Abstract
Arachidonic acid (AA) is the precursor of a series of bioactive lipids with relevant cell signaling and pathophysiological actions. Arachidonic acid signaling needs the first step of release from the membrane, being the released AA the substrate of four possible enzymatic pathways: prostaglandin endoperoxide H synthase (PGHS), lipoxygenase (LOX), cytochrome p450 (CYP 450), and anandamide pathways which lead to the formation of the bioactive 20-carbon oxygenated polyunsaturated fatty acids. The analysis of the different bioactive lipids formed in platelets, with AA as their precursor, is of relevance to the study of the mechanisms involved in platelet aggregation as well as for the development of novel antiplatelet and antithrombotic drugs. In this chapter, we will discuss the state of the art to detect and quantify different metabolites in resting and activated platelets.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Arachidonic acid, all-cis-5,8,11,14-eicosatetraenoic acid (AA), is the precursor of a series of enzymatic and nonenzymatic oxidized-derived products with relevant cell signaling and pathophysiological actions (Brash 2001; Das 2018a, b; Hanna and Hafez 2018; Tsai et al. 2011). Its presence at the cell membrane not only is essential and necessary for membrane fluidity but also for membrane flexibility and function. At platelet membranes up to 25% of phospholipid fatty acids are AA, reaching levels near to 5 mM in resting platelets (Neufeld and Majerus 1983) and usually localized in the glycerol backbone sn-2 position (Brash 2001; Das 2018a, b; Hanna and Hafez 2018; Tsai et al. 2011). Human body requirements of AA are higher than the concentration found in the human diet. Thus, intake of the AA precursor linoleic acid (LA 18:2n-6) supports AA synthesis regulated by the activity of Δ6 and Δ5 desaturases which convert LA to gamma-linolenic acid (GLA, 18:3), dihomo-GLA (DGLA, 20:3), and AA (Das 2018a; Hanna and Hafez 2018).
The analysis of the different bioactive lipids formed in platelets, with AA as their precursor, is of relevance to the study of the mechanisms involved in platelet aggregation in physiology and pathophysiology but also the development of novel antiplatelet and antithrombotic drugs. In the current review, we will discuss the enzymatic oxidation of AA, which products are formed, and the state of the art to detect and quantify different metabolites in resting and activated platelets. We will discuss the benefits of different analytical methodologies as well as the pitfalls of their use, in addition to the description of recent methods to evaluate platelet metabolism.
3.2 AA Metabolism in Platelets: COX and LOX
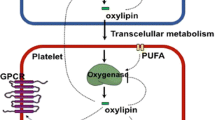
Arachidonic acid signaling needs the first step of release from the membrane by phospholipase A2 (PLA2) which hydrolyze the AA present at the sn-2 position on the phospholipid backbone (Brash 2001; Davi and Patrono 2007; Holinstat et al. 2011; Maskrey et al. 2007; Thomas et al. 2010). The released AA is the substrate of four possible enzymatic pathways: prostaglandin endoperoxide H synthase (PGHS) or cyclooxygenase (COX), lipoxygenase (LOX), cytochrome p450 (CYP 450), and anandamide pathways which lead to the formation of the bioactive 20-carbon oxygenated polyunsaturated fatty acids called eicosanoids. Esterified AA can also be oxidized, i.e., by LOX with the bioactive products released after PLA2 activity. In platelets, as well as in other tissues, the metabolic fate of AA depends on the pool of enzymes that catabolize the fatty acid-forming products with antagonistic function in different tissues, e.g., PGE2 (Brash 2001; Hanna and Hafez 2018). Cyclooxygenase (COX) oxygenate AA forming the hydroperoxide prostaglandin G2 (PGG2), and then reduces it to prostaglandin H2 (PGH2) (Smith et al. 2000). PGH2 is an intermediate hub that can be further metabolized by downstream enzymes to different eicosanoids in platelets as PGE2, PGD2, and PGF2α, or thromboxane A2 (TXA2). LOX pathway in platelets consists of AA oxidation mainly by the enzyme isoforms 12-LOX and 15-LOX, and then further transformed to leukotrienes (LTA4, LTB4, LTC4, LTD4, and LTE4) by other cell types (Brash 2001; Das 2018a, b; Hanna and Hafez 2018; Davi and Patrono 2007; Ikei et al. 2012; Marnett et al. 1999; Mollace et al. 2005; Murphy and Gijon 2007; Nascimento-Silva et al. 2005; O’Donnell et al. 2009) (Fig. 3.1).
