Abstract
Antibiotic-associated diarrhea (AAD) is an adverse impact of antibiotic therapy which alters the metabolic function of the host’s gut microbiota causing diarrhea (osmotic or infectious type), and significant infection by Clostridium difficile. C. difficile is an opportunistic pathogen which thrives in the gut when the colonization resistance conferred by gut microbiota is compromised, leading to pathogenesis ranging from mild diarrhea to serious conditions like pseudomembranous colitis, which could be fatal. The impact of antibiotic therapy on the composition of gut microbiota has been observed to extend beyond the clinically targeted bacterial species since removing populations of certain species of gut bacteria opens up niches for other microorganisms to colonize, subsequently resulting in gut dysbiosis. Multiple meta-analyses have elucidated the cumulative beneficial impact of orally administered probiotics for the effective prophylaxis of CDI. Probiotics also benefit the host by immunomodulation which is critical in management of inflammation in the gut. It has been hypothesized that AAD is caused by dysbiosis which probiotics beneficially modulate and assist in restoring the homeostasis of the unbalanced indigenous gut microbiota. Probiotics have been proven to reduce the risk of AAD by 51% without the risk of any adverse effects. The present chapter provides a comprehensive outlook on the current trends in the management of AAD by the intervention of various probiotic microorganisms, highlighting their merits and demerits to facilitate effective management of the dysbiosis of the gut and its allied metabolic and immunological ramifications that are critical factors contributing to the onset and development of AAD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Overview
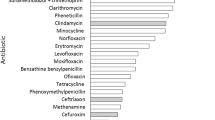
Antibiotic-associated diarrhea (AAD) is the most important variant of nosocomial diarrhea which is characterized by unexplained diarrhea observed in patients undergoing antibiotic therapy, subsequent to the exclusion of other possible aetiologies (Barlett 2002). Globally, the frequency of AAD varies widely ranging from 4.3 to 80% [median value: 22%], with the mean age of patients with AAD being in the range of 18–48 months. It has been observed that in children, the primary risk factors for AAD comprise the age of the child and also the specific type of antibiotic used (McFarland et al. 2016). The onset of AAD might take place within a range of a few hours or up to 8 weeks subsequent to the administration of antibiotics (Cote and Buchman 2006), and it contributes significantly to the aggravated suffering as well as contributing to additional costs and duration of hospitalization (McFarland 1998). The severity of AAD ranges from symptoms of mild diarrhea to progressing towards serious conditions such as fulminant pseudomembranous colitis (McFarland 2006). Children develop the symptoms much more rapidly compared with the adults. Remarkably, the recovery period in children is faster, and they also display lesser complications, and the overall duration of the disease is less, in comparison with adult patients. The specific mechanism of AAD is yet to be completely elucidated. Research reports have revealed that the putative mechanism of action for the onset of AAD is the direct impact of the administration of antibiotics on the gastrointestinal mucosa, which ultimately alters the composition of the gut microbiota and the proliferation of pathogenic microorganisms. Gastrointestinal disturbances are well documented as an adverse effect of administration of broad-spectrum antibiotics [e.g. Vancomycin, Amikacin, Gentamicin, and third generation β-lactams]. Clostridium difficile is the most prominent causative agent of AAD (Fox et al. 2015). However, other pathogenic microbes such as Staphylococcus spp., Candida spp., members of Enterobacteriaceae (e.g. Klebsiella), may also contribute to AAD.
The incidence of CDAD (C. difficile associated diarrhea) cases has recorded exponential growth during the past decade, with a larger proportion of cases being reported as “community-acquired” type. Critical illness among hospitalized patients has been strongly associated with gut microbial dysbiosis, potentially aggravating the susceptibility to developing infection, ultimately leading to organ failure. The risk of health complications linked to gut dysbiosis due to antibiotic therapy (viz. diarrhea) highlights the importance of rational administration of antibiotics (Kołodziej and Szajewska 2017).
The classic pathogenesis of CDAD is attributed to administration of antibiotics and the subsequent dysbiosis of the microbiota of the GI tract, ultimately causing colonization of the gut mucosa by C. difficile. Notably, the administration of anti-acidity therapy [viz. proton pump inhibitors (PPI) and H2-receptor antagonists] has been linked to aggravated risk towards CDAD. One possible hypothesis is that the elevated pH in the gastric contents could enhance the survival of C. difficile. In addition, the administration of PPI, irrespective of the duration of treatment, has been shown to alter the gene expression in human colon cell lines, leading to decreased integrity of the colonocytes (Biswal 2014). Therefore, a possible association has been proposed regarding PPIs and nonsteroidal anti-inflammatory drugs (NSAIDs), viz. diclofenac, and community-acquired CDAD in patients without a recent history of hospitalization or exposure to antimicrobial compounds has been hypothesized (Permpalung et al. 2016).
Imperatively, there is an ambiguity regarding the definition of dysbiosis, which is a widely used term in the field of probiotic research. Dysbiosis is primarily used to indicate an “abnormal” microbiome, that includes not only changes in microbial diversity, but also used to indicate the reduction of keystone taxa (chief microbial species that influence the composition and function of the microbial community), colonization by pathogens, and alterations in metabolic capacity, which contributes a functional aspect to the structural elements. Dysbiosis is hard to be specifically defined because the opposite term, eubiosis, which indicates a healthy microbiome under homeostasis, is a highly heterogeneous state. Interestingly, reports on the profiling of microbiomes among individuals have revealed unique inter-personal taxonomical patterns, which is even evident among twins. However, the existence of a stable core of functions and thus genes (the microbiome core) in an individual has been substantiated. The exact definition of what constitutes a “healthy” gut microbiota and the mechanism by which various strains of gut microbiota affect their host is still being developed. It has been very difficult to reach a consensus due to the variations observed in various studies, such as different strains, doses, and also the duration of the treatment.
6.2 Probiotics: Relevance in Management of AAD
Ever since the dawn of the concept of probiotics as proposed by Elie Metchnikoff (1907), the hypothesis that consumption of specific microorganisms could impart health benefits has fascinated the scientific community. The term “probiotics” itself first appeared in 1974 and has conceptually evolved with newer insights due to focussed research efforts to the current accepted definition, which is “live microorganisms that confer a health benefit when consumed in adequate amounts,” as per the mandate provided by the World Health Organization in 2002 (Hill et al. 2014). In the current scenario, probiotics constitute a multi-billion dollar industry projected to reach USD 65.87 billion by the year 2024 and are among the most popular food supplements consumed across the globe (Zion Market Research 2018). In order to impart health benefits, popular foods such as fermented dairy products (yogurt, cheese, etc.), ice cream, infant formulas, and nutrition bars been supplemented with probiotics. Probiotics have also been commercialized as lyophilized pills (Hoffmann et al. 2014).
Across the decades, the consumption of probiotics has received steady support by medical practitioners, specifically gastroenterologists. It is notable that despite the soaring popularity of probiotics among the medical fraternity as well as the consumers, insights from research efforts to elucidate the efficacy of probiotics for the management of various health conditions, can at times, point to ambiguous inferences. This is primarily due to the inter-species and even intra-strain variation that is observed among the probiotics that have been used in the clinical studies. Additionally, it is imperative to note that a probiotic strain would vary in its efficacy to impart specific health benefits among different population sub-groups (ethnic, dietary habits, age groups, etc.). Thus, microbiome and probiotic research has received an increased focus in recent years. Oral administration of probiotics provides a viable supply of beneficial bacteria for the management of gut health, enhancing nutrient absorption, and immunomodulation. Multiple in vivo studies as well as clinical trials have provided valuable insights regarding the efficacy of probiotics to effectively modulate the immune system. An open-labelled clinical study involved 18 healthy subjects, who were administered probiotic Bacillus subtilis HU58 capsules (2 × 109 CFU/Cap) once a day orally for 8 weeks. At the end of test period, it was observed that administration of probiotics was effective in reducing the levels of cytokines such as IL-6 and TNF-α by 45% and 55%, respectively.
