Abstract
Hypericum perforatum, generally recognized as St. John’s Wort (SJW), has been used as an alternative form of medicine for centuries in various traditions and cultures all over the world. Traditional uses for this herb include broad wound healing, gastrointestinal issues and pathologies, pain, and bronchitis, among other ailments. However, it has garnered the most attention today as a treatment for mental disorders, namely depression and bipolar disorder. Supplements of SJW are easily and widely accessible due to their commercial popularity. Though a multitude of therapeutic benefits has been reported, the efficacy of SJW remains to be documented clinically. Many studies have found it to have substantial adverse and toxic effects, and at times, potentially fatal drug interactions. The current chapter provides a brief history of H. perforatum, a summary of its active constituents, current and potential therapeutic uses, adverse drug effects, and drug interactions that should be taken into consideration when prophylactically and/or therapeutically using SJW with prescribed medications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Hypericum perforatum, also known as St. John’s Wort (SJW), Klamath weed, goatweed, and Tipton weed, is a flowering perennial plant in the Hypericaceae family. The plant is native to drier sites of humid and subhumid temperate parts of Europe and Asia. H. perforatum usually grows around 1 m in height, with branched, erect stems in the upper section and a clustered, depressed base. Stems are slender and grow superficially within the soil. The green–yellow leaves of the plant have translucent, glandular tissue dots that give the leaves a perforated appearance. Between late spring and early to midsummer, bright yellow flowers with five petals and three bundles of stamens appear (Fig. 1).
H. perforatum reproduces both sexually and asexually. The plant produces 33,000 seeds in a season, which are then dispersed by wind, water, or animals. It is believed that H. perforatum, a species with 32 chromosomes, is an allopolyploid species that resulted from the hybridization between two related species, each with 16 chromosomes, H. maculatum is found through western Europe extending to Siberia, and H. attenuatum is found in regions between Siberia and Korea and eastern China. The common name for the species finds its origin in the feast of St. John the Baptist on June 24th, which coincides with the blossoming and harvesting of the plant. The plant would be hung on doors to ward off evil spirits and protect men from harm. The name Hypericum is derived from Greek root words of “hyper,” meaning “over” and “eikon,” meaning “picture,” referring to traditional uses of the plant that hung the herb over religious pictures (Benzie and Wachtel-Galor 2011). Though the plant is native to temperate regions, its ability to invade plant communities and replace native vegetation in North and South America, India, New Zealand, Australia, and South Africa has led to its classification as a noxious weed. Now it is also the most common species of Hypericum over its native regions. The invasiveness of the plant is also problematic for livestock, as the plant causes damaging photosensitivity reactions in animals that consume the plant. Utilization of St. John’s Wort for medicinal purposes extends back to the ancient physicians of Galen, Dioscorides, and Hippocrates. The herb was commonly used as a diuretic, wound treatment, and for intestinal worms. In the middle Ages, association with priests and the ability to repel demons garnered the plant the name of Fuga demonum or the Scourge of the Devil (Fig. 2).
Oral administration of Hypericum was commonly used to treat mental distress, bronchitis, headaches, gastritis, diarrhea, and gout. In contrast, topical administration of the oil extract of the plant was used for burns and general wound treatment, as well as ulcers and hemorrhoids (Lee 1999). Extracts were prepared by macerating the flowering tops of the plant with olive oil, palm oil, sunflower oil, or wheat–germ oil and exposed to sunlight for 40 days. Today, commercially available pharmaceutical grade preparations of H. perforatum are made from dried aerial parts of the plant, typically using ethanol or methanol for extraction of active components (Benzie and Wachtel-Galor 2011).
2 Chemical Constituents of St. John’s Wort (H. perforatum)
The chemical composition of St. John’s Wort can vary in kind and quantity by the geographical origin of the plant material, growing conditions, harvest time, use of raw or dried plant materials, method of preparation, and storage conditions (Arsić 2016). However, the most common medicinally active classes of compounds consist of naphthodianthrones, phloroglucinols, flavonoids, and phenolic compounds. Notable compounds in the naphthodianthrone class include hypericin, psuedohypericin, protohypericin, and proto-pseudo hypericin (Fig. 3).
Hypericin is a pigment derived from anthraquinone. Found in the black dots in the flowers of H. perforatum, hypericin is also responsible for the red color of St. John Wort oils (Meruelo et al. 1988). Hypericin is highly photoreactive due to its structure. Four hydroxyl groups are adjacent to two carbonyl groups. Due to resonance, the hydrogen transfers between the hydroxyl groups and the carbonyl oxygen occur in the presence of fluorescent light (Fig. 4). This transfer of hydrogen atoms causes acidification of the environment (Fehr et al. 1995).
Flavonoids can be found in ranges of 7–12%, depending on the portion of the flower that is used for preparation (stem, flowers, leaves, etc.). Flavonoids are characterized by a planar structure and are notable for the free radical scavenging capabilities. Common flavonoids found in St John’s Wort include flavonols, flavones, glycosides, biflavones, amentoflavone, myricetin, hyperin, oligomeric proanthocyanidins, and miquelianin (Fig. 5). Flavonol glycosides are the largest group of secondary metabolites obtained from H. perforatum (Benzie and Wachtel-Galor 2011).
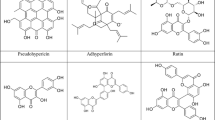
Phloroglucinols include hyperforin, adhyperforin, furohyperforin, and other hyperforin analogs (Fig. 6). Hyperforin has been isolated in concentrations ranging from 2 to 4.5%; however, the instability of the compound in the presence of oxygen and light has made the total synthesis of hyperforin difficult. Essential oils found in H. perforatum include mono- and sesquiterpenes and range in concentrations of 0.05–0.09% (Benzie and Wachtel-Galor 2011).
