Abstract
Assessing phenotypic traits associated with physiology, biochemistry, and plant health based on leaf spectral reflectance properties has become an important high throughput tool in agriculture research. Precise quantification and monitoring of plant responses to stresses (abiotic or biotic) help researchers’ phenotype different genetic resources, map genetic loci, and choose donors for trait development. Studies have shown the potential use of leaf hyperspectral reflectance in assessing the plant phenotype under biotic and abiotic stress conditions. We compiled wavebands or reflectance strongly related to the lab and field-based measurements for pigments, leaf nitrogen, and leaf water content. This chapter also highlights the recent applications of hyperspectral reflectance in plant phenotyping, stress diagnosis, species classification, and robust statistical methods. Furthermore, we discuss the need for advanced analytical tools and their potential applications in plant phenotyping.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Electromagnetic spectrum
- Diagnosis
- Hyperspectral reflectance
- Pigments
- Leaf nitrogen
- Phenotyping
- Remote sensing
- Sensors
15.1 Introduction
Based on evolutionary history, genetic background, and stress adaptation capacity, plants readily express differences in physiology and biochemistry, which can be detected using leaf or canopy spectral reflectance changes (Roitsch et al. 2019; Grzybowski et al. 2021). Along with the differences in morphology or ideotype at the plant level, plants have also evolved to synthesize different combinations of leaf pigments and other compounds to support growth and development under various environmental conditions. Improved detection and monitoring of plant health have been vital areas of research under a rapidly changing climate. Needless to say that high throughput non-invasive sensing-based phenotyping is faster, cheaper, reliable, unbiased, extendable, and robust.
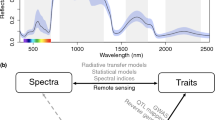
Remote sensing techniques have been utilizing leaf optical properties (reflectance, absorbance, and transmittance) to understand leaf pigments (visible range), cell structure (near-infrared), and biochemical properties (shortwaves) using the leaf or canopy signatures. When a leaf or canopy is exposed to electromagnetic (EM) radiation, the reflected radiation can be measured and recorded as reflectance spectrum. Sensor modalities to study reflectance can be grouped into two categories (based on the signal type): optical and EM. Other choices include acoustic (ultrasound), magnetic (MRI), X-ray, thermal, electronic (electron-microscope observations), and mechanical sensors. To measure object (e.g., leaf or canopy) properties, most light/sound-based signal processing sensors/algorithms rely on the signal frequency, and the transduction depends on the following:
-
Amplitude of the input/reflected/refracted signal
-
Directionality of the input/reflected/refracted signal
-
Time-of-flight measurement of the received signal
Let us recap a (conveniently biased) sampling of definitions from image processing. An image is the numerical representation of any real-world picture; it is a multi-dimensional array (or matrix) of numbers, each representing the amount of signal intensity sampled in a physical region, known as the pixel. There are a few choices to both describe the relative intensities of the different colors at each pixel (color space) and store images/parameters (Gonzalez and Woods 2018). Object-light interaction is dependent on both the incident signal characteristics (power level, frequency, bandwidth, etc.) and the structure and chemical constituents of the object. The signal’s frequency is invariant as it travels through a medium. Thus, the velocity (and wavelength) of light traveling from vacuum through a non-vacuum medium will decrease. The ratio of light velocities between the medium and vacuum is the medium’s refractive index (RI). Characterizing the object’s structure based on the reflection/refraction signal can be found in more detail in text describing Snell’s law or Fermat’s least time principle.
Recall the wavelength ranges of the EM spectrum (Fig. 15.1). The EM spectrum is divided into four major wavelengths (λ) bands: visible (350–700 nm), near-infrared (NIR, 700–2500 nm), mid-infrared (MIR, 2500–25,000 nm), and far-infrared (FIR, 25,000–100,000 nm) (Lin et al. 2004; Manley 2014). The EM wave (light/IR) penetrates the surface of the specimen, and its penetration depth is defined as the depth at which the incident light intensity is reduced by 99%. It has been observed (Lammertyn et al. 2000; Qi et al. 2010) that the penetration depth is inversely proportional to the wavelength, which implies that the IR signal can penetrate deeper into the cell structure.
When EM strikes on the matter or canopy, three interactions can occur: absorb, reflect, and transmit. In plants, the EM absorbed can be used for photosynthesis, converted to heat, and re-emitted as fluorescence (van Bezouw et al. 2019). The leaf surface can reflect part of the incident EM radiation, which can ultimately reach an observer to perceive as color. The remaining portion of the incident radiation can transmit through the leaf and leave from the other side. All these interactions are related to each other and can be denoted as in Eq. (15.1)
where Iλ is the incident radiation energy, Aλ is the absorbed energy, Rλ is the reflected energy, and Tλ is the transmitted energy at wavelength λ.