Bacillus subtilis produces a variety of antimicrobial molecules such as bacteriocins, subtilin, and subtilosin and have also been extensively studied at genetic and physiological levels. Reports have demonstrated the efficacy of B. subtilis in the effective management of traveller’s diarrhea caused by Citrobacter rodentium in murine model [14]. B. subtilis has been demonstrated to improve clinical, microbiological, and immunomodulatory efficacy in case of acute infectious diarrhea in young children. In poultry, B. subtilis has been shown be effective against pathogenic infections caused by Salmonella enterica, Clostridium perfringens, and E. coli. In vitro studies have also shown its potential efficacy against Helicobacter pylori. The HU58 strain of Bacillus subtilis, isolated from healthy human volunteers has been studied extensively and has been proven to be more stable in the highly acidic gastric environment. In addition, it can grow and sporulate in the anaerobic GI tract with high sporulation efficiency and enhance gut colonization through the development of biofilms and production of surfactant molecules that enhance adhesion to the gut mucosa (Mehta et al. 2020).
It is imperative to note that, since 2001, multiple B. subtilis strains, which were already commercially exploited as probiotics and also in other industries, were taxonomically reclassified as B. clausii (Khatri et al. 2019). Currently, B. clausii in commercially marketed as a probiotic formulation (Trade name: Enterogermina®), which is composed of four strains of B. clausii, based on their resistance to various antibiotics O/C (chloramphenicol), N/R (novobiocin/rifampicin), SIN (neomycin/streptomycin), and T (tetracycline). These four strains have been established as being derived from B. subtilis ATCC 9799, which originally was resistant to penicillin (Mazza et al. 1992). The intrinsic resistant to antibiotics is advantageous to restoring healthy gut function, especially in cases where probiotics are administered in combination with antibiotics (Varankovich et al. 2015). B. clausii O/C strain is proven to inhibit the cytotoxic effects induced by toxins secreted by C. difficile and B. cereus. The specific mechanism of action of strains of B. clausii is yet to be fully understood, but research insights have provided information about the secretion of specific proteins which play a vital role in colonization of human GIT, immunomodulation, etc. (Lopetuso et al. 2016; Pradhan et al. 2016). The sporeforming probiotic strains have important advantage over non-sporeforming probiotic strains in terms of commercial application due to enhanced resistance to heat, desiccation, and exposure to chemicals, which are encountered during product development.
The genetic difference among probiotic bacterial strains is significant, and leads to the inability to extrapolate the function of one strain to another. This unique quality among bacterial strains, termed as strain specificity, and is well established with research studies, such as carried out by Douillard et al. (2013), where 100 strains of probiotic Lactobacillus rhamnosus, originally isolated from human as well as dairy sources, were proven to possess remarkable different probiotic attributes (viz. tolerance to bile acids, carbohydrate metabolism, and the ability to produce mucus-binding pili). The results provide more clarity to the concept that taxonomic profiling is not sufficient as a measure of functional efficacy of the strain, and the need for probiotic strains to be selected based on the evidence available to prove their functional attributes as opposed to total reliance on the popularity of the name of their particular species.
The symbiotic component of the intricate relationship between humans and their microbiome has garnered increased research focus during the past decade. The major component of the microbial population is located in the gastrointestinal system, and is known as gut microbiome, which includes their collective genomes (Marchesi and Ravel 2015). The results from the extensive research highlight the pivotal role played by the gut microbiome to contribute to the homeostasis of the human host, in health and also in the onset and development of disease. Beneficial attributes of gut microbiota have been classically linked with improved gastrointestinal function and healthy immune response (Thaiss et al. 2016; Azcarate-Peril et al. 2017; Fetissov 2017; van de Wouw et al. 2017). Also, important adverse effects were reported that range across a variety of health conditions, with the primary cause pointing to an altering of the gut microbiota composition and the subsequent interaction of metabolites that are the by-product of bacterial metabolism of the dietary components (Miele et al. 2015; Budden et al. 2017; Yu and Schwabe 2017; Schirmer et al. 2018). Consequently, there has been a heightened interest about probiotic products for their ability to promote wholesome health and also for effective therapeutic modulators of the onset and development of a variety of diseases.
Despite compelling evidence for the administration of probiotics for their efficacy in the effective management of a variety of health issues ranging from improved digestion to neurological health, relatively few probiotic strains are available in the market and probiotics are yet to become part of routine clinical practice (Van den Nieuwboer et al. 2016). In addition, commercially available probiotic strains are claimed to provide a wide variety of health benefits spanning multiple health conditions without being substantiated using standardized study models (Day et al. 2019).
In order to counter it, regulatory agencies of various countries have developed their own framework to protect the interests of the consumers with respect to consumption of probiotic foods. The EFSA (European Food Safety Authority) has provided three critical regulatory parameters that need compliance prior to making a health claim. As a consequence, many health benefits claimed for probiotics have been rejected, and have resulted in the restriction of the use of the term “probiotic,” as popular consumer sentiment implies that the very use of the term confers a health benefit. The regulatory issues discussed above mandate necessitate the demonstrated evidence of the health benefit (i.e., proof that the biomarker under investigation contributes to the claimed health benefit) and the capability to apply the same to the general population. Health claims about probiotics need to have specific details and general, vague claims involving phrases like “strengthens the immune system” are considered insufficient. Therefore, research studies have been focussed towards establishing the impact of probiotics on key biomarkers. It is important to note that, an established set of biomarkers with wide approval has not been finalized, which are well-correlated and validated by clinical inferences. As per requirements of EFSA, any health claims need to be clearly demonstrated using a minimum of two randomized, placebo-controlled clinical studies, with a scientifically validated mechanism of action including cause and effects of the probiotic efficacy (Rijkers et al. 2011).
In this perspective, poor study design, paucity of consistent research funding, and a lacunae in the understanding of the key mechanisms of action has resulted in “pilotitis”—where small studies without a specific focus have been unable to generate a robust evidence base, which is essential to obtain regulatory approval (Van den Nieuwboer et al. 2016). For example, in a recent systematic review with special emphasis on randomized controlled trials (RCTs), which deals with evaluating the efficacy of probiotic strains in the effective management of irritable bowel syndrome (IBS). Among the various RCTs (35 nos.) that were considered for the review, only three RCTs had a minimum of 100 participants, approximately and about 75% were pilot studies (McKenzie et al. 2016). Therefore, the need of the hour is well designed Phase III clinical trials to prove the efficacy of probiotics which is essential to provide the evidence base in order to generate confidence among health practitioners regarding the efficacy of probiotics.
6.3 Diversity of Gut Microbiota and Dysbiosis
The Human Microbiome Project (HMP1), a pioneering project, involving 250 healthy volunteers, under the aegis of National Institute of Health, concluded that the human microbiome is composed of 3500–35,000 species, which was based on operational taxonomic units (Morgan et al. 2013). The total count of bacteria harbored by the human body has been estimated to be hundred trillion (1013), a value close to the total count of cells present in the human body (Sender et al. 2016). Thus, the human body accommodates a rich, highly diverse and unique microbial population. It is pertinent to note that there is significant variation in the diversity of microflora present in different body sites, e.g. the oral cavity and the colon have the greatest number of bacterial cells, with the least quantity of microflora observed in the vaginal region (Morgan et al. 2013). Interestingly, superior diversity of microflora in the gastrointestinal tract has been reported in individuals from primal hunter and gatherer populations, viz. Hadza from Tanzania (Turroni et al. 2016) and the Yanomami in Brazil (Clemente et al. 2015), which are potential indicators of the composition of our ancestral gut microbiota. These isolated and often dwindling ethnic communities display evidence for robust health contrary to the urbanized Westernized populations which are characterized by colonization by far less diverse microbiota (Moeller 2017). Low diversity of the intestinal microbiome is linked with a range of serious health issues, viz. Irritable Bowel Syndrome (IBS), Crohn’s disease, colorectal cancer and also in obesity (Mosca et al. 2016). However, high diversity of microflora does not directly correlate with a healthy microbiome across various sites, as there are notable exceptions such as the vaginal region, where it is characteristic of bacterial vaginosis (Charbonneau et al. 2016), which is the most widespread cause of vaginitis.
