Abstract
Functional dyspepsia (FD) is defined as a clinical condition in which pain arises from the gastroduodenal area in the absence of any organic, systemic, or metabolic disease that could explain the symptoms. Dyspeptic symptoms must be present for the previous 3 months with symptom onset at least 6 months before diagnosis according to the Rome III criteria. Helicobacter pylori (H. pylori) infection is considered a predisposing factor for FD, because H. pylori is known not only to induce pathologic change of the gastric mucosa but also to affect several gastrointestinal hormones. Furthermore, recent studies showed that H. pylori could influence molecular biology causing motility disorders. Unfortunately, the results are still controversial, and further studies are needed to evaluate the role of H. pylori. H. pylori eradication is suggested as one of the treatment options for FD. In countries with a high prevalence of H. pylori infection, such as Korea, H. pylori eradication might improve the symptoms of FD patients. Several meta-analyses reported that H. pylori eradication could improve symptoms in patients with FD compared to placebo; however, there are limitations, including heterogeneity of enrolled studies. Therefore, well-designed, prospective studies are required to confirm the effect of H. pylori eradication in FD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Dyspepsia is one of the most prevalent gastrointestinal diseases, and its prevalence is presumed to be about 5 % of all patients who visit primary healthcare clinics. Patients with dyspeptic symptoms usually suffer from heartburn, epigastric pain, postprandial discomfort, bloating, and a heavy feeling in the upper abdominal area. These symptoms are generally chronic and can decrease quality of life. Some patients with dyspeptic symptoms are diagnosed with functional dyspepsia (FD), which is defined as chronic and recurrent gastroduodenal discomfort without evidence of organic or systemic disease, such as peptic ulcer, gastrointestinal malignancy, gastroesophageal reflux disease, or pancreatobiliary disease. Of patients who were referred to the department of gastroenterology at a tertiary hospital in Korea, about 8–20 % had organic diseases, and about 70–92 % had functional gastrointestinal disorders (FGIDs). FD was the most common disorder among the patients with FGIDs [1]. According to the National Health Insurance Corporation database in Korea, FGIDs lead to a significant socioeconomic burden [2].
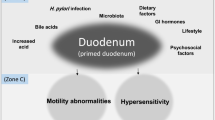
Several factors have been suggested to induce the symptoms of FD, including disturbed gastroduodenal motility, visceral hypersensitivity, psychological factors, and diet. Among them, the direct connection between Helicobacter pylori (H. pylori) infection and FD remains controversial. Given this background, the aims of this chapter are to evaluate the relationship between FD and H. pylori infection, to assess the role of H. pylori in FD pathogenesis, and to assess the effect of H. pylori eradiation on treatment of FD.
2 Definition of Functional Dyspepsia
Prior to investigating the relationship between H. pylori infection and FD, we reviewed the definition of FD in detail. According to the Rome III criteria, FD is defined as the existence of symptoms seeming to originate in the gastroduodenal area but with a negative diagnostic workup for organic or metabolic diseases that would explain the pain, including upper endoscopy, abdomen ultrasonography, laboratory findings, computed tomography, or other modalities [3]. FD was divided into four symptoms based on the Rome III criteria as follows: epigastric pain, epigastric burning, bothersome postprandial fullness, and early satiation (prevents finishing regular-sized meals). These symptoms must have been present for the previous 3 months with symptom onset at least 6 months before diagnosis. In addition, they presented two novel subcategories of FD based on cohort and population-based studies: postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) [4–6]. PDS must include one or both of the following symptoms: bothersome postprandial fullness after regular-sized meals at least several times per week and early satiety preventing finishing a regular-sized meal at least several times per week. In addition, patients with PDS can experience bloating in the upper abdominal area, postprandial nausea, or belching. Furthermore, PDS may coexist with EPS. EPS includes epigastric pain or burning of at least moderate severity at least once per week. This epigastric pain or burning should be intermittent, should not be generalized or localized to other abdominal or chest areas, should not be alleviated by defecation or passage of flatus, and should not be associated with gallbladder or sphincter of Oddi disorders. Additionally, the pain is usually induced or resolved by intake of a meal but may also happen during a fasting state [3, 7] (Table 18.1).
