Abstract
The Palanan lowland forests are being utilized in many ways. It has been a research site for determining ecosystem resiliency. To the communities especially the Agtas, it is an area where they get resources from. Among its ecosystem services is pollination and seed dispersal which can be understood by studying the reproductive phenological development of plant species considering there is little knowledge on biotic interactions involved. The collection of reproductive phenological data was performed in the four sites within the lowland forests of the municipality of Palanan: Brgy. Dipogen, PFDP, and Brgy. Alomanay and Mangrove area in Maharlika. A modified reproductive phenology scoring was utilized. Individual tree count in PFDP is high due to the high recruitment rate brought upon by the frequent freeing up of space in the forest canopy every typhoon, which temporarily removes light limitation for seedlings to grow. The majority of the reproducing species during the study period were categorized as supra-annuals. Of these families, Meliaceae, Euphorbiaceae, Lauraceae, Annonaceae, Lamiaceae, Rubiaceae, Arecaceae, and Dipterocarpaceae, all of which are among the most species-rich families inside the plot with flowering and fruiting occurring during the hot dry conditions. Almost forty percent of the species reproduce sub-annually (≈20%) and annually (≈18%). The less rich group of species reproduces continuously. Current data shows flowering was mildly affected by temperature and moderately affected by rainfall. Fruiting was not really affected by temperature and rainfall.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
With the increasing population of the Philippines, more space is needed for agricultural lands to meet the demand and increase food security. Local practices such as Swidden agriculture (kaingin) clear forest patches for the planting of crops on a smaller scale. Timber, on the other hand, is harvested for the building of houses of locals. Housing and infrastructure for the public such as clinics, hospitals, and schools require space. It is common for developing countries to expand land use to these basic services. Inevitably, a lot of green spaces are removed to accommodate these needs. Along with the removal of forest cover is also the loss of habitat for a part of the wildlife population. Additionally, illegal logging is still a big problem, especially in less monitored forests. With the increasing rate of deforestation in the country, it is of primary concern to replenish the lost green spaces wherever needed and possible. Aside from the lowlands and mountains, the mangrove ecosystems also suffer from indiscriminate logging and conversions. In response to this, reforestation programs have been put up by the government, non-government organizations, and the private sector; through laws that required private companies to help in restoring lost habitats, especially for mining companies, as part of corporate social responsibility. All the aforementioned affects the ecosystem services provided by forest ecosystems. One of the most important of which is pollination and seed dispersal. However, in order to evaluate this, one must understand the reproductive phenological development of plant species. It is, therefore, imperative that floral phenology be considered in literature considering there is little knowledge on biotic interactions involved.
Plant reproductive phenology is the study of the periodicity of repeating biological events in plants, in relation to short-term climatic change (Sakai et al. 2005). Long-term disturbances brought about by anthropogenic factors can directly affect floral phenology and ecology as these may cause a decrease in the population and diversity of plant species (Hussein et al. 2021) and plant community structure (Wolf et al. 2017) which in turn cause habitat loss for the fauna. Climatic conditions also directly affect the floral ecology and in return, if reproduction is successful, it affects the microclimatic environment through vegetation enhancement. Conservation and management can be performed when all aspects are considered.
In the Philippines, a long-term research area has been established in Palanan, Isabela. Palanan, Isabela is a first-class municipality whose population in 2021 is at 17,260 and estimated revenue of PhP2,6M (DTI 2021). It is a remote and isolated coastal town, separated by the Sierra Madre Mountains along its western borders from most of the towns of Isabela. As early as 2020, the Ilagan-Divilacan Road was opened to enable travel by land to the remote towns of Divilacan, Maconacon, Palanan, and Dinapigue.
Palanan’s forest is the only forest patch in the Philippines whose monitoring or census is mainly supported by the Smithsonian Tropical Research Institute (STRI) through its Forest Global Earth Observatory Network (ForestGEO). A 16-ha area, the Palanan Forest Dynamics Plot (PFDP), was established in 1994 for this endeavor. Since the Palanan Forests is quite vast, several communities of indigenous Agta live in it. However, their culture has been threatened by socio-economic pressures added to the general ecosystem being affected by illegal logging, resource exploitation, and land-use change (ForestGEO 2022).