Platelet aggregation can be activated by different agonists by interacting with receptors at the cell membrane. As a consequence, AA is released from the membrane being metabolized intracellularly, i.e., by COX and LOX. Lipid metabolites can be detected by HPLC-UV or HPLC-MS/MS studies as indicated. Figure modified from Exp Ther Med. 2012 Apr; 3(4): 577–584
Arachidonic acid metabolism is relevant for platelet function upon activation or returns the platelet bulk to the resting state. Platelet function is regulated by many agents with a central role being played by eicosanoids, i.e., TxA2 (Jennings 2009). The hemeprotein COX oxidizes AA to PGH2 being COX-1 the major isoform present in platelets (Rouzer and Marnett 2003; Boutaud et al. 2001; Kurumbail et al. 2001). Traces of the COX-2 isoform is present in platelets as a result of the transcription of residual mRNA into protein or carried over from the platelet precursor cells (Maskrey et al. 2007; Marnett et al. 1999; Marnett 2002). In platelets, PGH2 is further metabolized by TxA2 synthase forming the pro-aggregant mediator TxA2 which is released and autocatalytically induces platelet aggregation (Rouzer and Marnett 2003; Trostchansky et al. 2011). More recently, other relevant products from the COX-1 pathway in platelets have been described (Rauzi et al. 2016). In addition to the wide variety of eicosanoids formed by the COX pathway (Kirkby et al. 2015) (Fig. 3.1), other products such as 11-hydroxyeicosatetraenoic acid (11-HETE) and 15(S)-HETE are produced when AA is inserted at the active site of COX-1 in a different structural arrangement than the one necessary for PGH2 synthesis (Rauzi et al. 2016). When high concentrations of AA are released from platelet membranes, e.g., at platelet hyperactivation both products can be formed at similar levels to TxA2.
As shown in Fig. 3.1, AA can also be oxidized by the non-heme iron-containing enzymes LOXs. Hydroperoxy- (HpETE) and hydroxy- (HETE) eicosatetraenoic acids are the products formed by this enzymatic activity (Trostchansky et al. 2021; Wood et al. 2020). Different isoforms of LOXs are found depending on the carbon where the hydroperoxyl (–OOH) group is added. The main isoform present in platelets is 12-LOX, also known as p12-LOX, which oxidizes de fatty acid at C-12 forming the 12S-hydroperoxy-5Z,8Z,10E,14Z-eicosatetraenoic acid [12(S)HpETE] (Trostchansky et al. 2021; Wood et al. 2020; Brash 1999), being reduced to its hydroxyl derivative 12-HETE due to the highly reducing environment (Wood et al. 2020; Brash 1999). The biological activity of 12-HETE in platelets in vivo remains under discussion with a suggested anti-aggregant and anti-inflammatory action, i.e., by lowering the release of AA from the membrane while others propose a prothrombotic activity (Kalgutkar et al. 1998a, b).
3.3 Analytical Techniques to Detect and Quantify Bioactive Lipids in Platelets
Due to their pivotal role, PG and Tx have been extensively studied in platelet function. The extremely low concentration of PG and Tx in biological fluids and the great variety and similarity between the different types of AA-derived molecules have been the main problems for their determination and identification and the need for the use of complex and sensitive techniques for its estimation. High-resolution gas chromatography (GC) in combination with tandem mass spectrometry (GC-MS/MS) has played a relevant role in the identification and quantification of bioactive lipids in biological samples (Kuksis 2000). However, in the last decade the simpler, rapid, and powerful high-performance liquid chromatography (HPLC)-MS/MS has displaced GC-MS and GC-MS/MS in the area of instrumental analysis of physiological substances, drugs, and their metabolites (O’Donnell et al. 2014). Also, spectrophotometric and fluorimetric detection upon HPLC separation can be used in combination with LC-MS/MS for the analysis of PGs and Txs. Below we will describe different aspects and approximations for the detection and quantification of eicosanoids in platelets, discussing their benefits and pitfalls.
3.3.1 HPLC with Ultraviolet Detection
This method has been in use for several decades and has been refined ever since (Terragno et al. 1981). The standard ultraviolet (UV) detector HPLC samples, measures the absorbance of monochromatic light of fixed wavelength in the UV (190 nm) or visible wavelength range (400 nm in blue light) against a reference beam, relating the magnitude of the absorbance to the concentration of an analyte. This technique functions with molecules that contain unsaturated bonds, aromatic groups, or functional groups containing heteroatoms. In this sense, PGs and TXs, in contrast with leukotrienes, are particularly difficult to measure by UV. Spectral UV analysis of PGs reveals that they have a wavelength maximum of 192.5 nm (Puppolo et al. 2014). Now, UV detectors capable of working at low UV wavelengths are available, making it possible to detect nanogram quantities of PGs without the necessity to modify them chemically. However, chemical modification or “derivatization,” is still in use in HPLC combined with fluorimetric methods (see below). Besides, PGs and TXs do not have strong chromophores and thus spectrophotometric detection after chromatography is difficult. Most PGs do not absorb UV light, so they have to be chemically converted before their determination, commonly in the carboxyl group to UV-absorbing phenacyl esters (Salari et al. 1987). The detection is then in order of nanograms to picograms.
