extract of a plant called Echinaforce® was tested for Anti-Influenza A virus action. Echinaforce consists of an extract in ethanol of herb and root (95:5) of plant E. purpurea. The extract contains most of the caffeic acid derivatives and alkyl amides and traces of polysaccharide [42]. Human H1N1-type IV, extremely infectious avian IV (HPAIV) of the H5- and H7-types and swine-origin IV (S-OIV, H1N1), were all deactivated in MDCK cell culture assays by the Echinaforce extract at recommended doses for oral ingestion. A thorough study indicated that with the H5N1 HPAIV strain, before infection, straight interaction between Echinaforce and virus was compulsory for utmost suppression in virus replication. The extract hinders with the viral entry into cells as indicated by Hemagglutination assay that the extract suppressed the receptor-binding action of the virus. Echinaforce did not produce any type of resistance as compared to Tamiflu which produced resistance in viruses.
-
(h)
Caesalpinia sappan L. [31]: C. sappan is a plant belonging to the Leguminosae family, also known as Brazil or Sappan wood having distribution in Asia. The heartwood of this plant has been used traditionally for various medicinal purposes. Chemical constituents present sappan wood includes various phenolic compounds like xanthone, coumarin, chalcones, flavones, homoisoflavonoids, protosappanin A, 3-deoxysappanchalcone, sappanchalcone, rhamnetin, brazilein, and brazilin. The anti-influenza activity of these constituents was studied with help of the cytopathic effect (CPE) reduction method in vitro on the A/Guangdong/243/72 (H3N2) virus strain with the help of using MDCK cell line. Antiviral actions of brazilein, brazilin, and protosappanin A were <50% at their maximal non-cytotoxic concentrations (MNCC) as indicated by results of the CPE assay, while rhamnetin showed anti-influenza activity lesser than that of ribavirin and oseltamivir acid. 3-deoxysappanchalcone showed substantial in vitro anti-influenza virus activity. IC50 value was approximately eight times lesser than ribavirin and almost 15 times more than oseltamivir acid. Sappanchalcone showed a potency similar to that of 3-deoxysappanchalcone. Oxysappanchalcone and sappanchalcone displayed the maximum anti-influenza virus (H3N2) activity with IC50 values of 1.06 and 2.06 μg/mL, correspondingly.
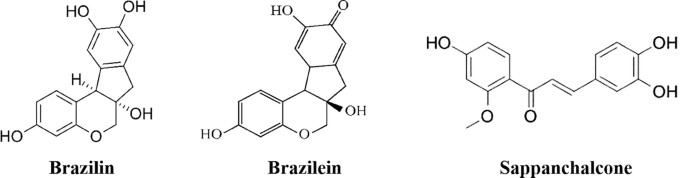
-
(i)
Glycyrrhiza uralensis [39, 51]: Glycyrrhiza uralensis is a member of the Leguminosae family commonly called as licorice has been used for various medicinal purposes traditionally like treatment of fever, liver diseases, constipation, ulcer, etc. The major constituents present in the root are flavonoids, pentacyclic triterpene saponins like liquiritin, isoliquiritin, glycyrrhizin, and glycyrrhizic acid. Among 18 isolated polyphenols from methanol extract of roots of the plant shown neuraminidase inhibitory activity. 18 polyphenols are from different groups like chalcones, flavonoids, coumarins, and phenylbenzofuran. Among these 18 polyphenols isoliquiritigenin (IC50 = 9.0 lM) and glycyrol (IC50 = 3.1 lM) had strong inhibitory activity. From the structure-activity relationship, it became clear that the furan ring present in polyphenols is essential for the activity of neuraminidase inhibition. Also, this activity is increased by the apioside group on the chalcone and flavone backbone. Also, in another study, glycyrrhizin present in licorice root protected mice exposed to a lethal amount of influenza virus through stimulation of IFN-Ƴ production by T cells.

