Abstract
Frontline healthcare workers will play a central role in encouraging COVID-19 vaccination. Many studies have found that physicians are the most important influencers of vaccine decision making. To substantially reduce morbidity and mortality from COVID-19, an efficacious and safe vaccine must be delivered swiftly and broadly to the public as soon as it is available. However, the mere availability of a vaccine is insufficient to guarantee broad immunological protection; the vaccine must also be acceptable to both the health community and general public. Vaccine hesitancy is a major barrier to vaccine uptake and the achievement of herd immunity, which is required to protect the most vulnerable populations. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population. This chapter outlines the structure of coronavirus and vaccine development, different approaches of COVID-19 vaccines, whole virus vaccines, protein subunit vaccines, DNA vaccines, and composition of novel corona vaccine. It also illustrates the steps for corona vaccine development with preclinical, clinical evaluation, large-scale production of vaccine, quality control, packaging, storage shipping, and marketing. It describes the key challenges to scale up vaccine production, overcoming key challenges related to vaccine scale-up with business plan for novel corona vaccine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
19.1 Introduction
Coronavirus is a zoonotic virus, a new positive-strand RNA virus which belongs to the family Coronaviridae of the order Nidovirales (Padron-Regalado 2020). The types of coronavirus known to date are as follows: the alpha coronaviruses HCoV-229E and HCoV-NL63; the beta coronaviruses HCoV-OC43 and HCoV-HKU1; SARS-CoV, which causes severe acute respiratory syndrome (SARS); MERS-CoV, which causes Middle East respiratory syndrome (MERS); and SARS-CoV-2, a new coronavirus which was first reported in December 2019 in China (Padron-Regalado 2020), which causes the disease known as coronavirus disease 2019 (COVID-19). The full genome sequence of coronavirus was first shared by China on January 12, 2020, for countries to use in developing specific diagnostic kits (Kyriakidis et al. 2021). By September 2020, GISAID (Global Initiative on Sharing All Influenza Data), a large international repositories of SARS-CoV-2 uploaded and shared almost 100,000 full SARS-CoV-2 genomic sequences on GSAID SARS-CoV-2 Genomic Epidemiology platform (Peera 2020). According to WHO Report October 2021, globally over 23.7 crore people were affected from COVID-19 with 48.5 lakh confirmed deaths that were solely attributable to this disease (Peera 2020). Most people infected with COVID-19 reported respiratory tract illness which can range from very mild rhinorrhea to severe acute respiratory distress syndrome and death (Guan et al. 2020a; Chen et al. 2020; Bhatraju et al. 2020). However, other nonrespiratory symptoms have also been associated with COVID-19 such as anosmia, diarrhea, rash, thromboembolic disorders, myocarditis, and vasculitis (Chen et al. 2020; Bhatraju et al. 2020; Wang et al. 2020; Pan et al. 2020; Huang et al. 2020; Giacomelli et al. 2020; Zhou et al. 2020; Song et al. 2020a; Recalcati 2020). After exposure to the virus in 2–14 days, one will get persistent fever, on an average of 5–6 days. The mean incubation period is estimated to be 5 days (2–7), with a majority developing symptoms by 11.5 days (Lauer et al. 2020; Zou et al. 2020). Prolonged exposure for at least 15 minutes within 6 ft to an infected person and briefer exposures to individuals who are symptomatic (e.g., coughing) are associated with higher risk for transmission, while brief exposures to asymptomatic individuals are less likely to result in transmission of COVID-19. The death rate of COVID-19 is consistently reported to be age dependent, with a higher percentage in elderly (aged >70 years) cases dying, although other factors are also associated with intensive care admission and mortality (Global Polio Eradication Initiative 2020; Guan et al. 2020b) including sex (male > female), hypertension, obesity, and diabetes.
According to WHO, on October 12, 2021, there are currently 194 candidate vaccines that are in preclinical development worldwide. There are 40 vaccines which are in Phase I trial undergoing safety tests in healthy young individuals, 35 vaccines are in Phase II trial which are being tested in broader groups of people, 34 vaccines are in Phase III trial which are in large international trials to test their impact on COVID-19, and 8 vaccines are being monitored in the wider population after being approved (WHO 2020). Most of these trials are enrolling healthy adults (from age 18 years) only, with the upper age limit of inclusion ranging from 50 to 60 years.