Concerning the stationary phase, separation in reversed-phase (RP)-HPLC relies on the hydrophobic properties of the analytes and therefore remains the main method for the separation of the metabolites of AA (Puppolo et al. 2014). For Txs determination by HPLC-UV, chemical alterations are also commonly required, with panacyl bromide and methoxyamine to form methoxime-panacyl ester derivatives (Pullen et al. 1987). The normal plasma concentrations of thromboxane B2 (TxB2), the decay-stable TxA2 end-product, are very low (10–370 pg/mL) and manipulation during the blood sampling alters the release from platelets (Nyyssönen et al. 1993).
3.3.2 HPLC with Fluorimetric Detection
This technique is based on the same principles of HPLC-UV, but with the use of a fluorescent detector after the HPLC separation. As mentioned above, PGs and TXs do not contain aromatic or natural fluorescent groups thus chemical derivatization of these lipid molecules led to the formation of fluorescent complexes, before the HPLC separation, allowing their fluorescent detection. However, this makes the analysis more expensive and time-consuming (Liakh et al. 2020; Yue et al. 2004) but still less expensive than GC-MS. Some of the derivatizing agents used are p-(9-anthroyloxy)phenylacil bromide and anthryldiazomethane (Yamaki and Oh-Ishi 1986) for TxB2 and bromomethyl 7-acetoxycoumarin for PGs (Tsuchiya et al. 1982).
3.3.3 MS Analysis of AA-Derived Metabolites
In the last decade, the improvement of sensitivity of mass liquid chromatography–MS (LC/MS) instruments, for example, electrospray ionization coupled to tandem (triple quadrupole or MS/MS) led to a better capacity to detect and quantify small amounts of lipids in diverse biological samples (O’Donnell et al. 2014). An advantage to GC-MS, LC/MS does not need sample derivatization before analysis with the increased use of LC/MS/MS methods in studies where the detection and quantitation of specific lipids are of interest for researchers (O’Donnell et al. 2014; Tacconelli et al. 2020; Tsikas and Zoerner 2014). Besides, LC-MS/MS methods show several-fold lower limits of quantitation values than reported GC-MS/MS methods (Tsikas and Zoerner 2014).
LC/MS/MS has been applied to the quantitation of eicosanoids and the identification of sphingolipids molecular species (O’Donnell et al. 2014; Tsikas and Zoerner 2014; Kornilov et al. 2019). When analyzing platelets’ lipids, the use of prostacyclin or indomethacin should be avoided during cell purification procedures to avoid interference with lipid-sensitive signaling pathways (O’Donnell et al. 2014; Cebo et al. 2020a, b). Also, care has to be taken during the purification process to avoid undesired platelet activation (Trostchansky et al. 2011, 2019; O’Donnell et al. 2014). After cell purification, platelets’ lipids can be isolated using several different methods. Among the most used are the Bligh and Dyer or the hexane/isopropanol-based solvent mixtures methods (Trostchansky et al. 2011, 2019; O’Donnell et al. 2014). The advantage of using the hexane method is that the organic phase is the upper one and less contamination with precipitated proteins is shown. Besides, a solid phase extract step, with C18 columns, can be included to obtain a cleaner lipid sample (Tsikas and Zoerner 2014; Cebo et al. 2020a). There exists a wide variety of labeled eicosanoids that are useful for identification and quantitation by LC-MS/MS (Trostchansky et al. 2011, 2018; O’Donnell et al. 2014; Kornilov et al. 2019). The standards have to be added before initiating the extraction procedure. Importantly, calibration curves have to be constructed in the same biological fluid that is analyzed to reduce the errors arising from ion suppression and ensuring accurate quantification (O’Donnell et al. 2014; Trostchansky et al. 2018).
Sample preparation is very important as artifactual formation of eicosanoid metabolites can be formed affecting lipid quantification. For example, ex vivo formation of TxA2 by platelets can occur during blood sampling and processing (Maskrey and O’Donnell 2008) leading to overestimation of platelet eicosanoids levels. Improvement of the sensitivity of LC-MS/MS methods would also be obtained by using larger sample volumes.
One of the most analyzed platelet-generated eicosanoids is the potent proaggregatory TxA2. TxA2 is extremely unstable and rapidly rearranges to TxB2, which is released at ng amounts per 2 × 108 cells as measured by LC-MS/MS (Trostchansky et al. 2011, 2018; O’Donnell et al. 2014; Kornilov et al. 2019). We and others follow TxB2 formation in the negative ion mode by following the m/z 369/169 transition (Fig. 3.1). When analyzing in vivo systemic changes in Tx formation, and due to TxA2 short plasma half-life, generation of platelet Tx is best measured by quantifying the urinary metabolites, 11-dehydroTxB2, or 2,3-dinor TxB2 through LC-MS/MS (Trostchansky et al. 2011, 2018; O’Donnell et al. 2014; Kornilov et al. 2019). This is considered a reliable way for in vivo platelet reactivity measurement.