This chapter shall discuss the reproductive phenology of plant species within selected sites of the Palanan Forest. Methodologies utilized will also be presented. This data assists in describing the area’s long-term dynamics as well as in the understanding of how forests respond to continuous disturbances, such as typhoons and human interventions.
2 Gathering Reproductive Phenological Data
This is an offshoot study from the larger objective of assessing the bat pollen diet of phytophagous bats in the lowland rainforests of Palanan, Isabela which is a part of a 2.5 years research program (2016–2018) in understanding fruit bats and their interaction with plants in the said area. The underlying criteria for choosing the sites are based on different populations of phytophagous bats determined through the capture and recapture method which this phenological study is supporting.
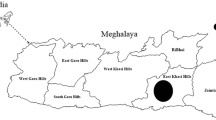
The collection of reproductive phenological data was performed in the municipality of Palanan. A total of four sites were utilized for the study. The three study sites in the lowland forests were Brgy. Dipogen, Palanan Forest Dynamics Plot (PFDP), and Brgy. Alomanay (Fig. 14.1). PFDP is a reserved area; Brgy. Alomanay site is at a higher elevation and Brgy. Dipogen is at a mid-elevation of the two. The Agta communities are nearest to Brgy. Dipogen site. The fourth site in the lowland forest was later replaced by a mangrove site in Barangay Maligaya due to the similarity of plant assemblages with PFDP. The selection of the mangrove site was to give an array of information regarding the reproductive phenology of mangrove and mangrove-associated species. These sites were visited one after another. The monitoring of the transects was conducted from April 2016 to November 2017.
Map of the three study sites. The mangrove site is not represented in the figure. (https://www.darrinward.com/lat-long)
Diurnal phenological observation and data collection were performed, in between nocturnal nettings. In each site, three 20 m x 80 m transects were set up in the middle of the 20 nets utilized for the bat research. This rendered a sum of 4800 m2 of surveyed area for each site and 19,200 m2 for all four sites. The presence of flowers and fruits was recorded using a reproductive phenology scoring (Table 14.1) devised by Yap (2015), which was used in her previous studies in the PFDP. It includes a recording of flowering and fruiting intensity by eye estimation of its percent canopy cover. This phenological scoring is qualitative, simpler, and more practical in field studies without spending long hours gathering absolute counts of flowers and fruits. This was modified from crown estimates of flower and fruit cover within the canopy, methods which were first used in other studies such as Koelmeyer (1959), Sinhaseni (2008), and Elliott et al. (2013). However, the work of Yost et al. (2018) on reproductive phenology scoring using digitized protocol may be utilized later as herbarium specimens have been collected.
Field plant identification was mostly done by long-term field guides and locals. These guides were trained from the previous censuses of the PFDP to identify plants using plant codes representative of the scientific names of the plants in the Palanan plant code guide. Voucher and flower collection were simultaneously done with a gathering of field phenology data (Fig. 14.2). Voucher collections were gathered for identification of individuals that cannot be readily identified in the field, thus were pressed, and brought to the UP Diliman herbarium. Different flora was also utilized for identification and name verification (Pelser et al. 2011; Madulid 2021; Pancho 1983; Pancho and Gruezo 2006; Pancho and Gruezo 2012; Slik 2009). Flower collections were done in conjunction with pollen-type identification.
Phenology monitoring in the field (a and g) field guides assist in spotting reproductively active individuals, (b) field guides inspecting tag number of trees, (c and d) climbing trees to get flowers and fruits, (e) inspection of buds/fruits, and (f) gathering individual flowers/florets for mounting of pollen
Data on monthly rainfall and temperature were also gathered through the Davis Weather Station and Vantage Pro™ that was set in the PFDP bunkhouse. This data was utilized in determining the general conditions in the sites which then is correlated to the reproductive phenology.