Phenolic acids are biologically valuable for their antioxidant, anticancer, and anti-inflammatory activities. Using HPLC on dried crude plant material of H. perforatum, 22 different phenolic compounds have been identified. Notable phenolic acids obtained from St. John’s Wort include chlorogenic, caffeic, p-coumaric, ferulic, p-hydroxybenzoic, and vanillic acids (Kwiecień et al. 2015) (Fig. 7).
3 Pharmacodynamic Effects in the Central Nervous System
St. John’s Wort is primarily known for its use as a supplement in treating depression and other depressive disorders. Though depression is typically diagnosed through a patient’s clinical symptoms, there have been hypotheses as to the root cause of the major depressive disorder. The monoamine hypothesis states that deficiencies of monoaminergic transmitters norepinephrine, serotonin, and/or dopamine are responsible for the main symptoms of depression, such as depressed mood, anhedonia, and fatigue. Thus, current pharmacological therapies focus on increasing the concentration of serotonin and/or norepinephrine (tricyclic antidepressants, monoamine oxidase (MAO) inhibitors, and selective serotonin reuptake inhibitors). However, such treatments are not without side effects, and there are severe cases of depression that are resistant to current treatments (Brigitta 2002). Thus, alternative therapies may be used to reduce the number or severity of side effects or to act as supplements to be used in tandem with current therapies. The efficacy of SJW in treating depression has been confirmed in a variety of clinical studies. Öztürk et al. (1996) found that extracts of H. perforatum were as effective as desipramine and trimipramine, two antidepressant drugs, in behavioral models including swimming time, locomotor activity, tail-flick, and hold-board experiments. Various in vitro studies support the antidepressive effects of H. perforatum; however, no single constituent or specific pharmacodynamic effect has been identified, primarily due to the lack of well-understood mechanisms underlying depression itself. It is believed to be the multiple compounds found in the herb that contribute to its effects. Hyperforin is one such compound, which was found to inhibit the reuptake of serotonin, dopamine, noradrenaline, GABA, and l-glutamate (Chatterjee et al. 1998). Hyperforin non-competitively inhibits the reuptake of various neurotransmitters by increasing intracellular Na+ ion concentration. The elevation in Na+ concentration results in a decrease in H+ ion concentrations, which may be due to secondary activation of Na+/H+ exchanger. The sodium ionophore monensin acts as an inhibitor of the synaptosomal reuptake of monoamines. The chronic treatment induces changes on the receptor level, as is often seen with long-term usage of most antidepressants. Hypericum has been shown to downregulate β1-adrenoceptor in the frontal cortex without changing receptor affinity. Chronic administration of hypericum has also been shown to enhance 5-HT neurotransmission by upregulating 5-HT1A receptors in the dorsal hippocampus and forebrain and upregulating 5-HT2 receptors (Nathan 2001).
Flavonoids obtained from standard extracts of St. John’s Wort are effective in protecting PC12 cells from hydrogen-peroxide-induced cell apoptosis by preventing DNA fragmentation and cell shrinkage (Lu et al. 2004). Specifically, the flavonoids quercetin and kaempferol decrease oxidation of the mitochondrial lipid membrane, providing neuroprotective action (Silva et al. 2008). A similar in vitro study has found H. perforatum to be effective against glutamate-induced cellular apoptosis in HT22 hippocampal cell lines. Glutamate is known to increase intracellular Ca2+ concentration, which results in depolarization of the mitochondria membrane and subsequent accumulation of reactive oxygen species. Incubation of cells with glutamate and H. perforatum extracts has been shown to prevent cell death (Breyer et al. 2007). Butterweck has found that SJW is capable of inhibiting uptake of serotonin, dopamine, and norepinephrine with comparable affinity and a significant affinity for adenosine, gamma-aminobutyric acid (GABA), and glutamate receptor. In vivo extracts of H. perforatum were found to cause changes in neurotransmitter concentration in areas of the brain typically afflicted by depression. SJW has also been suggested in gene regulation of the hypothalamic–pituitary–adrenal axis function. Again, the pharmacological effects of SJW have been attributed to hypericin, hyperforin, and flavonoids (Butterweck 2003).
Several studies have investigated potential uses for St. John’s Wort in neurological disorders beyond major depression. Specifically, the herb’s ability to mitigate debilitating symptoms of neurodegenerative diseases like Alzheimer’s disease (AD) and Parkinson’s disease (PD) are of particular interest due to the growing global prevalence of such conditions. Alzheimer’s, the most common neurodegenerative disease, is characterized by the presence of β-amyloid (Aβ) plaque depositions in the cerebral cortex. β- and γ-secretases proteolytically cleave amyloid precursor protein to produce Aβ. Aβ aggregates to form the pathologic hallmark plaques of AD. Both oligomeric and monomeric forms of Aβ are toxic to neurons, resulting in neuronal death and synaptic dysfunction. In a study conducted by Hofrichter et al. extracts of SJW were found to reduce the size and number of Aβ plaques, restore neurons, restore cognition, and activate microglia both in vitro and in vivo of APP-transgenic mice. The actions are believed to be consequences of increased “ATP Binding Cassette Subfamily C Member 1” (ABCC1) transporter activity to export Aβ across the blood–brain barrier and excrete it into the peripheral circulation (Hofrichter et al. 2013). Similar results were obtained in a study investigating the activity of “ATP Binding Cassette Subfamily B Member 1” (ABCB1) transporter in double-transgenic mice treated with SJW extracts over 60 and 120 days, which showed increased activity of ABCB1 expression and reduction in Aβ accumulation (Brenn et al. 2014).
Parkinson’s disease (a movement disorder), the second highest prevalent neurodegenerative disease, is characterized by a loss of dopaminergic neurons in the substantia nigra (SN) and the presence of Lewy bodies in all affected brain regions. Currently, PD is treated primarily with l-DOPA (l-3,4-dihydroxyphenylalanine), a precursor to dopamine that can cross the blood–brain barrier. The dopamine precursor l-DOPA undergoes decarboxylated and thus converted to dopamine by dopa decarboxylase in the presence of pyridoxal phosphate to generate dopamine and alleviate the effects of PD. However, alternative therapies such as MAO inhibitors have been investigated for their antioxidant and neuroprotective effects due to the extensive side effects exhibited by current therapies. Extracts of SJW have been found to inhibit apoptotic cascades and reduce neuronal cell death in the substantia nigra against rotenone in rats by decreasing Bax levels, an apoptotic protein gene (del Rio et al. 2013).