In contrast to TxA2, which comes from the COX pathway, 12-HETE generated by 12-LOX is quantitatively more abundant (Fuentes et al. 2021; Mendez et al. 2020; Paes et al. 2019). However, the effects of 12-HETE remain in discussion. As discussed earlier, LOX products can be detected by UV absorbance (Bonilla et al. 2013; O’Donnell et al. 2000). To analyze by LC-MS/MS, lipids extracted from platelets are separated by HPLC employing a Spherisorb ODS2 column (5 μm, 150 × 4.6 mm; waters). HPLC settings at a flow rate of 0.5 mL/min are 50–90% in 40 min with mobile phase A = H2O/acetonitrile/acetic acid (75:25:0.1 v/v), and B = methanol/acetonitrile/acetic acid (60:40:0.1 v/v) (Trostchansky et al. 2011, 2018; O’Donnell et al. 2000). These chromatographic conditions allow also us to identify and quantify other platelets’ positional HETE isomers by following m/z 319/179 (12-HETE) and m/z 319/219 (15-HETE) (Murphy et al. 2005).
Small amounts of PGs, particularly PGE2 and prostaglandin D2 (PGD2) are formed in platelets. Although both bioactive lipids present the same transitions m/z 351/189, both compounds present different retention times in the column allowing their identification and quantitation (Trostchansky et al. 2018).
3.4 Analysis of Arachidonic Acid-Derived Metabolites on Platelet Mitochondria
Arachidonic acid is an omega-6 polyunsaturated fatty acid (PUFA); it is a structural part of the cell membrane and is necessary for membrane fluidity, flexibility, and function in all cell types (Paes et al. 2019). Fatty acids fulfill structural, signaling, and energy storage functions; phospholipids are the main structural lipids of platelets and their metabolism produces very important secondary mediators for the regulation of platelet activation (Lepropre et al. 2018). AA is released from phospholipid membranes and acts as a precursor to eicosanoids (Olechowski et al. 2017). When platelets are activated, signal transduction generates the mobilization of intracellular calcium, which increases and activates phospholipases, which catalyze the release of phospholipids, such as AA (Morel et al. 2016). As discussed, eicosanoids are produced in response to different cellular stimuli such as hormones, stress, and cytokines (Boer et al. 2018); with different effects such as pro- and anti-inflammatory (Trostchansky et al. 2019).
Current evidence suggests that LOX- and COX-generated AA metabolites can induce ROS generation by stimulating NAD(P)H oxidase (NOX) and that there is a potential signaling connection between LOX/COX and NOX metabolites (Cho et al. 2011). Platelet aggregation has been reported to exponentially increase reactive oxygen species (ROS), such as hydrogen peroxide, which acts as a second messenger and stimulates AA metabolism and the phospholipase C pathway (Trostchansky et al. 2019). Excess ROS of NOX and/or mitochondria are related to vascular dysfunction and hypertension (Martinez-Revelles et al. 2013). The COX pathway is important for platelet activation, specifically in prothrombotic activity and the production of pro-inflammatory mediators (Bijak and Saluk-Bijak 2017).
Among the main metabolites of AA, we find PGs, which are synthesized in response to various physiological stimuli (Fang et al. 2004). Among the evidenced effects of PGs on mitochondria, we found that the physiological increase of PGE2 increased mitochondrial function and autophagy (Palla et al. 2021). In dendritic cells, PGE2 has a protective effect on the mitochondrial membrane and it generates a decrease in the activity of caspase 3 and granzymes, regulating several pro-apoptotic molecules (Vassiliou et al. 2004). On the contrary, the authors point out that PGE2 decreases the mitochondrial membrane potential in cells that carry out cellular respiration, associated with a reduction in oxidative phosphorylation, but does not show damage to the mitochondria (Sanin et al. 2018). In this case, prostaglandin E1 (PGE1) has been used as a pretreatment for ischemic reperfusion injury in various biological systems, mainly due to a protective effect on the mitochondria (Zhu et al. 2017).
In the case of HETE eicosanoids, it has been described that 12-HETE in isolated mitochondria increases the concentration of intramitochondrial ionized calcium, stimulates the activity of mitochondrial nitric oxide (NO) synthase (mtNOS), which causes mitochondrial dysfunction by decrease respiration and transmembrane potential, which ultimately induces the release of cytochrome c and stimulates the aggregation of mitochondria (Nazarewicz et al. 2007). About 15-HETE has been shown that increases the generation of mitochondrial ROS, especially in the electron transport chain (Li et al. 2016). 20-HETE is characterized by increasing the production of mitochondrial superoxide (Lakhkar et al. 2016); to the point of generating mitochondrial dysfunction and apoptosis in neurons (with traumatic brain injuries) (Cui et al. 2021). Besides, 20-HETE induces apoptosis of cardiomyocytes, since it induces a decrease in the mitochondrial membrane potential and stimulates the activity of caspase-3 (Bao et al. 2011).