19.2 Structure of Coronavirus and Vaccine Development
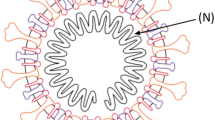
Coronaviruses have a large (30 + kb) single-stranded positive sense RNA genome which belongs to Coronaviridae family. The genome encodes several structural proteins like nucleocapsid phosphoprotein (N) which forms the helical capsid which is further surrounded by an outer envelope comprised of matrix protein (M), envelope protein (E), and spike proteins (S) (Boopathi et al. 2020). Other transcribed nonstructural proteins include orf1ab, ORF3a, ORF6, ORF7a, ORF10, and ORF8. The S protein, which naturally occurs in a trimetric form, contains the receptor-binding domain (RBD) responsible for binding onto the angiotensin converting enzyme 2 (ACE2) and entry primarily into the lung cells (Fig. 19.1).
With the availability of genome sequence of SARS-CoV-2 on January, 2020, different research groups across the world were employed to develop a vaccine against the coronavirus by using a variety of platform technologies that could stimulate the immune system against several viral antigens. Traditionally, vaccine development strategies such as live attenuated viruses (e.g., measles, mumps, rubella), inactivated viruses (e.g., inactivated polio vaccine), protein or polysaccharide conjugated subunit vaccines (protein: acellular pertussis, hepatitis B; polysaccharide conjugated: pneumococcus, meningococcus), and virus-like particles have been used in vaccines licensed in humans, but over the last decade, new sophisticated technology platforms to expedite vaccine development have been explored which include vaccines composed of nucleic acid (DNA and RNA) and viral vectors and recombinant proteins (Rauch et al. 2018). At present, the majority of the candidate vaccines for SARS COVID-19 that employ administration of viral antigens or viral gene sequences aim to elicit neutralizing antibodies against the viral spike protein (S), preventing uptake through the human ACE2 receptor and, therefore, blocking infection (Buchholz et al. 2004; Walls et al. 2020). Currently, there are four approaches of COVID-19 vaccines according to the platform technology used in their development: whole virus, protein subunit, viral vector, and nucleic acid (RNA and DNA). Some of them try to smuggle the antigen into the body; others use the body’s own cells to make the viral antigen.
19.3 Different Approaches of COVID-19 Vaccines (Fig. 19.2)
19.3.1 Whole Virus Vaccines
Whole virus vaccines use a weakened (attenuated) or deactivated form of the pathogen that causes a disease to trigger protective immunity to it. There are two types of whole virus vaccines, i.e., live attenuated and inactivated vaccines.
Live attenuated vaccines use a weakened form of the virus, which can still grow and replicate, but cause no or only very mild disease. These vaccines trigger the immune system by mobilizing a range of defenses against it, including killer T cells (which identify and destroy infected cells), helper T cells (which support antibody production), and antibody-producing B cells (which target pathogens lurking elsewhere in the body, e.g., the blood). This immune response continues until the virus is cleared from the body, meaning there is plenty of time for memory cells against the virus to develop (Koirala et al. 2020) (Fig. 19.2).
However, they may be unsuitable for people with compromised immune systems (e.g., those with HIV) and pregnant women though, because even a weakened virus may trigger disease in these individuals.
They are used for rubella virus, varicella zoster virus, and influenza virus and measles virus. Live attenuated SARS-CoV-2 vaccine candidates include: Cold-adapted mutants, temperature-sensitive mutants, and codon deoptimized mutants. According to Okamura S et al. 2021, cold-adapted mutants used for SARS-CoV-2 virus replicate more slowly in the lower respiratory tract and lungs, compared to the wild-type strain, whereas temperature-sensitive (TS) mutants could replicate not replicate at these locations. However, Mueller Steffen and his coworkers reported that codon deoptimized mutants of virus show lower proliferation rates than the wild-type strain, at any location (Okamura and Ebina 2021).