-
(j)
Sambucus nigra L. [24]: Elderberry has been used traditionally for the treatment of influenza and colds. S. nigra belongs to the family Caprifoliaceae. Elderberry is known to have a high content of polysaccharides and phenolic compounds like phenolic acid, flavonoids, proanthocyanidins, and catechins [56]. The anti-influenza activity of these phenolic compounds is suggested due to their direct action on IVA. The study reported IC50 value of elderberry concentrated juice is 720 ± 79 μg/mL and the selectivity index is 36 ± 6.7. The anti-IVA mechanism of action of elderberry juice can be through inhibition of haemagglutination and viral proliferation. The constituent from elderberry juice said to stop the adhesion of the virus-cell to the host cell receptors. In addition to this, it also stimulates the production of cytokines and monocytes.

-
(k)
Ma-Huang-Tang [54]: Also known as ephedra in common language belongs to the family Ephedraceae. Ma-Huang-Tang (MHT) is used for various purposes like bronchitis, asthma, and influenza. Various chemical constituents present in MHT include L-methylephedrin (LMEP), L-ephedrine (LEP), and D-pseudo-ephedrine (DPEP). These chemicals were found to be safe in cytotoxic studied on MDCK cells. These constituents are said to hinder the proliferation of influenza A virus in vitro. These ingredients significantly inhibited different gene signaling pathways related to mRNAs like TLR3, TLR4, and TLR7. Hence three ephedra alkaloids were found to be effective against virus in vitro. Animal research indicated that LEP and DPEP significantly decreased lung index, lung injury, virus load in the lung, level of IL-1β, and stopped viral mRNA expression and protein expression. These all findings suggest that MHT alkaloids can be used for effective management of the Influenza A virus.
-
(l)
Syzygium nervosum [19]: This plant is also known as Cleistocalyx operculatus native to Asia belonging to the family Myrtaceae. During the study neuraminidase, inhibitory activity was shown by ethanolic extract of leaves. Chemical constituents of leaves include acetophenones, flavanones, and others. Isolated components showed maximum enzymatic prohibition on several neuraminidases from diverse influenza viruses, like H1N1, H9N2, and oseltamivir-resistant novel H1N1 with H274Y mutation expressed in HEK293 cells. IC50 values of these constituents’ ranges from 5.07 ± 0.94 μM to 9.34 ± 2.52 μM.
-
(m)
Pinus densiflora [20]: Plant is usually recognized as Korean red pine and from the family Pinaceae. The plant is extensively spread to East Asia, Korea, China, and Japan. Traditionally P. densiflora is used for the treatment of stroke, fatigue, depression, anxiety, and cancer. Chemical components present in the leaves of the plant include essential oil (α-pinene, β-pinene, camphene, limonene), bornyl acetate, borneol, benzoic acid, cinnamic acid, flavonoids, diterpenoids, and stilbenoid. Cytotoxic and cytopathic studies of plant leave extract were performed on DCK cells. Extracted components were studied for Anti-influenza activity by CPE inhibition and NA inhibition assay method. The mechanism of these components includes a decrease in the synthesis of HA and NA depending upon the dose, which was supported by immunofluorescence assay. In the viral infected cell, green fluorescence was observed of tagged NA while fluorescence was not found in the treated cell. Flavonoids exerted anti-influenza activity by direct NA inhibition while diterpenoids affect the gene expressions of various proteins which are essential for viral replication.
-
(n)
Mosla scabra [58]: M. scabra is a tomentose plant of the family Labiatae native to southeast China. It is used for antiviral, antipyretic effects for lung disease and demonstrated to be useful in cold, fever, inflammation, and bronchitis. Crude drug extract primarily contains flavonoids, such as apingenin, 5-hydroxy-6,7-dimethoxyflavone, 5,7-dihydroxy-4-methoxyflavone, and acacetin. In this study antiviral effect of M. scabra herbal extract is studied against the influenza virus A/PR/8/34 virus (H1N1 subtype). After administration of the extract at a concentration of 0.3–30.0 mg/kg in allantoic fluid of egg showed a survival rate of more than 80%. This suggested no toxic effect at the therapeutic dose. The IC50 value of extract was determined to be 0.15 μg/mL. Flavonoids present in the extract are mainly responsible for the antiviral activity which can affect the membrane synthesis and budding of viral particles by the prohibition of IVA sialidase.