In heart failure, HETEs open the mitochondrial permeability transition pore, increasing mitochondrial calcium that triggers mitochondrial inflammation and myocyte death. At the same time, in a healthy myocardial model, phospholipase A2 produces AA for the generation of protective epoxyeicosatrienoic acids (EETs) (Wolf 2018). Studies have reported that platelets activated by endogenous agonists release AA metabolites, such as EET and 20-HETE (Jarrar et al. 2013).
ETTs are epoxygenase metabolites of AA by the activity of cytochrome P450 that are recognized for their cardioprotective role, specifically for the prevention of calcium overload and maintenance of mitochondrial function (Batchu et al. 2012). ETTs trigger a protective response that limits mitochondrial dysfunction and reduces cell death, by regulating the autophagic response, resulting in a healthier pool of mitochondria in starved heart cells (Samokhvalov et al. 2013). It has also been described that ETTs are involved in the maintenance of homeostasis and protection against cell injury, mainly by counteracting the loss of mitochondrial membrane potential (El-Sikhry et al. 2011). Specifically, 14,15-EET can promote cell survival during ischemia/reperfusion in neurons through a decrease in the mitochondrial apoptotic pathway (Geng et al. 2017) promoting mitochondrial biogenesis (Wang et al. 2014). In a model of mitochondrial damage in cardiomyocytes by dronedarone, it decreases the mitochondrial membrane potential, inhibits the mitochondrial complex I, and uncouples the electron transport chain; In this context, the exogenous pretreatment of H9c2 cells with 11,12-EET and 14,15-EET improved cytotoxicity, the decrease in ATP, and the alteration of the mitochondrial membrane potential (Karkhanis et al. 2018).
3.5 Concluding Remarks
The increased use of LC-MS/MS methodologies is having an impact on the study of platelet function and the development of antithrombotic drugs targeted to the AA-metabolizing enzymes. The techniques discussed in this chapter are faster, cheaper, and easiest to apply compared to GC-MS, increasing the number of laboratories capable of performing lipid analysis. Also, LC-MS/MS benefits allow the study of large cohort sample sets, and new data with the potential to obtain information to understand disease mechanisms. As data is being collected, continuous work is still required to improve separation and analytical conditions to analyze large data sets from clinical studies to increase the potential of these techniques in terms of understanding disease mechanisms.
References
Bao Y, Wang X, Li W, Huo D, Shen X, Han Y et al (2011) 20-Hydroxyeicosatetraenoic acid induces apoptosis in neonatal rat cardiomyocytes through mitochondrial-dependent pathways. J Cardiovasc Pharmacol 57:294–301
Batchu SN, Chaudhary KR, El-Sikhry H, Yang W, Light PE, Oudit GY et al (2012) Role of PI3Kalpha and sarcolemmal ATP-sensitive potassium channels in epoxyeicosatrienoic acid mediated cardioprotection. J Mol Cell Cardiol 53:43–52
Bijak M, Saluk-Bijak J (2017) Flavonolignans inhibit the arachidonic acid pathway in blood platelets. BMC Complement Altern Med 17:396
Boer RE, Gimenez-Bastida JA, Boutaud O, Jana S, Schneider C, Sulikowski GA (2018) Total synthesis and biological activity of the arachidonic acid metabolite hemiketal E2. Org Lett 20:4020–4022
Bonilla L, O’Donnell VB, Clark SR, Rubbo H, Trostchansky A (2013) Regulation of protein kinase C by nitroarachidonic acid: impact on human platelet activation. Arch Biochem Biophys 533:55–61
Boutaud O, Li J, Chaurand P, Brame CJ, Marnett LJ, Roberts LJ et al (2001) Oxygenation of arachidonic acid by cyclooxygenases generates reactive intermediates that form adducts with proteins. Adv Exp Med Biol 500:133–137
Brash AR (1999) Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem 274:23679–23682
Brash AR (2001) Arachidonic acid as a bioactive molecule. J Clin Invest 107:1339–1345
Cebo M, Fu X, Gawaz M, Chatterjee M, Lammerhofer M (2020a) Micro-UHPLC-MS/MS method for analysis of oxylipins in plasma and platelets. J Pharm Biomed Anal 189:113426
Cebo M, Schlotterbeck J, Gawaz M, Chatterjee M, Lammerhofer M (2020b) Simultaneous targeted and untargeted UHPLC-ESI-MS/MS method with data-independent acquisition for quantification and profiling of (oxidized) fatty acids released upon platelet activation by thrombin. Anal Chim Acta 1094:57–69
