Abstract
Chemical composition of river water is vital to assessment of water quality for irrigation, agriculture and domestic usage. Chemical attributes of riverine water primarily governed by natural weathering of rocks have become overshadowed by increasing range of anthropogenic activities. Progressive pollution of the river waters are critical as rivers in floodplain zones recharge ground water; a vital source of drinking water in India. The degree of impact of the anthropogenic activities within the surface waters of the developing countries has expanded significantly amid the past decades. Hence identification and quantification of natural as well as anthropogenic impact and understanding the source of contaminant is fundamental. In addition continuous monitoring of river water quality is required in order to maintain freshwater assets. The Brahmaputra River is the lifeline of Assam. People residing along its bank directly or indirectly are heavily dependent on the river for their livelihood. Majority of the population in the region is dependent on an agricultural economy. In the present study different multivariate statistical techniques such as cluster analysis (CA), principal component analysis (PCA) and factor analysis (FA) were applied for evaluation of spatial and temporal variations of water quality of the Brahmaputra River for two years (2011–2014) by monitoring nine sampling sites from upstream to downstream along the Assam stretch. The present study highlights the usefulness and need of multivariate statistical assessment of the database in identification of main process that influence the water quality of the river and probable source of contamination.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Rivers are progressively changed in terms of flow and form, and those changes are not only both events of the anthropocene but also worldwide threats to water security (Poff and Matthews 2013) During the past decades the impact of the anthropogenic activities in the surface waters has increased significantly. The impact of humans in river ecologies over the past 250 years has been intense. Freshwater pollution problems are receiving much attention worldwide because of their impacts on social, economic and cultural life of the people (Kannel et al. 2007). Anthropogenic activities and natural processes have lead to serious decline in surface waters impairing their use for agricultural, drinking, recreational, other purposes (Carpenter et al. 1998).
Progressive pollution of the river waters are critical as rivers in floodplain zones recharge ground water; a vital source of drinking water in India. Chemical composition of river water is vital to assessment of water quality for irrigation, agriculture and domestic usage. Chemical attributes of riverine water primarily governed by natural weathering of rocks have become overshadowed by increasing range of anthropogenic activities. Rivers are major part of the worldwide water cycle which plays a vital role in the geochemical cycling of elements. The composition of surface water is subordinate on common components (geographical, meteorological, hydrological and organic) within the drainage basins and shifts with regular contrast in runoff volumes, climate conditions and water levels (Sarin 2001; Krishnaswami and Singh 2005). The water chemistry of rivers relies upon contributions from the atmosphere, the geology through which it travels and from anthropogenic activities. Study of chemical composition of river water is of great importance for quantifying the dissolved load and various sources of major ions to rivers and estimation of weathering and CO2 consumption rate (Gaillardet et al. 1999; Grasby and Hutcheon 2000). Investigation of chemical composition of river water is of incredible significance for evaluating the dissolved load and different sources of major ions to rivers and estimation of weathering and CO2 consumption rate (Jha et al. 2009; Singh et al. 2005). In addition weathering of rocks contributes majority of ions in the river system. Therefore seasonal variation in major ion composition in the river reflects dominant weathering process.
In India, a few researchers discussed on major ion chemistry within the Himalayan and Peninsular river basins and set up geochemical budget due to catchment weathering in expansive waterway frameworks. (Subramanian 1983; Sarin et al. 1989; Ramesh and Subramanian 1988; Sharma and Subramanian 2008). However, still long term understanding of hydro-geochemical process within the Himalayan River like Brahmaputra River is extremely important. Therefore the main objectives of the present study (1) To understand spatial and temporal variations in major ion chemistry of the Brahmaputra River (BR) (2) To evaluate freshwater quality through multivariate statistical tool.
