Abstract
The present study aims at the in vitro cytotoxic effect of epiphytic orchids, viz., Acampe praemorsa and Aerides odorata. Plants were collected from the Eastern Ghats of the Visakhapatnam district, Andhra Pradesh, India. Leaf materials were shade dried; ethyl acetate and methanolic leaf extracts were prepared for this investigation. The leaf extracts were tested for their inhibitory effect on HeLa and MCF-7 cell lines, which were evaluated by the MTT assay; these plants showed good anticancer activity and did not show any adverse effect to normal cells. Both extracts showed good anticancer activity on the MCF-7 cell line than on the HeLa cell line. The methanolic leaf extract of Aerides odorata has significant cytotoxicity effect on the MCF-7 with concentration ranging from 5 to 100 μg/ml, with an IC 50 (μg/ml) value 26.211. The findings from this study showed that the methanolic extract of the A. odorata leaf possesses vast potential as a medicinal drug in breast cancer treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The Orchidaceae is one of the largest families with more than 30,000 species spread over to 750 genera distributed throughout the world from the tropics to the Alpine states (White and Sharma 2000). They are the most diverse among the flowering plant families with over 184 genera and 1331 species in India (Kumar and Manilal 1994). The usage of orchids in medicine has a long history, as indicated in ancient records stating that these plant parts have been utilised to treat various diseases by the Chinese, Indians, Sumerians and Egyptians (Bulpitt 2005). In many countries like China and in some parts of Europe, America, Australia and Africa, orchids have been used as traditional drugs since times immemorial (Dash et al. 2008). The earliest Middle East report of plant remedies is in a 4000-year-old Sumerian clay tablet, which included some orchids (Kong et al. 2003). They are also one of the ingredients in ancient Indian systems of medicine called Ayurveda (Bijaya pant 2013).
The present study deals with the in vitro cytotoxic effect of two orchid species, viz., Acampe praemorsa and Aerides odorata, against HeLa and MCF-7 cancer cell lines. In Andhra Pradesh (India), the Koya community uses the pulverised plant A. praemorsa, mixed with egg white and calcium, to produce a paste for application on fractured limbs to promote healing (Akarsh 2004). Ten drops of warm butter extracted from cow milk taken on the leaf of this plant are bandaged to the legs of kids to cure tetanus (Behera et al. 2013). The leaf juice is applied over the nipple for stomach ache, and it is also used for earache and for controlling body temperature (Shanavaskan et al. 2012). The forest dwellers in Araku valley showed that leaf paste, along with a piece of garlic, taken seven days is effective for the relief of chest pain and stomach disorder caused by hyperacidity (Padal et al. 2013). The leaf juice of this plant has been used to control mild tuberculosis (Dash et al. 2008); the leaf paste of this plant is used to treat cuts and wounds (Bijaya Pant 2013) and also to cure boils in the ear and nose (Hossain 2009). The whole plant and leaves have been used to treat pneumonia, dyspepsia, epilepsy, paralysis, inflammation, waist ache and fractures (Akhtar et al. 2017). The leaves of A. praemorsa and Luisia zeylanica and the aerial roots of Cymbidium aloifolium are used to fix human bone fractures (Behera et al. 2013). A wide range of chemical compounds have been isolated from various parts of orchids. These compounds are believed to be effective in reducing fevers, increasing WBC count, curing eye infection, treating fatigue and headache and, most importantly, functioning as an anticancer agent (Bulpitt 2005). Indian Vanda orchids have antiproliferative effects against various types of cancer (Ho and Chen 2003).
The leaves of Acampe praemorsa and Aerides odorata were collected from Paderu, Visakhapatnam, Eastern Ghats of Andhra Pradesh, India, and were washed and shade dried; the dried material was made into a coarse powder. The dried powdered leaf material was extracted in ethyl acetate and methanol solvents; the resulting extracts were filtered and then concentrated.
1.1 Human Cell Lines
The HeLa (cervical cancer) and MCF-7 (breast cancer) cell lines were obtained from NCCS, Pune (India), and the cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% foetal bovine serum (FBS) and antibiotics such as penicillin/streptomycin (0.5 mL−1), in 5% CO2/95% air atmosphere at 37 °C temperature.