-
(o)
Alpinia katsumadai [17, 27]: A. katsumadai is a traditional Chinese medicine belonging to the Zingiberaceae family used as anti-emetic and stomachic. Major constituents present in the plant include diarylheptanoids, monoterpenes, flavonoids, sesquiterpenes, and chalcones. A study was performed to find out the anti-influenza outcome of two plant seed extract and five fractions on virus strains like A/PR/8/34 (H1N1) and A/Chicken/Korea/MS96/96 (H9N2) in MDCK cells. One of the extracts demonstrated dose-dependent antiviral activity against A/PR/8/34 (H1N1) at a dose of 12.5 μg/mL. From quantitative real-time PCR, the extract showed activity by the mechanism of obstruction of viral attachment and virus replication after entry.
18.6 Phytochemicals Used in Influenza A Virus Infections
-
(a)
Polyphenols:
Polyphenols show a wide range of antiviral activity through various mechanisms like the prohibition of NA activity, inhibition of viral protein or mRNA synthesis, or inhibition of membrane fusion. Polyphenols show activity in the early stages of viral infection. Polyphenols extracted from fruits of Chaenomeles sinensis showed activity against influenza A by inhibition of HA activity as well as by suppression of NS2 protein synthesis [40]. Polyphenols from leaves extract of Folium isatidis cause a reduction in the pulmonary index and reduced mortality rate in mice [22]. Polyphenols from plant extract Reynoutria elliptica showed high inhibitory activity for NA [29]. Extract of plant Geranium sanguineum is rich in polyphenolic complexes showed strong anti-influenza activity in vitro as well as in vivo in mice [15]. Cistus incanus extract having polyphenolic content showed strong antiviral activity in MDCK and A549 cell cultures infested with avian and human influenza strains [9]. Flavonoids are a group of polyphenols that have shown antiviral activity against the influenza virus. Houttuynia cordata contains flavonoid quercetin 3-rhamnoside which is reported to have an inhibitory result on the replication of Influenza A virus [7]. Juice of the root of plant Agrimonia pilosa has been used for the treatment of cough and colds traditionally, later the juice was found to contain different flavonoids like catechin, hyperoside, quercitrin, quercetin, and rutin and are useful for the treatment of influenza A virus [43].
-
(b)
Alkaloids:
Alkaloids are present abundantly in different medicinal plants. Alkaloids possess a variety of pharmacological activities. The isoquinoline alkaloid thalimonine which is isolated from the Thalictrum simplex has exhibited antiviral activity against influenza strain H7N7 and H7N1 in cell cultures. Thalimonine distinctly stopped the influenza virus replication in vitro by decreasing the activity of viral glycoproteins HA and NA on the surface of infected cells [41]. Extract of plant Commelina communis contains alkaloids which shown antiviral activity against the influenza virus H1N1 in vitro as well as in vivo [2]. Total alkaloids like β-carbolines: harmine, harmalol, harmaline, and harman and quinazoline derivatives: vasicine and vasicinone present in the extract of Peganum harmala seeds exhibited in vitro anti-influenza activity [34]. Isoquinoline alkaloid berberine derived from plant Hydrastis canadensis strong inhibitory effect on the growth of H1N1 influenza A strains PR/8/34. Berberine acts post-transitionally and stops virus protein maturation and hence stop viral growth [5]. Hirsutine is a type of corynanthe indole alkaloid derived from the plant Uncaria rhynchophylla. Hursutine showed a potent anti-influenza activity [47].