Diversity is a key component in definitions of dysbiosis, a popular term referring to an“abnormal” microbiome. Microbiome profiling studies have recognized the presence of unique interpersonal patterns, which is evident even among the twins. Despite the unique features, the presence of an enduring core of specific functions and their corresponding genes has been established. The essential components of a ‘healthy’ gut microbiota and the mechanism of action regarding the impact of various species of gut microbiota and their interaction with the host is yet to be fully elucidated. This is further proving to be difficult due to the presence of variations among the strain, dose, and duration of treatment among different studies which hinder direct comparison and reaching a general consensus (Permpalung et al. 2016).
It has been well recognized that critical illness, particularly in hospitalized condition, has been linked with imbalance of gut microbiota, potentially causing increased susceptibility to contracting infection, which can lead to and ultimately, organ failure. During this scenario, the risk of developing antibiotic-related gut microbial dysbiosis, which also includes diarrhea, underscores the importance of rational administration of antibiotics and other antimicrobial agents (Appel-da-Silva et al. 2017).
6.4 Clostridium difficile Infection: Mechanism of Action
The key pathological conditions associated with Clostridium difficile infection (CDI) are primarily correlated with the activity of two toxins, TcdA and TcdB (Chandrasekaran and Lacy 2017). In C. difficile chromosome, the genes responsible for coding both TcdA and TcdB, in addition to the three additional toxins (Tcd C, TcdE, and TcdF) are found within the PaLoc (pathogenicity Locus), which has a size of 19.6 Kb (kilobase) (Kuehne et al. 2010). In terms of molecular nature, TcdA and TcdB share 49% homology in their amino acid composition (Navaneethan et al. 2010). The C-terminal domain of both the toxins binds to the intestinal epithelial cells, whereas the N-terminal domain is responsible for cytotoxic activity. The entry of the two toxins in to the epithelial cells is facilitated by the transmembrane domain. The gene products for the accessory toxins, TcdD upregulates transcription of the toxin, whereas TcdC, is a repressor for the genes coding for the toxin (Waligora et al. 2001). The gene product of TcdE is responsible for cell wall lysis and facilitating the release of TcdA and TcdB in to the intestinal lumen (Dicks et al. 2019).
Despite the fact that the respective roles played by these toxins in the pathogenesis have not been fully understood, various studies have provided insight regarding the synergistic action of the toxins. Other reports highlight the fact that many clinical isolates are unable to produce TcdA, supporting suggestions that TcdA is not essential for pathogenesis (Farrow et al. 2020). These clinical observations also concur with the in vivo studies, viz. infection studies using mouse and hamster models employing mutant strains for isogenic toxin have indicated that TcdB in itself is sufficient for the development of significant steps of the pathogenesis, including the key symptoms associated with the disease. In contrast, studies involving strains having the capability for the production of only TcdA (TcdA+TcdB-) have been either non-pathogenic or highly attenuated. The results of the studies provide insight into the significant development disease in case of the strains that are capable of producing TcdB (TcdA-TcdB+) and thereby clearly point to the central role played by TcdB in pathogenesis (Carter et al. 2015; Lyras et al. 2009). This is further substantiated by studies in gnotobiotic piglet model where an antibody against TcdB conferred protection against CDI (gastrointestinal and systemic types (Steele et al. 2013). This has also been further confirmed in clinical trials, where treatment with antibodies against TcdB was able to effectively reduce the incidence of relapse of CDI (Wilcox et al. 2017).
In terms of the enzymatic nature of the toxins, both the toxins (TcdA and TcdB) are homologous glucosyltransferases which can effectively alter and ultimately inactivate Rho family GTPases that are present in the host cell. The activity of these glucosyltransferases have been directly associated with disruption of the actin components of the cytoskeleton and eventually causes cytopathic changes such as disruption of the tight junctions. The disruption of tight junctions triggers the immune system and triggers the synthesis of pro-inflammatory cytokines (Pruitt and Lacy 2012). Additionally, along with these cytopathic changes, the toxin (TcdB) is an effective cytotoxin that eventually leads to necrosis of the affected host cells and tissue (Chumbler et al. 2012). The glucosyltransferase activity of the toxin is not linked with the necrotic response. The necrotic effects of the toxin (TcdB) are due to the induction of the assembly of relevant NOX complex (NADPH oxidase) and the concurrent secretion of ROS (reactive oxygen species) (Farrow et al. 2013), which at elevated levels can lead to damage of mitochondria, peroxidation of lipids, and oxidation of proteins. Cell and tissue-based studies have demonstrated that the necrotic effects is unique to TcdB and occurs at certain concentrations (100 pM) (Chumbler et al. 2016). As the development of necrotic lesions are characteristic feature of colitis caused by C. difficile, and levels of the toxins are directly linked with the severity of the disease. It has been hypothesized that these mechanisms of action of the toxin (TcdB) produced by C. difficile are responsible for the onset and development of the characteristic symptoms of the disease (Farrow et al. 2020).
Antibiotic-associated diarrhea (AAD) is characterized by the imbalance in the homeostasis of the gut microbiota, and particularly, decreased concentrations of secondary metabolites [viz. short chain fatty acid (SCFA)] in the intestinal lumen, and the concurrent increase in the presence of carbohydrates in the intestinal lumen and biliary acids in the colon, along with impaired water absorption, and eventually causing diarrhea. As discussed previously, probiotics present a viable solution in the management of AAD in numerous clinical trials by modulating the gut microbiota, influencing the metabolism of bile salts and nutrients, inducing the action of epithelial solute transporters, augmenting the function of the intestinal barrier, and imparting positive effects leading to the effective modulation of the immune system.
In a four-week clinical study involving patients treated for CDI, oral administration of probiotics [consortium of probiotic strains (4 nos.) of Lactobacillus and Bifidobacterium in combination with antibiotics showed significant improvement with respect to reducing the duration of C. difficile diarrhea (Barker et al. 2017). Microbiological analysis of the fecal contents revealed that patients who were administered with probiotics contained reduced levels of Verrucomicrobiaceae in the gut, in comparison with placebo-treated groups (De Wolfe et al. 2018). Although other variations with observed with administration of the probiotic consortium, in microbiological composition of the gut, the reduced levels of the presence of members of Verrucomicrobiaceae were also consistent with the direct association of the susceptibility of the family of the patient to developing infection by C. difficile (Bassis et al. 2014). In case of a clinical study carried out subsequent to antibiotic therapy for the treatment of infection by H. pylori, it was observed that in case of patients taking a probiotic consortium (multiple strains of Bacillus subtilis and Enterococcus faecium) there was a reduction in the changes to fecal microbial composition due to antibiotic therapy changes in comparison with placebo-treated group (Wu et al. 2019). It is notable that other clinical studies have reported similar beneficial effects of probiotic administration during the antibiotic treatment period for management of H. pylori infection (Mekonnen et al. 2020).
The major global medical regulatory bodies, viz. the European Food Safety Authority (Rijkers et al. 2011) and the Food and Drug Administration (USA) (Saldanha 2008) have not yet provided approval for the use of any probiotic formulation for administration as a therapeutic agent. Subsequently, the probiotics are being marketed as dietary supplements with importance focussed mainly on the safety, ability to survive the passage through the gastrointestinal tract, no adverse impact on the organoleptic attributes of the carrier product, instead of their unequivocal health-promoting effects. Therefore, the current scenario demands improved scientific proof of the key health benefits and adverse effects of the administration of probiotics (Sniffen et al. 2018).