10.2 Study Area
The Brahmaputra River originates on the Angsi Glacier, situated on the northern side of the Himalayas in Burang Province of Tibet, at an elevation of 5300 m above mean sea level (Sarin et al. 1989). The Brahmaputra River discharges high sediment and is the fifth largest river in the world (Berner et al. 1996) and second with respect to sediment transport per unit area (Milliman and Meade 1983). The River has high regular variety, high sediment load, and is described by continuous changes in channel position. The River has high seasonal variation, high sediment load, and is characterized by frequent changes in channel position (Thorne et al. 1993). The Brahmaputra River has a drainage area of 580,000 km2 and shared by China (50.5%), India (33.6%), Bangladesh (8.1%), Bhutan (7.8%), (Berner et al. 1996; Subramanian 2004). The width of the river varies from 3 km to 18 km with an average of 10 km in the plains of Assam and Bangladesh. Geologically, the Brahmaputra Basin is divided into Higher and Lesser Himalaya sequences consist of schists, marbles with amphibolites, and quartzites (Sarin et al. 1989). All over the Brahmaputra valley of Assam comprises of consists of older and newer alluvium deposits. Alluvium shaped amid the Pleistocene age (older alluvium) is found in marginally undulating zones on the two sides of the Brahmaputra River and the new alluvium soils close to the river comprise of alluvial materials washed down from the highland areas. On the northern side, alluvial plain of Assam abuts Siwalik ridges of the Himalayas, which are in turn overlain by highly tectonized Paleozoic sediments. On the northern side, alluvial plain of Assam adjoins Siwalik ridges of the Himalayas and overlain by exceptionally tectonized Paleozoic sediment. The alluvial deposits are occurring in the eastern side of Assam valley. The Tertiary rock sequences occur in Patkai and Naga Hills on the southern side of the alluvial plain and comprises of dark grey shales, sandstones and shales with coal seams, clay and conglomerates.
The Brahmaputra Valley is basically a Quaternary fill valley with many separated sedimentary residual hill in Upper Assam and inselbergs and hill of gneissic rocks within the Darrang, Kamrup, and Goalpara area. The Brahmaputra and its tributaries experience frequently changing meandering course due to lateral erosion, periodic, local and sudden changes in the basement levels due to tectonic activity.
10.3 Materials and Method
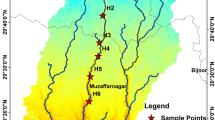
The water samples (n = 54) were collected from main stream of the Brahmaputra River in pre-monsoon, monsoon and post-monsoon consecutively in three different seasons during 2011–2012 and 2013–2014. Nine sampling locations were selected for this study covering upstream to downstream of the Brahmaputra River namely; Guijan (B1), Roumeria (B2), Dibrugarh (B3), Jorhat (B4), Dhansiri Mukh (B5), Tezpur (B6), Guwahati (B7), Jogighopa (B8), Dhubri (B9) are shown in Fig. 10.1. Water samples were collected in polypropylene bottles. Samples were brought to the laboratory and were filtered using 0.45μm Millipore filter papers and acidified with nitric acid. In- situ measurements included pH, electrical conductivity (EC), total dissolved solid (TDS) which were measured using Multiparameter Water Quality Portable Meter (Hanna model-HI 9828) and bicarbonate (HCO3−) was measured by potentiometric titration method in unfiltered samples. For anion analysis, samples were stored below 4 °C until analysis. All chemicals used in the study were obtained from Merck, India and were of analytical grade. All glass- wares and other sample containers were cleaned with milli-Q water. The major ion analyses were carried out as per standard methods given in American Public Health Association (1995), {Calcium (Ca2+) and Magnesium (Mg2+) ICP-OES}; {Sodium (Na+) and Potassium (K+), Flame Photometer}; {Phosphate (PO43−)}, {Dissolved silica (H4SiO4), Sulphate (SO42−), Nitrate (NO3−), UV Spectrophotometer};{Chloride (Cl−), titration method}. Statistical analyses (ANOVA and PCA) were performed using SPSS Version 22.0.