Some studies have investigated the efficacy of H. perforatum in treating epilepsy; however, results reported in the literature are conflicting. One study investigating the effect of different fractions of H. perforatum on kindling epilepsy in chinchilla rabbits found that polar fractions of the extract were able to repress epileptic discharges. In contrast, nonpolar lipid fractions potentiated epileptic activity (Ivetic et al. 2002). Another study by Hosseinzadeh et al. (2005) confirmed H. perforatum’s anticonvulsant activity in pentylenetetrazole and maximum electric shock tests, suggesting a potential benefit of SJW for treating petit mal seizures. However, the use of SJW for treatment of epilepsy is generally cautioned against due to interactions with prescribed anticonvulsants used concomitantly with SJW.
4 Other Indications
Since St John Wort is an herbal remedy, it is classified as a dietary supplement by the Food and Drug Administration, United States of America. Thus, it is not strictly regulated for safety and efficacy compared to pharmaceutical drugs. However, St. John’s Wort can be found in the form of tablets, capsules, teas, and tinctures and is widely available as a dietary supplement for treating depressive disorders and their associated symptoms such as anxiety, fatigue, poor appetite, or insomnia. Various studies, including analyses on tissue samples, animal models, and human clinical trials, appear to verify the efficacy of St John’s Wort in treating depression. Because St John’s Wort supplements are most heavily commercially marketed as a treatment for depression, this has been the most extensively studied claim. However, multiple studies have found several other uses and indications for the herb.
4.1 Antibacterial and Antiviral Effects
According to the Centers for Disease Control and Prevention (CDC), there are 15.5 million physician office visits in the United States each year by patients who have a diagnosis of an infectious or parasitic disease. Every year, the influenza virus kills roughly 0.1% of those infected. Most current therapies rely on antibiotics, antivirals to eradicate the infectious pathogens, or focus heavily on alleviating symptoms of the disease. Extracts of H. perforatum have been found to exhibit antibacterial properties, with hyperforin being the main antibacterial component of the plant (Schempp et al. 1999). Hyperforin has been found to inhibit the growth of gram-positive bacteria, as well as methicillin-resistant and penicillin-resistant S. aureus specifically (Lyles et al. 2017). The hypericin compounds found in H. perforatum also have been shown to express viricidal against enveloped viruses by altering viral proteins and inhibiting the virus’s ability to fuse with the cell membrane (Diwu 1995). In vitro studies support the antiviral activities of hypericin, specifically in action against enveloped viruses in human blood of acquired immunodeficiency syndrome (AIDS) (Holden 1991). Recent studies have found 3-hydroxy lauric acid present in H. perforatum exhibits antihuman immunodeficiency virus (HIV) activity (Maury et al. 2009).
4.2 Anticancer Effects
Cancer is currently one of the prominent causes of global mortality. Current therapies include surgery, radiation therapy, chemotherapy, targeted therapy, hormonal therapy, and immunotherapy. Advances in detection and treatment have increased survival rates generally; however, the treatments are not without detrimental side effects, the most common of which include pain, fatigue, nausea, and vomiting (Miller et al. 2019). St. John’s Wort has been suggested as a potential alternative treatment due to the ability of its bioactive constituents to suppress cell proliferation and induce apoptosis.
Hyperforin and hypericin appear to inhibit tumor cell growth in vitro by inducing cell apoptosis through the activations of caspases and triggering the release of cytochrome c (Schempp et al. 2002). Hyperforin used in tandem with procyanidin B2 was effective in inhibiting in vitro growth of leukemia K562 and U937 cells and human brain glioblastoma cells LN229 (Hostanska et al. 2003). Hypericin has been shown to inhibit the growth of glioma, neuroblastoma, adenoma, mesothelioma, melanoma, carcinoma, sarcoma, and leukemia cells, which has been attributed to photodynamic properties of hypericin, specifically in its ability to generate superoxide radicals that kill tumor cells. Photoactivation of hypericin via white light or ultraviolet light was found to induce apoptosis in malignant cutaneous T cells and lymphoma T cells (Fox et al. 1998).
4.3 Anti-Inflammatory and Pain Effects
Inflammation is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants. Blood vessels dilate, blood flow increases, and neutrophils migrate to targeted areas. Macrophages and dendritic cells release cytokines such as IL-1 and TNF-α, and the pathogen or irritant is broken down. However, chronic inflammation can occur because of a failure to eliminate infectious organisms, recurring episodes of acute inflammation, oxidative stress, or autoimmune disorders. It can lead to a variety of diseases such as rheumatoid arthritis, lupus, asthma, and Crohn’s disease. Current treatments include the use of nonsteroidal anti-inflammatory drugs (NSAIDs), steroids, or disease-modifying immunosuppressant drugs as well as instructing patients to follow an anti-inflammatory diet (Pahwa et al. 2020).
Extracts of H. perforatum have been shown to possess analgesic properties in various animal models of nociception. Preparations of SJW were found to be effective in increasing both the thermal and chemical pain threshold in rats (reference). Specifically, hypericin, obtained through a chloroform fraction, was effective in mediating both thermal and chemical pain, and hyperforin, obtained through a methanol fraction, was effective in thermal antinociception. The same research group also found that thermal antinociception was reversed by naloxone and phorbol 12-myristrate 13-acetate (PMA), a protein kinase C activator. In contrast, chemical antinociception was prevented by PMA (Galeotti et al. 2010). In a different study investigating the efficacy of SJW on diabetic neuropathy in Sprague–Dawley rats with streptozotocin-induced diabetes, freeze-dried seed extracts of SJW were found to verse mechanical hyperalgesia in a dose-dependent manner. The antihyperalgesic effects of SJW in diabetes may be in part due to its observed antihyperglycemic effects (Galeotti et al. 2014). In vivo studies have shown that dosing rats with St John’s wort decreased blood and bowel enzymes associated with colonic inflammation and has less gastric ulcer incidences. This anti-inflammatory activity of H. perforatum can be attributed to quercetin and I3, II8-biapigenin, two oil extracts from SJW (Dost et al. 2009). It is believed the anti-inflammatory responses are a result of inhibition of pro-inflammatory genes (Tedeschi et al. 2003).