-
(c)
Aromatic organic compounds
Roots of plant Cynanchum stauntonii, belonging to the family Apocynaceae contain different volatile oils like decadienal, methypentanol, furanone, and dihydro-5-pentyl which showed antiviral action against the influenza virus in vivo and stopped deaths because of the virus in a dose-dependent manner [59]. Ferula assafoetida contains anti-influenza A components sesquiterpene coumarins and diterpenes. These components showed greater activity against Influenza A virus as compared to amantadine [50]. Glycyrrhizin is triterpenoid saponin present in roots of plant licorice. This triterpenoid saponin is found to have a protective action on the cells against influenza virus A, H3N2. Treatment with glycyrrhizin showed a strong decrease in the number of infested human lung cells [51].
-
(d)
Proteins/sugar derivatives
Sugar and sugar analogs have been tested for anti-influenza activity. These sugars act by disruption of glycosylation which is required for the synthesis of glycoprotein. These sugars inhibit various enzymes required for the synthesis of sugar chains inside the virus. Benzyl group attached sugar molecule has an antiviral effect against influenza A virus [50]. Codiaeum variegatum leaves contain bioactive cynoglucoside which is reported to have anti-influenza A activity [12] (Fig. 18.3).
Plant extracts and phytochemicals show anti-influenza activity by various mechanisms such as inhibition of acidification of viral membrane, by inhibition of viral attachment to host cell, by inhibition of entry of viral cell in a host cell, by inhibition of NA and HA present on viral cell, and by inhibition of viral replication through suppression of viral mRNA.
18.7 Conclusion and Future Prospectus
Influenza virus A is responsible for the pandemic in human history and it holds the dangerous potential to cause major pandemics and deaths in the future also. Influenza virus causes an acute respiratory infection which contributes to noteworthy morbidity and mortality in seasonal epidemics and pandemic eruptions throughout the world. Many new antiviral agents have been synthesized like oseltamivir and ribavirin for the cure of influenza infection. But these synthetic drugs have their own drawbacks like the development of resistance and side effects. Vaccination is another remedy for the stoppage of influenza virus contagion but the delay in the development of vaccines and changes in the genetic structure of the virus is a major problem associated with vaccination. Hence an effective and universal treatment against influenza virus species is still necessary. Such treatment against influenza A virus can be obtained from plants as plants hold a history of providing remedies against various diseases. Various studies are carried out concerning the anti-influenza virus potential of a number of plants and found to be effective. Physiological activities of various phytochemicals like polyphenols, flavonoids, alkaloids extracted from plant sources have shown promising activity against different strains of Influenza A virus in vitro and in vivo. In the future, a combination of modern high-throughput screening knowledge along with traditional knowledge of plants can lead to the discovery of a good anti-influenza candidate. This can prevent annual deaths occurring during pandemics throughout the world.
