Abstract
Large amounts of high level liquid waste (HLLW) has been generated from the reprocessing of spent nuclear fuel employing the PUREX process. It contains complex composition including various fission products (FPs), corrosion products and minor actinides (MAs). Some of these nuclides, such as strontium-90 (90Sr), can be used in military and medical fields and have commercial value. The separation of strontium is not only about to develop the value of HLLW but also reduce the radioactivity of the liquid waste for disposal. Separating of Sr from other elements including Ln, Y, Cs, Ru, Fe, Mo, Zr, etc. is essential to obtain available strontium products. In this work, N,N,N′,N′-Tetraoctyldiglycolamide (TODGA) was used for the purpose focusing on removing fission products and corrosion products other than Sr. Firstly, 0.05 mol/L TODGA was used as extractant to separate Sr from Ln, Zr and Y, by which process Ln, Zr and Y were extracted to the organic phase and Sr entered the raffinate. Meanwhile, Ru, Fe and Cs were in the aqueous phase together with Sr. 0.2 mol/L TODGA was further used to contact with the raffinate, by which Sr was extracted and Ru, Fe and Cs were still in the aqueous phase being separated. After the Sr was stripped from the organic phase, Sr product was obtained. The separation method has been verified with non-radioactive simulated HLLW and radioactive genuine HLLW as feed liquid, proving its reliability.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
HLLW generated from reprocessing of spent nuclear fuel by PUREX process is highly acidic (HNO3 about 3 mol/L) and complex in composition. The elements in HLLW include minor actinides (Americium (Am), Curium (Cm), etc.), fission products, including Lanthanides (Lanthanum (La), Neodymium (Nd), Europium (Eu), etc.), Strontium (Sr), Cesium (Cs), Ruthenium (Ru), Palladium (Pd), Niobium (Nb), Zirconium (Zr), etc., as well as many corrosion products (Iron (Fe), Nickel (Ni)) [1,2,3,4]. Since radio strontium 90Sr is one of the major heat generators in nuclear waste, the severe conditions for waste repository design governed by decay heat would be reduced on its removal. Besides, 90Sr (half-life: 28.79 year), as a pure beta emitter, whose decay daughter is 90Y (half-life: 2.67 day), is used in medical radiotherapy [5,6,7,8].
Since the 1940s, people have been working to remove and recover 90Sr from HLLW. The earliest reagents used include di(2-ethylhexyl)phosphoric acid, chlorinated cobalt dicarbollide and various derivatives of macrocyclic polyethers. The further modification of these works has led to the development of modern extraction methods, such as SREX, FPEX and UNEX, which are suitable for extracting 90Sr from HLLW. SREX (strontium extraction) process uses di-t-butylcyclohexano-18-crown-6 (DtBu18C6) as extractant to efficiently and selectively extract Sr from acidic HLLW containing a variety of fission products and inert components [9,10,11]. FPEX (fission product extraction) process is based on the simultaneous extraction of Cs and Sr from HLLW, the extraction of Sr with DtBu18C6 and the extraction of Cs with BOBCalixC6 [12,13,14]. UNEX (universal extraction) process implemented by INL, America and KRI, Russia, uses 0.08 mol/L chlorinated cobalt dicarbollide, 0.5% polyethylene glycol 400 and 0.02 mol/L diphenyl-N,N-dibutylcarbamoyl methyl phosphine oxide as extractants [15, 16].
N,N,N′,N′-tetra octyldiglycolamide (TODGA) is a widely concerned reagent in the treatment of HLLW because of its good extraction performance, excellent stability and easy to be obtained. Some studies have shown that TODGA displayed an affinity toward extraction of strontium from 2 to 3 mol/L nitric acid solutions [17,18,19,20,21]. In this work, for the HLLW after the removal of minor actinides, TODGA was used as the extractant to separate Sr from other fission products and corrosion products by batch extraction. Firstly, HLLW solution was extracted by a lower-concentrated TODGA to separate Sr from those with high distribution ratios such as Ln, Y and Zr. The raffinate was retained for a subsequent extraction with a higher-concentrated TODGA to separate Sr from elements with low distribution ratios such as Cs, Ru and Fe and Sr products was obtained after stripping. A preliminary hot test was conducted to verify the separation process.
