Abstract
Ovarian clear cell carcinoma (OCCC), one of five major histological subtypes of epithelial ovarian cancer (EOC), has unique clinical and molecular features. There is no specific targeted therapy for OCCC, and studies on translational genomics underlying OCCC pathogenesis are still ongoing. This chapter focuses on the molecular landscape in the OCCC tumor and its microenvironment. Our findings will help in the stratification of OCCC patients who may benefit from precision medicine for this unique histological subtype of EOC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Ovarian clear cell carcinoma (OCCC) is one of five major histotypes of epithelial ovarian cancer (EOC). The clinicopathological and biological features of OCCC include hypercalcemia, thromboembolism, a close association with endometriosis, and a higher prevalence in Eastern Asian women [1, 2]. In addition, compared to other histological subtypes, OCCC patients present at a relatively younger age and an early stage [3]. The 5-year survival rate for stage I OCCC is ~90%, with differences depending on the substage: patients with stage IA or IC1 OCCC have a favorable clinical outcome, while patients with stage IC2 or IC3 have a statistically poorer prognosis [4,5,6]. In addition, advanced-stage OCCC is resistant to conventional platinum-based front-line chemotherapy, resulting in poor prognosis [6, 7].
Because of the low prevalence of OCCC in Western countries, there is a lack of large randomized controlled clinical trials targeting OCCC with chemotherapy including molecular medicine. The current standard treatment is a one-size-fits-all approach, which includes debulking surgery with platinum agent + paclitaxel combination chemotherapy. A randomized phase III clinical trial conducted by the Japanese Gynecologic Oncology Group compared irinotecan and cisplatin (CPT-P) with paclitaxel plus carboplatin (TC) in stage I–IV OCCC patients. The authors reported no significant survival benefit in the CPT-P group [8]. In addition, studies on translational genomics underlying OCCC pathogenesis are ongoing [1, 9,10,11,12]. These findings highlight that other therapeutic approaches might improve survival in OCCC patients.
In this chapter, we reviewed recent advances in molecular profiling related to carcinogenesis and molecular targets of OCCC.
9.2 Mutational Landscape
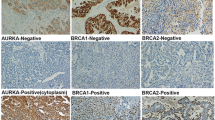
Several studies on large-scale genome-wide gene profiling for OCCC have reported actionable gene alterations that could lead to the development of a target therapy for OCCC (Table 9.1) [13,14,15,16,17,18,19,20]. Both AT-rich interactive domain 1A (ARID1A) and phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit α (PIK3CA) are most frequently mutated and often coexist in OCCC [19]. The coexistence of these mutations initiated OCCC tumor formation in a genetically engineered mouse model [28]. Since cancer cells are vulnerable to ARID1A deficiency, synthetic lethal approaches to ARID1A mutation in OCCC are of considerable clinical interest [1, 12]. PIK3CA and phosphatase and tensin homolog deleted from chromosome 10 (PTEN) mutations highly activate the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signal [14,15,16, 18,19,20]. In addition, KRAS and protein phosphatase 2 scaffold subunit A alpha (PPP2R1A) mutations have been found in 5–20% and 10–20% of OCCC patients, respectively [14,15,16, 18, 19]. Notably, mutations of these genes, where the mitogen-activated protein kinase (MAPK) signal could be a target candidate, also often coexist [18]. Compared to high-grade serous ovarian cancer (HGSOC), the most common form of EOC, germline mutations of germline breast cancer susceptibility 1 (BRCA1) and BRCA2 are infrequent and are found in 2–6% of OCCC patients [21, 22], indicating that only a small percentage of OCCC patients might benefit from a newly innovated treatment strategy using poly-adenosine diphosphate (ADP)-ribose polymerase (PARP) inhibitors.
