Abstract
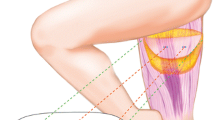
For patients who need autologous breast reconstruction after mastectomy, it is a common choice to use the perforator flap of inferior abdominal artery based on abdominal free flap. However, not all patients have sufficient abdominal tissue, either because of low body mass index, scars, inappropriate perforations, or because they have had DIEP or abdominal wall plastic surgery before [1]. An alternative is the transverse crescent-shaped upper gracilis myocutaneous flap (TUG) composed of upper tissue above the thigh [2–4]. However, TUG flap is considered to have some disadvantages. The amount of tissue available in the thigh is scarce, the vascular pedicle is short (6–7 cm in length), and the donor area is prone to wound dehiscence and infection [5–7]. As reported in the successful cases, a number of solutions against these constraints have been described from many aspects, among which the profunda artery perforator flap (PAP) reported by Allen is the most interesting refinement [8]. PAP flap is based on the musculocutaneous or septal perforator of the second or third perforator of profunda femoral artery. They converge behind the gracilis muscle and supply blood to the skin and subcutaneous fat under the gluteal sulcus. According to clinical observation and preoperative imaging studies, about 97% of the thighs have at least one suitable perforating branch with an average diameter of 1.9 mm in this area. The medial perforator is close to the adductor magnus, with an average distance of 3.8 cm from the midline and 5 cm below the gluteal sulcus, and the lateral perforator is close to the biceps femoris and lateral femoral muscle, with an average distance of 1.2 cm from the midline and 5 cm below the gluteal sulcus [9, 10]. When the abdominal tissue is insufficient, the flap can also be used as an alternative to provide soft tissue from the medial thigh donor area with relatively abundant tissue to complete breast reconstruction. It can be regarded as an evolutionary form of TUG flap. According to the evaluation of reconstructed breast and donor area, PAP flap should have more advantages than TUG flap in theory [8].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
For patients who need autologous breast reconstruction after mastectomy, it is a common choice to use the perforator flap of inferior abdominal artery based on abdominal free flap. However, not all patients have sufficient abdominal tissue, either because of low body mass index, scars, inappropriate perforations, or because they have had DIEP or abdominal wall plastic surgery before [1]. An alternative is the transverse crescent-shaped upper gracilis myocutaneous flap (TUG) composed of upper tissue above the thigh [2,3,4]. However, TUG flap is considered to have some disadvantages. The amount of tissue available in the thigh is scarce, the vascular pedicle is short (6–7 cm in length), and the donor area is prone to wound dehiscence and infection [5,6,7]. As reported in the successful cases, a number of solutions against these constraints have been described from many aspects, among which the profunda artery perforator flap (PAP) reported by Allen is the most interesting refinement [8]. PAP flap is based on the musculocutaneous or septal perforator of the second or third perforator of profunda femoral artery. They converge behind the gracilis muscle and supply blood to the skin and subcutaneous fat under the gluteal sulcus. According to clinical observation and preoperative imaging studies, about 97% of the thighs have at least one suitable perforating branch with an average diameter of 1.9 mm in this area. The medial perforator is close to the adductor magnus, with an average distance of 3.8 cm from the midline and 5 cm below the gluteal sulcus, and the lateral perforator is close to the biceps femoris and lateral femoral muscle, with an average distance of 1.2 cm from the midline and 5 cm below the gluteal sulcus [9, 10]. When the abdominal tissue is insufficient, the flap can also be used as an alternative to provide soft tissue from the medial thigh donor area with relatively abundant tissue to complete breast reconstruction. It can be regarded as an evolutionary form of TUG flap. According to the evaluation of reconstructed breast and donor area, PAP flap should have more advantages than TUG flap in theory [8].
As for the flap design, placing the perforator vessel in the center of the flap can optimize the blood supply of the flap to the greatest extent. Generally, it can carry 300–400 g tissue, which is equal to the weight of resected tissue after most mastectomies, so it is suitable for most patients. The skin island that can be dissected is long, and the general safety range is about 27 cm × 7 cm, which has prominent advantages for secondary breast reconstruction and remedial breast chest wall reconstruction. The vascular pedicle is longer, with an average length of 9.9 cm, which makes the tissue flap implantation more flexible. Also, the vessels are well matched with those in the receiving area. As for the donor area, the scar is well hidden in the hip sulcus. There is no need to sacrifice the muscle like dissection TUG flap, and the anatomical range is farther away from lymphatic vessels, which can reduce the potential risk of subcutaneous seroma. The operation is more complicated than gracilis myocutaneous flap, but you can master it quickly after understanding the anatomical basis. The anatomy and surgical methods have been previously elaborated.