As Eq. (15.1) indicates, all these phenomena are wavelength-dependent since the absorbance depends on the properties of the leaf, more specifically, different chemical compounds or bonds present in the leaf. Chemical bonds absorb EM photons at specific wavelengths causing vibrational and rotational changes in the molecules (Türker-Kaya and Huck 2017). These absorbances at different wavelengths, called spectral signatures or fingerprints, can effectively estimate the leaf’s other compounds or related properties. For example, the leaf nitrogen (N) and chlorophyll molecules have spectral signatures at 650 and 940 nm, which are used to estimate leaf nitrogen and chlorophyll contents (Wood 1993; Blackmer et al. 1994; Daughtry et al. 2000). Most fundamental spectral signatures occur in the mid-infrared region (MIR). The overtones and combinational bands can extend to near-infrared or visible spectrum regions. A compilation of these spectral signatures and relevant properties can be found in Türker-Kaya and Huck (2017).
15.2 Common Types of Remote-Sensing Tools Used in Plant Phenotyping
Cameras/sensors with different illumination ranges and capture capabilities (red-green-blue, multispectral, hyperspectral) have been routinely used in diverse studies.
15.2.1 Visible Light Sensor
This is a cheap and ubiquitously available sensor. The output (in its simplest form) is an 8 bits per pixel grayscale image. An RGB color image has 24 bits per pixel (one byte for each primary color, red, blue, and green). The range of human eye color perception is quite large, and unsurprisingly, the RGB space does not span the human color gamut. A filter attenuates (or entirely blocks) a subset of wavelengths and allows the complementary subset without attenuation. Many pre-processing options at the sensor and post-processing procedures on the image are available (Pereira et al. 2017; Ngugi et al. 2021).
15.2.2 Infrared Sensor
The leaf typically reflects 40–60% of incident near-IR energy from its spongy mesophyll. In terms of data capture/storing data, the IR is identical to a standard camera (visible light). The difference is in the light–object interaction. For example, an IR spectrum can replace commonly used laboratory methods for moisture content measurements like using a gravimetric oven or performing Karl Fischer titration.
15.2.3 Hyperspectral Sensor
While visible/infra-red imaging uses a wide range of frequencies (broadband) in the illuminating light source, the hyperspectral imaging sensor generates a series of images, each one representing the intensity distribution within a specific narrow-spectral band. For example, let us consider an object observed through the visible-IR wavelength range between 550 and 850 nm in steps of 5 nm. This set of 61 images, one for each frequency band, is arranged to form a three-dimensional (3D) hypercube used for further processing. This grouping of the frequency data in each pixel region using narrow bands (hyperspectral imaging) generates a number of independent measurements, thus strengthening any classification/detection procedures (Huang et al. 2014; Schmilovitch et al. 2014).
The most common types of remote-sensing cameras (RGB, multispectral, hyperspectral, light detection and ranging, LiDAR, and thermal) used in agricultural research are unmanned aerial vehicles (UAVs) based or handled spectroradiometers.
-
RGB (red, green, and blue) cameras are cheap, most widely used across the platform, and have a high spatial resolution.
-
Multispectral cameras: this platform consists of sensors with different lenses. Typically, there will be five sensors (red, green, blue, red-edge, and near-infrared), with each sensor sensitive in one spectral region.
-
Hyperspectral cameras cover the 400–1000 nm spectral region with relatively narrow bands (<20 nm).
-
Spectrometers cover the 350–2500 nm spectral region with high spectral resolution (1 nm).
The difference between hyperspectral and multispectral imaging is in their spectral resolution. A multispectral image is a collection of independent data from non-overlapping spectral bands. Usually, the number of bands is few (up to ten). A list of different sensors and their application has been complied in the Index Database (https://www.indexdatabase.de/; Roitsch et al. 2019). A spectrometer (or spectroradiometer) is used to measure both the wavelength and amplitude of the light reflected from the leaf’s surface. Wavelength is detected by the position on the sensor that the signal hits. The detected light intensity for each frequency band is obtained using refraction (prism) or diffraction (grating). On the other hand, hand-held spectrometers (Fig. 15.2a) are relatively low cost, easy to collect data and process compared to UAVs.