1.2 Preparation of Crude Leaf Extract and MTT Assay
For MTT [3-(4, 5- dimethylthiazol-2-yl)-2, 5- diphenyl tetrazolium bromide] assay, each leaf extract was weighed separately and dissolved in dimethyl sulphoxide. With the media, make up the final concentration to 1 mg/ml and the cells were treated with series of concentrations from 10 to 100 μg/ ml. MTT assay is a colorimetric assay that measures the reduction of yellow MTT by mitochondrial succinate dehydrogenase. The assay depends both on the number of cells present and on the assumption that dead cells or their products do not reduce tetrazolium. The MTT enters the cells and passes into the mitochondria, where it is reduced to an insoluble, dark-purple-coloured formazan crystals. The cells are then solubilised with a DMSO, and the released, solubilised formazan reagent is measured spectrophotometrically at 570 nm.
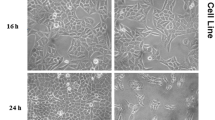
Cell viability was evaluated by the MTT assay with three independent experiments with six concentrations of crude leaf extract in triplicates. HeLa and MCF-7cell lines were trypsinised, and the Tryphan Blue assay was performed to identify viable cells in cell suspension. Cells were counted by the haemocytometer and seeded at a density of 5.0 × 103 cells/well in 100 μl media in 96 well plate culture media and incubated overnight at 37 °C. After incubation, the old medium was taken off, and the 100 μl fresh media with different concentrations of crude leaf extract in represented wells in 96 plates was added. After 48 h, the crude leaf extract was discarded, the fresh media with MTT solution (0.5 mg/mL−1) was added to each well, and plates were incubated at 37 °C for 3 h. At the end of incubation time, precipitates were formed as a result of the reduction of the MTT salt to chromophore formazan crystals by the cells with metabolically active mitochondria. The optical density of solubilised crystals in DMSO was measured at 570 nm on a microplate reader. Percentage growth inhibition was calculated using the following formula, and the concentration of test drug needed to inhibit cell growth by 50% in value is generated from the dose-response curves for each cell line using with ORIGIN software:
2 In Vitro Cytotoxic Effect Against Cancer Cell Lines
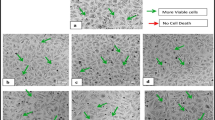
MTT assay for the in vitro cytotoxicity assessment of the ethyl acetate and methanolic leaf extracts of A. praemorsa and A. odorata was carried out at six different concentrations of 5, 10, 25, 50, 75 and 100 μg/ml on two different cell lines, MCF-7 (breast cancer) and HeLa (cervical cancer) (Figs. 27.1 and 27.2). At 100 μg/ml concentration of the crude leaf extract, the inhibition rate of cancer cell lines is high, which means that the percentage of viable cancer cells is less (Tables 27.1 and 27.2). The percentage of viable cancer cells decreases with the increase in the concentration of the crude leaf extract from 5 to 100 μg/ml (Figs. 27.3 and 27.4). IC50 values were recorded for all orchid leaf extracts. In vitro cytotoxic activity is measured in terms of IC50 value, which is half maximal inhibitory concentration that measures the potency of crude leaf extract (drug) in inhibiting the cancer cell line.
2.1 Acampe praemorsa
At 100% concentration of the ethyl acetate leaf extract of Acampe praemorsa, 61.128% of MCF-7 cells were inhibited (38.872% cells are viable) with an IC50 value of 49.276 μg/ml; 57.44% of HeLa cells were inhibited (42.56% of cells are viable) with an IC50 value of 61.68 1 μg/ml. Similarly, at 100% concentration of methanolic extract, 58.795% of MCF-7 cells were inhibited (41.205% of cells are viable) with an IC50 value of 55.904 μg/ml; 53.984% of HeLa cells were inhibited (46.016% of cells are viable) with an IC50 value of 76.94 μg/ml (Table 27.1). Here, MCF-7 cell lines were more prone to death to ethyl acetate extract. Hence, the highest in vitro cytotoxicity was seen in the ethyl acetate extract against the MCF-7 cell line (Fig. 27.1) with the least IC50 value of 49.276 μg/ml (Table 27.1), which was enough to kill 50% of MCF-7 cancer cells. In other cases, poor in vitro cytotoxicity was seen in methanolic extracts against the HeLa cell line.
2.2 Aerides odorata
At 100% concentration of the ethyl acetate leaf extract of Aerides odorata, 61.759% of MCF-7 cells were inhibited (38.241% of cells are viable) with an IC50 value of 41.094 μg/ml; 58.072% of HeLa cells were inhibited (41.928% of cells are viable) with an IC50 value of 59.061 μg/ml. In the case of 100% concentration of methanolic extract, 60.69% of MCF-7 cells were inhibited (39.31% of cells are viable) with an IC50 value of 26.211 μg/ml; 58.70% of HeLa cells were inhibited (41.3% of cells are viable) with an IC50 value of 52.167 μg/ml (Table 27.2). Here also, MCF-7 cells were more prone to death to methanolic extract when compared to HeLa cell lines. Hence, in vitro cytotoxicity of methanolic extract was highest against the MCF-7 cell line with the least IC50 value of 26.211 μg/ml (Table 27.1), which was enough to kill 50% of MCF-7 cancer cells (Fig. 27.2). Poor in vitro cytotoxicity was seen in the ethyl acetate extract against the HeLa cell line with an IC50 value of 59.061 μg/ml.