4.4 Antioxidant Activity
LC–MS separation and identification of H. perforatum fractions have shown significant antioxidant activity that surpasses that of synthetic antioxidants with specific ability to scavenge DPPH (1,1-Diphenyl-2-picrylhydrazyl) radical, 2,2-Diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl) radical, NO radical, 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH), and inhibition of lipid peroxidation (Orčić et al. 2011). Antioxidant properties primarily stem from the phenolic compounds found in H. perforatum. Specifically, the flavonoids quercetin and hyperoxide were most effective in scavenging the DPPH radical. The two compounds were not as effective in scavenging AAPH.
5 Adverse Drug Effects
A review of data obtained from randomized controlled studies, surveillance studies, and national and international drug safety monitoring bodies indicates H. perforatum to be well-tolerated (Ernst et al. 1998). Adverse reactions are reported in 1–3% of patients taking St. John’s Wort, and typically these effects are mild, including reports of gastrointestinal symptoms (dyspepsia, anorexia, diarrhea, nausea, and constipation), dizziness, confusion, sedation, or tiredness. In certain clinical trials, subjects exhibited dermatologic effects, including rash, itching, and pruritis (Hammerness et al. 2003; Schrader 2000). Hypericin and pseudo-hypericin, chemical constituents of St. John’s Wort, are known photosensitizing agents, and H. perforatum usage can lead to hypericism, or sensitivity to sunlight, by lowering light sensitivity thresholds. Photosensitivity due to the ingestion or topical application of St. John’s Wort has been reported for both humans and animals; however, presentation of symptoms of photosensitivity in humans has been rare. Lane-Brown (2000) has described three such isolated incidents of photosensitivity in humans in which all three patients exhibited bulla, erythema, and/or pain after sun exposure following oral or topical use of St. John’s Wort.
Livestock is particularly vulnerable to the phototoxic effects of H. perforatum, especially grazing animals, light-colored skin that consumes large quantities of the plant. Symptoms following consumption include skin blisters, psychomotor agitation, and in extreme cases, death (Ernst et al. 1998).
5.1 Adverse Drug Effects in the Central Nervous System
Reports of the adverse drug effects of St. John’s Wort in the central nervous system are limited to isolated case studies of patients with complicated past medical histories or concomitant prescription drug use. There have been no studies exhibiting adverse effects in the central nervous system on a wide sample of patients. In a double-blind, randomized, placebo-controlled clinical trial of 200 adults diagnosed with major depression, administration of St. John’s Wort extract for 4 weeks was associated with headaches for 10% or more of patients in the treatment groups with a significant difference in the incidence of headaches in treatment and placebo groups (Shelton et al. 2001). However, multiple other studies have not found the headache to be an adverse effect.
Mania induced by St. John’s Wort has been described in several case studies. Most subjects exhibiting mania, seemingly induced by H. perforatum, had histories of major depression or bipolar disorder. A 28-year-old female with a history of depression exhibited mania induced by high doses of St. John’s Wort (Fahmi et al. 2002). Another female patient with depression resulting from a traumatic brain injury exhibited hypomania after adding St. John’s Wort and Gingko biloba, another herbal supplement, to fluoxetine and buspirone. These symptoms ceased after discontinuation of herbal supplements (Spinella and Eaton 2002). Symptoms indicative of serotonin syndrome have also been reported in association with St. John’s Wort usage. In one case, a 40-year-old man with a history of anxiety disorder, depression, and selective serotonin reuptake inhibitors (SSRI-induced) mania, but not currently on any other medications, reported diaphoresis, hypertension, confusion, dyspnea, weakness, and tremors after ingesting 450 mg of St. John’s Wort daily for 10 days (Parker et al. 2001).
Isolated incidences of psychosis and psychotic symptoms have been observed in patients self-administering SJW. In one case study, a 25-year-old white male with no past medical history of psychiatric disorders and a positive family history of psychiatric disorders presented to an emergency psychiatry service with symptoms of disorganized speech, paranoia, and delusions. The patient had been self-medicating for 4 g of aqueous infusions of SJW four times daily for 3 months before admission into psychiatric services to treat a Helicobacter pylori infection discovered by his general practitioner. The patient self-reported exacerbation of psychotic symptoms during this time (Ferrara et al. 2017).
6 Drug Interactions
St. John’s Wort has been found to reduce the therapeutic efficacy of several prescribed medications when used concurrently. Various studies suggest the primary mechanism for drug interactions between St. John’s Wort and regular prescription drugs lies in hyperforin, one of the plant’s main active ingredients. The most clinically important effect of SJW is its effect on the expression of genes mediating metabolism, calcium regulation, cell proliferation, and apoptosis. Hyperforin is known to activate the pregnane X receptor, leading to the activation of genes expressing the metabolizing enzyme cytochrome P450 3A4 (CYP3A4) and the efflux transporter p-glycoprotein (Krusekopf and Roots 2005). Specifically, St. John’s Wort can promote significant induction of CYP3A4 (Gurley et al. 2005), Cytochrome P450 Family 2 Subfamily E Member 1CYP2E1 (Gurley et al. 2002), and CYP2C9 activities (Yue et al. 2000), and therefore enhance the metabolism of drugs processed by these enzymes. St John’s Wort is also believed to induce P-glycoprotein expression in isolated intestinal cells, lowering plasma concentrations for P-glycoprotein substrates such as digoxin, fexofenadine, and talinolol. Thus, many of the drug interactions observed with SJW are with those prescription drugs that are substrates of CYP enzymes and p-glycoprotein.