The key advantage of using hand-held spectrometers is (a) free from confounding effects of illumination, water band, and leaf or camera angle, (b) data processing and analysis require less time than data collected using the UAVs, and (c) data can easily be collected from controlled and field experiments. With the growing interest in sensors application in agriculture, several studies demonstrated the use of hyperspectral data collected from the spectrometer. The spectral bands are often used to establish an empirical relationship between given leaf properties and spectral data. Genomic techniques have been significantly advanced; however, throughput phenotyping remains the major bottleneck to molecular breeding. To take advantage of genomic resources to dissect complex stress tolerance, hyperspectral spectrometers could be used for high throughput phenotyping (Grzybowski et al. 2021). The following sections will provide information on how we can use spectrometers to take advantage of hyperspectral reflectance in plant stress physiology or phenotyping.
15.3 Leaf Reflectance Relationship with Plant Phenotypes in Different Crops
A typical leaf hyperspectral reflectance (350–2500 nm) spectrum is shown in Fig. 15.2b. It has low reflectance in the visible region (350–700 nm). Most of the energy is absorbed by the pigments such as chlorophyll and carotenoids for photosynthesis (Zur et al. 2000). There is a peak in the green region (~550 nm, Fig. 15.2b), which has comparatively less absorbance than other colors in the spectrum, causing the leaf to be perceived as the color “green” to the human eye. With changes in leaf pigments due to senescence or species differences, these spectra can show different peaks in the visible region (Sims and Gamon 2002; Morley et al. 2020), showing different colors to the human eye. A comparison of RGB and visible light reflectance implies the possibility of using both to classify plant species (Fig. 15.2b). The first part of the NIR region (700–1200 nm) is dominated by the light scattering at air-cell wall interfaces and characterized by high reflectance and transmittance. An increase in leaf reflectance at the transition from red to NIR, producing a spectral feature called red edge (Gitelson et al. 1996). Researchers have observed correlations between the red-edge position and chlorophyll content, plant stress, plant phenological stages (Yendrek et al. 2017; Silva-Perez et al. 2018; Mir et al. 2019; Reynolds et al. 2020). The second part of the NIR region (1200–2500 nm) is predominantly the light absorption by water. Two leading water absorption bands are at 1450 and 1950 nm (Hoffer and Johannsen 1969), shown in Fig. 15.2b. Leaf absorbance (reflectance) of green light is far less (more) than red light when the plants are stressed.
Observed variations in spectral signatures among the species (Fig. 15.2b) depend on the genetic, physiological, and biochemical properties of the leaf such as temperature, ion-concentration, pigments, concentrations of organic acids, and so on. Chlorophyll pigments reflect strongly around the green band with a green/green-yellow color. Anthocyanin pigments, red in color, provide a high reflectance around the red band. Sugars have a higher reflectance at the NIR. An obvious indication of the temporal change in absorption/reflectance characteristics of visible light on a leaf is color change in response to species or stress or age and nutrient deficiency. Thus, biologists have been perfecting relationships between individual ions and plant health.
15.3.1 Nitrogen and Pigments Associated Leaf Reflectance Spectra
Plants accumulate specific pigments during the courses of growth and development under different stresses. The pigments and their ratios determine the physiological characteristics and plant health status. Chlorophylls are the dominant pigment of green leaves and an expensive molecule because each ring contains four nitrogen atoms. Chlorophyll molecules absorb sunlight at different wavelengths (chlorophyll-a absorbs red-orange light and chlorophyll-b absorbs blue-purple light). As a result, chlorophyll index has been used as a proxy indicator of leaf or canopy nitrogen content and plant health. Traditional methods of determining pigments and tissue nitrogen are costly and time consuming.
Current advances in remote sensing have allowed timely data collection to assess plants’ crop growth and nutrient (nitrogen) status. Studies highlighted in Table 15.1 used traditional and sensor-based approaches and identified spectral bands associated with pigments and nitrogen content. Studies reported in Table 15.1 used leaf spectral reflectance data collected using a portable ASD Field Spec FR spectroradiometer (Analytical Spectral Devices Inc., Boulder, CO, USA) compared with the wet lab measured observations (shoot or leaf nitrogen). These studies tested the relationship between the measured values and spectral formulations or indices (Table 15.1). Leaf nitrogen and chlorophyll concentrations of sorghum leaves were significantly associated with the reflectance (R) ratios of R405/R715 (R2 = 0.68) and R1075/R735 (R2 = 0.66) (Zhao et al. 2005a).