In the two orchids studied here, the higher in vitro cytotoxicity was observed against the MCF-7 cell line through the methanolic extract of A. odorata; poor in vitro cytotoxicity was seen against the HeLa cell line through the methanolic extract of A. praemorsa.
2.3 Orchid Chemicals as Anticancer Agents
Cancer is associated with abnormal, uncontrolled cell growth. It is a group of diseases caused by the loss of cell cycle control. In the contemporary world, breast cancer is the most commonly occurring cancer among women around the world, and the current available therapies are not safe as they are toxic to normal cells, along with cancerous cells. The plant extracts’ preparation shows a potential anticancer effect for the treatment of different types of cancer (Sivaraj et al. 2014). Experimental works have been conducted for many years across the world, and various chemical compounds were isolated from these plant extracts, which possess pharmacological activities. A wide variety of orchid chemicals, such as alkaloids, flavonoids, terpenoids, tannins, steroids, phenols and glycosides, have been isolated (Mari Suji and Christudas 2016). Denbinobin, a naturally occurring phenanthroquinone isolated from the genus Dendrobium, is known to have antioxidant activity against lung carcinoma, human ovary adenocarcinoma and human promyelocytic leukemia cell lines (You et al. 1995). Phytochemical analysis of A. praemorsa was done earlier by Maridas et al. (2008), and they found that cyanogenic glycosides and flavonoids were present in A. praemorsa. Further, A. praemorsa has been reported to contain a phenanthropyran derivative, i.e. praemorsin (1, 7-dihydroxy-3-methoxy-9, 10-dihydrophenanthropyran) (Anuradha and Prakash 1994).
A bibenzyl derivative, moscatilin, isolated from Dendrobium loddigesii, has anticancer properties (Ho and Chen 2003). This compound was originally purified from the Indian orchid D. moschatum (Majumder and Sen 1987). Another compound, kinsonoside isolated from Anoectochilus formosanus, has got diverse pharmacological effects, including the repression of tumour growth (Du et al. 2000, Yoon et al. 2007). Similarly, bibenzylgigantol extracted and purified from D. draconis, prevents the development of stem-like phenotypes in human lung cancer cells and adversely affects tumour cell viability (Bhummaphan and Chanvorachote 2015). Further, terpenoid and phenolic groups of compounds isolated from D. lasianthera and Arachnis flas-aeris have cytotoxic efficacy against T47D breast cancer cells. Prasad and Koch (2014) studied the antitumour properties of the ethanolic extract of D. formosum and suggested an alternative in the treatment of cancer. Prasad et al. (2017) observed that the ethanolic extract of D. chrysanthum showed dose-dependent cytotoxic effect against the HeLa cell line. In the present study, the methanolic extract of Aerides odorata showed the highest cytotoxic effect on the MCF-7 cell line, whereas poor cytotoxicity was recorded in regard to the methanolic leaf extract of Acampe praemorsa against the HeLa cell line.
3 Conclusion and Future Perspectives
The results of our study evidently demonstrated the cytotoxic activity of the ethyl acetate and methanolic leaf extracts of A. praemorsa and A. odorata. Thus, both the extracts seem to possess profound cytotoxic activity against cancer cell lines. The methanolic extract of A. odorata against MCF-7 cell lines reveals that the plant had the highest anticancer activity, whereas the methanolic extract of A. praemorsa against HeLa cell lines had shown poor anticancer activity. Further research is to be carried out to fractionate and purify the extract to find the molecules responsible for the anticancer activity. More effort is needed to explore drugs that attack cancerous cells without causing damage to normal cells and to save humans.