6.1 Anticoagulants
In 1998, the Swedish bulletin of the Medical Products Agency (MPA) published findings of four cases of decreased efficacy of warfarin during concomitant St. John Wort usage. Patients exhibited a decreased INR value that was stabilized and returned to baseline levels before SJW usage after discontinuation of SJW (Yue et al. 2000). Though no in vitro studies have been conducted, it is believed SJW induces CYP2C9 activity, which results in an enhanced rate of warfarin inactivation.
6.2 Oral Contraceptives
In a single-blind sequential trial of 16 healthy women treated with a daily low-dose oral contraceptive (norethindrone and ethinyl estradiol) and 300 mg of St. John’s Wort (three times daily), there was a 13–15% reduction in the contraceptive exposure, and women exhibited an increased instance of breakthrough bleeding compared to the control. It is believed SJW increases the metabolic inactivation of norethindrone and ethinyl estradiol (Murphy et al. 2005). Vlachojannis, Cameron, and Chrubasik have obtained similar results. Concomitant use of SJW and “Ortho-Novum 1/35,” an oral contraceptive, led to an increase in the clearance of norethindrone and a reduction in the half-life of ethinyl estradiol. This increased metabolism is believed to be due to increased CYP3A activity (Vlachojannis et al. 2011).
6.3 Antiviral (Anti-HIV) Drugs
SJW has also been found to decrease plasma concentrations of the HIV protease inhibitor, indinavir, by 57% in 16 healthy volunteers (Piscitelli et al. 2000). Indinavir is a substrate for CYP3A4 and P-glycoprotein. Similar results of decreased plasma concentrations of prescription drugs were found in patients taking SJW with nevirapine, a substrate of CYP3A4 and CYP2B6 (de Maat et al. 2001). In one isolated case, a patient experienced an increase in HIV RNA viral load after concomitant use of hypericum with indinavir and lamivudine (Chiba et al. 1996).
6.4 Immunosuppressants
The interactions between SJW and cyclosporine are well documented in several case studies and clinical trials (Breidenbach et al. 2000; Dresser et al. 2003; Mai et al. 2004). SJW taken at therapeutic doses with cyclosporine following heart, renal, or liver transplants showed decreased blood levels, and in some cases, patients experienced acute rejection episodes. Patients clinically improved following discontinuation of the herbal supplement. Cyclosporine is a substrate of P-glycoprotein and is metabolized by CYP3A4. Dresser et al. also believe SJW may also induce multidrug-resistant gene (MDR1) activities. In addition to cyclosporine, blood levels of tacrolimus, another immunosuppressive drug, have also been found to decrease when taken with SJW. Interactions of tacrolimus with other drugs are of special interest, for low levels can result in rejection of transplants, while high levels can lead to nephrotoxicity. In one case, a 65-year-old renal transplant recipient showed a sharp decrease in plasma levels of tacrolimus after 1 month of taking 600 mg SJW to self-treat a depressive mood disorder (Bolley et al. 2002).
6.5 Statins
Simvastatin and atorvastatin are two statins metabolized by CYP34 and are substrates for P-glycoprotein. SJW has been shown to decrease plasma levels of simvastatin and reduce the efficacy of atorvastatin (Sugimoto et al. 2001). Patients with hyperlipidemia taking atorvastatin with SJW experienced a significant increase in serum levels of low-density lipoprotein (LDL) cholesterol and total cholesterol (Andrén et al. 2007).
6.6 Beta-Adrenergic and Calcium Blockers
Talinolol is a beta1-adrenergic blocker and P-glycoprotein substrate. A 12-day treatment of SJW resulted in a 93% increase in clearance of talinolol, which is believed to be due to inductions or MDR1 and P-glycoprotein levels modulated by SJW (Schwarz et al. 2007). SJW has also been shown to decrease the area under the plasma drug concentration–time curve (AUC) of nifedipine and verapamil, and these two calcium channel blockers are used to treat hypertension in healthy volunteers. Both these compounds are metabolized by CYP3A4 (Tannergren et al. 2004).
6.7 Antidepressants
Case reports of mania induced by SJW usage as well as symptoms of central serotonin syndrome show that SJW interacts with 5-HT reuptake inhibitors, such as paroxetine, sertraline, venlafaxine, and nefazodone. Though CYP enzymes of P-glycoprotein do not metabolize these selective serotonin reuptake inhibitors (SSRI), a pharmacodynamic mechanism is believed to be responsible (Lantz et al. 1999; Prost et al. 2000). Other antidepressants that are metabolized by CYP enzymes have shown decreased efficacy when used with SJW. In a study investigating the concomitant use of 75 mg of amitriptyline, a tricyclic antidepressant, twice a day, and 900 mg hypericum extract a day for 12–14 days by 12 participants, the patients were found to have a decreased plasma AUC of amitriptyline by 22%. The same study also found that the AUC for nortriptyline decreased by 41% (Johne et al. 2002).
6.8 Benzodiazepines
CYP3A4 enzymes metabolize alprazolam, midazolam, and quazepam. Accordingly, SJW has shown significant interactions with these medications. Concomitant oral administration of 2 mg alprazolam and one SJW tablet was found to increase the alprazolam clearance by twofold, decrease the alprazolam plasma concentration AUC by twofold, and decrease the half-life for alprazolam from 3.9 to 2.4 h in 12 healthy volunteers (Markowitz et al. 2003). Similar results of reduced plasma levels after use with SJW were observed with midazolam in healthy volunteers (Gurley et al. 2002). Thirteen participants taking 15 mg of quazepam after treatment with 900 mg a day of SJW for 14 days also exhibited a decreased plasma concentration and a significantly reduced AUC (Kawaguchi et al. 2004).