Likewise, measured leaf chlorophyll or carotenoids showed a stronger relationship with the reflectance ratios of R575/R526 (R2 = 0.69) or R712/R809 (R2 = 0.50) in maize (Zea mays), respectively (Zhao et al. 2003). In cotton (Gossypium hirsutum), R715/R405, R795/R755, R415/R710, R915/R515, and R915/R705 showed a stronger association with measured leaf or shoot nitrogen in cotton (Table 15.1). In general, spectral bands R405, R415, R515, R705, R710, R755, and R915 are associated with leaf nitrogen content or concentration across cotton studies (Tarpley et al. 2000; Read et al. 2002; Zhao et al. 2005b). Further, reflectance ratios of R555/R455 and R705/R675 showed strong correlations with total chlorophyll (R2 = 0.93) and carotenoids (R2 = 0.80) in castor bean (Ricinus communis L.), respectively (Reddy and Matcha 2010). The weakest relation was noted in Bermuda grass between forage nitrogen and R915/R515 (R2 = 0.44). The nitrogen and pigment-specific bands (Table 15.1) could be used for non-destructive phenotyping. This information can be utilized to make appropriate nitrogen application decisions in the field.
15.3.2 Correlations of Mid-Day Leaf Water Potential with Spectral Indices
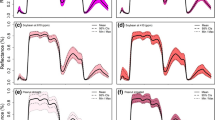
Drought is one of the most significant abiotic stress factors limiting the production of crops. Most field crops are sensitive to drought stress or soil moisture deficit during growth and development (Galieni et al. 2021). High throughput detection or quantification of plant responses to drought stress is one of the most critical crop improvement programs. Here we discuss a study that utilized reflectance indices such as normalized difference vegetation index (NDVI), normalized water index (NWI) in response to evapotranspiration-based irrigation treatments (100, 80, 60, 40, and 20% ET) in soybean. Mid-day leaf water potential was measured 42–57 days after sowing (DAS) using a pressure chamber method (Wijewardana et al. 2019). On the same plants, leaf reflectance measurements were taken between 1100 and 1200 h using a portable ASD spectroradiometer. Soil moisture stress increased soybean canopy reflectance in the visible spectrum range (400–700 nm), especially for 60, 40, and 20% ET compared to the control and 80% ET (Fig. 15.3a). The lower reflectance in the NIR region further confirmed the soil moisture stress-induced reduction in transpiration and stomatal conductance. Beyond the NIR region (1300–2500 nm), which is a function of leaf-water content and leaf thickness, reported to increase spectral reflectance in soil moisture stressed plants. The midday leaf water potential was strongly correlated (Fig. 15.3a) with NDVI (R2 = 0.82) and NWI16 (R2 = 0.72). Soil moisture stress-induced reduction in chlorophyll and canopies size resulted in lower NDVI values. These findings suggest that the NIR region could be used as a proxy to describe the canopy mass under water-deficit conditions (Lobos et al. 2014; Elsayed et al. 2015).
Soybean leaf hyperspectral reflectance of the five soil moisture stress treatments (a). The relationships between leaf water potential and leaf spectral indices (Normalized Difference Vegetation Index-NDVI, b; Normalized Water Index-NWI, c) for soybean subjected to five evapotranspiration-based irrigation treatments. For experimental details refer Wijewardana et al. (2019)
15.4 Advancement in the Analysis of Hyperspectral Reflectance Data
Conventionally, the spectral signatures are used to calculate simple indices, which are then used for testing the correlations among indices and plant phenotype (Table 15.1 and Fig. 15.3). However, this does not necessarily work for all the properties of interest or yield (Grzybowski et al. 2021). Estimating such leaf properties is not a simple regression task since overtones and combinational bands often contaminate these signatures, especially in the near-infrared (NIR) region. Hence, the leaf spectrum is a complex graph consisting of many overlapping peaks requiring complex multivariate modeling techniques for property estimations (Manley 2014).
The independent information from each band of the hyperspectral image increases the data dimension. While using the full spectral information in a classifier, we can only use a finite number of training samples to estimate many parameters necessary to describe the large data dimension. When the cardinality of the training samples is less than the number of parameters, the estimator performance naturally declines (Dalponte et al. 2009); this effect is called the Hughes phenomenon. An optimal pair of training samples, data dimensions exist in most practical circumstances. A dimensionality reduction step (Principal Component Analysis, PCA; Fisher’s Linear Discriminant Analysis, LDA; and Stepwise LDA, SLDA) would help bring down redundant features to reduce the dimensions of the hyperspectral observations (Duda et al. 2021). In brief, PCA obtains the best projections of the data. Only a few of the projected components have significant variance (information), and only these fewer “principal” components are used. While PCA is unsupervised and not classification-centered, the LDA (can also be run in supervised mode) maximizes the class separability between the two classes. In SLDA, the discrimination model is refined at each step where a decision on whether to retain or discard a subset of features is taken.