References
Beveridge WIB (1977) Influenza: the last great plague. An unfinished story of discovery. Heinemann Educational Books, London
Bing FH, Liu J, Li Z, Zhang GB, Liao YF, Li J, Dong CY (2009) Anti-influenza-virus activity of total alkaloids from Commelina communis L. Arch Virol 154(11):1837–1840. https://doi.org/10.1007/s00705-009-0503-9
Blümel J, Burger R, Drosten C, Gröner A, Gürtler L, Heiden M, Hildebrandt M, Jansen B, Klamm H, Montag-Lessing T, Offergeld R, Pauli G, Seitz R, Schlenkrich U, Schottstedt V, Willkommen H, Von König CHW, Schweiger B (2009) Influenza virus. Transfus Med Hemother 36(1):32–39. https://doi.org/10.1159/000197314
Cai W, Li Y, Chen S, Wang M, Zhang A, Zhou H, Chen H, Jin M (2015) 14-Deoxy-11,12-dehydroandrographolide exerts anti-influenza A virus activity and inhibits replication of H5N1 virus by restraining nuclear export of viral ribonucleoprotein complexes. Antivir Res 118(March):82–92. https://doi.org/10.1016/j.antiviral.2015.03.008
Cecil CE, Davis JM, Cech NB, Laster SM (2011) Inhibition of H1N1 influenza A virus growth and induction of inflammatory mediators by the isoquinoline alkaloid berberine and extracts of goldenseal (Hydrastis canadensis). Int Immunopharmacol 11(11):1706–1714. https://doi.org/10.1016/j.intimp.2011.06.002
Chen DY, Shien JH, Tiley L, Chiou SS, Wang SY, Chang TJ, Lee YJ, Chan KW, Hsu WL (2010) Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem 119(4):1346–1351. https://doi.org/10.1016/j.foodchem.2009.09.011
Choi HJ, Song JH, Park KS, Kwon DH (2009) Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur J Pharm Sci 37(3–4):329–333. https://doi.org/10.1016/j.ejps.2009.03.002
Dao TT, Nguyen PH, Won HK, Kim EH, Park J, Won BY, Oh WK (2012) Curcuminoids from Curcuma longa and their inhibitory activities on influenza A neuraminidases. Food Chem 134(1):21–28. https://doi.org/10.1016/j.foodchem.2012.02.015
Ehrhardt C, Hrincius ER, Korte V, Mazur I, Droebner K, Poetter A, Dreschers S, Schmolke M, Planz O, Ludwig S (2007) A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antivir Res 76(1):38–47. https://doi.org/10.1016/j.antiviral.2007.05.002
Ehrhardt C, Dudek SE, Holzberg M, Urban S, Hrincius ER, Haasbach E, Seyer R, Lapuse J, Planz O, Ludwig S (2013) A plant extract of Ribes nigrum folium possesses anti-influenza virus activity in vitro and in vivo by preventing virus entry to host cells. PLoS One 8(5):e63657. https://doi.org/10.1371/journal.pone.0063657
Fauci AS (2006) Seasonal and pandemic influenza preparedness: science and countermeasures. J Infect Dis 194(SUPPL. 2):73–76. https://doi.org/10.1086/507550
Forero JE, Avila L, Taborda N, Tabares P, López A, Torres F, Quiñones W, Bucio MA, Mora-Pérez Y, Rugeles MT, Joseph-Nathan P, Echeverri F (2008) In vitro anti-influenza screening of several Euphorbiaceae species: structure of a bioactive Cyanoglucoside from Codiaeum variegatum. Phytochemistry 69(16):2815–2819. https://doi.org/10.1016/j.phytochem.2008.09.003
Gasparini R, Amicizia D, Lai PL, Panatto D (2012) Clinical and socioeconomic impact of seasonal and pandemic influenza in adults and the elderly. Human Vaccines Immunother 8(1):21–28. https://doi.org/10.4161/hv.8.1.17622
Goel A, Kunnumakkara AB, Aggarwal BB (2007) Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol 75(4):787–809
Govorkova EA, Webster RG (2010) Combination chemotherapy for influenza. Viruses 2(8):1510–1529. https://doi.org/10.3390/v2081510