2 Experimental
TODGA was synthesized in our lab using a new method which hasn’t been published, and the purity analyzed by HPLC was > 96% (mobile phase: methanol, peak area: TODGA of 96.4%, ethyl acetate of 3%, di-n-octylamine of 0.6%). The organic TODGA solutions were prepared by dissolving precisely weighed amounts of TODGA and phase modifier into the kerosene diluent. The simulated aqueous solutions were prepared by dissolving appropriate amounts of nitrates into HNO3 solutions. All the metal nitrates are commercially available without further purification. The radioactive genuine HLLW was obtained from CNNC 404 Co., Ltd.
In the experiments, 10 ml of organic and 10 ml of aqueous phases were contacted in a 20 mL glass-stoppered tube and mixed by an oscillator with 250 rmp for 15 min. Preliminary experiments showed that the equilibrium was reached after five minutes. After stewing for 5 min with adequate phase separation, an aliquot of each phase was taken for the measurement of the distribution ratio (DM) that equals to the ratio of the concentration of M in the organic phase over that in the aqueous phase. All the extraction experiments were performed at 25 + 0.2 ℃.
Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-OES, Agilent 5110, USA) and Inductively Coupled Plasma Mass Spectrometer (ICP-MS, PerkinElmer NexlON 2000, USA) were used to measure the concentrations of metals in both phases for cold tests. Nuclear Magnetic Resonance Spectromete (NMR, Bruker 400MHz, Germany) was used to check the structure of TODGA. The activity of 90Sr was determined by measuring the activity of its daughter 90Y. The sample was put aside for 14 days and then 90Y was extracted with an equal volume of 30% TRPO in kerosene. The activity was determined by use of a liquid scintillation analyzer (Packard 2200 CA). The 137Cs and 152Eu activity was determined with a Ge-Li detector (EG&G, USA).
3 Results and Discussion
The extraction ability of strontium by TODGA increases with the rising of TODGA concentration, as shown in Fig. 1a. To provide insight into the composition of species of Sr2+ formed in the extraction, the dependences of DSr on the concentrations of TODGA and initial concentrations of HNO3 in aqueous phase were determined, respectively. Figure 1a shows the plot of logDSr and log[TODGA], and the slope is 2.28 ± 0.03. The experiments were implemented by 0–0.4 mol/L TODGA-0.5 mol/L TBP-kerosene with two phases of O:A = 1 and 3 mol/L HNO3 in aqueous phases. Figure 1b shows the plot between logDSr and log[HNO3]. The experiments were carried out by 0.2 mol/L TODGA-0.5 mol/L TBP-kerosene with two phases of O:A = 1. Based on the above results, the possible mechanism of strontium extraction by TODGA is as follows:
Figure 2 shows the distribution ratios of different fission and corrosion products extracted by TODGA under the condition of 3 mol/L HNO3 and O:A = 1. Kerosene was used as diluent and 0.5 mol/L TBP was used as phase modifier. As shown in Fig. 2a and b, the distribution ratio of Eu is significantly higher than that of Sr with the TODGA concentrations range of 0–0.4 mol/L. Specifically, when the TODGA concentrations range of 0.025–0.05 mol/L, DSr is very low (DSr < 0.1), and the distribution ratio of Eu is much higher (DEu > 10), which indicates that Sr will be in aqueous phase while Eu can be extracted into organic phase. Lanthanide elements usually have similar extraction properties and the performance of europium can be used as a reference for other lanthanides, which indicates that they have the same extraction trend under the same condition. Therefore, Sr can be separated from lanthanides by this simple TODGA contact. Figure 2c shows the distribution ratios of Sr and Zr, which indicates the much higher distribution ratio of Zr compared to that of Sr. Like Ln, Zr would be extracted and separated from Sr. In Fig. 2d, e, f comparisons of distribution ratios of Sr and Ru, Cs and Fe can be seen. As shown, the distribution ratios of Ru, Cs and Fe have been very low even when that of Sr has gone to an ascent. According to the distribution ratios, TODGA can be used to separate Sr from Ru, Cs and Fe when the using TODGA concentration is high enough to extract Sr. Based on the extraction properties, the separation of Sr from a large amount of other fission products and corrosion products can be achieved by adjusting the TODGA concentrations.