3 Temperature and Rainfall Readings
Mean temperature together with the total rainfall every month was plotted (Fig. 14.3). Some missing data (e.g., months of February to April 2017) were filled via imputation and interpolation method while data for April 2016 to July 2016 cannot be filled artificially. Examining the timing of reproduction of the species in the lowland forests of Palanan, at least two thirds of the monitored species reproduced during the hot months of April to September. In October 2017, the mean temperature reached 26.06 °C and rainfall hit 500.61 mm from a low of 56.83 mm the preceding month. Almost the same scenario can be seen from the interpolated data for October 2016 (μ temp. = 26.39 °C, total rainfall = 527.03 mm).
4 Richness and Diversity of Reproducing Tree Species
Individual tree count in PFDP is high due to the high recruitment rate. The high recruitment in the plot is brought upon by the frequent freeing up of space in the forest canopy every after typhoon, which temporarily removes light limitation for seedlings to grow. This demonstrates good forest resiliency (Yap et al. 2015). The proportion of the reproductive species > and <5.0 cm in DBH from the 429 individuals observed to have flowered was examined. This was computed from the data from the first year. It is remarkable that the proportion is 1:1, however even if the individual effort is 1:1, the intensity per individual is greater in bigger conspecifics.
A total of 151 species of fruiting species from 98 genera in 45 families were inside the transect used. Of these, only 107 species from 79 genera in 40 families were observed to have entered at least one episode of reproduction either in the form of flowering, fruiting, or both. Individuals that have been observed to be fruiting but not observed flowering the previous month were considered to have flowered during the 2-week fieldwork break. Of these families, Meliaceae has the most species representation. This was followed by the Euphorbiaceae, Lauraceae, Annonaceae, Lamiaceae, Rubiaceae, Arecaceae, and Dipterocarpaceae, all of which are among the most species-rich families inside the plot. Table 14.2 shows the five most represented families that flowered in PFDP along with their representative species. Their reproductive representation in the phenology data reflect how these families were the most successful and abundant in the lowland forests of Palanan. Litsea and Polyathia species were observed to be prolific flower producers as they were observed to form flower buds in several months. It is also worth noting that members of Euphorbiaceae such as from the genus Macaranga are fast-growing species. Some of them reproduce more than once in a year while members of the family Meliaceae reproduce once or less in a year.
5 General Flowering and Fruiting Phenology
The plant species monitored were placed in different phenological classes following the classification system devised by Newstrom et al. (1994). This system standardizes the comparisons on the phenological patterns as tropical plant species may vary geographically. Of the observed species to have reproduced during the observation period, the majority of the species were categorized as supra-annuals meaning that these plants reproduce in a scale greater than a year. Typically, though, the reproductive bouts are less than one per year. Those reproducing yearly and less than a year were labeled as annual and sub-annual, respectively. Those observed to flowering entirely were labeled continual.
However, since the duration of the observation is less than two years, the count of species reproducing supra-annually may indeed still consist of species flowering irregularly or those restricted to flower in general flowering (GF) periods which happens irregularly. Sakai (2000) presented that general flowering is a unique phenomenon occurring in lowland dipterocarp forests in Southeast Asia. In this phenomenon, most dipterocarp species may have profuse flowering in a span of several years in long periods and in synchronicity. Such can contribute to forest diversity because it can disrupt florivory, nectivory, and frugivory due to possible fluctuations in flower and fruit availability. The separation of those reproducing irregularly and strictly in GF events will be made with continuous observation of the species in the lowland forests of Palanan.
Among the monitored species, almost forty percent reproduce sub-annually (≈20%) and annually (≈18%). The less rich group of species reproduce continuously. This includes Pinanga insignis, Musa textilis, Ficus congesta, F. fistulosa, and Caryota cumingii. Musa textilis and its congeners are known to be visited by phytophagous bats for flowers, such as members of the genera Rousettus, Macroglossus, and Eonycteris (Marshall 1985). A phenology calendar was also constructed out of the data. Table 14.3 shows the full phenophase calendar for the two-year study which shows the timing of bud appearance, floral anthesis, and fruiting.
