Abstract
Recent discoveries supporting a functional and structural link between the brain and the heart emphasize the importance of understanding the cross talk for cardio- and cerebrovascular health. Stress has been shown to play a crucial role in the generation of cardiovascular diseases and has a major impact on neurodegenerative diseases and mental disorders presumably through activation of the sympathetic branch of the autonomic nervous system. It is well established that overactivity of the sympathetic nervous system plays a central role in the development of cardiovascular disease and constitutes an important risk factor for cardiovascular morbidity and mortality. Further, increasing evidence suggests the overactivity of the sympathetic branch is a common phenomenon linking major cardiac pathologies seen in association with several primarily neurological conditions, such as cerebral infarction and subarachnoid hemorrhage. Modulating brain activity in humans for otherwise treatment resistant disorders has been demonstrated to affect cardiovascular parameters. Direct electrical stimulation of specific midbrain areas in humans for pain relief regulates human cardiovascular reflex control and can evoke panic and anxiety, by modulating the activity of the autonomic nervous system.
This chapter examines the connection between the brain and the heart the autonomic nervous system provides. Evidence of a linking between emotional stresses and cardiovascular risk is explored, more specifically, stress-induced cardiomyopathy and a plausible explanation for the female predisposition of the condition.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Cardio- and cerebrovascular health is based on a complex relationship between the heart and the brain, with the sympathetic division of the autonomic nervous system (ANS) being the key regulator. In 1932 Walter Cannon suggested that two of the ANS principal divisions, the sympathetic and parasympathetic nervous systems, had a primary role in regulating our internal environment (milieu interieur, Claude Bernard 1878) by means of negative feedback. He also introduced the term homeostasis to describe the tendency toward stability in the body and portrayed the ANS as central to its regulation. Although the whole brain is more or less involved in the maintenance of homeostasis, the control is governed by an area comprising less than 1 % of its total volume, the hypothalamus. Despite its modest size, the hypothalamus controls cardiovascular functions, respiration, metabolism, and temperature regulation and mobilizes our fight and flight and defeat reaction by regulating and modifying autonomic output and endocrine function to face a challenge and restore homeostasis. Cannon showed that when an animal is strongly aroused, the sympathetic division of the ANS “mobilizes the animal for an emergency response of fight or flight. The sympathico-adrenal system orchestrates changes in blood supply, sugar availability, and the blood’s clotting capacity in a marshaling of resources keyed to the violent display of energy.” The key regulator in the finely tuned beat-to-beat orchestration of normal cardiovascular function from quiet rest to extreme activation is the sympathetic nervous system (SNS). It has been known for well over a century that the brain exerts a powerful effect over sympathetic outflow. It is therefore of pivotal importance to be able to measure sympathetic nervous function and its response to changes in the internal environment posed by external and internal physiological perturbations, when investigating the complex relationship between the brain and heart.
Assessing Human Sympathetic Nervous Function
With the aid of modern techniques to assess SNS activity, we now have better understanding of how the SNS maintains normal cardiovascular function on a beat-to-beat basis. In contrast to somatic motor nerves, sympathetic nerves are shown to be tonically active (Adrian et al. 1932), so all innervated blood vessels remain under some degree of continuous constriction. By rapidly regulating the level of activity, the degree of vasoconstriction in the blood vessels of many key organs around the body is altered. As the SNS plays such an important role in peripheral vascular resistance and shows a close relation to baroreceptor function, its involvement in the development of cardiovascular diseases has been vastly studied.
The different components in sympathetic nervous control of the cardiovascular system have provided a basis for various techniques for assessing sympathetic cardiovascular control in humans. These techniques are complimentary rather than competing methodologies and provide different aspects of sympathetic nervous function.