References
Akarsh (2004) Newsletter of ENVIS NODE on Indian Medicinal Plants 1(2)
Akhtar M, Hoque MM, Rahman M, Hossain MK (2017) Ethnobotanical investigation of some orchids used by five communities of Cox’s bazaar and Chittagang hilly tracts districts of Bangladesh. J Med Plant Stud 5:265–268
Anuradha V, Prakash NSR (1994) Praemorsin a new phenanthropyran from Acampe praemorsa. Phytochemistry 37:909–910
Behera D, Rath CC, Tayung K, Mohapatra UB (2013) Ethnomedicinal uses and antibacterial activity of two orchid species collected from Simlipal Biosphere Reserve, Odisha, India. J Agric Technol 9:1269–1283
Bhummaphan N, Chanvorachote P (2015) Gigantol suppresses cancer stem cell-like phenotypes in lung cancer cells. Evid Based Compliment Alternat Med 836564. https://doi.org/10.1155/2015/836564
Bijaya Pant (2013) Medicinal orchids and their uses: tissue culture a potential alternative for conservation. Afr J Plant Sci 7:448–467
Bulpitt CJ (2005) The uses and misuses of orchids in medicine. QJM 98:625–631
Dash PK, Sahoo S, Bal S (2008) Ethnobotanical studies on orchids of Niyamgiri Hill ranges, Orissa, India. Ethnobot Leafl 12:70–78
Du XM, Sub NY, Irino N, Shoyama Y (2000) Glycoside constituents from in vitro Anoectochilus formosanus. Chem Pharm Bull 48:1803–1804
Ho CK, Chen CC (2003) Moscalitin from the orchid Dendrobium loddigessi is a potential anticancer agent. Cancer Investig 21:729–736
Hossain MM (2009) Traditional therapeutic uses of some orchids of Bangladesh. Med Aromat Plant Sci Biochem 3:100–106
Kong JM, Goh NK, Chia LS, Chia TF (2003) Recent advances in traditional plant drugs and orchids. Acta Pharmacol Sin 24:7–21
Kumar CS, Manilal SK (1994) A catalogue of Indian orchids. Bishan Singh Mahendra Pal Singh, Dehradun
Majumder PL, Sen R (1987) Moscatilin, a bibenzyl derivative from the orchid Dendrobium moschatum. Phytochemistry 26:2121–2123
Maridas MMI, Zahir Hussain MI, Raju G (2008) Phytochemical survey of orchids in the Tiruelveli Hills of South India. Ethnobot Leafl 12:705–712
Mary Suji R, Christudas W (2016) Micropropagation, phytochemical studies and antioxidant potential of a wild epiphyte orchid. Acampe praemorsa (Roxb.) of Kanyakumari district, India. Eur J Pharm Med Res 3:572–576
Padal SB, Sandhyasri B, Chandrasekhar P (2013) Traditional uses of monocotyledon plants of Aruku valley Mandalam, Visakhapatnam District, Andhra Pradesh, India. IOSR J Pharm Biol Sci 6:12–16
Prasad R, Koch B (2014) Antitumour activity of ethanolic extract of Dendrobium formosum in T-cell lymphoma: an in vitro and in vivo study. Bio Med Res Int. https://doi.org/10.1155/2014/753451
Prasad R, Rana NK, Koch B (2017) Dendrobium chrysanthum ethanolic extract induces apoptosis via p53 up-regulation in HeLa cells and inhibits tumor progression in mice. J Complement Integrative Med 14(2)
Shanavaskhan AE, Sivadasan M, Alfarhan AH, Thomas J (2012) Ethnomedicinal aspects of angiospermic epiphytes and parasites of Kerala, India. Indian J Tradit Knowl 11:250–258
Sivaraj R, Rahman PKSM, Rajiv P, Vanathi P, Venkatesh R (2014) Biosynthesis and characterization of indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc 129:255–258
White KJ, Sharma B (2000) Wild orchids in Nepal: the guide to the Himalaya orchids of the Tribhuvan Rajpath and Chitwan Jungle. White Lotus Press, The University of Michigan, USA, Bangkok
Yoon YJ, Murthy HN, Hahn EJ, Pack KY (2007) Biomass production of Anoectochilus formosanus Hayata in a bioreactor system. J Plant Biol 50:573–576
You HL, Park JD, Baek NI, KIM S, Ahh BZ (1995) In vitro and in vivo antimural phenanthrenes from the aerial parts of Dendrobium nobile. Planta Med 61:178–180
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jhansi, K., Khasim, S.M. (2020). Anticancer Property in Acampe praemorsa and Aerides odorata (Orchidaceae), an In Vitro Approach. In: Khasim, S., Hegde, S., González-Arnao, M., Thammasiri, K. (eds) Orchid Biology: Recent Trends & Challenges . Springer, Singapore. https://doi.org/10.1007/978-981-32-9456-1_27
Download citation
DOI: https://doi.org/10.1007/978-981-32-9456-1_27
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-32-9455-4
Online ISBN: 978-981-32-9456-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)