9.3 Copy Number Landscape
Several molecular technologies, including single-nucleotide polymorphism (SNP) array, comparative genomic hybridization (CGH) array, and exome sequencing, have revealed copy number alterations (CNAs) in OCCC. Frequent amplification has been observed in chr8q, chr17q, and chr20q loci, while deletion has been observed in chr9q, chr13q, chr18q, and chr19p loci [16, 23, 29]. Notably, the whole-arm-CNA ratio is higher in OCCC compared to HGSOC, although fewer CNAs are found in OCCC patients [29]. Interestingly, whole chr8q and chr20q13.2 amplification, including zinc finger protein 217 (ZNF217), is more prevalent in Japanese OCCC patients compared to Korean or German OCCC patients [23]. Amplification or deletion of certain loci that contain several cancer-related genes might affect intracellular signals as potential therapeutic targets (Table 9.1).
9.4 Signaling Pathway Landscape
9.4.1 IL6/STAT3 Pathway
OCCC-specific expression signatures have been obtained using global gene expression assays. Compared to HGSOC, OCCC shows an enhanced interleukin 6 (IL6)/signal transducer and activator of transcription 3 (STAT3) pathway [30,31,32]. In addition, high tumor and serum IL6 levels are significantly correlated with poor prognosis in OCCC patients [5, 31]. IL6 is a pleiotropic pro-inflammatory cytokine that mediates critical processes, including cell proliferation, angiogenesis, and chemoresistance. IL6 signal inhibition has antitumor effects in OCCC, indicating that this pathway is a promising therapeutic target [33]. Although anti-IL6 antibody (siltuximab) has shown clinical activity in a phase II clinical trial of 18 patients with platinum-resistant ovarian cancer (1 OCCC patient) [34], no clinical trials specific for OCCC-targeting IL6/STAT3 signals have been conducted.
9.4.2 Angiogenesis
Intertumoral hypoxia with high hypoxia-inducible factor 1-alpha (HIF-1α) expression, in which a malignant tumor commonly develops, leads to an increase in the activity of various angiogenesis-related signals. In OCCC, increased HIF-1α expression increases the intracellular glycogen content, causing cell chemoresistance [35]. In addition, in OCCC, IL6 signals via STAT3 activates the expression of downstream genes, including HIF1A [31]. Therefore, vascular endothelial growth factor (VEGF), induced by HIF-1α, is overexpressed in >90% of OCCC patients, and VEGF expression is correlated with the patient’s survival [36]. Notably, bevacizumab, a monoclonal human VEGF antibody, has antitumor effects in OCCC both in vitro and in vivo.
On the basis of the findings that OCCC and renal CCC have similar gene expression profiles, one of which is characterized by the activated HIF pathway [37], researchers have focused on anti-angiogenetic therapy for OCCC (Table 9.2). The GOG-254 phase II study on sunitinib, which targets vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR), for recurrent or persistent OCCC treatment reported a median progression-free survival (PFS) and overall survival (OS) of 2.7 and 12.8 months, respectively [38]. The NRG-GY001 phase II study on cabozantinib, which targets VEGFR, MET, and RET, for recurrent or persistent OCCC reported that a single administration of cabozantinib leads to a median PFS and OS of 3.6 and 8.1 months, respectively [39]. In addition, a phase II study on ENMD-2076, which targets Aurora A and VEGFR, for recurrent OCCC reported a median PFS of 3.7 months, and 22% of evaluable patients had a 6-month PFS, which did not meet the preset bar for efficacy [40]. Most of these trials showed limited efficacy. A randomized phase II international NiCCC (ENGOT-GYN1) study on nintedanib (BIBF 1120), which targets VEGFR, PDFGR, and fibroblast growth factor receptor (FGFR), versus chemotherapy in recurrent or persistent OCCC is ongoing (NCT02866370) [41].
9.4.3 PI3K/Akt/mTOR Pathway
The PI3K/Akt/mTOR pathway plays a crucial role in the malignancy of human tumors and is involved in OCCC pathogenesis via frequent genetic alterations [14,15,16, 18,19,20]. Notably, comprehensive genomic profiling of OCCC shows that ~70% of samples harbor mutations in at least one component of the PI3K/Akt/mTOR pathway [46]. In addition, inhibitors of the PI3K/Akt/mTOR pathway shows antitumor effects in OCCC cells with high pathway activity [47]. These findings indicate the potential benefits of therapies targeting the PI3K/Akt/mTOR pathway in OCCC patients.