Microneurography: This electrophysiological technique, developed in Uppsala, Sweden, in the early 1960s, by Hagbarth and Vallbo (Vallbo et al. 1979), provides direct measure of sympathetic nerve firing from efferent postganglionic unmyelinated “C” nerve fibers in limb nerves of awake, unanesthetized humans. Postganglionic sympathetic nerves are composed of hundreds to thousands of unmyelinated fibers whose individual contributions to the recorded signal are exceedingly small. However, accumulation of these thinnest of all human nerve fibers makes it possible to lodge the tip of an electrode in their vicinity and record their ongoing activity. Virtually any human mixed nerve is accessible to the recording electrode, but studies have been confined mainly to the peroneal, tibial, or median nerves. Though visceral sympathetic and parasympathetic activity is still inaccessible, two sympathetic subdivisions are accessible, which are those to the skin vascular bed (SSNA) made up of a mixture of sudomotor and vasomotor impulses and to the muscle vascular bed (MSNA) which is dominated by vasoconstrictor impulses. The marked differences in temporal patterns of sympathetic activity in human skin and muscle nerves have challenged the concept of a “common sympathetic tonus,” as the findings indicate that there are different populations of sympathetic neurons subjected to their own homogenous supraspinal drive which may be different from that of other populations.
The noradrenaline spillover measurements: The methodology, which was introduced by Murray Esler and colleagues in Melbourne, Australia, in 1979, is based on the use of a radiotracer (radiolabeled noradrenaline (NA)) employed to measure the rates of turnover of tissue NA and organ-specific spillover of NA to plasma. The method has aided insight into the sympathetic neuronal outflow to internal organs, the inaccessibility of which has been a limitation in cardiovascular research (Esler et al. 1979, 1988, 1990).
Power spectral analysis of circulatory rhythms: With this method, which is based on mathematical partitioning, individual, superimposed rhythms producing cyclical variations in heart rate or arterial pressure can be separated and quantified (Pagani et al. 1986). The variability in heart rate, which is largely attributable to the influence of the autonomic nervous system, can be divided into high (0.3 Hz) and low (0.1 Hz) frequency components, proposed to reflect the vagal parasympathetic and cardiac sympathetic drive, respectively. While the high frequency component is shown to be a good marker for vagal activity, the low frequency component is not regarded a reliable measure of sympathetic activity (Kingwell et al. 1994).
The Sympathetic Nervous System and Stress
Clinical observations in patients with various primarily neurological conditions such as stroke, subarachnoid hemorrhage, epilepsy, traumatic brain injury, or central nervous system infections, with concomitant ECG abnormalities and in some cases cardiac pathology with wall motion abnormalities as in neurogenic stress cardiomyopathy (NSC) , support the connection between the brain and the heart (Mazzeo et al. 2014).
These cardiac abnormalities in acute neurological conditions are mediated by the autonomic nervous system.
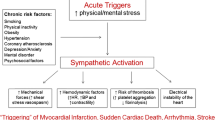
Acute mental stress can induce myocardial ischemia in patients with coronary artery disease and induce cardiac electrical instability leading to life-threatening arrhythmias (Kop et al. 2004).
While a flight and flight response to an external stressor is vital for survival, psychological distress, such as intense emotions, anger, and mental stress, can trigger acute coronary syndromes and sudden cardiac death (Samuels 2007).
Dysregulation of the autonomic nervous system, resulting in sympathetic activation and/or parasympathetic withdrawal, is the major pathophysiological mechanism of mental stress-induced cardiac events. Whether our lifestyle or responsiveness to emotional or other stimuli plays a mechanistic role is somewhat uncertain and remains to be elucidated. It is however shown that sympathetic nerve activity and blood pressure tend to increase in emotionally stressful environments (white coat hypertension), while staying stable over time in populations (cloistered nuns) living in secluded and unchanging environments (Timio et al. 2001).
Sudden Cardiac Events Associated with Increased Sympathetic Activity
It is well established that acute coronary syndromes and sudden cardiac death can be triggered by acute psychological distress, such as intense emotions, anger, and mental stress (Samuels 2007). The SNS is known to be active in sudden cardiovascular death. Though not fully understood by which mechanism increased SNS activity to different organs can lead to such events, the parallel increase in SNS activity and morning peaks in acute MI, transient ischemia, and stroke indicates that increased SNS activity may be a trigger for sudden cardiovascular events.
The interaction between central and autonomic nervous system responses may have implications for further investigations of the brain-heart associations and mechanisms by which acute and chronic psychological distress increase the risk of myocardial infarction, cardiac arrhythmias, and sudden cardiac death.