10.4 Results and Discussion
10.4.1 Hydro-geochemical status of the Brahmaputra River
Spatio- temporal variations of major ions of the Brahmaputra River are shown in Table 10.1 and Fig. 10.2.
All units are in µM except EC (µS cm−1), TDS (mgL−1) and pH; *SD-standard deviation.
10.4.2 Spatial variation
pH of river water showed slightly neutral to alkaline in nature and there was no so much in variation. EC and TDS concentration was observed to be high in downstream location (B7 and B9) amid pre-monsoon and post-monsoon seasons which might be because of different anthropogenic activities such as dredging, sand recuperation, transportation of ship, fishing, cremation on the banks and cultivation. High HCO3− concentration at S1 and S7 might be because of seasonal and anthropogenic factors. SO42− concentration was maximum at B5 and B8; its increase towards downstream of the river showing anthropogenic impact. Cl− content was found to be highest at B5 and B6. NO3− and PO43− content found to be highest in upstream locations might be attributed to occurrence of several tea gardens and populated villages with cultivable land along the river bank. H4SiO4 content was found to be high at downstream location. B9. Na+ concentration was found to be high at B2 and B6, K+ at B1 and B8. The concentration of Ca and Mg show increasing trend from upstream to downstream.
10.4.3 Temporal variation
A comparative statistical summary of the Brahmaputra River water chemistry from different seasons during 2011–2012 and 2013–2014 is showed in Table 10.1. Average pH value of water samples in all year in different seasons varied from 7.48 to 7.91 indicates alkaline in nature. Variation in pH values during various period of the year were ascribed to factors like exclusion of CO2 by photosynthesis through bicarbonate degradation, dilution of water with fresh water influx, decrease in temperature and decay of natural matter (Rajasegar 2003).
EC is an important water quality parameter shows presence of dissolved ions in the water. The average value of EC was maximum in pre-monsoon season followed by post- monsoon season attributed to high concentrations of dissolved salts and presence of more alluvial-derived soil (entisol, inceptisol, alfisol) and less resistant minerals in the catchment area. Low EC values during monsoon might be owing to dilution effect by rain water. In general, the TDS values are likely to be diluted by surface runoff in monsoon season and for most rivers there is an inverse correlation between discharge rate and TDS (Charkhabi and Sakizadeh 2006). TDS value in post monsoon season was increased which may be due to increase in dissolved minerals.
Temporal change in the major ion concentration of the Brahmaputra River from 2011 to 2014 is shown in Fig. 10.2. Seasonal difference of average anion content in water samples in decreasing order are as follow: HCO3− > SO42− > Cl− > H4SiO4− > NO3− > PO43−. Average cations concentrations in all seasons in decreasing order are as follows: Ca2+ > Mg2+ > Na+ > K+.
Among dissolved anion, HCO3− was present in high concentration in pre–monsoon and monsoon seasons for both year. High bicarbonate concentration in river water samples indicate bicarbonate is formed via mineral weathering and influence of anthropogenic activity. On the contrary decreased HCO3− concentration was caused by low pH in post-monsoon season. Average Cl− content was maximum in monsoon season might be due to mixing of runoff water which bring larger amount of salts from catchment area. Other probable sources of Cl− are release of domestic and industrial waste to the river. EC is a good marker for the chloride and patterns appear that low Cl− concentration in 2013–2014 was due to low EC. Average NO3− content was highest in monsoon season and PO43− content was highest in pre-monsoon season for all the year. Leaching of fertilizers from the agriculture overwhelmed zones; sewage effluents and decay of organic matter are potential source of NO3− and PO43−.