Gregory V, Bennett M, Orkhan MH, Al Hajjar S, Varsano N, Mendelson E, Lin YP (2002) Emergence of influenza A H1N2 reassortant viruses in the human population during 2001. Virology 300(1):1–7. https://doi.org/10.1006/viro.2002.1513
Grienke U, Schmidtke M, Kirchmair J, Pfarr K, Wutzler P, Dürrwald R, Wolber G, Liedl KR, Stuppner H, Rollinger JM (2010) Antiviral potential and molecular insight into neuraminidase inhibiting diarylheptanoids from Alpinia katsumadai. J Med Chem 53(2):778–786. https://doi.org/10.1021/jm901440f
Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB (2019) Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - the United States, 2019-20 influenza season. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep 68(3):1–21. https://doi.org/10.15585/mmwr.rr6803a1
Ha TKQ, Dao TT, Nguyen NH, Kim J, Kim E, Cho TO, Oh WK (2016) Antiviral phenolics from the leaves of Cleistocalyx operculatus. Fitoterapia 110:135–141. https://doi.org/10.1016/j.fitote.2016.03.006
Ha TKQ, Lee BW, Nguyen NH, Cho HM, Venkatesan T, Doan TP, Kim E, Oh WK (2020) Antiviral activities of compounds isolated from Pinus densiflora (pine tree) against the influenza A virus. Biomol Ther 10(5):711. https://doi.org/10.3390/biom10050711
Haber P, Moro PL, Ng C, Dores GM, Lewis P, Cano M (2019) Post-licensure surveillance of trivalent adjuvanted influenza vaccine (aIIV3; Fluad), vaccine adverse event reporting system (VAERS), United States, July 2016–June 2018. Vaccine 37(11):1516–1520. https://doi.org/10.1016/j.vaccine.2019.01.052
Jiang LL, Lu YL, Jin JH, Dong LL, Xu FL, Chen SS, Wang ZY, Liang G, Shan XO (2015) n-Butanol extract from folium isatidis inhibits lipopolysaccharide-induced inflammatory cytokine production in macrophages and protects mice against lipopolysaccharide-induced endotoxic shock. Drug Design. Dev Ther 9:5601–5609. https://doi.org/10.2147/DDDT.S89924
Kenmoe S, Tcharnenwa C, Monamele GC, Kengne CN, Ripa MN, Whitaker B, Alroy KA, Balajee SA, Njouom R (2019) Comparison of FTD® respiratory pathogens 33 and a singleplex CDC assay for the detection of respiratory viruses: A study from Cameroon. Diagn Microbiol Infect Dis 94(3):236–242. https://doi.org/10.1016/j.diagmicrobio.2019.01.007
Kinoshita E, Hayashi K, Katayama H, Hayashi T, Obata A (2012) Anti-influenza virus effects of elderberry juice and its fractions. Biosci Biotechnol Biochem 76(9):1633–1638. https://doi.org/10.1271/bbb.120112
Knox YM, Suzutani T, Yosida I, Azuma M (2003) Anti-influenza virus activity of crude extract of Ribes nigrum L. Phytother Res 17(2):120–122. https://doi.org/10.1002/ptr.1053
Krau SD (2013) The impact of heat on morbidity and mortality. Crit Care Nurs Clin North Am 25(2):243–250. https://doi.org/10.1016/j.ccell.2013.02.009
Kwon HJ, Kim HH, Yoon SY, Ryu YB, Chang JS, Cho KO, Rho MC, Park SJ, Lee WS (2010) In vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virol J 7:1–9. https://doi.org/10.1186/1743-422X-7-307
Lamb R, Krug R (2001) Orthomyxoviridae: the viruses and their replication. In: Field’s virology. Lippincott-Raven Press, Philadelphia, pp 1487–1532
Lee CH, Kim SI, Lee KB, Yoo YC, Ryu SY, Song KS (2003) Neuraminidase inhibitors from Reynoutria elliptica. Arch Pharm Res 26(5):367–374. https://doi.org/10.1007/BF02976693
Lichtblau D, Berger JM, Nakanishi K (2002) Efficient extraction of ginkgolides and bilobalide from Ginkgo biloba leaves. J Nat Prod 65(10):1501–1504. https://doi.org/10.1021/np0201974