3 Pathophysiology of Functional Dyspepsia
FD is a complex disease that can arise from a variety of causes. Unfortunately, as stated above, the association between FD and H. pylori infection has not been clearly established. From the perspective of the Rome committee, H. pylori infection is not included as an organic cause of FD. Thus, H. pylori-infected patients with dyspeptic symptoms can be considered to have FD. Recently, several studies have suggested that the relationship between FD and H. pylori infection may play a role in FD pathophysiology.
Several studies have reported that some patients acquired irritable bowel syndrome (IBS) symptoms after a gastrointestinal infection. Similarly, several studies showed that a subset of patients developed FD following an episode of gastrointestinal infection [9, 10]. A recent meta-analysis reported that the odds ratio (OR) for the occurrence of post-infectious FD was 2.54 (95 % confidence interval [CI], 1.76–3.65) more than 6 months after acute gastroenteritis compared with the control [11]. Another meta-analysis found an OR of 2.18 (95 % CI, 1.70–2.81) for FD risk following acute gastroenteritis [12]. Taken together, gastrointestinal infection is associated with an increased risk of FD, supporting an inflammatory and immunological mechanism in the pathogenesis of FD [13]. H. pylori infection is the main cause of gastroduodenal inflammation [14] and provokes activation of a complex cytokine and chemokine response in the gastric mucosa [15], which may induce dyspepsia [16].
H. pylori infection induces gastric acid hypersecretion in the gastric antral mucosa [17]. Antral-predominant gastritis occurs in about 10–15 % of patients with H. pylori infection, likely due to gastric acid hypersecretion [18]. Patients with antral-predominant gastritis due to H. pylori infection showed decreased somatostatin release in the antral gland area, which leads to increased gastrin release with subsequent rise in acid secretion. This mechanism may explain dyspepsia.
In addition to clinical symptoms, H. pylori infection induces macroscopic and microscopic changes in the gastric mucosa that, along with lifestyle factors, may increase the risk of atrophic gastritis and intestinal metaplasia, leading to gastric cancer [19–21]. However, the results of studies that investigated the relationship between severity of histological gastritis and dyspepsia symptoms were varied. Czinn et al. [22] revealed an association between the severity of inflammation and epigastric pain. Likewise, another study group also revealed an indirect association between the severity of inflammation on the gastric corpus and the severity of symptoms [23]. However, there was no significant difference between the severity of dyspepsia symptoms and the severity of histological gastritis in other studies [24, 25]. Consequently, it is difficult to draw firm conclusions about the relationship between the severity of gastritis and the severity of dyspeptic symptoms, and further studies are needed to investigate this association.
Among the gastrointestinal hormones studied so far, ghrelin and leptin are representative hormones controlling appetite. Ghrelin promotes appetite and food intake and stimulates gastric emptying and gastric acid secretion [26]. These functions are partially achieved via vagal nerve pathways [27]. Plasma ghrelin concentrations are elevated in gastroduodenal mucosal injury for the purpose of gastroduodenal cytoprotection [28, 29]. However, plasma ghrelin levels decrease in the severe gastric mucosal atrophic state induced by H. pylori infection [30, 31]. Thus, H. pylori infection may be related to induction of gastric motor dysfunction and reduction of appetite by suppression of ghrelin secretion [16]. Ghrelin may therefore play a role in the occurrence of FD, especially that related to H. pylori. Studies have revealed that patients with FD experience changing plasma ghrelin levels, which are frequently related to FD symptom scores [32, 33]. A few studies reported that plasma ghrelin levels were significantly decreased in dysmotility-like FD patients with postprandial fullness and/or early satiety [34, 35]. A recent study revealed that the Leu72Met (408C > A) single nucleotide polymorphism of the ghrelin gene was significantly related to early-phase gastric emptying in patients with FD [36]. Akamizu et al. [37] revealed that repeated ghrelin administration increased food intake in patients with FD; however, another study showed that there was no significant difference between patients with dysmotility-like FD and healthy controls [32]. Increased plasma ghrelin levels in patients with FD were also reported [38]. Leptin also activates vagal nerve terminals, decreases appetite, and enhances gastric mucin secretion [39]. Similar to ghrelin, leptin may induce the occurrence of FD. A study in Iran reported that serum leptin levels were higher in patients with dysmotility-like dyspepsia [40]. Moreover, H. pylori-infected patients had increased serum leptin concentrations and leptin mRNA expression in the gastric mucosa [40, 41], suggesting that H. pylori infection could cause decreased appetite by stimulating serum leptin secretion. Unfortunately, there are only a few studies investigating the correlation of H. pylori-positive FD patients with ghrelin or leptin. Thus, the association between gastrointestinal hormones and H. pylori-associated dyspepsia remains speculative.