Cho KJ, Seo JM, Kim JH (2011) Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol Cells 32:1–5
Cui W, Wu X, Shi Y, Guo W, Luo J, Liu H et al (2021) 20-HETE synthesis inhibition attenuates traumatic brain injury-induced mitochondrial dysfunction and neuronal apoptosis via the SIRT1/PGC-1alpha pathway: a translational study. Cell Prolif 54:e12964
Das UN (2018a) Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus: a review. J Adv Res 11:43–55
Das UN (2018b) Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review. J Adv Res 11:57–66
Davi G, Patrono C (2007) Platelet activation and atherothrombosis. N Engl J Med 357:2482–2494
El-Sikhry HE, Miller GG, Madiyalakan MR, Seubert JM (2011) Sonodynamic and photodynamic mechanisms of action of the novel hypocrellin sonosensitizer, SL017: mitochondrial cell death is attenuated by 11,12-epoxyeicosatrienoic acid. Investig New Drugs 29:1328–1336
Fang KM, Shu WH, Chang HC, Wang JJ, Mak OT (2004) Study of prostaglandin receptors in mitochondria on apoptosis of human lung carcinoma cell line A549. Biochem Soc Trans 32:1078–1080
Fuentes E, Trostchansky A, Reguengo LM, Marostica MR Jr, Palomo I (2021) Antiplatelet effects of bioactive compounds present in tomato pomace. Curr Drug Targets 22(15):1716–1724
Geng HX, Li RP, Li YG, Wang XQ, Zhang L, Deng JB et al (2017) 14,15-EET suppresses neuronal apoptosis in ischemia-reperfusion through the mitochondrial pathway. Neurochem Res 42:2841–2849
Hanna VS, Hafez EAA (2018) Synopsis of arachidonic acid metabolism: a review. J Adv Res 11:23–32
Holinstat M, Boutaud O, Apopa PL, Vesci J, Bala M, Oates JA et al (2011) Protease-activated receptor signaling in platelets activates cytosolic phospholipase A2alpha differently for cyclooxygenase-1 and 12-lipoxygenase catalysis. Arterioscler Thromb Vasc Biol 31:435–442
Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR et al (2012) Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation. J Lipid Res 53:2546–2559
Jarrar YB, Cho SA, Oh KS, Kim DH, Shin JG, Lee SJ (2013) Identification of cytochrome P450s involved in the metabolism of arachidonic acid in human platelets. Prostaglandins Leukot Essent Fatty Acids 89:227–234
Jennings LK (2009) Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost 102:248–257
Kalgutkar AS, Crews BC, Rowlinson SW, Garner C, Seibert K, Marnett LJ (1998a) Aspirin-like molecules that covalently inactivate cyclooxygenase-2. Science 280:1268–1270
Kalgutkar AS, Kozak KR, Crews BC, Hochgesang GP Jr, Marnett LJ (1998b) Covalent modification of cyclooxygenase-2 (COX-2) by 2-acetoxyphenyl alkyl sulfides, a new class of selective COX-2 inactivators. J Med Chem 41:4800–4818
Karkhanis A, Leow JWH, Hagen T, Chan ECY (2018) Dronedarone-induced cardiac mitochondrial dysfunction and its mitigation by epoxyeicosatrienoic acids. Toxicol Sci 163:79–91
Kirkby NS, Reed DM, Edin ML, Rauzi F, Mataragka S, Vojnovic I et al (2015) Inherited human group IVA cytosolic phospholipase A2 deficiency abolishes platelet, endothelial, and leucocyte eicosanoid generation. FASEB J 29:4568–4578
Kornilov A, Kennedy PD, Aldrovandi M, Watson AJA, Hinz C, Harless B et al (2019) Revising the structure of a new eicosanoid from human platelets to 8,9-11,12-diepoxy-13-hydroxyeicosadienoic acid. J Biol Chem 294:9225–9238
Kuksis A (2000) Lipids | gas chromatography. In: Wilson ID (ed) Encyclopedia of separation science. Academic Press, Cambridge, pp 3219–3237
Kurumbail RG, Kiefer JR, Marnett LJ (2001) Cyclooxygenase enzymes: catalysis and inhibition. Curr Opin Struct Biol 11:752–760
Lakhkar A, Dhagia V, Joshi SR, Gotlinger K, Patel D, Sun D et al (2016) 20-HETE-induced mitochondrial superoxide production and inflammatory phenotype in vascular smooth muscle is prevented by glucose-6-phosphate dehydrogenase inhibition. Am J Physiol Heart Circ Physiol 310:H1107–H1117
Lepropre S, Kautbally S, Octave M, Ginion A, Onselaer MB, Steinberg GR et al (2018) AMPK-ACC signaling modulates platelet phospholipids and potentiates thrombus formation. Blood 132:1180–1192
Li Q, Mao M, Qiu Y, Liu G, Sheng T, Yu X et al (2016) Key role of ROS in the process of 15-lipoxygenase/15-hydroxyeicosatetraenoiccid-induced pulmonary vascular remodeling in hypoxia pulmonary hypertension. PLoS One 11:e0149164