The health benefits attributed to the consumption of probiotics in humans has received extensive research by scientific community as well as the food and pharmaceutical industry for decades. As a result, a myriad of health claims has been suggested encompassing both therapeutic and prophylactic health approaches, such as management of acute, ADI & CDI, IBS, IBD (Inflammatory bowel disease) and decreased risk of sepsis (late-onset type) and necrotizing colitis in neonatal subjects (Suez et al. 2019). Other health claims include, management of Helicobacter pylori infection, respiratory tract infections, neurological health (alleviation of depression, mood swings, etc.), and decreased cardiovascular risk factors linked with the cardio-metabolic syndrome. It should be noted that in spite of data from many clinical studies positively affirming the health benefits mentioned above, comply with sound methodology and scientific validation (Gao et al. 2010; Panigrahi et al. 2017) for the major portion of these health conditions, there are also clinical studies of equal scientific validation that have featured contrasting negative results, thereby contributing to the development of rather ambiguous and inconclusive scientific ambience.
C. difficile flourishes in the gut mucosa in situations where colonization resistance due to microbial homeostasis is adversely affected (viz. due to antibiotic treatment among inpatients).
Through the outcome of focussed research in recent years, it is evident from several meta-analyses that administration of probiotics provides an augmentative positive effects for orally administered probiotics, both prophylactically and regarding the effective management of associated morbidity (Goldenberg et al. 2017), especially when the probiotics are administered close to antibiotic exposure (Shen et al. 2017). Additionally, subsequent follow-up of the meta-analysis involving 8672 cases involving various probiotic strains, age groups, dosage and duration of administration provided insights in to the ability to impart reasonable prophylactic effect in patients undergoing antibiotic therapy. In contrast, the results showed the presence of considerable heterogeneity between the clinical trials. Furthermore, post hoc analysis of the results failed to reveal significant beneficial impact of administration of probiotics on the protection of CDAD during clinical studies with subjects having low to medium risk of CDAD (Goldenberg et al. 2017). Similar conclusions have also been revealed with respect to other probiotic strains. For example, one meta-analysis showed that among different probiotic strains, only Saccharomyces boulardii was efficient against C. difficile. In contrast, other meta-analysis specially related to S. boulardii concluded that the strain was beneficial at decreasing the susceptibility to develop CDAD in children but not among adult groups (Szajewska et al. 2016).
Detailed analysis of the individual clinical studies that formed the basis for these meta-analyses has elucidated the incidence of C. difficile infection during the duration of the trial was non-existent or observed to be predominantly decrease in studies among the placebo and treatment groups. Also, majority of the studies that were considered for the meta-analyses did not provide clear evidence regarding the efficacy of various strains against infection by C. difficile or CDAD. This conclusion may be accounted for by the insufficient power of these clinical studies to provide clear evidence regarding the decreased incidence of infection caused by C. difficile. In contrast, among a couple of random controlled trials (RCTs) where special emphasis was directed on studying population groups with a high observed rates of C. difficile infection. The RCTs also featured one of the largest clinical studies of probiotic administration for the particular indication, and the results revealed no significant difference among the placebo and treatment groups (Allen et al. 2013; Szajewska et al. 2016). Therefore, the clinical evidence for prophylactic effects of the administration of probiotics to tackle CDAD is primarily supported by only minority of studies (Suez et al. 2019).
Research findings have also provided insight regarding the role of probiotic strains on the expression levels of specific immune-related genes, activity of key inflammatory pathway, and levels of important immune markers, which include modulation of NF-κB (intestinal epithelial cell), mitogen-activated protein kinase (MAPK), Akt [i.e. phosphoinositide 3-kinase PI3K], peroxisome proliferator-activated receptor-γ, CRP, interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, IL-1β and interferon-γ (IFN-γ). Probiotics have been purported to act through multiple mechanisms that are primarily driven by interaction with the right target cells (Thomas and Versalovic 2010). Interestingly, certain studies involving viable and dead probiotic bacteria have shown a variance about their effect on the expression of genes, providing insight regarding the importance of the surface of the bacterial cell as well as the secreted molecules on the intestinal transcriptome (Van Baarlen et al. 2009). In addition, studies have reported on the ability for immunomodulation in the host by probiotics which is evidenced through the TLR2 (toll-like receptor 2)-dependent stimulation of the secretion of TNF-α secretion in conjunction with lipoteichoic acid (LTA), observed in the case of Lactobacillus sp. (Matsuguchi et al. 2003), contact-dependent secretion of IL-10 mediated by B. longum (Medina et al. 2007), stimulation of TNF-α response by sortase-dependent pili in Bifidobacterium (Turroni et al. 2013), cell surface exopolysaccharide (sEPS) of B. longum 36,524 able to modulate secretion of pro-inflammatory cytokines and response of T-helper cell 17 (TH17) in colon as well as in the lungs (Schiavi et al. 2016). Also, in in vitro models, immunostimulatory cell surface appendages (SpaCBA), in Lactobacillus rhamnosus GG have been shown to be capable of mediating adhesion to mucus present in the human intestine as well as TLR2-dependant immunomodulation of the secretion of cytokines (TNF-α, IL-6, IL-10, and IL-12) (von Ossowski et al. 2013). Using in vivo models, LGG has also been shown to be effective in elevated production of ROS (reactive oxygen species) and the subsequent inhibition of that is induced by TNF-α via adhesion to the intestinal epithelium mediated by SpaC appendages (Ardita et al. 2014) (Table 6.1).
In vivo studies (mice model) have also shown that cell wall peptidoglycan from L. salivarius Ls33 is effective in prevention of colitis (chemically induced) through the association with nucleotide-binding oligomerization domain-containing protein 2(NOD2)–IL-10-dependent mechanisms (Fernandez et al. 2011). However, the same beneficial effects were not reported in L. acidophilus NCFM. Similar studies have provided insight regarding the ability of L. acidophilus L-92 to bind to M cells (microfold cells), and providing immunomodulation via its surface layer protein A (SlpA); other examples include the ability of B. infantis 35,624 to induce TLR2-depended T-regulatory cells in clinical studies, and also inducing the secretion of IgA by B. animalis lactisBb-12 (Yanagihara et al. 2017).
It is noteworthy that in majority of the studies quoted above, there is a need for the occurrence of physical contact(proximity) between probiotic bacteria and the host cells for the potential induction of both pro- and anti-inflammatory responses, which provides special relevance about the mode of administration of probiotics. Therefore, further clinical studies are required to establish their beneficial outcomes in humans where these probiotic strains are capable of successful colonization of the gut mucosa. Additionally, understanding the molecular mechanisms of probiotic action in the gut is critical for effective use of the existing probiotic strains with specific focus on the recommended dosage, frequency and the total duration of administration, the significance of employing a consortium involving multiple probiotic strains, and the optimal protocols and the components for the manufacturing of the probiotic products. In addition, understanding the molecular mechanisms of action can also help in select in the next-generation probiotics.
6.5 Short Chain Fatty Acid (SCFA) Metabolism and AAD
Among the research studies on probiotic efficacy, there is good consensus on the hypothesis that the modification of the metabolism of nutrients caused by the remodelling of the gut microbiome that is exposed to antibiotic therapy can significantly alter the intestinal metabolome. The chief factor that contributes to the metabolite alteration in the lumen is the decrease in the levels of short chain fatty acids (SCFA) (Binder, 2010; Theriot et al. 2014). SCFAs (viz. acetate, butyrate, and propionate) are the principal metabolic by-products of bacterial metabolism of carbohydrate in GIT and take up to 10% of the daily energy requirement in humans (van der Beek et al. 2017). A reduction in the biosynthesis of SCFAs in the gut might lead to the development of AAD because they promote absorption of water and sodium chloride.
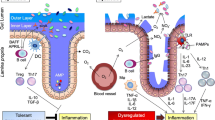
SCFAs are efficiently taken up in the large intestine and stimulate fluid absorption (sodium-dependent) through a cyclic AMP-independent mechanism with exchanges of Sodium-Hydrogen, SCFA-Chloride, and SCFA-bicarbonate moieties (Fig. 6.1).