The GOG-268 phase II study on temsirolimus, which targets mTOR complex 1, in combination with paclitaxel and carboplatin, followed by temsirolimus, as a first-line therapy in stage III–IV OCCC patients did not show an improved PFS compared to historical controls (Table 9.2; NCT01196429) [42].
9.4.4 HNF-1β Pathway
Hepatocyte nuclear factor 1β (HNF-1β), a transcription factor, is commonly expressed in OCCC and is therefore used as an OCCC diagnostic marker [1, 9, 11, 12]. A decrease in HNF-1β expression is associated with a favorable clinical outcome in OCCC patients [48]. Epigenetic silencing via hypomethylation is one of the mechanisms underlying high HNF-1β expression [30]. In OCCC, HNF-1β plays an important role in cancer cell survival and chemoresistance by modulating glucose metabolism and internal oxidative stress [49, 50].
9.5 Synthetic Lethal Approaches for ARID1A
As mentioned before, cancer cells are vulnerable to ARID1A deficiency. Therefore, synthetic lethal approaches to targeting this vulnerability of OCCC cells are being developed. The small-molecule inhibitor of the enhancer of zeste homolog 2 (EZH2) methyltransferase (GSK126) inhibits growth in ARID1A-mutated ovarian cancer cells because ARID1A and EZH2 have an antagonistic association with regard to PI3K-interacting protein 1 (PIK3IP1) expression that promotes apoptosis via negative PI3K/Akt signaling regulation [51]. As another epigenetic concept of ARID1A deficiency, modulation of histone deacetylase 6 (HDAC6) activity using the HDAC6 inhibitor (ACY1215) has a therapeutic effect in ARID1A-mutated tumors [52]. ARID1A transcriptionally represses HDAC6, so ARID1A mutation inactivates the apoptosis-promoting function of P53 via HADC6 upregulation. Notably, high HDAC6 expression, as shown by immunohistochemistry (IHC) assay, is correlated with unfavorable prognosis in OCCC with ARID1A loss [53]. In addition, HDAC6 inhibition can synergize with anti-programmed death-ligand 1 (PD-L1) immune checkpoint blockade in an ARID1A-inactivated genetic OCCC mouse model [54].
ARID1A-deficient tumors show therapeutic vulnerability to PARP inhibitors [55]. ARID1A is recruited to the site of double-stranded DNA breaks (DNA DSBs) via interaction with ataxia–telangiectasia and RAD3-related protein (ATR), in addition to facilitating DNA DSB end processing and sustaining ATR activation for DNA damage signaling. Therefore, impaired DNA damage checkpoint regulation in ARID1A-deficient tumors sensitizes cancer cells to PARP inhibitors. In addition, high-throughput RNA interference (RNAi) chemosensitization screening shows that ARID1A is a synthetic lethal partner of the ATR inhibitor [56]. ARID1A deficiency delays the cell cycle because of the inability to recruit topoisomerase II to chromatin, increasing dependency on ATR checkpoint activity. Therefore, ATR inhibition in ARID1A-deficient tumors induces premature mitosis, triggering genomic instability and cancer cell death.
A high-throughput drug screen targeting ARID1A synthetic lethal effects revealed dasatinib, a multitarget kinase inhibitor, as a clinically used selective drug for ARID1A-mutated OCCC cell lines [57]. The sensitivity of dasatinib in ARID1A-mutated cancer cells is characterized by G1 cell cycle arrest, followed by p21- and Rb-associated apoptosis. Studies focusing on cellular metabolism as a new concept of synthetic lethal approaches have shown that ARID1A-deficient tumors are vulnerable to glutathione (GSH) metabolism [58]. ARID1A maintains GSH homeostasis by modulating SLC7A11 expression (a transporter of cysteine, a key source for GSH) and therefore maintains an intricate balance between GSH and reactive oxygen species (ROS). Inhibition of the GSH metabolic pathway using either APR-246 or buthionine sulfoximine (BSO, a rate-limiting enzyme in GSH synthesis) in ARID1A-deficient tumors collapses the GSH-ROS balance, followed by apoptosis by ROS.