A recent study evaluated the role of microRNAs (miRNAs) in gastric motility disorders related to H. pylori infection [42]. Expression levels of muscle-specific miRNAs in the stomach of H. pylori-infected mice, such as miR-1, miR-133a, and miR-133b, were downregulated. On the other hand, the expression profiles of histone deacetylase 4 and serum response factor, which are target genes of miR-1 and miR-133 to reinforce muscular hyperproliferation, were accelerated. Furthermore, notable thickening of the muscular layer in the gastric corpus of H. pylori-infected mice was identified on histologic examination. Additionally, gastric emptying was significantly enhanced in H. pylori-infected mice. Taken together, chronic H. pylori infection influences the expression of muscle-specific miRNAs, histone deacetylase 4, and serum response factor, which might induce hyperplasia of the gastric muscular layer and acceleration of gastric emptying in H. pylori-infected mice. Though no study has tested this proposed mechanism in humans, these results suggest new molecular biology perspectives and provide a theoretical underpinning for H. pylori-associated dyspepsia to be an organic disease rather than a functional disorder.
Current literature suggests increased duodenal acid secretion in patients with dyspeptic symptoms [43] and a relationship between duodenal eosinophilia and FD. When eosinophil counts in patients with FD and asymptomatic controls were investigated, the OR for FD patients with high eosinophil counts in the duodenal bulb and duodenal second portion were 11.7 (95 % CI, 3.9–34.9) and 7.3 (95 % CI, 2.9–18.1), respectively, compared with asymptomatic controls [44]. H. pylori infection induces eosinophil aggregation in the gastric mucosa [45]. Another recent study also reported that eosinophilia in the duodenal second portion was significantly increased in FD patients with postprandial fullness, early satiety, and abdominal pain [46]. Therefore, H. pylori infection might be related to duodenal eosinophilia alongside the occurrence of FD. Intraepithelial lymphocytes in patients with FD and healthy controls were also evaluated by a study in France [47]. The number of intraepithelial lymphocytes was significantly higher in H. pylori-positive FD patients compared to healthy controls, but there was no significant difference between H. pylori-negative FD patients and healthy controls. The expression of CD95/Fas and HLA-DR expressing CD3+ intraepithelial lymphocytes was significantly lower in H. pylori-negative FD patients than in healthy controls. These results suggest that the phenotypic characteristics of intraepithelial lymphocytes might explain the difference between H. pylori-positive FD and H. pylori-negative FD.
Taken together, H. pylori-positive dyspepsia may represent a new area of study for FD. Recent studies suggested that H. pylori-positive dyspepsia, or H. pylori-associated dyspepsia, is not a functional disorder but an organic disease with many and various mechanisms of pathophysiology. Therefore, some study groups proposed that H. pylori-associated dyspepsia is different from FD and should be separated [16, 19]. A recent report from Kyoto global consensus clarified that H. pylori infection is the cause of dyspepsia in a subset of patients, and H. pylori-associated dyspepsia is a different entity compared to FD [48]. Further studies are needed to evaluate the true association between H. pylori infection and symptoms of FD.
4 Diagnostic Approach to Functional Dyspepsia
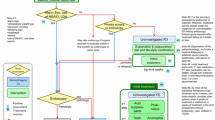
The committee of the American College of Gastroenterology issued guidance on the evaluation of FD patients [8]. Dyspeptic patients more than 55 years old or those with alarm features (Table 18.2) should undergo prompt endoscopy to rule out peptic ulcer disease, esophagogastric malignancy, and other rare upper gastrointestinal tract diseases. In patients aged 55 years or younger with no alarm features, the clinician may consider two approximately equivalent management options: (i) test and treat for H. pylori using a validated noninvasive test and a trial of acid suppression if eradication in successful but dyspeptic symptoms do not resolve, or (ii) an empiric trial of acid suppression with a proton pump inhibitor (PPI) for 4–8 weeks. The test-and-treat option is preferable in populations with a moderate-to-high prevalence of H. pylori infection (≥10 %), whereas the empirical PPI strategy is preferable in low prevalence situations (Fig. 18.1).