A schematic model of the potential molecular mechanisms of probiotic action towards prophylaxis of AAD (Mekonnen et al. 2020)
Antibiotic therapy also disrupts the GI tract microbiota via loss of homeostasis, which causes amplified colonization by opportunistic pathogens, the build-up of unutilized carbohydrates and decreased concentration of secondary bile acids and SCFAs. Probiotics could effectively tackle these changes by direct antagonism towards pathogens or through inducing changes in the gut microbiota composition, chiefly by the elevated production of SCFAs, production of secreted metabolites viz. bacteriocins, reduction of luminal pH and oxygen concentrations. Probiotic strains may also influence the composition of biliary acids in addition to positive interaction with intestinal epithelium and the immune system of the host ultimately leading to enhanced gut barrier function besides the effective regulation of water and solute transport.
In mice model, L. rhamnosus GG was effective with an efficiency matching tributyrin (a derivative of butyrate) in providing prophylactic effects in case of intestinal injury induced by antibiotic therapy and reductions the levels of SCFA receptor (GPR109a) and transporters(SLC5A8) (Cresci et al. 2013). Since Lactobacillus spp. do not have the pathways that are needed for the production of butyrate, the increased luminal butyrate levels of the administration of L. rhamnosus GG has been hypothesized to be a case of cross-feeding with other components of the gut microbiota, induced by probiotics. Clinical trials have also provided evidence of the positive impact of probiotics regarding the intestinal SCFA levels. L. plantarum 299V has been proven to be effective in arresting a decrease in SCFA during treatment with metronidazole (Wullt et al. 2007).
In cell line models, metabolites secreted by L. acidophilus ATCC4537 were able to prevent the inhibition of the uptake of butyrate by Caco-2 cells mediated by enteropathogenic E. coli through the inhibition of MCT1 (monocarboxylate transporter isoform 1) endocytosis. Imperatively, the same capability has not been observed in case of heat-killed L. acidophilus or by viable cells of three other Lactobacillus strains tested (Kumar et al. 2015). Thus, there is a strong indication of strain-specific factors being responsible for the observed effects.
Probiotics might also directly influence the SCFA levels in the intestinal lumen by the production of organic acids (viz. acetate, lactate, etc.) and by encouraging the presence of SCFA-producing gut bacteria. The production of SCFA (viz. acetate) by Bifidobacterium spp. in GIT has been proven to effectively decrease the risk for infection by enteropathogenic E. coli (Fukuda et al. 2011). Probiotic metabolism and production of organic acids (e.g. lactate and acetate) in situ could lower luminal pH and oxygen levels in addition to acting as substrates used for the synthesis of butyrate and propionate by microbial components of the gut microbiota (Louis and Flint 2017). In addition, the colonization of probiotics in GIT might decrease the levels of undigested carbohydrates, leading to a reduced risk of developing diarrhea caused by osmogradient modifications (Binder 2010).
6.6 Modulation of the Secretion of Electrolyte Secretion and Absorption Efficacy
The inefficient absorption as well as the active secretion of electrolytes (solutes) by the intestinal epithelial cells contribute to the clinical manifestation of watery diarrhea. Electrolyte concentrations in GIT are regulated by multiple baso-lateral and apical channels along with transporters regulating the transport of chloride (Cl-) as well as active transport of sodium (Na+) through the epithelial barrier with parallel absorption of chloride (Cl–) or bicarbonate (HCO3–)ions (Camilleri et al. 2017; Thiagarajah et al. 2015). In case of in vivo mice model, B. subtilis CU1 (CNCM I2745) was shown to stimulate the expression of elevated levels of NHE3 (epithelial Na+/H+ exchanger-3 protein), which enhances fluid absorption, and reduced levels of cystic fibrosis transmembrane conductance regulator (CFTR), a protein component which plays a pivotal role in the secretion of chloride ions (Urdaci et al. 2018). The similar effects were not reported in L. plantarum CNCM I-4547.
In another in vivo study (mice model), L. acidophilus ATCC4357 was able to prevent diarrhea by effectively tackling the inhibition of NHE3 protein caused by infection of Citrobacter rodentium. Additionally, the Cl/HCO3exchanger protein (DRA) was also reported to retain its activity subsequent to treatment with L. acidophilus (Kumar et al. 2016). Analogous results have been demonstrated involving other studies, such as Bacteroides fragilis ZY-312 (Zhang et al. 2018) & L. rhamnosus GG (Cresci et al. 2013), which resulted in the upregulation in the expression of genes specifically coding for membrane proteins that act as aquaporin water channels. Therefore, the alterations mediated by probiotics with respect to the various electrolyte transporters present in the GIT might represent an effective mechanism for the efficient management of AAD.
Besides SCFAs, other metabolites secreted by probiotics might also confer similar beneficial effects such as gassericin A, a bacteriocin produced by L. gasseri and L. frumenti. In vitro tests have shown that gassericin A is effective at increasing intestinal fluid absorption by inducing higher levels of cellular cyclic nucleotide observed in epithelial cells through mechanisms that involves binding to the membrane protein Keratin 19 (KRT19) and effectively activating mTOR (mechanistic Target of Rapamycin) phosphodiesterase activity. In vivo studies using piglets have also demonstrated the efficacy of gassericin A producing L. gasseri in prevention of diarrhea (Hu et al. 2018).
6.7 Increase in the Concentration of Secondary Bile Acids
In healthy humans, an estimated 95% of the luminal biliary acids are subject to reabsorption in distal region of ileum (Winston and Theriot 2020), with the remaining bile acids being subject to modification by intestinal bacteria and subsequently passively absorbed or excreted. Antibiotic therapy disrupts this balance and causes an increase in the levels of primary biliary acids present in the gut, which inhibit activity of epithelial ion transport proteins. It has also been shown that decrease in the microbially altered, secondary biliary acids elevates the susceptibility to develop infection by C. difficile (Buffie et al. 2015). Clinical studies have demonstrated the efficacy of S. boulardii CNCM I-745 to alter the biliary acid composition during antibiotic therapy among healthy volunteers. Higher quantities of primary biliary acids (cholic acids), and lower levels of secondary biliary acids have been reported in the fecal samples from subjects on treatment with antibiotics (amoxicillin-clavulanate). Administration of S. boulardii CNCM I-745 was also reported to reverse these changes in clinical studies (Kelly et al. 2019).
Deconjugation of bile acids by the secretion of bile salt hydrolases (BSH) by probiotics (viz. species of Bifidobacterium, Lactobacillus, and Clostridium) is already well established. BSH deconjugate biliary acids which can be additionally metabolized to secondary and tertiary biliary acids by the action of other components of gut microbiota (Winston and Theriot 2020).
In vivo studies (mice model) have demonstrated the efficacy of enhanced BSH activity in ameliorating the cardiometabolic impacts of high lipid diet (Joyce et al. 2014). Research insights have revealed that genes coding for BSH are selectively enriched among Lactobacillus spp. that are associated with vertebrates (O’Flaherty et al. 2018). Significantly, gut-associated BSH phylotype with the most effective enzymatic activity was primarily reported in Lactobacillus spp. and not other components of the human gut microbiome (Song et al. 2019).
6.8 Augmenting the Intestinal Barrier Function
The integrity of intestinal epithelial barrier has been showed to be very crucial for the pathologenesis of a variety of intestinal as well as other systemic diseases (König et al. 2016). As per in vivo studies carried out using rodent model, it has been shown that antibiotic therapy induces deficits in barrier function, also termed as a “leaky gut,” but its severity varies based on the specific type of antibiotic that has been employed for the therapy (Tulstrup et al. 2015). In animal studies, particular strains of probiotics have been demonstrated to thwart these disruptions to the intestinal epithelium that are induced by antibiotics. For example, Bacillus amyloliquefaciens was reported to provide enhanced structural and functional attributes of the epithelium in piglets that were administered aureomycin (Du et al. 2018). Also, similar effects were observed in case of Lactobacillus casei CGMCC 12435 and a combination of Lactobacillus and Bifidobacterium strains in mice subjected to ampicillin treatment. The results were substantiated by the observation of enhanced transcripts for tight junction proteins (Shi et al. 2018). Administration of B. fragilis ZY-312 (109 CFU/day) was observed to increase ZO-1 as well as occluding, tight junction proteins, production of mucin, and cell markers for the proliferation of epithelial cells in the colon (Zhang et al. 2018). Additional research efforts are needed to expound the role of specific metabolites produced by probiotics that impact epithelial barrier function (Bron et al. 2017). Recent reports involving in vitro models have demonstrated that Plantaricin EF, a bacteriocin secreted by L. plantarum can effectively thwart pro-damage to the barrier integrity caused by the action of inflammatory cytokines. Additional extracellular proteins secreted by bacteria such as the outer membrane, pilus-associated protein synthesized by Akkermansia mucinophilia could also provide improvements to the effectiveness of the intestinal barrier (Plovier et al. 2017) (Fig. 6.1).