The ARID1A deficiency status is used for OCCC patient stratification in both clinical settings and trials. OCCC with ARID1A mutation shows selective sensitivity to gemcitabine, although the underlying molecular mechanism is unclear [59]. Gemcitabine is commonly available for EOC treatment, so this finding might directly contribute to precision medicine for OCCC. In addition, a phase II retrospective study on dasatinib for recurrent or persistent ovarian and endometrial clear cell carcinoma to evaluate antitumor effects with regard to the ARID1A expression status is ongoing (NCT02059265) [43].
9.6 Immunological Landscape
Pembrolizumab is a humanized monoclonal antibody against programmed death-1 (PD-1) and is approved for any unresectable or metastatic solid tumor with microsatellite instability (MSI). MSI with a high tumor mutation burden arises from mismatch repair (MMR) deficiency caused by either germline mutations in MMR gene components in Lynch syndrome patients or somatic hypermethylation of the MLH1 promoter region in tumors. Histological subtypes of EOC associated with Lynch syndrome include endometrioid carcinoma and OCCC [60]. The frequency of aberrant MMR expression, as assayed by IHC, is 6–13% in OCCC [61,62,63], and MMR expression and MSI status are correlated [63, 64].
In the KEYNOTE-100 phase II study on pembrolizumab in 376 recurrent EOC patients, the objective response rate (ORR) of OCCC was 15.8%, although the overall ORR was 8% [65]. Importantly, this study also showed that higher PD-L1 expression in tumors is correlated with a higher ORR; ~50% of OCCC patients showed positive PD-L1 expression regardless of the MMR status [62, 63], indicating that a large percentage of OCCC patients might benefit from this new therapeutic approach of immune checkpoint blockade. ARID1A deficiency induced impaired MMR via interaction with MSH2, followed by increased PD-L1 expression, in a syngeneic ovarian cancer mouse model [66]. In another phase II study on the anti-PD-1 antibody nivolumab in 20 platinum-resistant EOC patients, 2 patients (one with OCCC) showed a durable complete response [67]. Other ongoing clinical trials targeting immune checkpoint blockade for OCCC include the MOCCA phase II randomized study on durvalumab, which targets PD-L1, versus chemotherapy (NCT03405454) [44] and the BrUOG 354 phase II randomized study on nivolumab plus ipilimumab, which target CTLA4, versus only nivolumab (NCT03355976) [44] (Table 9.2).
9.7 Conclusion
The clinical need for OCCC treatment is still unmet. Alternative therapeutic strategies using targeted therapies based on the molecular characteristics of OCCC might significantly affect the clinical outcome in OCCC patients. Given its low prevalence, the proof-of-concept via adequately designed clinical trials with international collaboration on the basis of the OCCC molecular landscapes are required in order to develop precision medicine for OCCC.
References
Khalique S, Lord CJ, Banerjee S, Natrajan R. Translational genomics of ovarian clear cell carcinoma. Semin Cancer Biol. 2020;61:121–31. https://doi.org/10.1016/j.semcancer.2019.10.025.
Takahashi K, Takenaka M, Kawabata A, Yanaihara N, Okamoto A. Rethinking of treatment strategies and clinical management in ovarian clear cell carcinoma. Int J Clin Oncol. 2020;25:425–31. https://doi.org/10.1007/s10147-020-01625-w.
Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370–6. https://doi.org/10.1016/j.ygyno.2008.02.006.
Suzuki K, Takakura S, Saito M, Morikawa A, Suzuki J, Takahashi K, et al. Impact of surgical staging in stage I clear cell adenocarcinoma of the ovary. Int J Gynecol Cancer. 2014;24:1181–9. https://doi.org/10.1097/IGC.0000000000000178.
Kawabata A, Yanaihara N, Nagata C, Saito M, Noguchi D, Takenaka M, et al. Prognostic impact of interleukin-6 expression in stage I ovarian clear cell carcinoma. Gynecol Oncol. 2017;146:609–14. https://doi.org/10.1016/j.ygyno.2017.06.027.