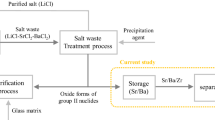
According to the extraction performance, the process of separating Sr from other fission products and corrosion products in simulated HLLW by TODGA was designed and verified. Kerosene was used as diluent and 0.5 mol/L TBP was used as phase modifier in this process. The concentration of HNO3 in the feed liquid was 3 mol/L to simulate the HLLW. The initial metal concentrations can be seen in Table 1. Firstly, 0.05 mol/L TODGA was contacted with feed solution with O:A = 1. As seen in the result, DSr was 0.03, which indicated that Sr hadn’t been extracted remaining in aqueous phase. Meanwhile, the distributions ratios of Zr, Y and Eu, Gd, Nd were much higher (DZr > 700, DY > 500, DEu > 200, DGd > 400, DNd = 7.9), signifying that these elements were extracted being separated from Sr. However, the distributions of Cs, Ru, Fe were low (DCs < 0.01, DFe = 0.39, DRu = 0.6), implying Cs, Ru and Fe would keep in aqueous phase along with Sr. Thus, further separation was needed. The raffinate obtained from this process was further contacted with 0.2 mol/L TODGA, by which operation Sr could be extracted into organic phase (Dsr = 4.5) and Cs, Ru, Fe and other analogous fission and corrosion products were separated by keeping in aqueous phase according to the distribution ratios (DCs < 0.01, DFe = 0.39, DRu = 0.6). The final striping composition is shown in the Table 1. The schematic diagram of the whole process is shown in the Fig. 3.
To demonstrate the reliability of this process, further hot-tested verification using radioactive genuine HLLW as feed liquid has been implemented according to Fig. 3. Due to the limitation of experimental time and characterization means, only three elements of Sr, Cs and Eu were traced. Separation of Sr from Eu and Cs were testified to be consistent with that of cold test, with Dsr of 0.01, DCs of < 0.01 and DEu of > 750 in the first contact and Dsr of 4.3, DCs of < 0.01 in the second contact, respectively.
4 Conclusions
As a fission product in HLLW, strontium-90 finds extensive use in nuclear medicine and industry, and its research on separation is of great significance. Sr was successfully separated from other fission and corrosion products in HLLW using TODGA as extractant in this work. Two concentrations of TODGA were employed in a flow sheet with two contacts, separating Sr from Ln, Y, Zr, Cs, Ru and Fe etc. Specifically, with a low TODGA concentration (0.05 mol/L) extraction, Sr had a low distribution ratio remaining in the aqueous phase, while Ln, Zr and other elements with high distribution ratio were extracted into the organic phase, resulting in their separation. At a higher TODGA concentration (0.2 mol/L), Sr showed a high distribution ratio and could be extracted, Cs, Ru and Fe went into the raffinate separating from Sr. After two batch extractions by TODGA, a Sr product could be obtained getting rid of a large amount of fission and corrosion elements. This method has been tested using the genuine hot HLLW as feed liquid with verification of its consistency with that of cold test. The hot experiment will be further elaborated in the future work.
References
Veliscek-Carolan, J.: Separation of actinides from spent nuclear fuel: a review. J. Hazard. Mater. 318, 266–281 (2016)
Ewing, R.C.: Long-term storage of spent nuclear fuel. Nat. Mater. 14, 252–257 (2015)
Miguirditchian, M., Vanel, V., Marie, C., Pacary, V., Charbonnel, M.-C., Berthon, L., Hérès, X., Montuir, M., Sorel, C., Bollesteros, M.-J., Costenoble, S., Rostaing, C., Masson, M., Poinssot, C.: Americium recovery from highly active PUREX raffinate by solvent extraction: The EXAm process. a review of 10 years of R&D. Solvent Extr. Ion Exc. 38, 365–387 (2020)
Chitnis, R.R., et al.: Separation and recovery of uranium, neptunium, and plutonium from high level waste using tributyl phosphate: countercurrent studies with simulated waste solution. Sep. Sci. Technol. 33, 1877–1887 (1998)