7 Future Recommendations for Prophylactic and Therapeutic Use
Though readily available in US commercial markets, St. John’s Wort does not appear to be a homeopathic panacea that many manufacturers tout it to be. Despite many clinical, in vivo, and in vitro studies, key questions regarding SJW’s molecular mechanisms, efficacy, and adverse effects remain unanswered. Even its therapeutic value in the treatment of depression remains in question, as pointed out by Linde et al. (2008). They identified contradictions in studies investigating the efficacy of SJW in treating major depression conducted by German-speaking and non-German-speaking countries (Linde et al. 2008). Further research is essential in elucidating major adverse impacts, especially when used concomitantly with other drugs. Isolated studies investigating adverse drug interactions must be replicated in rigorous pharmacokinetic trials to deem as sufficient evidence of its adverse effects. Studies investigating the potential neurotoxicity of SJW should have larger sample sizes to determine if observed incidences were isolated or can be generalized. Finally, research on the different formulations, different preparations, and the effects this has on its therapeutic value must also be conducted for safe usage.
Abbreviations
- AAPH:
-
2,2′-Azobis(2-amidinopropane) dihydrochloride
- AD:
-
Alzheimer’s disease
- AIDS:
-
Acquired immunodeficiency syndrome
- AUC:
-
Area under the curve
- Aβ:
-
Amyloid-β
- CDC:
-
Centers for Disease Control and Prevention
- CYP2C9:
-
Cytochrome P450 Family 2 Subfamily C Member 9
- CYP2E1:
-
Cytochrome P450 Family 2 Subfamily E Member 1
- CYP3A4:
-
Cytochrome P450 3A4
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- GABA:
-
Gamma-aminobutyric acid
- HIV:
-
Human immunodeficiency virus
- LC–MS:
-
Liquid chromatography–mass spectrometry
- LDL:
-
Low-density lipoprotein
- l-DOPA:
-
l-3,4-Dihydroxyphenylalanine
- MAO:
-
Monoamine oxidase
- MDR1:
-
Multidrug-resistant gene
- MPA:
-
Medical products agency
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- PD:
-
Parkinson’s disease
- PMA:
-
Phorbol 12-myristate 13-acetate
- SJW:
-
St. John’s Wort
- SN:
-
Substantia nigra
- SSRI:
-
Selective serotonin reuptake inhibitor
References
Andrén L, Andreasson A, Eggertsen R (2007) Interaction between a commercially available St. John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol 63:913–916. https://doi.org/10.1007/s00228-007-0345-x
Arsić I (2016) Preparation and characterization of St. John’s Wort herb extracts using olive, sunflower and palm oils. Acta Facultatis Medicae Naissensis 33:119–126. https://doi.org/10.1515/afmnai-2016-0013
Benzie IFF, Wachtel-Galor S (eds) (2011) Herbal medicine: biomolecular and clinical aspects, 2nd edn. CRC Press, Boca Raton
Bolley R, Zülke C, Kammerl M et al (2002) Tacrolimus-induced nephrotoxicity unmasked by induction of the CYP3A4 system with St John’s wort. Transplantation 73:1009. https://doi.org/10.1097/00007890-200203270-00035
Breidenbach T, Hoffmann MW, Becker T et al (2000) Drug interaction of St John’s wort with cyclosporin. Lancet 355:1912. https://doi.org/10.1016/s0140-6736(05)73359-6
Brenn A, Grube M, Jedlitschky G et al (2014) St. John’s wort reduces Beta-amyloid accumulation in a double transgenic Alzheimer’s disease mouse model-role of P-glycoprotein: St. John’s wort and Alzheimer’s disease. Brain Pathol 24:18–24. https://doi.org/10.1111/bpa.12069
Breyer A, Elstner M, Gillessen T et al (2007) Glutamate-induced cell death in neuronal HT22 cells is attenuated by extracts from St. John’s wort (Hypericum perforatum L.). Phytomedicine 14:250–255. https://doi.org/10.1016/j.phymed.2007.02.001
Brigitta B (2002) Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci 4:7–20
Butterweck V (2003) Mechanism of action of St John’s wort in depression: what is known? CNS Drugs 17:539–562. https://doi.org/10.2165/00023210-200317080-00001
Chatterjee SS, Bhattacharya SK, Wonnemann M et al (1998) Hyperforin as a possible antidepressant component of hypericum extracts. Life Sci 63:499–510. https://doi.org/10.1016/s0024-3205(98)00299-9
Chiba M, Hensleigh M, Nishime JA et al (1996) Role of cytochrome P450 3A4 in human metabolism of MK-639, a potent human immunodeficiency virus protease inhibitor. Drug Metab Dispos 24:307–314
Diwu Z (1995) Novel therapeutic and diagnostic applications of hypocrellins and hypericins. Photochem Photobiol 61:529–539. https://doi.org/10.1111/j.1751-1097.1995.tb09903.x
Dost T, Ozkayran H, Gokalp F et al (2009) The effect of Hypericum perforatum (St. John’s wort) on experimental colitis in rat. Dig Dis Sci 54:1214–1221. https://doi.org/10.1007/s10620-008-0477-6
Dresser GK, Schwarz UI, Wilkinson GR, Kim RB (2003) Coordinate induction of both cytochrome P4503A and MDR1 by St John’s wort in healthy subjects. Clin Pharmacol Ther 73:41–50. https://doi.org/10.1067/mcp.2003.10
Ernst E, Rand JI, Barnes J, Stevinson C (1998) Adverse effects profile of the herbal antidepressant St. John’s wort (Hypericum perforatum L.). Eur J Clin Pharmacol 54:589–594. https://doi.org/10.1007/s002280050519
Fahmi M, Huang C, Schweitzer I (2002) A case of mania induced by Hypericum. World J Biol Psychiatry 3:58–59. https://doi.org/10.3109/15622970209150602
Fehr MJ, McCloskey MA, Petrich JW (1995) Light-induced acidification by the antiviral agent Hypericin. J Am Chem Soc 117:1833–1836. https://doi.org/10.1021/ja00111a024