Shu CA, Zhou Q, Jotwani AR, Iasonos A, Leitao MM Jr, Konner JA, et al. Ovarian clear cell carcinoma, outcomes by stage: the MSK experience. Gynecol Oncol. 2015;139:236–41. https://doi.org/10.1016/j.ygyno.2015.09.016.
Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–74. https://doi.org/10.1038/sj.bjc.6603116.
Sugiyama T, Okamoto A, Enomoto T, Hamano T, Aotani E, Terao Y, et al. Randomized phase III trial of irinotecan plus cisplatin compared with paclitaxel plus carboplatin as first-line chemotherapy for ovarian clear cell carcinoma: JGOG3017/GCIG trial. J Clin Oncol. 2016;34:2881–7. https://doi.org/10.1200/JCO.2016.66.9010.
Mabuchi S, Sugiyama T, Kimura T. Clear cell carcinoma of the ovary: molecular insights and future therapeutic perspectives. J Gynecol Oncol. 2016;27:e31. https://doi.org/10.3802/jgo.2016.27.e31.
Jang JYA, Yanaihara N, Pujade-Lauraine E, Mikami Y, Oda K, Bookman M, et al. Update on rare epithelial ovarian cancers: based on the Rare Ovarian Tumors Young Investigator Conference. J Gynecol Oncol. 2017;28:e54. https://doi.org/10.3802/jgo.2017.28.e54.
Oda K, Hamanishi J, Matsuo K, Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: Unique opportunities for management. Gynecol Oncol. 2018;151:381–9. https://doi.org/10.1016/j.ygyno.2018.09.001.
Kuroda T, Kohno T. Precision medicine for ovarian clear cell carcinoma based on gene alterations. Int J Clin Oncol. 2020;25:419–24. https://doi.org/10.1007/s10147-020-01622-z.
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43. https://doi.org/10.1056/NEJMoa1008433.
Jones S, Wang TL, Shih IeM, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–31. https://doi.org/10.1126/science.1196333.
Itamochi H, Oishi T, Oumi N, Takeuchi S, Yoshihara K, Mikami M, et al. Whole-genome sequencing revealed novel prognostic biomarkers and promising targets for therapy of ovarian clear cell carcinoma. Br J Cancer. 2017;117:717–24. https://doi.org/10.1038/bjc.2017.228.
Murakami R, Matsumura N, Brown JB, Higasa K, Tsutsumi T, Kamada M, et al. Exome sequencing landscape analysis in ovarian clear cell carcinoma shed light on key chromosomal regions and mutation gene networks. Am J Pathol. 2017;187:2246–58. https://doi.org/10.1016/j.ajpath.2017.06.012.
Wang YK, Bashashati A, Anglesio MS, Cochrane DR, Grewal DS, Ha G, et al. Genomic consequences of aberrant DNA repair mechanisms stratify ovarian cancer histotypes. Nat Genet. 2017;49:856–65. https://doi.org/10.1038/ng.3849.
Shibuya Y, Tokunaga H, Saito S, Shimokawa K, Katsuoka F, Bin L, et al. Identification of somatic genetic alterations in ovarian clear cell carcinoma with next generation sequencing. Genes Chromosomes Cancer. 2018;57:51–60. https://doi.org/10.1002/gcc.22507.
Kim SI, Lee JW, Lee M, Kim HS, Chung HH, Kim JW, et al. Genomic landscape of ovarian clear cell carcinoma via whole exome sequencing. Gynecol Oncol. 2018;148:375–82. https://doi.org/10.1016/j.ygyno.2017.12.005.
Takenaka M, Saito M, Iwakawa R, Yanaihara N, Saito M, Kato M, et al. Profiling of actionable gene alterations in ovarian cancer by targeted deep sequencing. Int J Oncol. 2015;46:2389–98. https://doi.org/10.3892/ijo.2015.2951.
Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63. https://doi.org/10.1200/JCO.2011.39.8545.