6.9 Modulation of Intestinal Immune Response
Antibiotic therapy has also been proven to adversely affect the homeostasis of the immune system of the host. In clinical studies, antibiotic therapy has been associated with impaired responses to vaccination, among subjects with reduced prevalent antibody titres (Hagan et al. 2019). In mice model, antibiotics have also been known to induce chronic, macrophage-dependent elevation of the inflammatory T-helper 1 (TH1) and heightened susceptibility to certain infections (Scott et al. 2018).
Putative and proven probiotic strains of microorganisms are demonstrated to effectively protect against antibiotic-associated activation of inflammatory pathways in both in vivo models (mic and piglets) as well as in humans (Suez et al. 2018). These are supported by observed reductions in the quantities of C-reactive protein, complement proteins (C3), and antibodies (IgG) which indicate the efficacy of probiotics to limit the systemic effects of antibiotics. The reports concur with the strain-dependent immunomodulatory activities of probiotics among healthy subjects and also individuals with immune system mediated chronic diseases (e.g. allergy, asthma) (Galdeano et al. 2019; Peters et al. 2019). Although additional research efforts are necessary to bring to light the role of specific components of the probiotic cell that directly alter the immune function during antibiotic therapy, recent reports have suggested that the exopolysaccharides produced by Bifidobacterium (Schiavi et al. 2016) and Lactobacillus S-layer proteins (Lightfoot et al. 2015) have immunomodulatory function. Thus, immunomodulation by probiotics during antibiotic therapy could be mediated by the secretion of multiple compounds.
Despite the large quantum of studies which indicate the efficacy of probiotics to effectively treat AAD, it is significant to note that very few studies have investigated the molecular mechanisms of their action. It is particularly to be noted that very few mechanistic studies using in vivo models and clinical trials have directly examined the most popular choice of probiotics for clinical trials, viz. L. rhamnosus GG or S. boulardii CNCM I-745. It has been hypothesized that the positive effects imparted by probiotic strains are multi-factorial and strongly dependent on the probiotic strain as well as the health status of the host (Goldenberg et al. 2015).
The presence of mechanistic overlap among probiotic strains (e.g. beneficial effects caused by the secretion of SCFAs), in addition to host–microbe interactions that are strain-specific in nature (e.g. beneficial effects due to secretion of key enzymes) have also been hypothesized (Hill et al. 2014). These beneficial effects need to be assessed in aptly regulated, multi-center clinical studies where responses of the intestinal and gut microbiota are analyzed and validated by supporting in vivo studies applying the same protocols.
Therefore, it is of paramount importance that the elucidation of the molecular mechanisms of action of probiotics in GIT is of chief importance for developing viable recommendations for current probiotic strains in specific areas (viz. recommended dose, frequency and total duration of the administration of probiotics). In addition, it also provides insight regarding the value of using a consortium comprising multiple strains of probiotics and the optimal protocols for its manufacturing and carrier delivery. It can also be applied for developing appropriate techniques for the selection of next-generation of probiotics with enhanced efficacy to facilitate the management of AAD.
6.10 Conclusion
The fascinating research insights summarized in the present chapter provide clear understanding of the holistic and comprehensive role played by a variety of probiotic strains in the effective management of AAD. The global incidence of AAD is only going to increase owing to the widespread and unscientific administration of broad-spectrum antibiotics as well as the rising trend of high stress, coupled with imbalanced diet and unhealthy lifestyle among the population. These changes are primarily due to rampant urbanization and the subsequent migration of rural population to the urban centers in India as well across the developing world. The overall scenario provides strong impetus to the addition of probiotics in the arsenal to effectively tackle AAD. This would be further hastened with specific research efforts targeting the lacunae discussed in the present chapter especially the need for comprehensive, well-planned clinical studies to provide unequivocal evidence for the efficacy of specific probiotic strains, which would enable the authorized regulatory agencies to approve its administration to benefit the patients affected by AAD.
References
Allen SJ, Wareham K, Wang D, Bradley C, Hutchings H, Harris W et al (2013) Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 382(9900):1249–1257
Appel-da-Silva MC, Narvaez GA, Perez LR, Drehmer L, Lewgoy J (2017) Saccharomyces cerevisiae var. boulardii fungemia following probiotic treatment. Med Mycol Case Rep 18:15–17
Ardita CS, Mercante JW, Kwon YM, Luo L, Crawford ME, Powell DN et al (2014) Epithelial adhesion mediated by pilin SpaC is required for Lactobacillus rhamnosus GG-induced cellular responses. Appl Environ Microbiol 80(16):5068–5077
Azcarate-Peril MA, Ritter AJ, Savaiano D, Monteagudo-Mera A, Anderson C, Magness ST, Klaenhammer TR (2017) Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc Natl Acad Sci 114(3):E367–E375
Barker AK, Duster M, Valentine S, Hess T, Archbald-Pannone L, Guerrant R, Safdar N (2017) A randomized controlled trial of probiotics for Clostridium difficile infection in adults (PICO). J Antimicrob Chemother 72(11):3177–3180
Bartlett JG (2002) Clinical practice. Antibiotic associated diarrhea. N Engl J Med 346:334e9
Bassis CM, Theriot CM, Young VB (2014) Alteration of the murine gastrointestinal microbiota by tigecycline leads to increased susceptibility to Clostridium difficile infection. Antimicrob Agents Chemother 58(5):2767–2774
Binder HJ (2010) Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol 72:297–313
Biswal S (2014) Proton pump inhibitors and risk for Clostridium difficile associated diarrhea. Biom J 37(4):178
Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT et al (2017) Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 117(1):93–107
Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM (2017) Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol 15(1):55
Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A et al (2015) Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517(7533):205–208
Camilleri M, Sellin JH, Barrett KE (2017) Pathophysiology, evaluation, and management of chronic watery diarrhea. Gastroenterology 152(3):515–532
Carter GP, Chakravorty A, Pham Nguyen TA, Mileto S, Schreiber F, Li L et al (2015) Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. MBio 6:e00551–e00515
Chandrasekaran R, Lacy DB (2017) The role of toxins in Clostridium difficile infection. FEMS Microbiol Rev 41(6):723–750
Charbonneau MR, Blanton LV, DiGiulio DB, Relman DA, Lebrilla CB, Mills DA, Gordon JI (2016) A microbial perspective of human developmental biology. Nature 535(7610):48–55
Chumbler NM, Farrow MA, Lapierre LA, Franklin JL, Haslam D, Goldenring JR, Lacy DB (2012) Clostridium difficile toxin B causes epithelial cell necrosis through an autoprocessing-independent mechanism. PLoS Pathog 8(12)
Chumbler NM, Farrow MA, Lapierre LA, Franklin JL, Lacy DB (2016) Clostridium difficile toxins TcdA and TcdB cause colonic tissue damage by distinct mechanisms. Infect Immun 84(10):2871–2877
Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B et al (2015) The microbiome of uncontacted Amerindians. Sci Adv 1:e1500183
Cote GA, Buchman AL (2006) Antibiotic-associated diarrhoea. Expert Opin Drug Saf 5:361e72