Ferrara M, Mungai F, Starace F (2017) St John’s wort (Hypericum perforatum)-induced psychosis: a case report. J Med Case Rep 11(1):137. https://doi.org/10.1186/s13256-017-1302-7
Fox FE, Niu Z, Tobia A, Rook AH (1998) Photoactivated Hypericin is an anti-proliferative agent that induces a high rate of apoptotic death of normal, transformed, and malignant t lymphocytes: implications for the treatment of cutaneous lymphoproliferative and inflammatory disorders. J Invest Dermatol 111:327–332. https://doi.org/10.1046/j.1523-1747.1998.00278.x
Galeotti N, Vivoli E, Bilia AR et al (2010) A prolonged protein kinase c-mediated, opioid-related antinociceptive effect of St John’s wort in mice. J Pain 11:149–159. https://doi.org/10.1016/j.jpain.2009.06.013
Galeotti N, Maidecchi A, Mattoli L et al (2014) St. John’s wort seed and feverfew flower extracts relieve painful diabetic neuropathy in a rat model of diabetes. Fitoterapia 92:23–33. https://doi.org/10.1016/j.fitote.2013.10.003
Gurley BJ, Gardner SF, Hubbard MA et al (2002) Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther 72:276–287. https://doi.org/10.1067/mcp.2002.126913
Gurley BJ, Gardner SF, Hubbard MA et al (2005) Clinical assessment of effects of botanical supplementation on cytochrome p450 phenotypes in the elderly: St John’s wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging 22:525–539. https://doi.org/10.2165/00002512-200522060-00006
Hammerness P, Basch E, Ulbricht C et al (2003) St. John’s wort: a systematic review of adverse effects and drug interactions for the consultation psychiatrist. Psychosomatics 44:271–282. https://doi.org/10.1176/appi.psy.44.4.271
Hofrichter J, Krohn M, Schumacher T et al (2013) Reduced Alzheimer’s disease pathology by St. John’s wort treatment is independent of hyperforin and facilitated by ABCC1 and microglia activation in mice. Curr Alzheimer Res 10:1057–1069. https://doi.org/10.2174/15672050113106660171
Holden C (1991) Treating AIDS with Worts. Science 254:522
Hosseinzadeh H, Karimi GR, Rakhshanizadeh M (2005) Anticonvulsant effect of Hypericum perforatum: role of nitric oxide. J Ethnopharmacol 98:207–208. https://doi.org/10.1016/j.jep.2004.12.007
Hostanska K, Reichling J, Bommer S et al (2003) Hyperforin a constituent of St John’s wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cell lines. Eur J Pharm Biopharm 56:121–132. https://doi.org/10.1016/S0939-6411(03)00046-8
Ivetic V, Popovic M, Mimica-Dukic N et al (2002) St. John’s wort (Hypericum perforatum L.) and kindling epilepsy in rabbit. Phytomedicine 9:496–499. https://doi.org/10.1078/09447110260572985
Johne A, Schmider J, Brockmöller J et al (2002) Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St. John’s wort (Hypericum perforatum). J Clin Psychopharmacol 22:46–54. https://doi.org/10.1097/00004714-200202000-00008
Kawaguchi A, Ohmori M, Tsuruoka S-I et al (2004) Drug interaction between St John’s wort and quazepam. Br J Clin Pharmacol 58:403–410. https://doi.org/10.1111/j.1365-2125.2004.02171.x
Krusekopf S, Roots I (2005) St. John’s wort and its constituent hyperforin concordantly regulate expression of genes encoding enzymes involved in basic cellular pathways. Pharmacogenet Genomics 15:817–829. https://doi.org/10.1097/01.fpc.0000175597.60066.3d
Kwiecień I, Szydłowska A, Kawka B et al (2015) Accumulation of biologically active phenolic acids in agitated shoot cultures of three Hypericum perforatum cultivars: ‘Elixir’, ‘Helos’ and ‘Topas. Plant Cell Tiss Organ Cult (PCTOC) 123:273–281. https://doi.org/10.1007/s11240-015-0830-3
Lane-Brown MM (2000) Photosensitivity associated with herbal preparations off St John’s wort (Hypericum perforatum). Med J Aust 172:302. https://doi.org/10.5694/j.1326-5377.2000.tb123965.x
Lantz MS, Buchalter E, Giambanco V (1999) St. John’s wort and antidepressant drug interactions in the elderly. J Geriatr Psychiatry Neurol 12:7–10. https://doi.org/10.1177/089198879901200103
Lee MR (1999) Saint John’s wort (Hupericum perforatum). A balm for hurt minds? Proc R Coll Physicians Edinb 29:253–257
Linde K, Berner MM, Kriston L (2008) St John’s wort for major depression. Cochrane Database Syst Rev 2008:CD000448. https://doi.org/10.1002/14651858.CD000448.pub3
Lu Y-H, Du C-B, Liu J-W et al (2004) Neuroprotective effects of Hypericum perforatum on trauma induced by hydrogen peroxide in PC12 cells. Am J Chin Med 32:397–405. https://doi.org/10.1142/S0192415X04002053
Lyles JT, Kim A, Nelson K et al (2017) The chemical and antibacterial evaluation of St. John’s wort oil macerates used in Kosovar traditional medicine. Front Microbiol 8:1639. https://doi.org/10.3389/fmicb.2017.01639
de Maat MMR, Hoetelmans RMW, Mathôt RAA et al (2001) Drug interaction between St John’s wort and nevirapine. AIDS 15:420–421. https://doi.org/10.1097/00002030-200102160-00019
Mai I, Bauer S, Perloff ES et al (2004) Hyperforin content determines the magnitude of the St John’s wort-cyclosporine drug interaction. Clin Pharmacol Ther 76:330–340. https://doi.org/10.1016/j.clpt.2004.07.004
Markowitz JS, Donovan JL, DeVane CL et al (2003) Effect of St John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 290:1500–1504. https://doi.org/10.1001/jama.290.11.1500