Although live attenuated vaccines are considered as one of the most powerful vaccine modalities, there are several problems associated to their use. The most serious problem is that viruses may reacquire their toxicity due to mutations after vaccination. A possible solution to this problem is to construct strains with various mutations by using reverse genetic methods such as BAC DNA and CPER methods (Ye et al. 2020; Torii et al. 2021) and thus maintain live attenuation, even when one responsible mutation is replaced by the wild-type sequence. In addition, adverse reactions like coagulopathy in elderly COVID-19 patients and induction of cytokine storm due to the proliferation of the live attenuated vaccine strains in the nasal cavity have been reported due to SARS-CoV-2 infection (Yuan et al. 2020; Song et al. 2020b). The temperature-sensitive strains that we isolated were able to proliferate in the nasal cavity as the wild-type strain, thereby potentially disrupting the epithelial tissues. Therefore, its effects need to be evaluated in detail.
Inactivated vaccines contain viruses whose genetic material has been destroyed by heat, chemicals, or radiation to stop disease-producing capacity, but they can replicate and still trigger an immune response. For this reason, they are considered safer and more stable than live attenuated vaccines, and they can be given to people with compromised immune systems. The disadvantage of inactivated vaccines is that they only stimulate antibody-mediated responses, and this response may be weaker and less long-lived. To overcome this problem, inactivated vaccines are often given alongside adjuvants (agents that stimulate the immune system), and booster doses may be required. Moreover, production of attenuated or inactivated vaccines requires large amounts of virus or bacteria to grown, which must then be isolated and purified, depending on the vaccine. Each of these steps requires specific equipment, reagents, and stringent procedures to avoid, and check for, contamination, which can further increase costs.
An inactivated SARS-CoV-2 vaccine was first reported in July 2020 (Song et al. 2020b). Gao et al. inactivated the virus with betapropiolactone, and the vaccine candidate was evaluated in mouse, rat, and nonhuman primate models. This vaccine-induced anti-S and anti-RBD antibodies, and the serum from immunized animals showed neutralizing activity. Kaabi et al. investigated the effect of 2 inactivated SARS-CoV-2 vaccines (SARS-CoV-2 WIV04 and HB02) on symptomatic COVID-19 infection in adults. Xia et al. reported that β-propiolactone-inactivated whole virus vaccine provides important interim safety, tolerability, and immune response against COVID-19. To date, four SARS-CoV-2 inactivated vaccines (Sinopharm, Sinovac, Sinopharm-Wuhan, and Bharat Biotech) have been approved and are being used in China, U.A.E, and India (Kaabi et al. 2021).
19.3.2 Recombinant Vaccines/Viral Vectors
Viral vector-based vaccines differ from most conventional vaccines in that they do not actually contain antigens, but rather use the body’s own cells to produce them. They do this by using recombinant viruses which are constructed by inserting the coding sequence of an antigen into a viral genome or by replacing a part of it. In the case of COVID-19, spike proteins found on the surface of the virus are used as antigens (Feng et al. 2020). Upon infection, the antigen’s coding sequence can be delivered to the cytoplasm or the nucleus of the infected host cell and instructing them to make large amounts of antigen, which then trigger an immune response; the vaccine mimics what happens during natural infection with certain pathogens, especially viruses. This has the advantage of triggering a strong cellular immune response by T cells as well the production of antibodies by B cells. There are two main types of viral vector-based vaccines. Nonreplicating vector vaccines are unable to make new viral particles; they only produce the vaccine antigen. Replicating vector vaccines also produce new viral particles in the cells they infect, which then go on to infect new cells that will also make the vaccine antigen. The COVID-19 viral vector vaccines under development use nonreplicating viral vectors.