Liu AL, Shu SH, Qin HL, Lee SMY, Wang YT, Du GH (2009) In vitro anti-influenza viral activities of constituents from Caesalpinia sappan. Planta Med 75(4):337–339. https://doi.org/10.1055/s-0028-1112208
Memorandum WHO (1980) A revision of the system of nomenclature for influenza viruses: a WHO memorandum. Bull World Health Organ 58(4):585–591
Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J (2000) Clinical signs and symptoms predicting influenza infection. Arch Intern Med 160(21):3243–3247. https://doi.org/10.1001/archinte.160.21.3243
Moradi MT, Karimi A, Rafieian-Kopaei M, Fotouhi F (2017) In vitro antiviral effects of Peganum harmala seed extract and its total alkaloids against influenza virus. Microb Pathog 110:42–49. https://doi.org/10.1016/j.micpath.2017.06.014
Morais-Braga MFB, Carneiro JNP, Machado AJT, Sales DL, dos Santos ATL, Boligon AA, Athayde ML, Menezes IRA, Souza DSL, Costa JGM, Coutinho HDM (2017) Phenolic composition and medicinal usage of Psidium guajava Linn.: antifungal activity or inhibition of virulence? Saudi J Biol Sci 24(2):302–313. https://doi.org/10.1016/j.sjbs.2015.09.028
Nováková L, Pavlík J, Chrenková L, Martinec O, Červený L (2018) Current antiviral drugs and their analysis in biological materials – part II: antivirals against hepatitis and HIV viruses. J Pharm Biomed Anal 147:378–399. https://doi.org/10.1016/j.jpba.2017.07.003
Pleschka S, Stein M, Schoop R, Hudson JB (2009) Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol J 6:1–9. https://doi.org/10.1186/1743-422X-6-197
Rumschlag-Booms E, Rong L (2013) Influenza A virus entry: implications in virulence and future therapeutics. Adv Virol 2013:121924. https://doi.org/10.1155/2013/121924
Ryu YB, Kim JH, Park SJ, Chang JS, Rho MC, Bae KH, Park KH, Lee WS (2010) Inhibition of neuraminidase activity by polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorg Med Chem Lett 20(3):971–974. https://doi.org/10.1016/j.bmcl.2009.12.106
Sawai R, Kuroda K, Shibata T, Gomyou R, Osawa K, Shimizu K (2008) Anti-influenza virus activity of Chaenomeles sinensis. J Ethnopharmacol 118(1):108–112. https://doi.org/10.1016/j.jep.2008.03.013
Serkedjieva J, Velcheva M (2003) In vitro anti-influenza virus activity of the pavine alkaloid (−)-thalimonine isolated from Thalictrum simplex L. Antivir Chem Chemother 14(2):75–80. https://doi.org/10.1177/095632020301400202
Sharma M, Anderson SA, Schoop R, Hudson JB (2009) Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antivir Res 83(2):165–170. https://doi.org/10.1016/j.antiviral.2009.04.009
Shin WJ, Lee KH, Park MH, Seong BL (2010) Broad-spectrum antiviral effect of Agrimonia pilosa extract on influenza viruses. Microbiol Immunol 54(1):11–19. https://doi.org/10.1111/j.1348-0421.2009.00173.x
Simonsen L (1999) The global impact of influenza on morbidity and mortality. Vaccine 17(Suppl 1):S3–S10
Smith GJD, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ambaut A (2009) Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza an epidemic. Nature 459(7250):1122–1125. https://doi.org/10.1038/nature08182
Sriwilaijaroen N, Fukumoto S, Kumagai K, Hiramatsu H, Odagiri T (2012) Antiviral effects of Psidium guajava Linn. (guava) tea on the growth of clinical isolated H1N1 viruses: its role in viral hemagglutination and neuraminidase inhibition. Antivir Res 94(2):139–146. https://doi.org/10.1016/j.antiviral.2012.02.013