Cresci G, Nagy LE, Ganapathy V (2013) Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. J Parenter Enter Nutr 37(6):763–774
Day RL, Harper AJ, Woods RM, Davies OG, Heaney LM (2019) Probiotics: current landscape and future horizons. Fut Sci OA 5(4):FSO391
De Wolfe TJ, Eggers S, Barker AK, Kates AE, Dill-McFarland KA, Suen G, Safdar N (2018) Oral probiotic combination of lactobacillus and Bifidobacterium alters the gastrointestinal microbiota during antibiotic treatment for Clostridium difficile infection. PLoS One 13(9)
Dicks LM, Mikkelsen LS, Brandsborg E, Marcotte H (2019) Clostridium difficile, the difficult “kloster” fuelled by antibiotics. Curr Microbiol 76(6):774–782
Douillard FP, Ribbera A, Kant R, Pietilä TE, Järvinen HM, Messing M et al (2013) Comparative genomic and functional analysis of 100 lactobacillus rhamnosus strains and their comparison with strain GG. PLoS Genet 9(8)
Du W, Xu H, Mei X, Cao X, Gong L, Wu Y et al (2018) Probiotic Bacillus enhance the intestinal epithelial cell barrier and immune function of piglets. Benefic Microbes 9(5):743–754
Farrow MA, Chumbler NM, Lapierre LA, Franklin JL, Rutherford SA, Goldenring JR, Lacy DB (2013) Clostridium difficile toxin B-induced necrosis is mediated by the host epithelial cell NADPH oxidase complex. Proc Natl Acad Sci 110(46):18674–18679
Farrow MA, Chumbler NM, Bloch SC, King M, Moton-Melancon K, Shupe J et al (2020) A small molecule inhibitor screen reveals calcium channel signaling as a mechanistic mediator of Clostridium difficile TcdB-induced necrosis. ACS Chem Biol. https://doi.org/10.1021/acschembio.9b00906
Fernandez EM, Valenti V, Rockel C, Hermann C, Pot B, Boneca IG, Grangette C (2011) Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 60(8):1050–1059
Fetissov SO (2017) Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol 13(1):11
Fox MJ, Ahuja KDK, Robertson IK et al (2015) Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo controlled study. BMJ Open 5:e006474. https://doi.org/10.1136/bmjopen-2014-006474
Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K et al (2011) Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469(7331):543–547
Galdeano CM, Cazorla SI, Dumit JML, Vélez E, Perdigón G (2019) Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab 74(2):115–124
Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE (2010) Dose–response efficacy of a proprietary probiotic formula of Lactobacillusacidophilus CL1285 and Lactobacilluscasei LBC80R for antibiotic-associated Diarrhea and Clostridiumdifficile-associated Diarrhea prophylaxis in adult patients. Am J Gastroenterol 105(7):1636–1641
Global Probiotic Market Is Set For Rapid Growth and is Expected To Reach Value Around USD 65.87 Billion by 2024 (Zion Market Research, 2018)
Goldenberg JZ, Lytvyn L, Steurich J, Parkin P, Mahant S, Johnston BC (2015) Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev 12
Goldenberg JZ, Yap C, Lytvyn L, Lo CKF, Beardsley J, Mertz D, Johnston BC (2017) Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 12
Hagan T, Cortese M, Rouphael N, Boudreau C, Linde C, Maddur MS et al (2019) Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell 178(6):1313–1328
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B et al (2014) Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514
Hoffmann DE, Fraser CM, Palumbo F, Ravel J, Rowthorn V, Schwartz J (2014) Probiotics: achieving a better regulatory fit. Food Drug Law J 69(2):237
Hu J, Ma L, Nie Y, Chen J, Zheng W, Wang X et al (2018) A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe 24(6):817–832
Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F et al (2014) Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci 111(20):7421–7426
Kelly CP, Chong Nguyen C, Palmieri LJ, Pallav K, Dowd S, SEKSIK P et al (2019) Saccharomyces boulardii CNCM I-745 modulates the fecal bile acids metabolism during antimicrobial therapy in healthy volunteers. Front Microbiol 10:336
Khatri I, Sharma G, Subramanian S (2019) Composite genome sequence of Bacillus clausii, a probiotic commercially available as Enterogermina®, and insights into its probiotic properties. BMC Microbiol 19(1):1–15
Kołodziej M, Szajewska H (2017) Lactobacillus reuteri DSM 17938 in the prevention of antibiotic-associated diarrhoea in children: protocol of a randomised controlled trial. BMJ Open 7(1):e013928
König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A et al (2016) Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 7(10):e196
Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP (2010) The role of toxin a and toxin B in Clostridium difficile infection. Nature 467(7316):711–713
Kumar A, Alrefai WA, Borthakur A, Dudeja PK (2015) Lactobacillus acidophilus counteracts enteropathogenic E. coli-induced inhibition of butyrate uptake in intestinal epithelial cells. Am J Physiol-Gastrointest Liver Physiol 309(7):G602–G607
Kumar A, Anbazhagan AN, Coffing H, Chatterjee I, Priyamvada S, Gujral T et al (2016) Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium. Am J Physiol-Gastrointest Liver Physiol 311(5):G817–G826
Lightfoot YL, Selle K, Yang T, Goh YJ, Sahay B, Zadeh M et al (2015) SIGNR3-dependent immune regulation by lactobacillus acidophilus surface layer protein a in colitis. EMBO J 34(7):881–895
Lopetuso LR, Scaldaferri F, Franceschi F, Gasbarrini A (2016) Bacillusclausii and gut homeostasis: state of the art and future perspectives. Expert Rev Gastroenterol Hepatol 10(8):943–948
Louis P, Flint HJ (2017) Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 19(1):29–41
Lyras D, O’Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T et al (2009) Toxin B is essential for virulence of Clostridium difficile. Nature 458(7242):1176–1179
Marchesi JR, Ravel J (2015) The vocabulary of microbiome research: a proposal. Microbiome 3:31
Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, Yoshikai Y (2003) Lipoteichoic acids from lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through toll-like receptor 2. Clin Diagn Lab Immunol 10(2):259–266
Mazza P, Zani F, Martelli P (1992) Studies on the antibiotic resistance of Bacillus subtilis strains used in oral bacteriotherapy. Boll Chim Farm 131(11):401–408
McFarland LV (1998) Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis 16(5):292–307
McFarland LV (2006) Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol 101:812e22
McFarland LV, Ozen M, Dinleyici EC, Goh S (2016) Comparison of pediatric and adult antibiotic-associated diarrhea and Clostridium difficile infections. World J Gastroenterol 22:3078e104
McKenzie YA, Thompson J, Gulia P, Lomer MCE, (IBS Dietetic Guideline Review Group on behalf of Gastroenterology Specialist Group of the British Dietetic Association) (2016) British dietetic association systematic review of systematic reviews and evidence-based practice guidelines for the use of probiotics in the management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet, 29(5), 576–592
Medina M, Izquierdo E, Ennahar S, Sanz Y (2007) Differential immunomodulatory properties of Bifidobacterium logum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol 150(3):531–538
Mehta D, de Souza A, Jadhav SS, Devale M (2020) A study of probiotic Bacillus subtilis HU58 for the management of antibiotic-associated diarrhoea in adults. Indian Pract 73(4):22–28
Mekonnen SA, Merenstein D, Fraser CM, Marco ML (2020) Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr Opin Biotechnol 61:226–234
Miele L, Giorgio V, Alberelli MA, De Candia E, Gasbarrini A, Grieco A (2015) Impact of gut microbiota on obesity, diabetes, and cardiovascular disease risk. Curr Cardiol Rep 17(12):120
Moeller AH (2017) The shrinking human gut microbiome. Curr Opin Microbiol 38:30–35
Morgan XC, Segata N, Huttenhower C (2013) Biodiversity and functional genomics in the human microbiome. Trends Genet 29(1):51–58
Mosca A, Leclerc M, Hugot JP (2016) Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol 7:455