Maury W, Price JP, Brindley MA et al (2009) Identification of light-independent inhibition of human immunodeficiency virus-1 infection through bioguided fractionation of Hypericum perforatum. Virol J 6:101. https://doi.org/10.1186/1743-422X-6-101
Meruelo D, Lavie G, Lavie D (1988) Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: aromatic polycyclic diones hypericin and pseudohypericin. Proc Natl Acad Sci U S A 85:5230–5234. https://doi.org/10.1073/pnas.85.14.5230
Miller KD, Nogueira L, Mariotto AB et al (2019) Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 69:363–385. https://doi.org/10.3322/caac.21565
Murphy PA, Kern SE, Stanczyk FZ, Westhoff CL (2005) Interaction of St. John’s wort with oral contraceptives: effects on the pharmacokinetics of norethindrone and ethinyl estradiol, ovarian activity and breakthrough bleeding. Contraception 71:402–408. https://doi.org/10.1016/j.contraception.2004.11.004
Nathan PJ (2001) Hypericum perforatum (St John’s wort): a non-selective reuptake inhibitor? A review of the recent advances in its pharmacology. J Psychopharmacol 15:47–54. https://doi.org/10.1177/026988110101500109
Orčić DZ, Mimica-Dukić NM, Francišković MM et al (2011) Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem Cent J 5:34. https://doi.org/10.1186/1752-153X-5-34
Öztürk Y, Aydin S, Beis R et al (1996) Effects of Hypericum perforatum L. and Hypericum calycinum L. extracts on the central nervous system in mice. Phytomedicine 3:139–146. https://doi.org/10.1016/S0944-7113(96)80027-4
Pahwa R, Goyal A, Bansal P, Jialal I (2020) Chronic inflammation. In: StatPearls. StatPearls Publishing, Treasure Island, FL
Parker V, Wong AH, Boon HS, Seeman MV (2001) Adverse reactions to St John’s wort. Can J Psychiatr 46:77–79. https://doi.org/10.1177/070674370104600112
Piscitelli SC, Burstein AH, Chaitt D et al (2000) Indinavir concentrations and St John’s wort. Lancet 355:547–548. https://doi.org/10.1016/S0140-6736(99)05712-8
Prost N, Tichadou L, Rodor F et al (2000) Interaction millepertuis-venlafaxine [St. John’s wort-venlafaxine interaction]. Presse Med 29:1285–1286
del Rio MA, Sanchez-Reus MI, Iglesias I et al (2013) Neuroprotective properties of standardized extracts of Hypericum perforatum on rotenone model of Parkinson’s disease. CNS Neurol Disord Drug Targets 12:665–679. https://doi.org/10.2174/1871527311312050013
Schempp CM, Pelz K, Wittmer A et al (1999) Antibacterial activity of hyperforin from St John’s wort, against multiresistant Staphylococcus aureus and gram-positive bacteria. Lancet 353:2129. https://doi.org/10.1016/S0140-6736(99)00214-7
Schempp CM, Kirkin V, Simon-Haarhaus B et al (2002) Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John’s wort that acts by induction of apoptosis. Oncogene 21:1242–1250. https://doi.org/10.1038/sj.onc.1205190
Schrader E (2000) Equivalence of St Johnʼs wort extract (Ze 117) and fluoxetine: a randomized, controlled study in mildmoderate depression. Int Clin Psychopharmacol 15:61–68. https://doi.org/10.1097/00004850-200015020-00001
Schwarz UI, Hanso H, Oertel R et al (2007) Induction of intestinal P-glycoprotein by St John’s wort reduces the oral bioavailability of talinolol. Clin Pharmacol Ther 81:669–678. https://doi.org/10.1038/sj.clpt.6100191
Shelton RC, Keller MB, Gelenberg A et al (2001) Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA 285:1978. https://doi.org/10.1001/jama.285.15.1978
Silva B, Oliveira PJ, Dias A, Malva JO (2008) Quercetin, kaempferol and biapigenin from Hypericum perforatum are neuroprotective against excitotoxic insults. Neurotox Res 13:265–279. https://doi.org/10.1007/bf03033510
Spinella M, Eaton LA (2002) Hypomania induced by herbal and pharmaceutical psychotropic medicines following mild traumatic brain injury. Brain Inj 16:359–367. https://doi.org/10.1080/02699050110103319
Sugimoto K, Ohmori M, Tsuruoka S et al (2001) Different effects of St John’s wort on the pharmacokinetics of simvastatin and pravastatin. Clin Pharmacol Ther 70:518–524. https://doi.org/10.1067/mcp.2001.120025
Tannergren C, Engman H, Knutson L et al (2004) St John’s wort decreases the bioavailability of R- and S-verapamil through induction of the first-pass metabolism. Clin Pharmacol Ther 75:298–309. https://doi.org/10.1016/j.clpt.2003.12.012
Tedeschi E, Menegazzi M, Margotto D et al (2003) Anti-inflammatory actions of St. John’s wort: inhibition of human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1alpha (STAT-1alpha) activation. J Pharmacol Exp Ther 307:254–261. https://doi.org/10.1124/jpet.103.054460
Vlachojannis J, Cameron M, Chrubasik S (2011) Drug interactions with St. John’s wort products. Pharmacol Res 63:254–256. https://doi.org/10.1016/j.phrs.2010.11.011
Yue QY, Bergquist C, Gerdén B (2000) Safety of St John’s wort (Hypericum perforatum). Lancet 355:576–577. https://doi.org/10.1016/S0140-6736(05)73227-X
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Fujihashi, A. et al. (2021). St. John’s Wort: A Therapeutic Herb to Be Cautioned for Its Potential Neurotoxic Effects and Major Drug Interactions. In: Agrawal, D.C., Dhanasekaran, M. (eds) Medicinal Herbs and Fungi. Springer, Singapore. https://doi.org/10.1007/978-981-33-4141-8_15
Download citation
DOI: https://doi.org/10.1007/978-981-33-4141-8_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-4140-1
Online ISBN: 978-981-33-4141-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)