From April 2016 to March 2017 (Y1), a general trend of decreasing abundance (Fig. 14.4) in flowering and fruiting individuals was observed for the three sites in Palanan, this includes the sites in Brgy. Dipogen, PFDP in Brgy. Villa Robles and another site in Brgy. Alomanay. As in Table 14.3, most of the species monitored produced flowers and fruits during the observed hot dry conditions. Very few were observed during cold wet conditions.
General continuous (except July 2016 and January 2017) phenological data, and climatic condition observation from April 2016 to March 2017 (Y1) in the three sites, reveal quite interesting patterns (Fig. 14.5). Diversity of flowering species was observed to have at least positive weak (r = 0.190777816) correlation with average monthly temperature and negative moderate (r = −0.512106557) correlation with total monthly rainfall. The effect of temperature in the diversity of flowering species is very minimal but quite detrimental to rainfall. It is understandable the flowers being sink zones will not be produced at very high average monthly temperatures or when average monthly rainfall is high which will destroy the flowers directly. In both cases, plants are under stress. Similar observations were gathered by Ushio et al. (2020) in their study on GF in Bornean lowland rainforest. Using models and time series data from 1993 to 2011, they found out that GF is synergistic and not independent of cool air temperature and drought affecting it. On the other hand, the opposite was observed with the fruiting diversity (rtemp = −0.30969, rrain = 0.671168). Since most flowering occurred during hot dry conditions, it is more likely that fruiting will come during cold wet conditions. This is not evident, though, as some species were still with fruiting or with fruits during hot dry conditions. Rainfall may have also not affected the presence of fruits. In the research of Polansky and Boesch (2013), rainfall does not explain fruiting phenology and diversity even with long-term changes. Both flowering and fruiting may also be linked to seasonal patterns, pollination syndromes, and dispersal mechanisms (Mohandass et al. 2018) so long-term monitoring is important.
With these insights even without the integration of the current data with previous data gathered by Yap in 2015, some inferences can be deduced. Considering climate change is a major threat to conservation, organizing long-term and multi-taxa data on phenology is important to developing proper conservation strategies (Rosemartin et al. 2014), especially on reproductive phenology data as it will give information for seedling recruitment. Borghi et al. (2019) further presented that disruptions in plant–pollinator interactions reduce crop yields when drastic environmental conditions occur during flowering periods. Additionally, close monitoring may provide useful information on the behavior of Palanan lowland forests in phenomena such as general flowering.
6 Summary and Conclusion
Individual tree count in PFDP is high due to the high recruitment rate. A total of 151 species of fruiting species from 98 genera in 45 families were inside the transect used. Of these, only 107 species from 79 genera in 40 families were observed to have entered at least one episode of reproduction either in the form of flowering, fruiting, or both. The most reproductively active families were Meliaceae, Euphorbiaceae, and Lauraceae. The integration of previous phenological data with what has been gathered in this study is needed. The current information generated can be amended to the phenological schedules generated from February 2014 to March 2015. In general, the majority of the species reproduce during the relatively hot period of the year from April to September. The phenophase calendar constructed has shown that most of the plant species are supra-annuals with flowering and fruiting occurring during the hot dry conditions. This calendar can be continuously revised with previous and new incoming data.
The tropical lowland forests of the Philippines are indeed one of the most diverse in the region. The population of species, dynamics, and resiliency of these forests are well documented for some designated areas, but most are still under the pressure of conversion for human use. Furthermore, little is known of its reproductive phenology (Co et al. 2006), from species to community levels. With the ever-changing climate, gradual change in global temperatures, and increased fluctuations from established climatic patterns, it is imperative to study the responses and adaptability of these species. Palanan, a unique lowland forest, a frontier in the Philippines, diverse and resilient, may enable us to understand how to not replace but to “restore forests” (Ong 2017).