The efficacy of the maximum likelihood (ML) statistical classifier in combination with PCA/LDA/SLDA has been used to classify hyperspectral signatures (Samiappan et al. 2021). These analyses have the capabilities to investigate temporal misalignments between training and testing conditions. These modeling techniques can range from classical partial least squares regression (PLSR) to more modern machine learning techniques such as artificial neural networks, convolutional neural networks, and deep learning (Grzybowski et al. 2021).
15.4.1 Leaf Reflectance for Early Season Disease Diagnosis
Disease diagnosis and accurate estimation of disease incidence are fundamental in agriculture production. For example, the root-knot nematode (RKN) (Meloidogyne incognita) is a significant threat to cotton production. The RKN, a soilborne roundworm, threatens cotton and other crops. The formation of RKN affects a series of physiological traits, root system architecture, and final yield. Early diagnosis of RKN is meaningful in reducing disease spread, financial loss and facilitating real-time management practice. Leaf hyperspectral reflectance (350–2500 nm) has been used to understand early-season RKN damages on cotton (Fig. 15.4). Cotton genotypes (Rk-Rn-1, nematode-resistant and M8, nematode susceptible) with contrasting responses to nematode infection at the early vegetative stage were inoculated with nematodes. Temporal hyperspectral reflectance scans were taken 10, 30, and 60 days after the dose of nematode infestation (DAI) using PSR+ 3500 Spectroradiometer (Spectral Evolution, MD, USA) to discriminate the cotton plants with invisible RKN disease symptoms. We applied statistical supervised learning algorithms to classify RKN infested cotton from the control group using the spectral range is 350–2500 nm (2150 bands) with a spectral resolution of 2.8 nm at 700 nm, 8 nm at 1500 nm, and 6 nm at 2100 nm full width at half maximum (Fig. 15.4b). A maximum likelihood classifier with a dimensionality reducer revealed temporal hyperspectral changes in response to RKN before the plant’s visual symptoms appear. The classifier performed with >90% accuracy using hyperspectral data while classifying healthy cotton plants from RKN infected plants (Fig. 15.4). These results show the possibility of using leaf reflectance data to diagnose RKN infestation in cotton, and the same techniques could be used for other diseases in other crops. The effect caused by the diseases can be non-invasively analyzed using hyperspectral data at the early growth stage.
15.4.2 Crop Species Discrimination Using Hyperspectral Data
Leaf reflectance has been used for monitoring plant health, nutrient status, and stress tolerance in a range of crops (Yendrek et al. 2017; Silva-Perez et al. 2018; Mir et al. 2019; Reynolds et al. 2020; Grzybowski et al. 2021). Differences in leaf morph-physiological properties among different species lead to varying spectral signatures, which can be used for the classification of species (Fig. 15.2), abiotic (i.e., soil moisture stress treatments, Fig. 15.3) and biotic stresses (root-knot nematode, Fig. 15.4). For example, leaf spectral reflectance of showing a clear discrimination between corn and soybean in spectral-domain (Fig. 15.5a) and principal component domain (Fig. 15.5b). It is evident from the results that the two species: corn and soybean can be discriminated utilizing the leaf hyperspectral information (Fig. 15.5). However, this is not always guaranteed, especially when intraspecies spectral variability exceeds interspecies. Natural processes such as leaf senescence, nutrients, and stress can further complicate species separation (Reynolds et al. 2020).
Average corn and soybean leave spectra (a) and their separation in principal components (PCs) space (b). Corn and soybean leaf hyperspectral signatures were collected from plants grown under controlled environment conditions during the reproductive stage (Bheemanahalli et al., Plant Stress Physiology Lab, Mississippi State University, unpublished). Leaf reflectance observations were collected using the PSR+ 3500 Spectroradiometer (Spectral Evolution, MD, USA)
15.4.3 Leaf Reflectance and Soil Nutrients
Spectroscopy has been used in soil analysis for a few decades to estimate soil properties rapidly and cost-effectively. However, uncertainties are associated with estimated soil nutrient content as plant-available nutrients (Singh et al. 2019). The leaf level spectral measurements can bypass the soil nutrient analysis and show the direct impact of available nutrients on plants. Nutrients and minerals in plants in organic and inorganic fractions create spectral signatures used for estimations. For example, leaf N, mainly present in organic form in chlorophyll, absorbs light in the visible region due to the C–C and C=C bonds in the porphyrin rings and the magnesium ion (Sims and Gamon 2002). In addition, the mid-infrared (MIR) region has primary absorptions of many leaf properties such as cellulose, hemicellulose, lignin, xylan, cutin, carbohydrates, and proteins (Türker-Kaya and Huck 2017). Inorganic leaf nutrients such as Ca, K, and other micronutrients can also be derived from spectra due to their association with the organic compound functional groups or organic matrix (Yarce and Rojas 2012; Prananto et al. 2021). A compilation of past studies using near-infrared (NIR) to derive plant nutrients can be found in Prananto et al. (2020). In addition to NIR, the MIR region has also been utilized to derive numerous plant properties, including cell wall compounds (Jiang et al. 2009; Largo-Gosens et al. 2014), protein structure (Kumar et al. 2016), tissue and taxa differentiation (Huck-Pezzei et al. 2012), and water content (Ullah et al. 2012). Since the leaf level spectral measurements can better indicate the available nutrients in the soil; it can effectively be used in nutrient deficiency diagnosis or management. A study showed that leaf spectra could identify corn’s N, P, K, and Ca deficiencies (Al-Abbas et al. 1972). Similarly, other nutrients and physiological processes such as leaf senescence (Ivanova and Singh 2003) can be detected using the leaf-level spectra.