Adenoviruses are commonly considered backbone vectors for the development of SARS-CoV-2 vaccines. They are nonenveloped double-stranded DNA (dsDNA) viruses with a packaging capacity of up to 7.5 kb of foreign genes (Schiedner et al. 1998). In almost all cases, the deletion of genes such as E1 and E3 genes has been engineered for the expression of the SARS-CoV-2 spike (S) protein or epitopes of it such as the receptor-binding domain (RBD) (Feng et al. 2020) gene therapy. An example of a viral vector vaccine is the rVSV-ZEBOV vaccine against Ebola. The recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV) Ebola vaccine is currently the only vector vaccine that has been licensed and available for human use and only produced and used to a limited extent (Regules et al. 2017; Kennedy et al. 2017; World Health Organization 2019). A MERS-CoV vaccine (MVA-MERS-S_DF1) using modified vaccinia virus Ankara and expressing the spike (S) protein of MERS-CoV was evaluated in an open label, Phase 1 trial on 26 individuals aged 18–55. It showed a favorable safety profile without any severe adverse effects but induced only a relatively limited humoral and T-cell response to the MERS CoV (Koch et al. 2020). Reassuringly, the study showed that although vector specific neutralization antibody was elicited, the vaccine still elicited antibody responses against the transgene following booster immunization (Koch et al. 2020). Results of the Phase 1 clinical trial for an alternate vaccine, ChAdOx1 MERS vaccine that uses a replication-deficient simian adenoviral vector expressing the spike (S) protein in 24 individuals aged 18–50 years, showed that a single dose was able to elicit both humoral and cellular responses against MERS CoV. The majority of solicited and unsolicited adverse events (AEs) reported by participants were mild or moderate, and all were self-limiting, and there were no serious AEs related to vaccine administration, which supports progression into Phase 1b and Phase 2 trials (Folegatti et al. 2020).
In the context of viral vector-based vaccine candidates against SARS-CoV-2, a number of preclinical studies have been conducted (Lundstrom and Aljabali 2021). Among the 162 ongoing COVID-19 vaccine candidates, studies listed by the WHO (Folegatti et al. 2020), utilize viral vectors or virus like particles (VLPs). Adenovirus-based vectors have dominated the field including human Ad5 and Ad26 and the simian AdChOx1. For instance, in a preclinical study, the intramuscularly administered Ad5 vector was utilized for the expression of the codon-optimized SARS-CoV-2 spike protein (S) in rhesus macaques (Feng et al. 2020). The immunization with Ad5-S-nb2 elicited systemic S-specific antibody and cell-mediated immune (CMI) responses. Currently, four adenovirus vector vaccines are in widespread use. These are the products (in alphabetical order) of CanSino Biological Inc./Beijing Institute of Biotechnology, Janssen—Johnson & Johnson, Oxford–AstraZeneca, and The Gamaleya Institute Moscow.
19.3.3 Nucleic Acid Vaccines
This is a relatively new technology, which utilizes antigen/genetic material from a disease-causing virus or bacterium-encoding plasmid DNA or RNA, messenger RNA (mRNA), or viral replicons. The nucleic acid, once taken up by a cell, provides the instructions for making a specific protein from the pathogen, to which a humoral and cell-mediated immune response is expected to occur, similar to natural infection. Nucleic acid vaccines use (a pathogen) to stimulate an immune response against it.
So far, DNA and RNA vaccines are being developed against various diseases, including HIV, Zika virus, and COVID-19, but none of them have yet been approved for human use. Beside this, several DNA vaccines are licensed for animal use, including a horse vaccine against West Nile virus. Such vaccines have been trialed for veterinary infectious diseases and demonstrated immunogenicity, for example, for foot and mouth disease, deer Powassan virus, and rabies virus (Pulido et al. 2010; VanBlargan et al. 2018; Saxena et al. 2009). However, Phase I trials in humans are underway for nucleic acid vaccines against Ebola, influenza, and Zika virus (Rauch et al. 2018).