Average SO42− concentration was high in monsoon season for all the year. Oxidation of pyritic sediments and dissolution of gypsum or anhydrites is major donor of sulphate in water (Gansser 1964; Das et al. 2016a; Patel et al. 2019a, b). Other probable anthropogenic sources of SO42− are runoff from agricultural land and untreated effluents released within the river system. Average H4SiO4 content was found to be high in monsoon season which may be due to high discharge during monsoon season. In addition weathering of igneous and metamorphic rocks contributes higher concentrations of silica in water (Stallard et al. 1981; Das et al. 2016a) and below pH 9 silicon is released by weathering as silicic acid (H4SiO4) (Iler 1979). Hence weathering of minerals is the major source of silica in the freshwater system.
Average Na+ content was highest in pre-monsoon season for 2011–2012 and in monsoon for 2013–2014. Weathering of silicate minerals, halite dissolution and atmospheric precipitation are the probable source of sodium. Average potassium (K+) concentration was high in monsoon season for both years. It was found that there was not much variation in K+ concentration during the three seasons. Weathering of rocks and minerals in the catchment are the key contributor of K+ in water. Other potential sources of K+ are decomposition of organic matter and springs/groundwater (Krishnaswami and Singh 2005).
Among the cations, Ca2+ was the dominant ion concentration and average Ca2+ content was highest in pre-monsoon and post-monsoon season. Dissolution of carbonate rocks (limestones, dolomite) is the major source of Ca2+ in water (Nikanrov and Brazhnikova 2006). Average Mg2+content were high in pre-monsoon for all the year. The high concentration of Mg in non-monsoon season may be due to anthropogenic impact. It was reported that magnesium in natural water is primarily supply through ferromagnesian minerals (e.g. olivine, pyroxene, amphiboles) which are present in igneous rocks and sedimentary rocks.
In general, results show that ion concentration was high during pre-monsoon followed by monsoon and post-monsoon season. In 2011–2012 concentration of HCO3−, Ca2+, Mg2+, Na+, K+ was high in pre-monsoon season. In 2013–2014 concentration of Cl−, SO42−, H4SiO4, HCO3−, Na+, K+ was found to be high in monsoon season. For 2011–2012, pre-monsoon was dominant and for 2013–2014 monsoon and post-monsoon were dominant. The high Maximum ion concentration in non-monsoon season credited anthropogenic impact and in monsoon season was because of flow of runoff, influence through cyclic salts. Taking into account the most land use for agriculture, manures and pesticides are vital supply of NO3− and PO43− (Patel et al. 2019b).
The quality of hydro-geochemical data of the BR is measured using charge balance (µeq) between total dissolved cations (TZ+= Na+ + K+ + 2Ca2+ + 2Mg2+) and total dissolved anions (TZ− = Cl− + HCO3− + 2CO32− + 2SO42−). Water samples from the mainstream show specific charge balance typically higher than 10% which is evaluated using Eq. (10.1) (Friedman and Erdmann 1982):
10.5 Identification of Hydrogeo-Chemical Processes
Groundwater chemistry in the study area is regulated by various processes and mechanisms. Thus Gibbs plot is employed in the present study to understand the primary mechanism controlling water chemistry i.e. atmospheric precipitation, mineral/rock weathering and evaporation (Gibbs 1970). Various workers have applied Gibbs plot in tracing major mechanisms that control water chemistry for rivers like Ganga, Cauvery, Krishna, Amazon. In Fig. 10.3 Gibbs plots have been used to show the relationship between TDS and (Na + K)/(Na + K + Ca) or Cl/(Cl + HCO3−) ratios, so as to assess the significance of three major natural mechanisms controlling water chemistry. Figure 10.3 illustrates that most of the samples falls in the weathering zone except few samples in the monsoon 2011 and 2013, indicating that the chemical weathering of the rock-forming minerals is the primary mechanism controls the major ion chemistry of the Brahmaputra River. Monsoon season of both cycles seem to have differences in terms of cationic input.