15.5 Future Perspectives
Plant physiologists, geneticists, and breeders are interested in leaf biochemical and physiological properties, usually measured destructively. Destructive measurements limit the speed of phenotyping, where hundreds of samples are needed to characterize variations in nutrients, metabolites, and photosynthetic traits (Grzybowski et al. 2021). Genomic techniques have been significantly advanced; however, throughput phenotyping has remained the primary bottleneck. To take advantage of genomic resources to dissect complex stress tolerance, leaf hyperspectral combined with spectroscopy can serve as an effective and efficient tool for a comprehensive assessment of complex plant performance to stresses. In recent years considerable progress has been made in building spectral features for crop improvement and the technologies to deliver from hand-held instrumentation to drones-assisted platforms. Numerous studies have shown that leaf and canopy spectra can help derive proxies associated with physiological and biochemical traits, which is tremendously helpful in studying heritability, genetic loci discovery, and improving breeding efficiency. Nevertheless, spectral-assisted breeding and selection will gradually evolve as data analysis and tools become more accessible for breeders and physiologists. In the future, spectral-assisted breeding could be seen as a revolutionary approach to enhance genetic gain under various environments.
References
Al-Abbas AH, Barr R, Hall JD, Crane FL, Baumgardner MF (1972) Spectral characteristics of normal and nutrient-deficient maize leaves. Information note 111472. Purdue University, West Lafayette
Blackmer TM, Schepers JS, Varvel GE (1994) Light reflectance compared with other nitrogen stress measurements in corn leaves. Agron J 86:934–938
Dalponte M, Bruzzone L, Vescovo L, Gianelle D (2009) The role of spectral resolution and classifier complexity in the analysis of hyperspectral images of forest areas. Remote Sens Environ 113:2345–2355
Daughtry CST, Walthall CL, Kim MS, de Colstoun EB, McMurtrey JE (2000) Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens Environ 74:229–239
Duda RO, Hart PE, Stork DG (2021) Pattern classification, 2nd edn. John Wiley & Sons, New York
Elsayed S, Rischbeck P, Schmidhalter U (2015) Comparing the performance of active and passive reflectance sensors to assess the normalized relative canopy temperature and grain yield of drought-stressed barley cultivars. Field Crop Res 177:148–160
Galieni A, D’Ascenzo N, Stagnari F, Pagnani G, Xie Q, Pisante M (2021) Past and future of plant stress detection: an overview from remote sensing to positron emission tomography. Front Plant Sci 11:1975
Gitelson AA, Merzlyak MN, Lichtenthaler HK (1996) Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J Plant Physiol 148:501–508
Gonzalez RC, Woods RE (2018) Digital image processing, 4th edn. https://www.pearson.com/content/one-dot-com/one-dot-com/us/en/higher-education/program.html. Accessed 18 Dec 2021
Grzybowski M, Wijewardane NK, Atefi A, Ge Y, Schnable JC (2021) Hyperspectral reflectance-based phenotyping for quantitative genetics in crops: progress and challenges. Plant Commun 2:100209
Hoffer RM, Johannsen CJ (1969) Ecological potential in spectral signatures analysis. In: Johnson PO (ed) Remote sensing in ecology. University of Georgia Press, Athens, pp 1–16. Accessed 18 December 2021
Huang M, Wang Q, Zhang M, Zhu Q (2014) Prediction of color and moisture content for vegetable soybean during drying using hyperspectral imaging technology. J Food Eng 128:24–30
Huck-Pezzei VA, Pallua JD, Pezzei C, Bittner LK, Schönbichler SA, Abel G, Popp M, Bonn GK, Huck CW (2012) Fourier transform infrared imaging analysis in discrimination studies of St. John’s wort (Hypericum perforatum). Anal Bioanal Chem 404:1771–1778