Nishiyama, Y., Hanafusa, T., Yamashita, J., Yamamoto, Y., Ono, T.: Adsorption and removal of strontium in aqueous solution by synthetic hydroxyapatite. J. Radioanal. Nucl. Chem. 307(2), 1279–1285 (2015). https://doi.org/10.1007/s10967-015-4228-9
Lumetta, G.J., Wagner, M.J., Jones, E.O.: Separation of strontium-90 from hanford high-level radioactive waste. Sep. Sci. Technol. 30, 1087–1101 (1995)
Happel, S., Streng, R., Vater, P., Ensinger, W.: Sr/Y separation by supported liquid membranes based on nuclear track micro filters. Radiat. Meas. 36, 761–766 (2003)
Pichestapong, P., Sriwiang, W., Injarean, U.: Separation of yttrium-90 from strontium-90 by extraction chromatography using combined Sr resin and RE resin. Energy Procedia 89, 366–372 (2016)
Sharma, J.N., Khan, P.N., Dhami, P.S., Jagasia, P., Tessy, V., Kaushik, C.P.: Separation of strontium-90 from a highly saline high level liquid waste solution using 4,4′(5′)-[di-tert-butyldicyclohexano]-18-crown-6 + isodecyl alcohol/n-dodecane solvent. Sep. Sci. Technol. 229 (2019)
Horwitz, E.P., Dietz, M.L., Fisher, D.E.: Srex: a newprocess for the extraction and recovery of strontium from acidic nuclear waste streams. Solvent Extr. Ion Exc. 9, 1–25 (1991)
Bai, F., He, C., Chen, G., Wei, J., Wang, J., Ye, G.: Synthesis of alkyl substituted dicyclohexano-18-crown-6 homologues for strontium extraction in HNO3 media. Energy Procedia 39, 396–402 (2013)
Xu, C., Wang, J., Chen, J.: Solvent extraction of strontium and cesium: a review of recent progress. Solvent Extr. Ion Exc. 30, 623–650 (2012)
Wang, J.: Co-extraction of strontium and cesium from simulated high-level liquid waste (HLLW) by calixcrown and crown ether. J. Nucl. Sci. Technol. 52, 171–177 (2014)
Riddle, C.L., et al.: Fission product extraction (FPEX): development of a novel solvent for the simultaneous separation of strontium and cesium from acidic solutions. Solvent Extr. Ion Exc. 23, 449–461 (2005)
Romanovskiy, V.N., Smirnov, I.V., Babain, V.A., Todd, T.A., Herbst, R.S., Law, J.D., Brewer, K.N.: The universal solvent extraction (Unex) process. I. development of the unex process solvent for the separation of cesium, strontium, and the actinides from acidic radioactive waste. Solvent Extr. Ion Exc. 19, 1–21 (2001)
Kumar, V., et al.: Separation of strontium from the raffinate solution of TEHDGA-actinide partitioning process: batch extraction and process development. Sep. Sci. Technol. 98, 118–122 (2012)
Tachimori, S., Suzuki, S., Sasaki, Y., Apichaibukol, A.: Solvent extraction of alkaline earth metal ions by diglycolic amides from nitric acid solutions. Solvent Extr. Ion Exc. 21, 707–715 (2003)
Tian, G., Wang, J., Shen, Y., Rao, L.: Extraction of strontium from HLLW using N, N, N′, N′-tetraisobutyl 3-oxa-glutaramide. Solvent Extr. Ion Exc. 23, 519–528 (2005)
Zhu, Z.-X., Sasaki, Y., Suzuki, H., Suzuki, S., Kimura, T.: Cumulative study on solvent extraction of elements by N, N, N′, N′-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into n-dodecane. Anal. Chim. Acta 527, 163–168 (2004)
Dhami, P.S., et al.: Separation and purification of 90Sr from PUREX HLLW using N,N,N′,N′-tetra(2-ethylhexyl) diglycolamide. J. Radioanal. Nucl. Ch. 296, 1341–1347 (2012)
Suzuki, Y.S.H., Sugo, Y., Apichaibukol, A., Kimura, T.: Extraction and separation of Am(III) and Sr(II) by N,N,N′,N′-tetraoctyl-3-oxapentanediamide (TODGA). Radiochim. Acta 92, 463–466 (2004)
Author information
Authors and Affiliations
Contributions
Nong Shuying and Chai Youqi worked equally to this work.
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Shuying, N. et al. (2023). Separation of Strontium from Other Fission Products in High Level Liquid Waste by TODGA. In: Liu, C. (eds) Proceedings of the 23rd Pacific Basin Nuclear Conference, Volume 2. Springer Proceedings in Physics, vol 284. Springer, Singapore. https://doi.org/10.1007/978-981-19-8780-9_35
Download citation
DOI: https://doi.org/10.1007/978-981-19-8780-9_35
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8779-3
Online ISBN: 978-981-19-8780-9
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)