In 2012, an Asian consensus report on FD was developed [49]. All of the consensus members agreed that if patients have any alarm features including new onset dyspepsia in a patient over 40 years of age in a population with high prevalence of upper gastrointestinal malignancy, such as China, Korea, or Japan, they should undergo further testing (Fig. 18.2). Compared to the guidelines of the West, those in Asia recommend a younger patient age at which new onset of dyspepsia should prompt endoscopic examination.
Diagnostic algorithm of functional dyspepsia. *Alarm features can be found in Table 18.2. †The appropriate choice from the three options according to patient symptoms, patient wishes, risk of H. pylori infection, and gastric cancer in each country as well as primary healthcare settings. H. pylori Helicobacter pylori (Adapted from Miwa et al. [49], with permission from The Korean Society of Neurogastroenterology and Motility)
According to Korean guidelines for the treatment of FD in 2012, which compared endoscopy with “test and treat” for H. pylori, endoscopy may be a more effective initial strategy for managing patients with FD in Korea given the high incidence of gastric cancer and low cost of endoscopy [50]. The usefulness of H. pylori serology testing before endoscopy in patients with dyspepsia was investigated in Korea [51]. The sensitivity and negative predictive value of anti-H. pylori IgG for organic disease were 76.6 % and 85.5 %, respectively, in patients with dyspepsia under 40 years old. In patients with dyspepsia over 40 years old, the sensitivity and negative predictive value of anti-H. pylori IgG for organic disease were 61.9 % and 64.0 %, respectively. Although further study will be needed, the “test-and-treat” H. pylori approach might not the preferred initial step in treating patients with FD in Korea. Rather, as mentioned earlier, endoscopy may be the more effective initial strategy for managing patients with FD in Korea [50, 52].
Other diagnostic tests, such as serum testing for anemia, liver disease, and pancreatitis (amylase and lipase) and upper abdominal ultrasound or computed tomography, can be helpful in diagnosing FD [49].
5 Treatment of Functional Dyspepsia
There are several options for treating FD, including PPIs, histamine-2 receptor antagonists, prokinetic agents, and antidepressant and anxiolytic agents. Among them, we focus on H. pylori eradication.
Several epidemiologic studies have revealed that the H. pylori infection rate in patients with FD is higher than in matched control populations. A meta-analysis expressed a summary OR of 1.6 (95 % CI, 1.4–1.8) for H. pylori infection in FD [53]. Although this has not yet been confirmed, this result suggests that eradication of H. pylori could improve FD symptoms [54].
A Cochrane meta-analysis was performed on 17 randomized controlled trials and identified an association between H. pylori eradication and improvement in FD symptoms. A small but significant benefit of H. pylori eradication therapy was observed with a number needed to treat of 14 (95 % CI, 10–25) [55]. In another recent study, the number needed to treat was eight [43]. The cumulative long-term benefit of H. pylori eradication in patients with FD was also performed in UK. Dyspeptic symptoms in the H. pylori eradication group were significantly decreased compared to the placebo group, and OR was 0.84 (95 % CI, 0.71–1.00) [56]. The effect of H. pylori eradication on Asian FD patients might be different from FD patients in the West due to the varying prevalence of H. pylori strains including polymorphisms of cagA gene, gastric acid levels, and the severity of gastritis [57]. A systemic review and meta-analysis from China reported that the summary OR for improvement in FD patients after H. pylori eradication was 3.61 (95 % CI, 2.62–4.98) [58]. A study in Singapore demonstrated a 13-fold increased chance of FD symptom resolution in successfully eradicated H. pylori FD patients compared to those with persistent H. pylori infection (95 % CI, 1.1–17.7) [59]. In addition, a recent meta-analysis of randomized controlled trials with 12 months follow-up revealed that dyspeptic symptoms were significantly improved in eradication group (OR 1.38; 95 % CI, 1.18–1.62) [60]. Unfortunately, there are no randomized controlled studies evaluating the effect of H. pylori eradication on FD in Korea. Our study group showed that H. pylori eradication (OR 5.81; 95 % CI, 1.07–31.59) was related to improvement of FD at one year [61]. Studies in meta-analysis evaluated the effect of H. pylori eradication in patients with functional dyspepsia since 2000 and were summarized in Table 18.3.