10.5.1 Characterization of chemical facies
Piper diagram is the indicative of overall water type which indicates that Ca is the dominant cation in all samples and the difference among them are due to their anionic compositions. Overall, majority of BR river water samples fall in the category of Ca–HCO3 along with Ca–Mg–HCO3–SO4 type (Fig. 10.4). Chemical weathering of silicates with carbonic acid seems to be the dominant process here which releases Na, K, Mg, Ca from them to solution along with HCO3. SO4 being the second dominant anion attributed to the oxidation of pyrites and organic acids present in the upper layers of sediment, the humic and fulvic acids (Berner and Berner 1996).
10.5.2 Percent of sodium (Na%)
Sodium content expressed in terms of sodium percentage or soluble sodium percentage defined as
where, all ionic concentrations are expressed in meq/l
Table 10.2 shows the result of Na% values of the BR ranges in 2011 ranges from 11.7 to 21.1 (mean = 17.3 ± 3.22) in pre-monsoon, 15.4–34.5 (mean = 22.4 ± 5.88) in monsoon, 15.9–33.5 (mean = 24.9 ± 5.06) in post-monsoon. In 2013, % of Na varies from 16.5 to 25.8 in pre-monsoon (mean = 21.3 ± 4.17), 16.3–30.70 (22.0 ± 4.47) in monsoon, 14.2–30.2 (21.6 ± 4.67) in post-monsoon.
High level of percentage of sodium (%Na) in water (>15%) is vital for soil richness, plant development and better condition as it lessens the permeability of the soil (Todd 1980). In the present study it was found that.% of Na in BR is suitable for agriculture purposes and monsoon season is most suitable for irrigation purpose. In Table 10.3 most of the BR water samples in all seasons fall in excellent to good categories indicating their suitability for irrigation. No water sample of the BR is fall in unsuitable category.
10.5.3 Sodium Adsorption Ratio (SAR)
Sodium adsorption ratio, an important determining suitability for irrigation, is a measure of alkali/sodium hazard to crops (Thomas et al. 2014). The excess sodium or limited calcium and magnesium are evaluated by SAR which is expressed as
All concentrations are in meqL−1
Irrigation waters are classified based on sodium adsorption ratio (WHO 2011). As per Richards (1954), water with SAR ≤10 is considered as of excellent quality. In Table 10.3, mean SAR in BR was found to be is 0.332 ± 0.07 (0.26–0.44) in pre-monsoon, 0.43 ± 0.15 (0.25–0.71) in monsoon, 0.46 ± 0.13 (0.24–0.67) in post-monsoon. Based on the Bower (1978) classification, the water samples belong to ‘no problem category’ of irrigation water quality (i.e., SAR < 6), thus water is suitable for irrigation purpose. According to the SAR classification, 100% of BR water samples in all seasons fall in excellent category which can be used for irrigation on almost all soil.
10.6 Effective CO2 Pressure
In aquatic system the partial pressure of CO2 (pCO2) reveal both internal carbon dynamics and external biogeochemical processes in terrestrial ecosystems (Jones et al. 2003). When carbonate rocks weathers, alkalinity is produced leading to an increase in pH and a decrease in pCO2. The effective CO2 pressure (Log pCO2) for different seasons has been calculated from pH values and HCO3− concentration. The average pCO2 values was found to be 10−2.4 in pre-monsoon, 10−2.9 in monsoon and 10−3.2 in post-monsoon are higher than the atmospheric value, i.e. 10−3.5. The higher pCO2 in the river water could be a worldwide drift showing that the river water appears disequilibrium with the atmosphere (Garrels and Mackenzie 1971). This might be because of input of groundwater containing high CO2 and the slow rate of its re-equilibrium between surface water and the atmosphere by release of excess CO2 (Subramanian et al. 2006; Sharma and Subramanian 2008).