Currently, there are 4 DNA vaccines under clinical trials (World-Health-Organization 2020). Among these, Phase I clinical trials have been conducted on SARS-CoV and MERS-CoV DNA vaccine candidates. Phase I trial on SARS-CoV DNA vaccine INO-4800 was investigated in 10 adults which includes the use of a recombinant SARS DNA coding for the SARS-CoV N protein genome, developed by the National Institute of Allergy and Infectious Diseases (NIAID) (Martin et al. 2008), and MERS-CoV DNA vaccine (GLS-5300), developed by GeneOne Life Science/Inovio and coding for the full length S protein genome, which had a higher number of participants (n = 75) (Modjarrad et al. 2019). Both vaccines showed acceptable safety profiles and induced humoral and cellular responses; the MERS-CoV DNA vaccine has advanced into a Phase 2 clinical trials (ClinicalTrials.gov 2020). The only other SARS vaccine produced by Sinovac Biotech that has entered a Phase I trial is an inactivated vaccine (ISCV) (Lin et al. 2007). There were no reports of human studies in which vaccinated subjects were challenged by the natural virus. The advantage of nucleic acid vaccines is the ease with which it allows antigen manipulation and the speed of production, as manufacturing can be synthetic and entirely cell-free so circumventing the need for BSL2 laboratories. The disadvantages are that nucleic acid, especially mRNA, is so feeble than DNA and needs a continuous cold-chain process for transport and storage (Zhang et al. 2019). mRNA-1273 (Moderna/US NIAID) is the first mRNA-based vaccine developed by Boston-based Moderna Therapeutics partnered up with the National Institute of Allergy and Infectious Diseases (NIAID) that entered clinical trials in 63 days after the genome sequencing of SARS-CoV-2 (Kyriakidis et al. 2021).
19.3.4 Protein Subunit Vaccines
Rather than injecting a whole pathogen to trigger an immune response, subunit vaccines (sometimes called acellular vaccines) contain purified pieces of viral proteins which are generated through recombinant synthesis and purification methods after cultivating large amounts of the pathogen, such as bacteria and yeast. Beside this, strict hygiene should be maintained to avoid contamination with other organisms. The main advantages of subunit vaccines are that these fragments are incapable of causing disease, thus considered very safe. Moreover, these are suitable for people with compromised immune systems. The disadvantage is that these vaccines explicit weaker immune response than with other types of vaccines. To overcome this problem, subunit vaccines are sometimes delivered alongside adjuvants (agents that stimulate the immune system) and booster doses may be required. Further, these vaccines are relatively complex and more expensive to produce than chemically synthesized vaccines, such as RNA vaccines. Yang et al. constructed a subunit vaccine composed of residues 319–545 of the SARS-CoV-2 RBD and produced it through the baculovirus expression system. The preclinical study reported that the vaccine could protect the nonhuman primates from SARS-CoV-2 infection with little toxicity (Yang et al. 2020).. Several teams across the world are currently working on engineering protein-based vaccines; however, the clinical results have not been published to date.
19.4 Composition of Novel Corona Vaccine
A novel corona vaccine comprises tiny fragments of the disease-causing organism or antigen which may be a whole virus in attenuated or inactivated form, recombinant virus, nucleic acid (DNA/RNA), or protein subunit and other ingredients like adjuvants, preservatives, stabilizers, surfactants, and residual which keep the vaccine safe and effective. Each component of vaccine serves a specific purpose, and each ingredient is tested for safety in the manufacturing process. Preservatives prevent the vaccine from becoming contaminated once the vial has been opened. The most commonly used preservative is 2-phenoxyethanol. Stabilizers prevent chemical reactions from occurring within the vaccine and keep the vaccine components from sticking to the vaccine vial. Stabilizers can be sugars (lactose, sucrose), amino acids (glycine), gelatin, and proteins (recombinant human albumin, derived from yeast). Surfactants keep all the ingredients in the vaccine blended together. Residuals are tiny amounts of various substances used during manufacturing or production of vaccines that are not active ingredients in the completed vaccine. Substances will vary depending on the manufacturing process used and may include egg proteins, yeast, or antibiotics.