Enomoto T, Aoki D, Hattori K, Jinushi M, Kigawa J, Takeshima N, et al. The first Japanese nationwide multicenter study of BRCA mutation testing in ovarian cancer: CHARacterizing the cross-sectionaL approach to Ovarian cancer geneTic TEsting of BRCA (CHARLOTTE). Int J Gynecol Cancer. 2019;29:1043–9. https://doi.org/10.1136/ijgc-2019-000384.
Okamoto A, Sehouli J, Yanaihara N, Hirata Y, Braicu I, Kim BG, et al. Somatic copy number alterations associated with Japanese or endometriosis in ovarian clear cell adenocarcinoma. PLoS One. 2015;10:e0116977. https://doi.org/10.1371/journal.pone.0116977.
Kuo KT, Mao TL, Chen X, Feng Y, Nakayama K, Wang Y, et al. DNA copy numbers profiles in affinity-purified ovarian clear cell carcinoma. Clin Cancer Res. 2010;16:1997–2008. https://doi.org/10.1158/1078-0432.CCR-09-2105.
Yamashita Y, Akatsuka S, Shinjo K, Yatabe Y, Kobayashi H, Seko H, et al. Met is the most frequently amplified gene in endometriosis-associated ovarian clear cell adenocarcinoma and correlates with worsened prognosis. PLoS One. 2013;8:e57724. https://doi.org/10.1371/journal.pone.0057724.
Tan DS, Iravani M, McCluggage WG, Lambros MB, Milanezi F, Mackay A, et al. Genomic analysis reveals the molecular heterogeneity of ovarian clear cell carcinomas. Clin Cancer Res. 2011;17:1521–34. https://doi.org/10.1158/1078-0432.CCR-10-1688.
Tan DS, Lambros MB, Rayter S, Natrajan R, Vatcheva R, Gao Q, et al. PPM1D is a potential therapeutic target in ovarian clear cell carcinomas. Clin Cancer Res. 2009;15:2269–80. https://doi.org/10.1158/1078-0432.CCR-08-2403.
Chandler RL, Damrauer JS, Raab JR, Schisler JC, Wilkerson MD, Didion JP, et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun. 2015;6:6118. https://doi.org/10.1038/ncomms7118.
Uehara Y, Oda K, Ikeda Y, Koso T, Tsuji S, Yamamoto S, et al. Integrated copy number and expression analysis identifies profiles of whole-arm chromosomal alterations and subgroups with favorable outcome in ovarian clear cell carcinomas. PLoS One. 2015;10:e0128066. https://doi.org/10.1371/journal.pone.0128066.
Yamaguchi K, Mandai M, Oura T, Matsumura N, Hamanishi J, Baba T, et al. Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene. 2010;29:1741–52. https://doi.org/10.1038/onc.2009.470.
Anglesio MS, George J, Kulbe H, Friedlander M, Rischin D, Lemech C, et al. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin Cancer Res. 2011;17:2538–48. https://doi.org/10.1158/1078-0432.CCR-10-3314.
Yanaihara N, Anglesio MS, Ochiai K, Hirata Y, Saito M, Nagata C, et al. Cytokine gene expression signature in ovarian clear cell carcinoma. Int J Oncol. 2012;41:1094–100. https://doi.org/10.3892/ijo.2012.1533.
Yanaihara N, Hirata Y, Yamaguchi N, Noguchi Y, Saito M, Nagata C, et al. Antitumor effects of interleukin-6 (IL-6)/interleukin-6 receptor (IL-6R) signaling pathway inhibition in clear cell carcinoma of the ovary. Mol Carcinog. 2016;55:832–41. https://doi.org/10.1002/mc.22325.
Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083–96. https://doi.org/10.1158/1078-0432.CCR-11-0945.
Iida Y, Aoki K, Asakura T, Ueda K, Yanaihara N, Takakura S, et al. Hypoxia promotes glycogen synthesis and accumulation in human ovarian clear cell carcinoma. Int J Oncol. 2012;40:2122–30. https://doi.org/10.3892/ijo.2012.1406.