Ivanova DG, Singh BR (2003) Nondestructive FTIR monitoring of leaf senescence and elicitin-induced changes in plant leaves. Biopolymers 72:79–85
Jiang B, Shen Z, Shen J, Yu D, Sheng X, Lu H (2009) Germination and growth of sponge gourd (Luffa cylindrica) pollen tubes and FTIR analysis of the pollen tube wall. Sci Hortic 122:638–644
Kumar S, Lahlali R, Liu X, Karunakaran C (2016) Infrared spectroscopy combined with imaging: a new developing analytical tool in health and plant science. Appl Spectrosc Rev 51:466–483
Lammertyn J, Peirs A, De Baerdemaeker J, Nicolaı̈ B (2000) Light penetration properties of NIR radiation in fruit with respect to non-destructive quality assessment. Postharvest Biol Technol 18:121–132
Largo-Gosens A, Hernández-Altamirano M, García-Calvo L, Alonso-Simón A, Álvarez J, Acebes JL (2014) Fourier transform mid infrared spectroscopy applications for monitoring the structural plasticity of plant cell walls. Front Plant Sci 5:303
Lin M, Al-Holy M, Al-Qadiri H, Kang D-H, Cavinato AG, Huang Y, Rasco BA (2004) Discrimination of intact and injured listeria monocytogenes by Fourier transform infrared spectroscopy and principal component analysis. J Agric Food Chem 52:5769–5772
Lobos GA, Matus I, Rodriguez A, Romero-Bravo S, Araus JL, del Pozo A (2014) Wheat genotypic variability in grain yield and carbon isotope discrimination under Mediterranean conditions assessed by spectral reflectance. J Integr Plant Biol 56:470–479
Manley M (2014) Near-infrared spectroscopy and hyperspectral imaging: non-destructive analysis of biological materials. Chem Soc Rev 43:8200–8214
Mir RR, Reynolds M, Pinto F, Khan MA, Bhat MA (2019) High-throughput phenotyping for crop improvement in the genomics era. Plant Sci 282:60–72
Morley PJ, Jump AS, West MD, Donoghue DNM (2020) Spectral response of chlorophyll content during leaf senescence in European beech trees. Environ Res Commun 2:071002
Ngugi LC, Abelwahab M, Abo-Zahhad M (2021) Recent advances in image processing techniques for automated leaf pest and disease recognition—a review. Inf Process Agric 8:27–51
Pereira CS, Morais R, Reis MJCS (2017) Recent advances in image processing techniques for automated harvesting purposes: a review. In: 2017 intelligent systems conference (IntelliSys), pp 566–575
Prananto J, Minasny B, Weaver T (2020) Near infrared (NIR) spectroscopy as a rapid and cost-effective method for nutrient analysis of plant leaf tissues. Adv Agron 164:1–49
Prananto JA, Minasny B, Weaver T (2021) Rapid and cost-effective nutrient content analysis of cotton leaves using near-infrared spectroscopy (NIRS). PeerJ 9:e11042
Qi Y, Heisler GM, Gao W, Vogelmann TC, Bai S (2010) Characteristics of UV-B radiation tolerance in broadleaf trees in southern USA. In: Gao W, Slusser JR, Schmoldt DL (eds) UV radiation in global climate change: measurements, modeling and effects on ecosystems. Springer, Berlin, pp 509–530
Read JJ, Tarpley L, McKinion JM, Reddy KR (2002) Narrow-waveband reflectance ratios for remote estimation of nitrogen status in cotton. J Environ Qual 31:1442–1452
Reddy KR, Matcha SK (2010) Remote sensing algorithms for castor bean nitrogen and pigment assessment for fertility management. Ind Crop Prod 32:411–419
Reynolds M, Chapman S, Crespo-Herrera L, Molero G, Mondal S, Pequeno DNL, Pinto F, Pinera-Chavez FJ, Poland J, Rivera-Amado C, Saint Pierre C, Sukumaran S (2020) Breeder friendly phenotyping. Plant Sci 295:110396
Roitsch T, Cabrera-Bosquet L, Fournier A, Ghamkhar K, Jiménez-Berni J, Pinto F, Ober ES (2019) Review: new sensors and data-driven approaches—a path to next generation phenomics. Plant Sci 282:2–10
Samiappan S, Bheemanahalli R, Zhou M, Brooks J, Wubben M (2021) Early detection of root-knot nematode (Meloidogyne incognita) infestation in cotton using hyperspectral data. In: 2021 IEEE international geoscience and remote sensing symposium IGARSS, pp 5849–5852