References
Borghi M, de Souza LP, Yoshida T, Fernie AR (2019) Flowers and climate change: a metabolic perspective. New Phytol 224(4):1425–1441
Co LL, LaFrankie JV, Lagunsad DA, Pasion KAC, Consunji HT, Bartolome NA, Yap SL, Molina JE, Tongco MDC, Ferreras UF, Davies SJ, Ashton PS (2006) Forest trees of Palanan. A study of Population Ecology. Center for Tropical Forest Science, Philippines
Department of Trade and Industry 2021. Cities and Municipalities Competitive Index—Isabela Profile. Retrieved from https://cmci.dti.gov.ph/prov-profile.php?prov=Isabela&year=2021
Elliott SD, Blakesley D, Hardwick K (2013) Restoring tropical forests: a practical guide. Royal Botanic Gardens, Kew, p 344
Forest Global Earth Observatory Network (ForestGEO) (2022) Palanan Forest Dynamic Plot (PFDP). Smithsonian Tropical Research Institute (STRI). Retrieved from https://forestgeo.si.edu/sites/philippines/palanan
Hussein EA, Abd El-Ghani MM, Hamdy RS, Shalabi LF (2021) Do anthropogenic activities affect floristic diversity and vegetation structure more than natural soil properties in hyper-Arid Desert environments? Diversity 13(4):157, 23p. https://doi.org/10.3390/d13040157
Koelmeyer KO (1959) The periodicity of leaf change and flowering in the principal forest communities of Ceylon. Ceylon Forest 4:157–189, 308–364
Madulid DA (2021) A pictorial encyclopedia of Philippine ornamental plants, 3rd edn. The Bookmark, Inc, Makati City, 699 p
Marshall AG (1985) Old World phytophagous bats (Megachiroptera) and their food plants: a survey. Zool J Linnean Soc 83:351–369
Newstrom LE, Frankie GW, Baker HG (1994) A new classification for plant phenology based on flowering patterns in lowland tropical rain Forest trees at La Selva. Costa Rica Biotropica 26(2):141–159
Mohandass D, Campbell MJ, Chen XS, Li QJ (2018) Flowering and fruiting phenology of woody trees in the tropical-seasonal rainforest, Southwestern China. Curr Sci 114(11):2313–2322
Ong P (2017) Personal communication
Pancho JV (1983) Vascular Flora of mount Makiling and vicinity (Luzon: Philippines), part 1. In: Kalikasan, the Philippine journal of biology, suppl 1. Kalikasan Press, Manila
Pancho JV, Gruezo WS (2006) Vascular Flora of mount Makiling and vicinity (Luzon: Philippines), Part 2. Vascular Flora of Mount Makiling and Vicinity (Luzon:Philippines), Part 2. National Academy of Science and Technology (NAST) Philippines, Department of Science and Technology, Bicutan, Taguig City and Institute of Biological Sciences, University of the Philippines Los Baños, College, Laguna, Philippines (Publishers), 626 p
Pancho JV, Gruezo WS (2012) Vascular Flora of mount Makiling and vicinity (Luzon: Philippines), part 4. National Academy of Science and Technology (NAST) Philippines, Department of Science and Technology, Bicutan, Taguig City and Institute of Biological Sciences, College of Arts and Sciences, University of the Philippines Los Banos, College, Laguna, Philippines (Publishers), 405 p
Pelser PB, Barcelona JF, Nickrent DL. 2011 onwards. Co's Digital Flora of the Philippines. www.philippineplants.org
Polansky L, Boesch C (2013) Long-term changes in fruit phenology in a west African lowland tropical rain Forest are not explained by rainfall. Biotropica 45(4):434–440
Rosemartin AH, Crimmins TM, Enquist CAF, Gerst KL, Kellermann JL, Posthumus EE, Denny EG, Guertin P, Marsh L, Weltzin JF (2014) Organizing phenological data resources to inform natural resource conservation. Biol Conserv 173:90–97
Sakai S (2000) Plant reproductive phenology in tropical forests: implications of general flowering in a lowland dipterocarp forest. Japanese J Ecol 50(1):23–39