Deep Brain Stimulation, Sympathetic Outflow, and Cardiovascular Function
The main central nervous system components associated with autonomic regulation constitute the Central Autonomic Network (CAN). This is a functional network of cortical and subcortical central nervous system structures that receive information from humoral, visceral, and environmental sources and integrates these inputs to generate preganglionic autonomic, neuroendocrine, and behavioral outputs essential for survival (Benarroch 1993).
The primary brain areas involved in the autonomic modulation of the brain-heart association are the insula, medial prefrontal cortex, and cerebellum. Other areas involved in stress-induced autonomic modulation are the (anterior) cingulate cortex, parietal cortex, somatomotor cortex/precentral gyrus, and temporal cortex.
One of the main structures of the central autonomic network is the subcortical periaqueductal gray (PAG), a complex midbrain region involved in regulating bodily as well as behavioral functions and which is routinely used to treat chronic neuropathic pain. Electrical stimulation of this area has been shown to have cardiovascular effects in humans (Pereira et al. 2010; Green et al. 2005). The PAG contains four longitudinal columns, referred to as the dorsomedial (dmPAG), dorsolateral (dlPAG), lateral (lPAG), and ventrolateral (vlPAG) subdivisions, which collectively have a pivotal role in integrating behavioral and physiological responses to external stressors as well as other functions. Activation of the vlPAG column produces a reduction in cardiovascular activity, while activation of the dlPAG column, believed to be an important component of the central mechanisms that generate the defensive response to acute psychological stressors, is associated with an increased cardiovascular activation (Dampney et al. 2013). The dorsolateral, lateral, and ventrolateral columns of the PAG have distinct reciprocal connections with autonomic centers of the lower brainstem and hypothalamus that differentially regulate activity in neurons of the peripheral autonomic pathways (Bandler et al. 2000).
In the early twentieth century, development of techniques aimed to electrically stimulate specific areas of the brain provided a vital tool to illuminate the role of the brain in regulating autonomic function. With more widespread use of deep brain stimulation (DBS) for otherwise treatment-resistant disorders, reports of autonomic side effects became an area of attention and interest, providing a unique insight into central autonomic regulation and pathophysiology. In rats stimulation of the dlPAG was shown to evoke flight behavior and autonomic changes characteristic of panic (Yardley and Hilton 1986); in humans, DBS at midbrain sites for treating neuropathic pain reported the procedure often evoked intolerable side effects, which resembled the symptoms of panic (Kumar et al. 1997; Nashold et al. 1969; Richardson and Akil 1977). In a recent study by Sverrisdóttir and colleagues (2014), stimulation of the dorsolateral PAG in patients with neuropathic pain was shown to result in a distinctive sympathetic nerve firing pattern previously reported in conditions associated with anxiety and mental stress (Wilkinson et al. 1998; Donadio et al. 2002). An active coping response (“fight and flight”) occurs when an individual encounters a threatening stimulus that may be harmful or cause pain. This defense pattern is represented in the dmPAG and dlPAG and is classically associated with endogenous non-opioid analgesia, increased arterial blood pressure, and heart rate, although recent evidence in humans suggests that it may in fact be opioid-mediated (Pereira et al 2013, Wang et al.). In the absence of an external threat, Sverrisdóttir and co-workers showed that by directly stimulating the midbrain region where the defense pattern is orchestrated, the nerve firing pattern and cardiovascular response resemble that of anxiety, mental stress, and fight and flight response.
While emotional stress and emotion have long been associated with ventricular arrhythmias and sudden cardiac death, in the past decade, cases and conditions have been identified in which severe mental distress has resulted in physical changes to the heart muscle, resulting in a spectrum of stress-related neurogenic cardiomyopathy syndromes, which includes the takotsubo or broken heart syndrome.
Takotsubo Stress Cardiomyopathy or Broken Heart Syndrome: When an Emotion Changes the Shape of Your Heart

Never morning wore to evening but some heart did break
The English artist Walter Langley’s emotional painting from 1894, which title arises from Tennyson’s poem In Memoriam, is about loss and heart break. The painting portrays a young woman being comforted by an older woman as she holds her head in her hands and cries. The turmoil of human emotions displayed is in direct contrast to the flat calm sea in the background; it is the calm after the storm which took the life of the young woman’s beloved and broke her heart.