10.7 Multivariate Analysis
10.7.1 Principal Component Analysis (PCA)
Table 10.4 shows the result of factor analysis, for three seasons i.e. pre-monsoon, monsoon and post-monsoon seasons. In pre-monsoon four factors were identified which controls the river chemistry. Factor 1 accounted for 33.6% variance and dominant variables are pH, EC, TDS, HCO3−, Ca2+ and Mg2+ indicate contribution of ions through carbonate weathering. Factor 2 accounted for 15.2% variance and dominant variables are Cl− and SO 24 − indicating oxidation of pyretic sediments and gypsum or anhydrites were the source of sulphate in water. Negative loading of PO 3–4 indicate less anthropogenic input. Factor 3 accounts for 14.6% variance with high positive loading of Na+ indicate contribution through weathering process and negative loading of NO3− indicate influence of less anthropogenic activities. Factor 4 accounts for 12.4% with high positive loading of K+ and Mg2+ indicate anthropogenic influence.
In the monsoon season, four factors were identified. Factor 1 accounted for 27.3% variance and high positive loading of EC, TDS, K+ and Ca2+ as variables indicate contribution of weathered material in the river system. Negative loading of Cl− and Na+ indicate evaporation enrichment. Factor 2 accounted for 14.4% variance and dominant variables were PO 34 , H4SiO4 and K+ input of anthropogenic runoff. Factor 3 accounted for13.6% variance and dominant variables were HCO3− and SO42−. Factor 4 accounted for 13.2% and dominant variables were pH and negative loading of Mg2+.
In post-monsoon season, four factors were identified. Factor 1 accounted for 26.0% variance. The variables were HCO −3 , SO 2−4 , PO 3−4 , H4SiO4 and K+ indicates contribution of ion through anthropogenic activities. Factor 2 accounted for 22.2% and variables were EC, TDS, Cl− and Na+ indicated contribution from atmospheric input. Factor 3 accounted for 13.5% variance and dominant variables were pH and NO3− indicated pH regulated the contribution of ion and influence of anthropogenic input. Factor 4 accounted for 11.8% variance and dominant variables were Na+ and Mg2+.
10.7.2 Cluster analysis
Cluster analysis (CA) has been used to identify similarities and differences between water qualities of different sampling sites. Figure 10.5 illustrates the clustering method of groups of similar locations. In Fig. 10.5 CA is present in the form of dendrogram that classifies locations into three significant clusters: cluster 1 (locations 3, 9, 7) and cluster 2 (locations 1, 2 and 6) and cluster 3 (locations 4, 5 and 6). In cluster 1, sites 7 and 9 are located in downstream of the Brahmaputra River. These sites likely receive pollution from anthropogenic source such as industry effluents, oil spill etc. In cluster 2, sites 1 and 2 are located in upstream region which are slightly polluted and are less influenced by industrial discharge.
In cluster 3, sites 4 and 5 are closely associated which are located in upstream region and less polluted as compared to downstream sites (Das et al. 2016b).
10.8 Conclusion
The spatial-temporal variation of water quality from 2011 to 2014 was studied from upstream to downstream of the Brahmaputra River. Water quality of the Brahmaputra River was found to be neutral to alkaline in nature. High concentration of EC and TDS values in pre and post-monsoon season implies contribution of runoff water which carries larger amount of salts from catchment region. Concentrations of ions were higher during pre-monsoon followed by monsoon and post-monsoon season. For 2011–2012 pre-monsoon was dominant and for 2013–2014 monsoon and post-monsoon was dominant. The high concentration of ion in non-monsoon season was credited to anthropogenic impact; and in the monsoon season is due to influx of runoff. In the Brahmaputra River, temporal variation of average anion concentration is in order: HCO3− > SO42− > Cl− > H4SiO4 > NO3− > PO43− and cation concentration is in the Ca2+ > Mg2+ > Na+ > K+ in all the seasons. High average molar ratio of (Ca2+ + Mg2+)/(Na+ + K+) and (Na+ + K+)/Tz+ suggested contribution of ions through carbonate and silicate minerals. The average precipitation corrected molar ratio of (Ca2+/Na+), (HCO3−/Na+) and (Mg2+/Na+) reflected influence of silicate and carbonate weathering. Relatively lower ratio of (Ca2+ + Mg2+)/(Na+ + K+) during monsoon season of 2013–2014 signifies addition of Na+ and K+ ions through anthropogenic source, which may be due to high runoff.