Mabuchi S, Kawase C, Altomare DA, Morishige K, Hayashi M, Sawada K, et al. Vascular endothelial growth factor is a promising therapeutic target for the treatment of clear cell carcinoma of the ovary. Mol Cancer Ther. 2010;9:2411–22. https://doi.org/10.1158/1535-7163.MCT-10-0169.
Ji JX, Wang YK, Cochrane DR, Huntsman DG. Clear cell carcinomas of the ovary and kidney: clarity through genomics. J Pathol. 2018;244:550–64. https://doi.org/10.1002/path.5037.
Chan JK, Brady W, Monk BJ, Brown J, Shahin MS, Rose PG, et al. A phase II evaluation of sunitinib in the treatment of persistent or recurrent clear cell ovarian carcinoma: An NRG Oncology/Gynecologic Oncology Group Study (GOG-254). Gynecol Oncol. 2018;150:247–52. https://doi.org/10.1016/j.ygyno.2018.05.029.
Konstantinopoulos PA, Brady WE, Farley J, Armstrong A, Uyar DS, Gershenson DM. Phase II study of single-agent cabozantinib in patients with recurrent clear cell ovarian, primary peritoneal or fallopian tube cancer (NRG-GY001). Gynecol Oncol. 2018;150:9–13. https://doi.org/10.1016/j.ygyno.2018.04.572.
Lheureux S, Tinker A, Clarke B, Ghatage P, Welch S, Weberpals JI, et al. A clinical and molecular phase II trial of oral ENMD-2076 in ovarian clear cell carcinoma (OCCC): a study of the Princess Margaret Phase II Consortium. Clin Cancer Res. 2018;24:6168–74. https://doi.org/10.1158/1078-0432.CCR-18-1244.
Elvin JA, Chura J, Gay LM, Markman M. Comprehensive genomic profiling (CGP) of ovarian clear cell carcinomas (OCCC) identifies clinically relevant genomic alterations (CRGA) and targeted therapy options. Gynecol Oncol Rep. 2017;20:62–6. https://doi.org/10.1016/j.gore.2017.02.007.
Mabuchi S, Kawase C, Altomare DA, Morishige K, Sawada K, Hayashi M, et al. mTOR is a promising therapeutic target both in cisplatin-sensitive and cisplatin-resistant clear cell carcinoma of the ovary. Clin Cancer Res. 2009;15:5404–13. https://doi.org/10.1158/1078-0432.CCR-09-0365.
Takenaka M, Köbel M, Garsed DW, Fereday S, Pandey A, Etemadmoghadam D, et al. Survival following chemotherapy in ovarian clear cell carcinoma is not associated with pathological misclassification of tumor histotype. Clin Cancer Res. 2019;25:3962–73. https://doi.org/10.1158/1078-0432.CCR-18-3691.
Okamoto T, Mandai M, Matsumura N, Yamaguchi K, Kondoh H, Amano Y, et al. Hepatocyte nuclear factor-1β (HNF-1β) promotes glucose uptake and glycolytic activity in ovarian clear cell carcinoma. Mol Carcinog. 2015;54:35–49. https://doi.org/10.1002/mc.22072.
Amano Y, Mandai M, Yamaguchi K, Matsumura N, Kharma B, Baba T, et al. Metabolic alterations caused by HNF1β expression in ovarian clear cell carcinoma contribute to cell survival. Oncotarget. 2015;6:26002–17. https://doi.org/10.18632/oncotarget.4692.
Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21:231–8. https://doi.org/10.1038/nm.3799.
Bitler BG, Wu S, Park PH, Hai Y, Aird KM, Wang Y, et al. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat Cell Biol. 2017;19:962–73. https://doi.org/10.1038/ncb3582.
Yano M, Katoh T, Miyazawa M, Miyazawa M, Ogane N, Miwa M, et al. Clinicopathological correlation of ARID1A status with HDAC6 and its related factors in ovarian clear cell carcinoma. Sci Rep. 2019;9:2397. https://doi.org/10.1038/s41598-019-38653-0.