The idea of the heart as the vulnerable locus of emotion is as old as time. Irish mythology has Deirdre of the Sorrows and her lover Naoise; Greek mythology has the tragedy of Apollo and Daphne, Wagner’s opera of Tristan and Isolde, and Shakespeare’s Romeo and Juliet. Tales of death from a broken heart feature throughout history and are as genuine today as they were yesterday.
Sympathetic Outflow in Stress-Induced Cardiomyopathy
Takotsubo cardiomyopathy, a particular stress-induced cardiomyopathy, was first described by the Japanese in the early 1990s. They reported baffling cases of patients with cardiac failure presenting signs and symptoms of acute coronary syndrome without evidence of obstructive coronary artery disease recovering spontaneously within days or weeks. This reversible cardiomyopathy was most prevalent in postmenopausal women following a strong emotional or physical stressor such as death of a loved one or other catastrophic news. Angiograms showed that the heart had changed shape. The stunned organ, constricted at the top, ballooned at the bottom, resembled more an octopus trap (takotsubo in Japanese) than a normal heart. The physical change in the shape of the heart, triggered by a powerful emotional stressor, strongly suggests that mental stress can cause cardiovascular disease.
Though the definite pathophysiology of takotsubo stress-induced cardiomyopathy (SIC) remains to be identified, increased plasma levels of catecholamine have been found (Wittstein et al. 2005). Hence, exaggerated SNS activation is proposed to be a major contributor to the pathogenesis and forms the basis for treatment of this medical entity. In a recent study, SNS activity, recorded with microneurography, was found to be lower in women in the acute and chronic phase of SIC as compared to healthy controls (Sverrisdóttir et al. 2012). While the findings of a reduced SNA activity in SIC compared to healthy controls may seem contradictory to findings of increased plasma catecholamine, they may instead reflect the different phases of the condition. As portrayed in Langley’s painting, an initial intense sympathetic storm in the acute phase of SIC may cause excessive catecholamine release over the atria and distension of the ventricles. A reflex sympatho-inhibition, as demonstrated in direct nerve recordings, follows as a consequence of the storm and leaves behind a broken heart.
How would this come about? The left ventricular myocardium contains unmyelinated afferents with receptors that are excited both by mechanical and chemical stimuli. It has been proposed that in myocardial distress, these intracardiac receptors may function as protective nociceptors, which when activated can inhibit cardiac contraction and decrease peripheral resistance (Warltier et al. 2003). A distension of the ventricular myocardium, due to excessive catecholamine release over the atria in the acute phase, could increase the rate of discharge of these unmyelinated cardiac c-fiber afferents, resulting in reflex vagal bradycardia and widespread sympathetic inhibition (Folkow 1979). Due to such a reflex mechanism, even though SNS activity is recorded in the acute phase, it would not capture the early acute catecholamine release as the reflex would already have “kicked in.”
Why the Female Predisposition?
The underlying cause for the evident female predisposition of SIC is unknown, but may be related to gender differences in vulnerability to emotional stress (Orth-Gomér et al. 2000) and myocardial sensitivity to catecholamine toxicity (Kneale et al. 2000). That SIC seems to predominantly occur in postmenopausal women indicates that their declining levels of estrogen in the absence of testosterone may also explain their greater vulnerability to SIC (Zhou et al. 2010). In postmenopausal women, estradiol and estrogen levels are closer to those of healthy adult men than those of premenopausal women. Estradiol reduces the response to vasoconstrictors in both male and female arteries. Increasing estrogen levels by means of transdermal estrogen replacement therapy has been shown to decrease SNS activity in postmenopausal women (Vongpatanasin et al. 2001).
Equally, the lower incidence of SIC in healthy males suggests that androgens may play a protective regulatory role in the pathophysiology of SIC.