However, Zullo et al. [63] pointed out several problems related to meta-analyses studying the effect of H. pylori eradication in FD patients. The type and number of dyspeptic symptoms of patients in enrolled studies were different, and the definitions of symptom improvement or regression varied widely. Many studies were performed to verify the effectiveness of H. pylori eradication in FD patients; however, only 12 studies were well-designed, double-blind, placebo-controlled studies with at least 50 patients followed for more than 6–12 months with final confirmation of H. pylori status by endoscopy with biopsy or a 13C-urea breath test. Unfortunately, the type and number of symptoms and the definition of symptom improvement were different even in these 12 well-designed studies. Considering these findings, we could assume that the studies evaluating the effectiveness of H. pylori eradication in patients with FD are difficult to interpret. There is thus no definitive conclusion about the usefulness of H. pylori eradication in FD patients.
Despite these limitations, the American Gastroenterological Association recommends H. pylori eradication as the initial management strategy for uncomplicated dyspepsia in patients younger than 55 years, especially in countries with high prevalence (>10 %) of H. pylori infection, like Korea [7]. The Korean College of Helicobacter and Upper Gastrointestinal Research also reported that H. pylori eradication helps long-term dyspeptic symptom improvement in some patients with FD [64].
On another note, H. pylori eradication is useful in only some patients with FD. A recent study reported that FD patients with microscopic duodenitis showed greater symptom improvement after H. pylori eradication than those without microscopic duodenitis [65]. Sugano et al. [19] who proposed that H. pylori-associated FD should be separated from FD also suggested that FD patients with H. pylori infection should be treated before meeting criteria for a diagnosis of FD. Because H. pylori infection clearly influences pathological and physiological changes, residual dyspepsia symptoms after complete eradication of H. pylori infection might be regarded as real FD coexisting with H. pylori infection. Based on these considerations, the Kyoto global consensus also agreed that patients who remain symptomatic after successful H. pylori eradication should be regarded as having FD [48] (Fig. 18.3). In addition, H. pylori eradication is first-line treatment for dyspeptic patients with H. pylori infection, because eradication therapy for dyspeptic symptoms is better than placebo in H. pylori-infected dyspeptic patients.
Diagnostic algorithm of H. pylori-associated dyspepsia. H. pylori Helicobacter pylori (Adapted from Sugano et al. [48], with permission from BMJ publishing group Ltd.)
All things considered, H. pylori eradication is effective in some patients with dyspepsia. Considering the short duration and acceptable cost-benefit analysis for improving dyspeptic symptoms, H. pylori eradication could be proposed as the treatment for those with H. pylori infection.
6 Conclusions
FD is one of the most prevalent gastrointestinal disorders; however, many physicians are hard to diagnose FD due to the varied pathophysiology and symptoms. Among the many causes of FD, H. pylori infection is considered to have some effect on the onset of FD worldwide. H. pylori eradication is suggested as one of the therapeutic options for FD patients with H. pylori infection, and several meta-analyses reported that H. pylori eradication improved dyspeptic symptoms in FD patients compared to placebo. Thus, H. pylori eradication could be attempted for treatment of FD patients. Some limitations still exist, such as lack of well-designed, double-blinded, placebo-controlled studies to establish the effectiveness of H. pylori eradication in FD patients. Therefore, additional well-designed, prospective studies are needed to demonstrate the association between FD and H. pylori infection.
References
Kim JS, Lee KJ, Kim JH, Hahm KB, Cho SW. Functional gastrointestinal disorders in patients referred to specialist gastroenterologists in a tertiary hospital. Korean J Neurogastroenterol Motil. 2004;10:111–7.
Jung HK, Jang B, Kim YH, Park J, Park SY, Nam MH, et al. Health care costs of digestive diseases in Korea. Korean J Gastroenterol. 2011;58:323–31.
Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–79.
Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Talley NJ. Do distinct dyspepsia subgroups exist in the community? A population-based study. Am J Gastroenterol. 2007;102:1983–9.