Gibbs plot implied weathering as the major mechanism that controls the water chemistry of the Brahmaputra River. Dominant hydro-chemical facies in the BR are Ca–HCO3 and Ca–Mg–HCO3–SO4. High effective CO2 pressure (pCO2) in the river water shows that weathering of rocks that regulate the water chemistry of the River exerts a strong influence on the global climate. The water chemistry of the Brahmaputra River is chiefly governed by weathering of rocks with minor contributions from atmospheric and anthropogenic sources. Therefore regular monitoring of hydro-chemical composition of the river water is essential in the context of climate change.
References
Berner EK, Berner RA (1996) Global Environment water, air, and geochemical cycles. Prentice-Hall, New Jersey
Bower H (1978) Groundwater hydrology. McGraw Hill, New York
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharply AN, Smith VH (1998) Non-point pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8(3):559–568
Charkhabi AH , Sakizadeh M (2006) Assessment of spatial variation of water quality parameters in the most polluted branch of the Anzali Wetland, Northern Iran. Pol J Environ Stud15(3):395–403
Das P, Sarma K, Jha PK Jr, Herbert R, Kumar M (2016a) Understanding the cyclicity of chemical weathering and associated CO2 consumption in the Brahmaputra River Basin (India): the role of major rivers in climate change mitigation perspective, Aquat Geochem 1–27. Doi: 10.1007/s10498-016-9290-6
Das N, Deka JP, Shim J, Patel AK, Kumar A, Sarma KP, Kumar M (2016b) Effect of river proximity on the arsenic and fluoride distribution in the aquifers of the Brahmaputra floodplains, Assam, northeast India. Groundw Sustain Dev 2:130–142
Friedman LC, Erdmann DE (1982) Quality assurance practices for analyses of water and fluvial sediment, Tech. Water Resources Inc Book 5 (Ch. A6)
Gaillardet J, Dupre B, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Sci 4:170
Grasby SE, Hutcheon I (2000) Chemical dynamics and weathering rates of a carbonate basin Bow River, Southern Alberta. Appl Geochem 15:67–77
Garrels RM, Mackenzie F (1971) Evolution of sedimentary rocks. Norton
Gansser A (1964) Geology of the Himalayas. Wiley, London
Hren MT, Chamberlain CP, Hilley GE, Blisniuk PM, Bookhagen B (2007) Major ion chemistry of the Yarlung Tsangpo-Brahmaputra River: chemical weathering, erosion and CO2 consumption in the southern Tibetan plateau and eastern syntaxis of the Himalaya. Geochim Cosmochim Acta 71:2907–2935
Iler R (1979) The chemistry of silica: solubility, polymerization, colloid and surface properties and biochemistry. Wiley, New York
Jones JB, Stanley EH, Mulholland PJ (2003) Long-term decline in carbon dioxide supersaturation in rivers across the contiguous United States. Geophys Res Lett 30(10)
Jha PK, Tiwari J, Singh UK, Kumar M, Subramanian V (2009) Chemical weathering and associated CO2 consumption in the Godavari river basin, India. Chem Geol 264:364–374
Kannel PR, Lee S, Kanel SR, Khan SP, Lee YS (2007) Spatial–temporal variation and comparative assessment of water qualities of urban river system: a case study of the river Bagmati (Nepal). Environ Monit Assess 129(1–3):433–459
Krishnaswami S, Singh SK (2005) Chemical weathering in the river basins of the Himalaya, India. Curr Sci 89(5):841–849
Milliman JD, Meade RH (1983) World-wide delivery of river sediment to the oceans. J Geol 91(1):1–21
Nikanorov AM, Brazhnikova LV (2009) Water chemical composition of rives, lakes and wetlands. Types Prop Water 2:42–80