Liakh I, Pakiet A, Sledzinski T, Mika A (2020) Methods of the analysis of oxylipins in biological samples. Molecules 25:349
Marnett LJ (2002) Recent developments in cyclooxygenase inhibition. Prostaglandins Other Lipid Mediat 68:153–164
Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA (1999) Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J Biol Chem 274:22903–22906
Martinez-Revelles S, Avendano MS, Garcia-Redondo AB, Alvarez Y, Aguado A, Perez-Giron JV et al (2013) Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxid Redox Signal 18:51–65
Maskrey BH, O’Donnell VB (2008) Analysis of eicosanoids and related lipid mediators using mass spectrometry. Biochem Soc Trans 36:1055–1059
Maskrey BH, Bermudez-Fajardo A, Morgan AH, Stewart-Jones E, Dioszeghy V, Taylor GW et al (2007) Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. J Biol Chem 282:20151–20163
Mendez D, Urra FA, Millas-Vargas JP, Alarcon M, Rodriguez-Lavado J, Palomo I et al (2020) Synthesis of antiplatelet ortho-carbonyl hydroquinones with differential action on platelet aggregation stimulated by collagen or TRAP-6. Eur J Med Chem 192:112187
Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D (2005) Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev 57:217–252
Morel A, Miller E, Bijak M, Saluk J (2016) The increased level of COX-dependent arachidonic acid metabolism in blood platelets from secondary progressive multiple sclerosis patients. Mol Cell Biochem 420:85–94
Murphy RC, Gijon MA (2007) Biosynthesis and metabolism of leukotrienes. Biochem J 405:379–395
Murphy RC, Barkley RM, Zemski BK, Hankin J, Harrison K, Johnson C et al (2005) Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem 346:1–42
Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Villela CG, Fierro IM (2005) Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am J Physiol Cell Physiol 289:C557–C563
Nazarewicz RR, Zenebe WJ, Parihar A, Parihar MS, Vaccaro M, Rink C et al (2007) 12(S)-Hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch Biochem Biophys 468:114–120
Neufeld EJ, Majerus PW (1983) Arachidonate release and phosphatidic acid turnover in stimulated human platelets. J Biol Chem 258:2461–2467
Nyyssönen K, Seppänen K, Salonen JT (1993) High-performance liquid chromatographic assay of platelet-produced thromboxane B2. J Chromatogr 612:27–32
O’Donnell VB, Coles B, Lewis MJ, Crews BC, Marnett LJ, Freeman BA (2000) Catalytic consumption of nitric oxide by prostaglandin H synthase-1 regulates platelet function. J Biol Chem 275:38239–38244
O’Donnell VB, Maskrey B, Taylor GW (2009) Eicosanoids: generation and detection in mammalian cells. Methods Mol Biol 462:5–23
O’Donnell VB, Murphy RC, Watson SP (2014) Platelet lipidomics: modern day perspective on lipid discovery and characterization in platelets. Circ Res 114:1185–1203
Olechowski B, Ashby A, Mariathas M, Khanna V, Mahmoudi M, Curzen N (2017) Is arachidonic acid stimulation really a test for the response to aspirin? Time to think again? Expert Rev Cardiovasc Ther 15:35–46
Paes AMA, Gaspar RS, Fuentes E, Wehinger S, Palomo I, Trostchansky A (2019) Lipid metabolism and signaling in platelet function. Adv Exp Med Biol 1127:97–115
Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang AV, Kraft P et al (2021) Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science 371:eabc8059
Pullen RH, Howell JA, Cox JW (1987) High performance liquid chromatographic determination of thromboxane B2 in human serum as a methoxime-panacylester derivative. Prostaglandins Leukot Med 29:205–219
Puppolo M, Varma D, Jansen SA (2014) A review of analytical methods for eicosanoids in brain tissue. J Chromatogr B Analyt Technol Biomed Life Sci 964:50–64
Rauzi F, Kirkby NS, Edin ML, Whiteford J, Zeldin DC, Mitchell JA et al (2016) Aspirin inhibits the production of proangiogenic 15(S)-HETE by platelet cyclooxygenase-1. FASEB J 30:4256–4266
Rouzer CA, Marnett LJ (2003) Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem Rev 103:2239–2304
Salari H, Yeung M, Douglas S, Morozowich W (1987) Detection of prostaglandins by high-performance liquid chromatography after conversion to p-(9-anthroyloxy)phenacyl esters. Anal Biochem 165:220–229
Samokhvalov V, Alsaleh N, El-Sikhry HE, Jamieson KL, Chen CB, Lopaschuk DG et al (2013) Epoxyeicosatrienoic acids protect cardiac cells during starvation by modulating an autophagic response. Cell Death Dis 4:e885