Aro P, Talley NJ, Ronkainen J, Storskrubb T, Vieth M, Johansson SE, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology. 2009;137:94–100.
Zagari RM, Law GR, Fuccio L, Cennamo V, Gilthorpe MS, Forman D, et al. Epidemiology of functional dyspepsia and subgroups in the Italian general population: an endoscopic study. Gastroenterology. 2010;138:1302–11.
Lacy BE, Talley NJ, Locke 3rd GR, Bouras EP, DiBaise JK, El-Serag HB, et al. Review article: current treatment options and management of functional dyspepsia. Aliment Pharmacol Ther. 2012;36:3–15.
Talley NJ, Vakil N; Practice Parameters Committee of the American College of Gastroenterology. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100:2324–37.
Dizdar V, Gilja OH, Hausken T. Increased visceral sensitivity in Giardia-induced postinfectious irritable bowel syndrome and functional dyspepsia. Effect of the 5HT3-antagonist ondansetron. Neurogastroenterol Motil. 2007;19:977–82.
Hanevik K, Hausken T, Morken MH, Strand EA, Morch K, Coll P, et al. Persisting symptoms and duodenal inflammation related to Giardia duodenalis infection. J Infect. 2007;55:524–30.
Futagami S, Itoh T, Sakamoto C. Systematic review with meta-analysis: post-infectious functional dyspepsia. Aliment Pharmacol Ther. 2015;41:177–88.
Pike BL, Porter CK, Sorrell TJ, Riddle MS. Acute gastroenteritis and the risk of functional dyspepsia: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:1558–63.
Gwee KA. Post-infectious irritable bowel syndrome, an inflammation-immunological model with relevance for other IBS and functional dyspepsia. J Neurogastroenterol Motil. 2010;16:30–4.
Suzuki H, Hibi T, Marshall BJ. Helicobacter pylori: present status and future prospects in Japan. J Gastroenterol. 2007;42:1–15.
D’Elios MM, Andersen LP. Inflammation, immunity, and vaccines for Helicobacter pylori. Helicobacter. 2009;14 Suppl 1:21–8.
Suzuki H, Matsuzaki J, Hibi T. What is the difference between Helicobacter pylori-associated dyspepsia and functional dyspepsia? J Neurogastroenterol Motil. 2011;17:124–30.
Liu Y, Vosmaer GD, Tytgat GN, Xiao SD, Ten Kate FJ. Gastrin (G) cells and somatostatin (D) cells in patients with dyspeptic symptoms: Helicobacter pylori associated and non-associated gastritis. J Clin Pathol. 2005;58:927–31.
Konturek SJ, Brzozowski T, Konturek PC, Schubert ML, Pawlik WW, Padol S, et al. Brain-gut and appetite regulating hormones in the control of gastric secretion and mucosal protection. J Physiol Pharmacol. 2008;59 Suppl 2:7–31.
Sugano K. Should we still subcategorize Helicobacter pylori-associated dyspepsia as functional disease? J Neurogastroenterol Motil. 2011;17:366–71.
Azuma T. Helicobacter pylori CagA protein variation associated with gastric cancer in Asia. J Gastroenterol. 2004;39:97–103.
Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47:1077–83.
Czinn SJ, Bertram TA, Murray PD, Yang P. Relationship between gastric inflammatory response and symptoms in patients infected with Helicobacter pylori. Scand J Gastroenterol Suppl. 1991;181:33–7.
van der Schaar PJ, Straathof JW, Veenendaal RA, Lamers CB, Masclee AA. Does Helicobacter pylori gastritis affect motor function of proximal stomach in dyspeptic patients? Dig Dis Sci. 2001;46:1833–8.
Pereira-Lima JG, Scholl J, Pinheiro JB, Pereira-Lima L, Riemann JF. Helicobacter pylori-associated gastritis: does it play a role in functional dyspepsia? Z Gastroenterol. 1995;33:421–5.
Joshi A, Gupta SD, Ahuja V, Sharma MP. Symptom score does not correlate with gastritis grade and Helicobacter pylori infection in non ulcer dyspepsia. Trop Gastroenterol. 2001;22:194–6.
Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–30.
Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8.