Patel AK, Das N, Goswami R, Kumar M (2019a) Arsenic mobility and potential co-leaching of fluoride from the sediments of three tributaries of the Upper Brahmaputra floodplain, Lakhimpur, Assam, India. J Geochem Explor 203:45–58
Patel AK, Das N, Kumar M (2019b) Multilayer arsenic mobilization and multimetal co-enrichment in the alluvium (Brahmaputra) plains of India: A tale of redox domination along the depth. Chemosphere 224:140–150
Poff NLR, Matthews JH (2013) Environmental flows in the Anthropocence: past progress and future prospects. Curr Opin Environ Sustain 5:667–675
Ramesh R, Subramanian V (1988) Nature of the dissolved load of the Krishna river basin, India. J Hydrol 103:139–155
Rajasegar M (2003) Physico-chemical characteristics of the Vellar estuary in relation to shrimp farming. J Environ Biol 24(1):95–101
Richards LA (1954) Diagnosis and improvement of saline alkali soils, US Department of Agriculture, Hand Book 60, p 160
Sarin MM (2001) Biogeochemistry of Himalayan Rivers as an agent of climate change. Curr Sci 81(11):1446–1450
Sarin MM, Krishnaswami S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of the Ganga-Brahmaputra River system: weathering processes and fluxes to the Bay of the Bengal. Geochim. Cosmochim. Acta 53(5):997–1009
Sharma SK, Subramanian V (2008) Hydrochemistry of the Narmada and Tapti Rivers, India. Hydrol Process 22:3444–3455
Stallard RF, Edmond JM (1981) Geochemistry of the Amazon 1. Precipitation chemistry and the marine contribution to the dissolved load at the time of peak discharge. J Geophys Res 86(10):9844–58
Subramanian V (1983) Factors controlling the chemical composition of rivers of India. In: Dissolved loads of rivers and surface water quantity/quality relationships, Webb BW (ed) IAHS Publication no. 141. IAHS Press: Wallingford, pp 145–151
Subramanian V (2004) Water Quality in South Asia. Asian J Water Environ Pollut 1(1–2):41–54
Singh SK, Sarin MM, France-Lanord C (2005) Chemical erosion in the eastern Himalaya: major ion composition of the Brahmaputra and δ 13 C of dissolved inorganic carbon. Geochim Cosmochim Acta 69(14):3573–3588
Subramanian V, Ittekkot V, Unger D, Madhavan N (2006) Silicate weathering in South Asian Tropical River Basins. In: The silicon cycle: human perturbations and impacts on aquatic systems. SCOPE Series, vol 66. Island Press, Washington, D.C
Thorne CR, Russell APG, Alam MK (1993) Planform pattern and channel evolution of the Brahmaputra River, Bangladesh. In: Best JL, Bristow CS (eds) Braided Rivers. Geological Society of London, London, pp 257–276
Thomas J, Joseph S, Thrivikramji KP, Manjusree TM, Arunkumar KS (2014) Seasonal variation in major ion chemistry of a tropical mountain river, the southern Western Ghats, Kerala, India. Environ Earth Sci. 71(5):2333–2351
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York, p 535
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization. WHO Press, Geneva
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Das, P., Kumar, M. (2020). Assessment of Water Quality Using Multivariate Analysis—A Case Study on the Brahmaputra River, Assam, India. In: Kumar, M., Snow, D., Honda, R. (eds) Emerging Issues in the Water Environment during Anthropocene. Springer Transactions in Civil and Environmental Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-32-9771-5_10
Download citation
DOI: https://doi.org/10.1007/978-981-32-9771-5_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-32-9770-8
Online ISBN: 978-981-32-9771-5
eBook Packages: EngineeringEngineering (R0)