Fukumoto T, Fatkhutdinov N, Zundell JA, Tcyganov EN, Nacarelli T, Karakashev S, et al. HDAC6 Inhibition Synergizes with Anti-PD-L1 Therapy in ARID1A-Inactivated Ovarian Cancer. Cancer Res. 2019;79:5482–9. https://doi.org/10.1158/0008-5472.CAN-19-1302.
Shen J, Peng Y, Wei L, Zhang W, Yang L, Lan L, et al. ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov. 2015;5:752–67. https://doi.org/10.1158/2159-8290.CD-14-0849.
Williamson CT, Miller R, Pemberton HN, Jones SE, Campbell J, Konde A, et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat Commun. 2016;7:13837. https://doi.org/10.1038/ncomms13837.
Miller RE, Brough R, Bajrami I, Williamson CT, McDade S, Campbell J, et al. Synthetic lethal targeting of ARID1A-mutant ovarian clear cell tumors with dasatinib. Mol Cancer Ther. 2016;15:1472–84. https://doi.org/10.1158/1535-7163.MCT-15-0554.
Ogiwara H, Takahashi K, Sasaki M, Kuroda T, Yoshida H, Watanabe R, et al. Targeting the vulnerability of glutathione metabolism in ARID1A-deficient cancers. Cancer Cell. 2019;35:177–90.e8. https://doi.org/10.1016/j.ccell.2018.12.009.
Kuroda T, Ogiwara H, Sasaki M, Takahashi K, Yoshida H, Kiyokawa T, et al. Therapeutic preferability of gemcitabine for ARID1A-deficient ovarian clear cell carcinoma. Gynecol Oncol. 2019;155:489–98. https://doi.org/10.1016/j.ygyno.2019.10.002.
Vierkoetter KR, Ayabe AR, VanDrunen M, Ahn HJ, Shimizu DM, Terada KY. Lynch Syndrome in patients with clear cell and endometrioid cancers of the ovary. Gynecol Oncol. 2014;135:81–4. https://doi.org/10.1016/j.ygyno.2014.07.100.
Bennett JA, Morales-Oyarvide V, Campbell S, Longacre TA, Oliva E. Mismatch repair protein expression in clear cell carcinoma of the ovary: incidence and morphologic associations in 109 cases. Am J Surg Pathol. 2016;40:656–63. https://doi.org/10.1097/PAS.0000000000000602.
Willis BC, Sloan EA, Atkins KA, Stoler MH, Mills AM. Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium. Mod Pathol. 2017;30:1622–32. https://doi.org/10.1038/modpathol.2017.67.
Howitt BE, Strickland KC, Sholl LM, Rodig S, Ritterhouse LL, Chowdhury D, et al. Clear cell ovarian cancers with microsatellite instability: A unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology. 2017;6:e1277308. https://doi.org/10.1080/2162402X.2016.1277308.
Cai KQ, Albarracin C, Rosen D, Zhong R, Zheng W, Luthra R, et al. Microsatellite instability and alteration of the expression of hMLH1 and hMSH2 in ovarian clear cell carcinoma. Hum Pathol. 2004;35:552–9. https://doi.org/10.1016/j.humpath.2003.12.009.
Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30:1080–7. https://doi.org/10.1093/annonc/mdz135.
Shen J, Ju Z, Zhao W, Wang L, Peng Y, Ge Z, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med. 2018;24:556–62. https://doi.org/10.1038/s41591-018-0012-z.
Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33:4015–22. https://doi.org/10.1200/JCO.2015.62.3397.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
None of the authors have any conflict of interest to disclose.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yanaihara, N., Okamoto, A. (2021). Molecular Landscape in Ovarian Clear Cell Carcinoma. In: Isonishi, S., Kikuchi, Y. (eds) Molecular Diagnosis and Targeting for Gynecologic Malignancy. Current Human Cell Research and Applications. Springer, Singapore. https://doi.org/10.1007/978-981-33-6013-6_9
Download citation
DOI: https://doi.org/10.1007/978-981-33-6013-6_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6012-9
Online ISBN: 978-981-33-6013-6
eBook Packages: MedicineMedicine (R0)