22.1 A Typical Case of Breast Reconstruction with Single Vessel Pedicled Profunda Femoral Artery Perforator Flap
The patient was a 60-year-old female with right breast mass found in physical examination for 1 week. The pathological diagnosis is invasive ductal carcinoma, histological grade I–II. After admission, she underwent modified radical mastectomy for right breast cancer and breast reconstruction with left profunda femoral artery perforator flap. The operation was successful and the flap survived well. The patients was transferred to internal medicine department for chemotherapy. The appearance of reconstructed breast was satisfactory during follow-up, and no tumor recurrence was found (Figs. 22.1, 22.2, 22.3, 22.4, 22.5, 22.6, 22.7, 22.8, 22.9, 22.10, and 22.11).
Lift the flap from the anterior to the posterior, reserve as much subcutaneous adipose tissue as possible in the flap, then expose the large perforating vessels between the gracilis and adductor longus, and separate them retrogradely. (1) Medial femoral muscle. (2) Adductor longus. (3) Great saphenous vein. (4) Gracilis muscle. (5) Adductor major. (6) Anterior branch of obturator nerve. (7) Perforating vessels
After the retrograde separation of the vascular pedicle of the gracilis muscle branch to the plane of the profunda femoral vessel, we switch to lift the posterior edge of the flap to expose a perforator vessel between the great adductor muscle and the semimembranosus muscle. (1) Adductor longus. (2) Gracilis muscle. (3) Adductor major. (4) Semimembranosus muscle. (5) Adductor major perforator vessel
The single vessel pedicled profunda femoral artery perforator flap for breast reconstruction has its characteristics and precautions: (1) There is the largest and most consistent profunda femoral artery perforator on the surface of adductor major, so the design center point of the flap should be properly moved backward when dissecting the profunda femoral artery perforator flap. (2) As long as the site of the perforator is reasonably designed near the central area of the flap, the reliable blood supply of the larger flap can be ensured, because the blood supply of the medial femoral is abundant, which is different from the vascular distribution of the lower abdomen. Also, there is no obvious restriction on the vascular perforating body area. (3) Preoperative Doppler detection of the location of perforating vessels is often not highly accurate, especially in female patients with thick fat on the inner thigh. If possible, CTA of the inner thigh should be used to determine the size, quantity, location, and source of perforating vessels.
References
Hunter JE, Lardi AM, Dower DR, Farhadi J. Evolution from the TUG to PAP flap for breast reconstruction: comparison and refinements of technique. J Plast Reconstr Aesthet Surg. 2015;68(7):960–5.
Yousif NJ, Matloub HS, Kolachalam R, Grunert BK, Sanger JR. The transverse gracilis musculocutaneous flap. Ann Plast Surg. 1992;29(6):482–90.
Schoeller T, Huemer GM, Wechselberger G. The transverse musculocutaneous gracilis flap for breast reconstruction: guidelines for flap and patient selection. Plast Reconstr Surg. 2008;122(1):29–38.
Fattah A, Figus A, Mathur B, Ramakrishnan VV. The transverse myocutaneous gracilis flap: technical refinements. J Plast Reconstr Aesthet Surg. 2010;63(2):305–13.
Wong C, Mojallal A, Bailey SH, Trussler A, Saint-Cyr M. The extended transverse musculocutaneous gracilis flap: vascular anatomy and clinical implications. Ann Plast Surg. 2011;67(2):170–7.
Schoeller T, Wechselberger G. Breast reconstruction by the free transverse gracilis (TUG) flap. Br J Plast Surg. 2004;57(5):481–2.
Locke MB, Zhong T, Mureau MA, Hofer SO. Tug ‘O’ war: challenges of transverse upper gracilis (TUG) myocutaneous free flap breast reconstruction. J Plast Reconstr Aesthet Surg. 2012;65(8):1041–50.
Allen RJ, Haddock NT, Ahn CY, Sadeghi A. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg. 2012;129(1):16e–23e.
Haddock NT, Greaney P, Otterburn D, Levine S, Allen RJ. Predicting perforator location on preoperative imaging for the profunda artery perforator flap. Microsurgery. 2012;32(7):507–11.
Ahmadzadeh R, Bergeron L, Tang M, Geddes CR, Morris SF. The posterior thigh perforator flap or profunda femoris artery perforator flap. Plast Reconstr Surg. 2007;119(1):194–200; discussion 201–2.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Shanghai Scientific and Technical Publishers
About this chapter
Cite this chapter
Song, D., Mitsunaga, N. (2023). Single Pedicled Profunda Femoral Artery Perforator Flap for Breast Reconstruction. In: Li, Z., Song, D. (eds) Oncoplastic Flap Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-19-8926-1_22
Download citation
DOI: https://doi.org/10.1007/978-981-19-8926-1_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8925-4
Online ISBN: 978-981-19-8926-1
eBook Packages: MedicineMedicine (R0)