Fukuhara S, Suzuki H, Masaoka T, Arakawa M, Hosoda H, Minegishi Y, et al. Enhanced ghrelin secretion in rats with cysteamine-induced duodenal ulcers. Am J Physiol Gastrointest Liver Physiol. 2005;289:G138–45.
Suzuki H, Masaoka T, Hosoda H, Nomura S, Ohara T, Kangawa K, et al. Plasma ghrelin concentration correlates with the levels of serum pepsinogen I and pepsinogen I/II ratio--a possible novel and non-invasive marker for gastric atrophy. Hepatogastroenterology. 2004;51:1249–54.
Kawashima J, Ohno S, Sakurada T, Takabayashi H, Kudo M, Ro S, et al. Circulating acylated ghrelin level decreases in accordance with the extent of atrophic gastritis. J Gastroenterol. 2009;44:1046–54.
Osawa H. Ghrelin and Helicobacter pylori infection. World J Gastroenterol. 2008;14:6327–33.
Takamori K, Mizuta Y, Takeshima F, Akazawa Y, Isomoto H, Ohnita K, et al. Relation among plasma ghrelin level, gastric emptying, and psychologic condition in patients with functional dyspepsia. J Clin Gastroenterol. 2007;41:477–83.
Shinomiya T, Fukunaga M, Akamizu T, Irako T, Yokode M, Kangawa K, et al. Plasma acylated ghrelin levels correlate with subjective symptoms of functional dyspepsia in female patients. Scand J Gastroenterol. 2005;40:648–53.
Lee KJ, Cha DY, Cheon SJ, Yeo M, Cho SW. Plasma ghrelin levels and their relationship with gastric emptying in patients with dysmotility-like functional dyspepsia. Digestion. 2009;80:58–63.
Shindo T, Futagami S, Hiratsuka T, Horie A, Hamamoto T, Ueki N, et al. Comparison of gastric emptying and plasma ghrelin levels in patients with functional dyspepsia and non-erosive reflux disease. Digestion. 2009;79:65–72.
Yamawaki H, Futagami S, Shimpuku M, Shindo T, Maruki Y, Nagoya H, et al. Leu72Met408 polymorphism of the ghrelin gene is associated with early phase of gastric emptying in the patients with functional dyspepsia in Japan. J Neurogastroenterol Motil. 2015;21:93–102.
Akamizu T, Iwakura H, Ariyasu H, Hosoda H, Murayama T, Yokode M, et al. Repeated administration of ghrelin to patients with functional dyspepsia: its effects on food intake and appetite. Eur J Endocrinol. 2008;158:491–8.
Lanzini A, Magni P, Petroni ML, Motta M, Lanzarotto F, Villanacci V, et al. Circulating ghrelin level is increased in coeliac disease as in functional dyspepsia and reverts to normal during gluten-free diet. Aliment Pharmacol Ther. 2006;23:907–13.
Sanger GJ, Lee K. Hormones of the gut-brain axis as targets for the treatment of upper gastrointestinal disorders. Nat Rev Drug Discov. 2008;7:241–54.
Lankarani KB, Moghadami M, Masoumpoor M, Geramizadeh B, Omrani GR. Serum leptin level in patients with functional dyspepsia. Dig Liver Dis. 2004;36:717–21.
Azuma T, Suto H, Ito Y, Ohtani M, Dojo M, Kuriyama M, et al. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324–9.
Saito Y, Suzuki H, Tsugawa H, Suzuki S, Matsuzaki J, Hirata K, et al. Dysfunctional gastric emptying with down-regulation of muscle-specific microRNAs in Helicobacter pylori-infected mice. Gastroenterology. 2011;140:189–98.
Mazzoleni LE, Sander GB, Francesconi CF, Mazzoleni F, Uchoa DM, De Bona LR, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929–36.
Talley NJ, Walker MM, Aro P, Ronkainen J, Storskrubb T, Hindley LA, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1175–83.
Aydemir SA, Tekin IO, Numanoglu G, Borazan A, Ustundag Y. Eosinophil infiltration, gastric juice and serum eosinophil cationic protein levels in Helicobacter pylori-associated chronic gastritis and gastric ulcer. Mediators Inflamm. 2004;13:369–72.