Takayama H, Iimura Y, Kitajima M, Aimi N, Konno K, Inoue H, Fujiwara M, Mizuta T, Yokota T, Shigeta S, Tokuhisa K, Hanasaki Y, Katsuura K (1997) Discovery of anti-influenza A virus activity of a corynanthe-type indole alkaloid, hirsutine, in vitro and the structure-activity relationship of natural and synthetic analogs. Bioorg Med Chem Lett 7(24):3145–3148. https://doi.org/10.1016/S0960-894X(97)10154-8
Taubenberger JK, Morens DM (2006) 1918 influenza: the mother of all pandemics. Emerg Infect Dis 12(1):15–22. https://doi.org/10.3201/eid1209.05-0979
Thompson WW, Shay DK, Weintraub E, Cox N, Anderson LJ, Fukuda K (2003) Mortality associated with influenza and respiratory syncytial virus in the United States. J Am Med Assoc 289(2):179–186. https://doi.org/10.1001/jama.289.2.179
Tyrrell BE, Sayce AC, Warfield KL, Miller JL, Zitzmann N (2017) Iminosugars: promising therapeutics for influenza infection. Crit Rev Microbiol 43(5):521–545. https://doi.org/10.1080/1040841X.2016.1242868
Utsunomiya T, Kobayashi M, Pollard RB, Suzuki F (1997) Glycyrrhizin, an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus. Antimicrob Agents Chemother 41(3):551–556. https://doi.org/10.1128/aac.41.3.551
Webster RG (1998) Influenza: An emerging disease. Emerg Infect Dis 4(3):436–441. https://doi.org/10.3201/eid0403.980325
Webster RG (2002) The importance of animal influenza for human disease. Vaccine 20(SUPPL. 2):16–20. https://doi.org/10.1016/S0264-410X(02)00123-8
Wei W, Du H, Shao C, Zhou H, Lu Y, Yu L, Wan H, He Y (2019) Screening of antiviral components of Ma Huang Tang and investigation on the ephedra alkaloids efficacy on influenza virus type A. Front Pharmacol 10(September):1–22. https://doi.org/10.3389/fphar.2019.00961
Woolhouse M, Scott F, Hudson Z, Howey R, Chase-Topping M (2012) Human viruses: discovery and emergence. Philos Trans R Soc B Biol Sci 367(1604):2864–2871. https://doi.org/10.1098/rstb.2011.0354
Wu X, Gu L, Prior RL, McKay S (2004) Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J Agric Food Chem 52(26):7846–7856. https://doi.org/10.1021/jf0486850
Yang L, Chan KP, Wong CM, Chiu SSS, Magalhaes RJS, Thach TQ, Peiris JSM, Clements ACA, Hu W (2019) Comparison of influenza disease burden in older populations of Hong Kong and Brisbane: the impact of influenza and pneumococcal vaccination. BMC Infect Dis 19(1):1–8. https://doi.org/10.1186/s12879-019-3735-7
Yu C, Yan Y, Wu X, Zhang B, Wang W, Wu Q (2010) Anti-influenza virus effects of the aqueous extract from Mosla scabra. J Ethnopharmacol 127:280–285. https://doi.org/10.1016/j.jep.2009.11.008
Zai-Chang Y, Bo-Chu W, Xiao-Sheng Y, Qiang W (2005) Chemical composition of the volatile oil from Cynanchum stauntonii and its activities of anti-influenza virus. Colloids Surf B: Biointerfaces 43(3–4):198–202. https://doi.org/10.1016/j.colsurfb.2005.05.003
Zucs P, Buchholz U, Haas W, Uphoff H (2005) Influenza associated excess mortality in Germany, 1985 - 2001. Emerg Themes Epidemiol 2(August 1992):1–9. https://doi.org/10.1186/1742-7622-2-6
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jadhav, S.P. et al. (2021). Medicinal Plants Used in the Treatment of Influenza A Virus Infections. In: Dua, K., Nammi, S., Chang, D., Chellappan, D.K., Gupta, G., Collet, T. (eds) Medicinal Plants for Lung Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-33-6850-7_18
Download citation
DOI: https://doi.org/10.1007/978-981-33-6850-7_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6849-1
Online ISBN: 978-981-33-6850-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)