Schmilovitch Z, Ignat T, Alchanatis V, Gatker J, Ostrovsky V, Felföldi J (2014) Hyperspectral imaging of intact bell peppers. Biosyst Eng 117:83–93
Silva-Perez V, Molero G, Serbin SP, Condon AG, Reynolds MP, Furbank RT, Evans JR (2018) Hyperspectral reflectance as a tool to measure biochemical and physiological traits in wheat. J Exp Bot 69:483–496
Sims DA, Gamon JA (2002) Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens Environ 81:337–354
Singh K, Majeed I, Panigrahi N, Vasava HB, Fidelis C, Karunaratne S, Bapiwai P, Yinil D, Sanderson T, Snoeck D, Das BS, Minasny B, Field DJ (2019) Near infrared diffuse reflectance spectroscopy for rapid and comprehensive soil condition assessment in smallholder cacao farming systems of Papua New Guinea. Catena 183:104185
Tarpley L, Reddy KR, Sassenrath-Cole GF (2000) Reflectance indices with precision and accuracy in predicting cotton leaf nitrogen concentration. Crop Sci 40:1814–1819
Türker-Kaya S, Huck CW (2017) A review of mid-infrared and near-infrared imaging: principles, concepts and applications in plant tissue analysis. Molecules 22:168
Ullah S, Skidmore AK, Naeem M, Schlerf M (2012) An accurate retrieval of leaf water content from mid to thermal infrared spectra using continuous wavelet analysis. Sci Total Environ 437:145–152
Van Bezouw RFHM, Keurentjes JJB, Harbinson J, Aarts MGM (2019) Converging phenomics and genomics to study natural variation in plant photosynthetic efficiency. Plant J 97:112–133
Wijewardana C, Alsajri FA, Irby JT, Krutz LJ, Golden B, Henry WB, Gao W, Reddy KR (2019) Physiological assessment of water deficit in soybean using midday leaf water potential and spectral features. J Plant Interact 14:533–543
Wood C (1993) Relationships between chlorophyll meter readings and leaf chlorophyll concentration, N status, and crop yield: a review. Proc Agron Soc NZ 23:1–9
Yarce CJ, Rojas G (2012) Near infrared spectroscopy for the analysis of macro and micronutrients in sugarcane leaves. Sugar Industry/Zuckerindustrie 137:707–710
Yendrek CR, Tomaz T, Montes CM, Cao Y, Morse AM, Brown PJ, McIntyre LM, Leakey ADB, Ainsworth EA (2017) High-throughput phenotyping of maize leaf physiological and biochemical traits using hyperspectral reflectance. Plant Physiol 173:614–626
Zhao D, Reddy KR, Kakani VG, Read JJ, Carter GA (2003) Corn (Zea mays L.) growth, leaf pigment concentration, photosynthesis and leaf hyperspectral reflectance properties as affected by nitrogen supply. Plant Soil 257:205–218
Zhao D, Reddy KR, Kakani VG, Reddy VR (2005a) Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur J Agron 22:391–403
Zhao D, Reddy KR, Starks PJ (2005b) Assessment of N status of cotton and forage plant using remote sensing. In: Li CJ et al (eds) Plant nutrition for food security, human health and environmental protection. Tsinmingo University Press, Bejing, pp 1104–1105
Zhao D, Reddy KR, Kakani VG, Read JJ, Koti S (2007) Canopy reflectance in cotton for growth assessment and lint yield prediction. Eur J Agron 26:335–344
Zur Y, Gitelson A, Chivkunova O, Merzlyak M (2000) The spectral contribution of carotenoids to light absorption and reflectance in green leaves. Papers in natural resources 272. https://digitalcommons.unl.edu/natrespapers/272
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bheemanahalli, R., Krishnan, B.S., Wijewardane, N.K., Samiappan, S., Reddy, K.R. (2023). Remote Sensing Algorithms and Their Applications in Plant Phenotyping. In: Harohalli Masthigowda, M., Gopalareddy, K., Khobra, R., Singh, G., Pratap Singh, G. (eds) Translating Physiological Tools to Augment Crop Breeding. Springer, Singapore. https://doi.org/10.1007/978-981-19-7498-4_15
Download citation
DOI: https://doi.org/10.1007/978-981-19-7498-4_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7497-7
Online ISBN: 978-981-19-7498-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)