A big man with a broken heart. In a recent clinical observation of a takotsubo stress-induced cardiomyopathy in a morbidly obese man (Zhou et al. 2010), free testosterone level was found to be lower and estrogen level higher than for an adult male, probably due to aromatization of androgens to estrogens; hence the protective role of androgens may be lost in obese men.
The Midbrain Periaqueductal Gray (PAG), Estrogen, and Emotional Stress
Depression and panic disorders are more common in women than in men and occur at times of extreme hormonal change, such as the premenstrual period, following pregnancy, and during the menopause (Lovick 2014). In mammals, 17β-estradiol (E2) has powerful effects on numerous central neural networks, reduces the response of vasoconstrictors in both male and female arteries, and can modulate pain, anxiety, depression, and cognitive function (García-Villalón et al. 1996).
There exists a reciprocal relationship between the hypothalamic-pituitary-adrenal (HPA) stress axis and the hypothalamic-pituitary-gonadal (HPG) hormonal axes. Both testosterone and estrogen can modulate the response of the stress axis, whereas activation of the stress axis inhibits estrogen and testosterone secretion (Toufexis et al. 2014).
The dorsolateral part of the periaqueductal gray, where the fight and flight reaction resides, is shown to be dense with estrogen receptors. Whether the decline in estrogen levels associated with the menopause affects the function and density of estrogen receptors in the dlPAG is not known. However, one may speculate that without the protective shield of estrogen, a change in estrogen receptor function and density might affect women’s response to emotional stress, rendering them more vulnerable and defenseless in the event of a sympathetic storm.
Conclusion
This chapter has taken a closer look at the link the autonomic nervous system provides between the brain and the heart, serving as a “telephone line” between the two and forming a basis for the connection. Evidence of a linking between emotional stresses and cardiovascular risk has been explored and more specifically, stress-induced cardiomyopathy and a plausible explanation for the female predisposition of the condition have been discussed.
References
Adrian, E. D., et al. (1932). Discharges in mammalian sympathetic nerves. The Journal of Physiology, 74(2), 115–133.
Bandler, R., et al. (2000). Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Research Bulletin, 53(1), 95–104.
Benarroch, E. E. (1993). The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings, 68(10), 988–1001.
Dampney, R. A. L., Furlong, T. M., Horiuchi, J., Iigaya, K. (2013). Role of dorsolateral periaqueductal grey in the coordinated regulation of cardiovascular and respiratory function. Autonomic Neuroscience, 175, 17–25.
Donadio, V., et al. (2002). Interindividual differences in sympathetic and effector responses to arousal in humans. The Journal of Physiology, 544(1), 293–302.
Esler, M., Jackman, G., Bobik, A., Kelleher, D., Jennings, G., Leonard, P., Skews, H., & Korner, P. (1979). Determination of norepinephrine apparent release rate and clearance in humans. Life Science, 25, 1461–1470.
Esler, M., Jennings, G., Korner, P., Willett, I., Dudley, F., Hasking, G., Anderson, W., & Lambert, G. (1988). Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension, 11, 3–20.
Esler, M., Jennings, G., Lambert, G., Meredith, I., Horne, M., & Eisenhofer, G. (1990). Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiological Reviews, 70, 963–985.
García-Villalón, A. L., et al. (1996). Sex differences in the effects of 17β-estradiol on vascular adrenergic responses. European Journal of Pharmacology, 314(3), 339–345.
Green, A. L., et al. (2005). Deep brain stimulation can regulate arterial blood pressure in awake humans. NeuroReport, 16(16), 1741–1745.
Kingwell, B. A., Thompson, J. M., Kaye, D. M., McPherson, G. A., Jennings, G. L., & Esler, M. D. (1994). Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation, 90, 234–240.
Kneale, B. J., et al. (2000). Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. Journal of the American College of Cardiology, 36(4), 1233–1238.
Kop, W. J., et al. (2004). Effects of acute mental stress and exercise on T-wave alternans in patients with implantable cardioverter defibrillators and controls. Circulation, 109(15), 1864–1869.
Kumar, K., Toth, C., & Nath, R. K. (1997). Deep brain stimulation for intractable pain: a 15 year experience. Neurosurgery, 40, 736–746.
Lovick, T. A. (2014). “Sex determinants of experimental panic attacks.” Neuroscience & Biobehavioral Reviews, 46, 465–471.