Navaneethan U, Venkatesh PG, Shen B (2010) Clostridium difficile infection and inflammatory bowel disease: understanding the evolving relationship. World J Gastroenterol: WJG 16(39):4892
O’Flaherty S, Crawley AB, Theriot CM, Barrangou R (2018) The lactobacillus bile salt hydrolase repertoire reveals niche-specific adaptation. mSphere 3(3):e00140–e00118
Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS et al (2017) A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548(7668):407–412
Permpalung N, Upala S, Sanguankeo A, Sornprom S (2016) Association between NSAIDs and Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Can J Gastroenterol Hepatol 2016:7431838. https://doi.org/10.1155/2016/7431838
Peters VBM, van de Steeg E, van Bilsen J, Meijerink M (2019) Mechanisms and immunomodulatory properties of pre-and probiotics. Benefic Microbes 10(3):225–236
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L et al (2017) A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23(1):107
Pradhan B, Guha D, Ray P, Das D, Aich P (2016) Comparative analysis of the effects of two probiotic bacterial strains on metabolism and innate immunity in the RAW 264.7 murine macrophage cell line. Probiot Antimicrobial Proteins 8(2):73–84
Pruitt RN, Lacy DB (2012) Toward a structural understanding of Clostridium difficile toxins a and B. Front Cell Infect Microbiol 2:28
Rijkers GT, De Vos WM, Brummer RJ, Morelli L, Corthier G, Marteau P (2011) Health benefits and health claims of probiotics: bridging science and marketing. Br J Nutr 106(9):1291–1296
Saldanha LG (2008) US Food and Drug Administration regulations governing label claims for food products, including probiotics. Clin Infect Dis 46(Supplement_2):S119–S121
Schiavi E, Gleinser M, Molloy E, Groeger D, Frei R, Ferstl R et al (2016) The surface-associated exopolysaccharide of Bifidobacterium longum 35624 plays an essential role in dampening host proinflammatory responses and repressing local TH17 responses. Appl Environ Microbiol 82(24):7185–7196
Schirmer M, Franzosa EA, Lloyd-Price J, McIver LJ, Schwager R, Poon TW et al (2018) Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat Microbiol 3(3):337–346
Scott NA, Andrusaite A, Andersen P, Lawson M, Alcon-Giner C, Leclaire C et al (2018) Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci Transl Med 10(464):eaao4755
Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14(8):e1002533
Shen NT, Maw A, Tmanova LL, Pino A, Ancy K, Crawford CV et al (2017) Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology 152(8):1889–1900
Shi Y, Kellingray L, Le Gall G, Zhao J, Zhang H, Narbad A et al (2018) The divergent restoration effects of lactobacillus strains in antibiotic-induced dysbiosis. J Funct Foods 51:142–152
Sniffen JC, McFarland LV, Evans CT, Goldstein EJ (2018) Choosing an appropriate probiotic product for your patient: an evidence-based practical guide. PLoS One 13(12)
Song Z, Cai Y, Lao X, Wang X, Lin X, Cui Y et al (2019) Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome 7(1):9
Steele J, Mukherjee J, Parry N, Tzipori S (2013) Antibody against TcdB, but not TcdA, prevents development of gastrointestinal and systemic Clostridium difficile disease. J Infect Dis 207(2):323–330
Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S et al (2018) Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 174(6):1406–1423
Suez J, Zmora N, Segal E, Elinav E (2019) The pros, cons, and many unknowns of probiotics. Nat Med 25(5):716–729
Szajewska H, Canani RB, Guarino A, Hojsak I, Indrio F, Kolacek S et al (2016) Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr 62(3):495–506
Thaiss CA, Zmora N, Levy M, Elinav E (2016) The microbiome and innate immunity. Nature 535(7610):65–74
Theriot CM, Koenigsknecht MJ, Carlson PE Jr, Hatton GE, Nelson AM, Li B et al (2014) Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun 5:3114
Thiagarajah JR, Donowitz M, Verkman AS (2015) Secretory diarrhoea: mechanisms and emerging therapies. Nat Rev Gastroenterol Hepatol 12(8):446
Thomas CM, Versalovic J (2010) Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes 1(3):148–163
Tulstrup MVL, Christensen EG, Carvalho V, Linninge C, Ahrné S, Højberg O et al (2015) Antibiotic treatment affects intestinal permeability and gut microbial composition in Wistar rats dependent on antibiotic class. PLoS One 10(12)
Turroni F, Serafini F, Foroni E, Duranti S, Motherway MOC, Taverniti V et al (2013) Role of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in modulating bacterium–host interactions. Proc Natl Acad Sci 110(27):11151–11156
Turroni S, Rampelli S, Centanni M, Schnorr SL, Consolandi C, Severgnini M et al (2016) Enterocyte-associated microbiome of the Hadza hunter-gatherers. Front Microbiol 7:865
Urdaci MC, Lefevre M, Lafforgue G, Cartier C, Rodriguez B, Fioramonti J (2018) Antidiarrheal action of Bacillus subtilis CU1 CNCM I-2745 and lactobacillus plantarum CNCM I-4547 in mice. Front Microbiol 9:1537
Van Baarlen P, Troost FJ, van Hemert S, van der Meer C, de Vos WM, de Groot PJ et al (2009) Differential NF-κB pathways induction by lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci 106(7):2371–2376
Van de Wouw M, Schellekens H, Dinan TG, Cryan JF (2017) Microbiota-gut-brain axis: modulator of host metabolism and appetite. J Nutr 147(5):727–745
Van den Nieuwboer M, Van De Burgwal LHM, Claassen E (2016) A quantitative key-opinion-leader analysis of innovation barriers in probiotic research and development: valorisation and improving the tech transfer cycle. Pharma Nutr 4(1):9–18
van der Beek CM, Dejong CH, Troost FJ, Masclee AA, Lenaerts K (2017) Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr Rev 75(4):286–305
Varankovich NV, Nickerson MT, Korber DR (2015) Probiotic-based strategies for therapeutic and prophylactic use against multiple gastrointestinal diseases. Front Microbiol 6:685
von Ossowski I, Pietilä TE, Rintahaka J, Nummenmaa E, Mäkinen VM, Reunanen J et al (2013) Using recombinant Lactococci as an approach to dissect the immunomodulating capacity of surface piliation in probiotic Lactobacillus rhamnosus GG. PLoS One 8(5)
Waligora AJ, Hennequin C, Mullany P, Bourlioux P, Collignon A, Karjalainen T (2001) Characterization of a cell surface protein of Clostridium difficile with adhesive properties. Infect Immun 69(4):2144–2153
Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T et al (2017) Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 376(4):305–317
Winston JA, Theriot CM (2020) Diversification of host bile acids by members of the gut microbiota. Gut Microbes 11(2):158–171
Wu L, Wang Z, Sun G, Peng L, Lu Z, Yan B et al (2019) Effects of anti-H. pylori triple therapy and a probiotic complex on intestinal microbiota in duodenal ulcer. Sci Rep 9(1):1–11
Wullt M, Hagslätt MLJ, Odenholt I, Berggren A (2007) Lactobacillus plantarum 299v enhances the concentrations of fecal short-chain fatty acids in patients with recurrent Clostridium difficile-associated diarrhea. Dig Dis Sci 52(9):2082
Yanagihara S, Kanaya T, Fukuda S, Nakato G, Hanazato M, Wu XR et al (2017) Uromodulin–SlpA binding dictates Lactobacillus acidophilus uptake by intestinal epithelial M cells. Int Immunol 29(8):357–363
Yu LX, Schwabe RF (2017) The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol 14(9):527
Zhang W, Zhu B, Xu J, Liu Y, Qiu E, Li Z et al (2018) Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front Immunol 9:1040
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Henry, D.E., Venkateswara Rao, V. (2021). Antibiotic-Associated Diarrhea and Update on Probiotics Recommendations. In: Pawar, S.V., Rishi, P. (eds) Probiotic Research in Therapeutics. Springer, Singapore. https://doi.org/10.1007/978-981-33-6236-9_6
Download citation
DOI: https://doi.org/10.1007/978-981-33-6236-9_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6235-2
Online ISBN: 978-981-33-6236-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)