Sanin DE, Matsushita M, RIK G, Grzes KM, van Teijlingen Bakker N, Corrado M et al (2018) Mitochondrial membrane potential regulates nuclear gene expression in macrophages exposed to prostaglandin E2. Immunity 49(6):1021–1033
Smith WL, Dewitt DL, Garavito RM (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182
Tacconelli S, Contursi A, Falcone L, Mucci M, D'agostino I, Fullone R et al (2020) Characterization of cyclooxygenase-2 acetylation and prostanoid inhibition by aspirin in cellular systems. Biochem Pharmacol 178:114094
Terragno A, Rydzik R, Terragno R (1981) High performance liquid chromatography and UV detection for the separation and quantitation of prostaglandins. Prostaglandins 21:101–112
Thomas CP, Morgan LT, Maskrey BH, Murphy RC, Kuhn H, Hazen SL et al (2010) Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J Biol Chem 285:6891–6903
Trostchansky A, Bonilla L, Thomas CP, O’Donnell VB, Marnett LJ, Radi R et al (2011) Nitroarachidonic acid, a novel peroxidase inhibitor of prostaglandin endoperoxide H synthases 1 and 2. J Biol Chem 286:12891–12900
Trostchansky A, Mastrogiovanni M, Miquel E, Rodriguez-Bottero S, Martinez-Palma L, Cassina P et al (2018) Profile of arachidonic acid-derived inflammatory markers and its modulation by nitro-oleic acid in an inherited model of amyotrophic lateral sclerosis. Front Mol Neurosci 11:131
Trostchansky A, Moore-Carrasco R, Fuentes E (2019) Oxidative pathways of arachidonic acid as targets for regulation of platelet activation. Prostaglandins Other Lipid Mediat 145:106382
Trostchansky A, Wood I, Rubbo H (2021) Regulation of arachidonic acid oxidation and metabolism by lipid electrophiles. Prostaglandins Other Lipid Mediat 152:106482
Tsai IJ, Croft KD, Puddey IB, Beilin LJ, Barden A (2011) 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am J Phys Heart Circ Phys 300:H1194–H1200
Tsikas D, Zoerner AA (2014) Analysis of eicosanoids by LC-MS/MS and GC-MS/MS: a historical retrospect and a discussion. J Chromatogr B Analyt Technol Biomed Life Sci 964:79–88
Tsuchiya H, Hayashi T, Naruse H, Takagi N (1982) Sensitive high-performance liquid chromatographic method for prostaglandins using a fluorescence reagent, 4-bromomethyl-7-acetoxycoumarin. J Chromatogr 231:247–254
Vassiliou E, Sharma V, Jing H, Sheibanie F, Ganea D (2004) Prostaglandin E2 promotes the survival of bone marrow-derived dendritic cells. J Immunol 173:6955–6964
Wang L, Chen M, Yuan L, Xiang Y, Zheng R, Zhu S (2014) 14,15-EET promotes mitochondrial biogenesis and protects cortical neurons against oxygen/glucose deprivation-induced apoptosis. Biochem Biophys Res Commun 450:604–609
Wolf MJ (2018) “HETE”ing up mitochondria in human heart failure. J Biol Chem 293:130–131
Wood I, Trostchansky A, Rubbo H (2020) Structural considerations on lipoxygenase function, inhibition and crosstalk with nitric oxide pathways. Biochimie 178:170–180
Yamaki K, Oh-Ishi S (1986) 9-Anthryldiazomethane-HPLC method for detection of prostaglandins and thromboxane: an application to the measurement of the products of stimulated rabbit platelets. Chem Pharm Bull (Tokyo) 34:3526–3529
Yue H, Strauss KI, Borenstein MR, Barbe MF, Rossi LJ, Jansen SA (2004) Determination of bioactive eicosanoids in brain tissue by a sensitive reversed-phase liquid chromatographic method with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci 803:267–277
Zhu H, Ding Y, Xu X, Li M, Fang Y, Gao B et al (2017) Prostaglandin E1 protects coronary microvascular function via the glycogen synthase kinase 3beta-mitochondrial permeability transition pore pathway in rat hearts subjected to sodium laurate-induced coronary microembolization. Am J Transl Res 9:2520–2534
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mastrogiovanni, M., Fuentes, E., Wehinger, S., Méndez, D., Palomo, I., Trostchansky, A. (2023). Methods for the Analysis of Arachidonic Acid-Derived Metabolites in Platelets. In: Ribeiro de Araujo, D., Carneiro-Ramos, M. (eds) Biotechnology Applied to Inflammatory Diseases. Interdisciplinary Biotechnological Advances. Springer, Singapore. https://doi.org/10.1007/978-981-19-8342-9_3
Download citation
DOI: https://doi.org/10.1007/978-981-19-8342-9_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8341-2
Online ISBN: 978-981-19-8342-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)