Mazzeo, A. T., et al. (2014). Brain–heart crosstalk: The many faces of stress-related cardiomyopathy syndromes in anaesthesia and intensive care. British Journal of Anaesthesia, 112(5), 803–815.
Nashold, B. S., Wilson, W. P., & Slaughter, D. G. (1969). Sensations evoked by stimulation in the midbrain of man. Journal of Neurosurgery, 30, 14–24.
Orth-Gomér, K., et al. (2000). Marital stress worsens prognosis in women with coronary heart disease: The Stockholm female coronary risk study. JAMA, 284(23), 3008–3014.
Pagani, M., Lombardi, F., Guzzetti, S., Rimoldi, O., Furlan, R., Pizzinelli, P., Sandrone, G., Malfatto, G., Dell’Orto, S., Piccaluga, E., Turiel, M., Baselli, G., Cerutti, S., & Malliani, A. (1986). Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation Research, 59, 178–193.
Pereira, E. A. C., et al. (2010). Sustained reduction of hypertension by deep brain stimulation. Journal of Clinical Neuroscience, 17(1), 124–127.
Pereira, E. A. C., et al. (2013). Elevated gamma band power in humans receiving naloxone suggests dorsal periaqueductal and periventricular gray deep brain stimulation produced analgesia is opioid mediated. Experimental Neurology, 239(0), 248–255.
Richardson, D. E., & Akil, H. (1977). Pain reduction by electrical brain stimulation in man. Part 1: acute administration in periaqueductal and periventricular sites. Journal of Neurosurgery, 47, 178–183.
Samuels, M. A. (2007). The brain–heart connection. Circulation, 116(1), 77–84.
Sverrisdóttir, Y., et al. (2012). Sympathetic nerve activity in stress-induced cardiomyopathy. Clinical Autonomic Research, 22(6), 259–264.
Sverrisdóttir, Y. B., et al. (2014). Differentiated baroreflex modulation of sympathetic nerve activity during deep brain stimulation in humans. Hypertension, 63(5), 1000–1010.
Timio, M., et al. (2001). A link between psychosocial factors and blood pressure trend in women. Physiology & Behavior, 73(3), 359–363.
Toufexis, D., et al. (2014). Stress and the reproductive axis. Journal of Neuroendocrinology, 26(9), 573–586.
Vallbo, A. B., et al. (1979). Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiological Reviews, 59(4), 919–957.
Vongpatanasin, W., et al. (2001). Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation, 103(24), 2903–2908.
Warltier, D. C., et al. (2003). Clinical relevance of the Bezold–Jarisch reflex. Anesthesiology, 98(5), 1250–1260.
Wilkinson, D., Thompson, J. M., Lambert, G. W., et al. (1998). Sympathetic activity in patients with panic disorder at rest, under laboratory mental stress, and during panic attacks. Archives of General Psychiatry, 55(6), 511–520.
Wittstein, I. S., et al. (2005). Neurohumoral features of myocardial stunning due to sudden emotional stress. New England Journal of Medicine, 352(6), 539–548.
Yardley, C. P., & Hilton, S. M. (1986). The hypothalamic and brainstem areas from which the cardiovascular and behavioural components of the defence reaction are elicited in the rat. Journal of the Autonomic Nervous System, 15, 227–244.
Zhou, J. Q., et al. (2010). A big man with a broken heart: Stress-induced cardiomyopathy in a morbidly obese man. Mayo Clinic Proceedings, 85(9), 864–865.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this entry
Cite this entry
Sverrisdóttir, Y.B. (2016). Sympathetic Nerve Activity, Stress, and Cardiovascular Risk. In: Alvarenga, M., Byrne, D. (eds) Handbook of Psychocardiology. Springer, Singapore. https://doi.org/10.1007/978-981-287-206-7_37
Download citation
DOI: https://doi.org/10.1007/978-981-287-206-7_37
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-287-205-0
Online ISBN: 978-981-287-206-7
eBook Packages: Behavioral Science and PsychologyReference Module Humanities and Social SciencesReference Module Business, Economics and Social Sciences