Walker MM, Aggarwal KR, Shim LS, Bassan M, Kalantar JS, Weltman MD, et al. Duodenal eosinophilia and early satiety in functional dyspepsia: confirmation of a positive association in an Australian cohort. J Gastroenterol Hepatol. 2014;29:474–9.
Wu JC. Asian consensus report on functional dyspepsia: necessary and ready? J Gastroenterol Hepatol. 2012;27:624–5.
Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–67.
Miwa H, Ghoshal UC, Gonlachanvit S, Gwee KA, Ang TL, Chang FY, et al. Asian consensus report on functional dyspepsia. J Neurogastroenterol Motil. 2012;18:150–68.
Jee SR, Jung HK, Min BH, Choi KD, Rhee PL, Kang YW, et al. Guidelines for the treatment of functional dyspepsia. Korean J Gastroenterol. 2011;57:67–81.
Hwang IR, Kim JH, Lee KJ, Cho SW. Can Helicobacter pylori serology predict non-ulcer dyspepsia in young dyspeptic patients? Korean J Gastrointest Endosc. 2000;21:696–703.
Park MI. Diagnosis & management of dyspepsia. Korean J Med. 2009;76 Suppl 2:S298–303.
Jaakkimainen RL, Boyle E, Tudiver F. Is Helicobacter pylori associated with non-ulcer dyspepsia and will eradication improve symptoms? A Meta-Analysis BMJ. 1999;319:1040–4.
Miwa H, Hirai S, Nagahara A, Murai T, Nishira T, Kikuchi S, et al. Cure of Helicobacter pylori infection does not improve symptoms in non-ulcer dyspepsia patients-a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2000;14:317–24.
Moayyedi P, Soo S, Deeks J, Delaney B, Harris A, Innes M, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;2, CD002096.
Harvey RF, Lane JA, Nair P, Egger M, Harvey I, Donovan J, et al. Clinical trial: prolonged beneficial effect of Helicobacter pylori eradication on dyspepsia consultations – the Bristol Helicobacter Project. Aliment Pharmacol Ther. 2010;32:394–400.
Suzuki H, Masaoka T, Sakai G, Ishii H, Hibi T. Improvement of gastrointestinal quality of life scores in cases of Helicobacter pylori-positive functional dyspepsia after successful eradication therapy. J Gastroenterol Hepatol. 2005;20:1652–60.
Jin X, Li YM. Systematic review and meta-analysis from Chinese literature: the association between Helicobacter pylori eradication and improvement of functional dyspepsia. Helicobacter. 2007;12:541–6.
Gwee KA, Teng L, Wong RK, Ho KY, Sutedja DS, Yeoh KG. The response of Asian patients with functional dyspepsia to eradication of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2009;21:417–24.
Zhao B, Zhao J, Cheng WF, Shi WJ, Liu W, Pan XL, et al. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J Clin Gastroenterol. 2014;48:241–7.
Kim SE, Park YS, Kim N, Kim MS, Jo HJ, Shin CM, et al. Effect of Helicobacter pylori eradication on functional dyspepsia. J Neurogastroenterol Motil. 2013;19:233–43.
Gisbert JP, Calvet X, Gabriel R, Pajares JM. Helicobacter pylori infection and functional dyspepsia. Meta-analysis of efficacy of eradication therapy. Med Clin. 2002;118:405–9.
Zullo A, Hassan C, De Francesco V, Repici A, Manta R, Tomao S, et al. Helicobacter pylori and functional dyspepsia: an unsolved issue? World J Gastroenterol. 2014;20:8957–63.
Kim SG, Jung HK, Lee HL, Jang JY, Lee H, Kim CG, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol. 2014;29:1371–86.
Mirbagheri SS, Mirbagheri SA, Nabavizadeh B, Entezari P, Ostovaneh MR, Hosseini SM, et al. Impact of microscopic duodenitis on symptomatic response to Helicobacter pylori eradication in functional dyspepsia. Dig Dis Sci. 2015;60: 163–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Kim, S.E. (2016). Functional Dyspepsia. In: Kim, N. (eds) Helicobacter pylori. Springer, Singapore. https://doi.org/10.1007/978-981-287-706-2_18
Download citation
DOI: https://doi.org/10.1007/978-981-287-706-2_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-287-705-5
Online ISBN: 978-981-287-706-2
eBook Packages: MedicineMedicine (R0)