Sakai S, Momose K, Yumoto T, Nagamitsu T, Nagamasu H, Hamid Karim AA, Nakashizuka T, Inoue T (2005) Chapter 4. Plant reproductive phenology and general flowering in a mixed dipterocarp Forest. In: Roubik DW, Sakai S, Hamid Karim AA (eds) Pollination ecology and the rain Forest. Ecological studies, vol 174. Springer, New York, NY. https://doi.org/10.1007/0-387-27161-9_4
Sinhaseni K. 2008. Natural establishment of tree seedlings in Forest restoration trials at ban Mae Sa Mai, Chiang Mai Province. MSc thesis, Chiang Mai University, Thailand
Slik JWF 2009 Onwards. Plants of Southeast Asia. Retrieved from: http://www.asianplant.net/Lythraceae/Duabanga_moluccana.htm
Ushio M, Osada Y, Kumagai T, Kume T, Pungga RAS, Nakashizuka T, Itioka T, Sakai S (2020) Dynamic and synergistic influences of air temperature and rainfall on general flowering in a Bornean lowland tropical forest. Ecol Res 35:17–29. https://doi.org/10.1111/1440-1703.12057
Wolf AA, Zavaleta ES, Selmants PC (2017) Flowering phenology shifts in response to biodiversity loss. PNAS 114(13):3463–3468. https://doi.org/10.1073/pnas.1608357114
Yap SL (2015) Phenology and seed-seedling dynamics of the Palanan Forest Dynamics Plot. In: Research and Development Terminal Report 2015. Institute of Biology, University of the Philippines, Diliman, Quezon City. Unpublished
Yap SL, Davies SJ, Condit R (2015) Dynamic response of a Philippine dipterocarp forest to typhoon disturbance. J Veg Sci 27:133–143
Yost J, Sweeney P, Gilbert E, Nelson G, Guralnick R, Gallinat A, Ellwood ER, Rossington N, Willis CG, Blum SD, Walls RL, Haston EM, Denslow MW, Zohner CM, Morris AB, Stucky BJ, Carter JR, Baxter DG, Bolmgren K, Denny EG, Dean E, Pearson KD, Davis CC, Mishler BD, Soltis PS, Mazer SJ (2018) Digitization protocol for scoring reproductive phenology from herbarium specimens of seed plants. Appl Plant Sci 6(2):e1022. https://doi.org/10.1002/aps3.1022. Retrieved from https://escholarship.org/uc/item/3s12x2p1
Acknowledgments
This is part of a research funded by the University of the Philippines System-Emerging Interdisciplinary Research (UP-EIDR) Program and the Department of Science and Technology – Philippine Council for Agriculture and Aquatic Resources and Development (DOST-PCAAARD).
Our thanks to the whole team for assisting each other in all ways needed. We would like to thank our field guides Toni Donato, Emy Cortez, Diomedes Salazar, Rey Donato, Raffy Donato, and Julius Donato; the members and staff of the Biodiversity Research Laboratory for the assistance in data collection, especially to Nikki Yvette Mendoza, Christopher John Pueblo, Lystra Zyrill Dayapera, Dr. Mariano Roy Duya and former staff Dr. Sandra Yap. We also extend our gratitude to the people of Palanan, Isabela for warmly assisting us in the conduct of the research. We also would like to thank the Department of Natural Resources for permitting us to do research in the area. And of course, to the late Dr. Perry Ong, our program leader and mentor, for all their guidance and suggestions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Manila-Fajardo, A.C., Tubale, R.A., Estrada, J.L. (2023). Floral and Fruiting Phenology in the Lowland Forests of Palanan, Isabela, Philippines. In: Ramamoorthy, S., Buot Jr., I.E., Rajasekaran, C. (eds) Plant Diversity in Biocultural Landscapes. Springer, Singapore. https://doi.org/10.1007/978-981-19-8649-9_14
Download citation
DOI: https://doi.org/10.1007/978-981-19-8649-9_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8648-2
Online ISBN: 978-981-19-8649-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)