19.5 Steps for Corona Vaccine Development
Vaccine development is a complex and lengthy process that has evolved and expanded considerably over the last few decades. Early on, the focus of the vaccine development process was the immunogenicity and efficacy of the vaccines, which were generally developed for diseases with significant burdens of morbidity, often with high mortality as well. Nowadays, greater emphasis is shifted on benefit-risk profiles of the vaccines under development. Moreover, economic evaluation of a new vaccine is also considered before its implementation (Roels et al. 2011). A viral pandemic requires rapid manufacture of effective treatments and vaccines. Billions of vaccine doses are required across the globe and the manufacturing of vaccines needs to occur at scale and pace while maintaining consistent high standards in quality. The first initial step in vaccine development is determining the burden of disease and defining the target population for a new vaccine. Disease burden is the impact of a health problem in a region or population, dependent upon the frequencies of the disease, the impact on quality of life (mortality and morbidity), healthcare resource use, and other indicators such as financial cost to society. A health problem or disease can have a relatively low incidence, but have a high case-fatality or case-disability incidence and treatment costs, resulting in a high burden of disease (Roels et al. 2011). The disease burden also differs greatly between the developed countries and developing world, due to differences in sanitation, healthcare, and other contributing factors, such as socioeconomic factors, access to preventive measures of communicable diseases, supportive care, and antibiotics. Funding is most important part in production of vaccine. Fund for Vaccine Procurement was developed by the Pan American Health Organization in 1979 for the purchase of vaccines, syringes/needles, and cold-chain equipment for countries in Latin America and the Caribbean. A major benefit of the fund’s role has been to ensure access to vaccines and thereby significantly improve population health. This gives vaccine manufacturers a return on their development costs, followed by availability of the vaccine in the market at an affordable price. Governments of developing countries are able to budget and plan for immunization programs, knowing that vaccines will be available in sufficient quantity, at a price they can afford, for the long term. Hence, for the production of a vaccine on large scale and in short time period requires a collaborative approach across pharmaceutical companies, governments and international organisations, medical professionals, policy makers, public or private health maintenance organisations.
Further, the World Health Organization (WHO) CHOICE (CHOosing Interventions that are Cost-Effective) project has the objective of providing policymakers with the evidence for deciding on the interventions and programs which maximize health for the available resources (Roels et al. 2011).
Vaccines have many challenges to overcome before they become licensed products. Each vaccine under development has gone through many steps before it can be introduced in a country’s vaccine program. Generally, vaccine development is a long and complex process often lasting for 10–15 years. The process of vaccine production starts with exploratory work on design of vaccine and evaluation in animal models. This process is followed further in different phages in which more preclinical experiments are performed. The process of vaccine production is designed, and proper toxicology studies are carried out to check any toxic effect of vaccine; further, this stage can also last for several years. But, during the development of COVID-19 vaccines, the clinical trial phases are overlapped to speed up the process, so the vaccines could be used as quickly as possible to control the pandemic.
These steps for the production of new vaccine are broadly categorized into three stages: preclinical, clinical, and postlicensure development, which ensure the safety and immunogenicity/efficacy of the final licensed vaccine.
19.5.1 Preclinical Evaluation
Each vaccine under development must first undergo screenings and evaluations to determine which antigen should be used to invoke an immune response. Before a vaccine can be tested in humans, it is first tested in animals to assess reactogenicity and/or characterize further the action of the antigen and any adjuvant. At this point, the vaccine manufacturing process is also defined. Compulsory initial submissions are made to regulatory authorities, such as an Investigational New Drug (IND) application to the Food and Drug Administration (FDA) in the USA, in order to begin clinical development. Before initiation of human trials, pharmacological and toxicological effects of new vaccines must be assessed which include single-dose toxicity, primary pharmacodynamics, secondary pharmacodynamics, pharmacokinetics, and local tolerance of the experimental vaccine.
19.5.2 Clinical Evaluation
After initial development, vaccines go through three phases of clinical trials to make sure they are safe and effective. Prior to regulatory approval, a vaccine usually undergoes three phases of clinical trials in humans. But, during the development of COVID-19 vaccines, the clinical trial phases are overlapped to speed up the process, so the vaccines could be used as quickly as possible to control the pandemic. The clinical trials for COVID-19 vaccines have involved tens of thousands of volunteers of different ages, races, and ethnicities. Clinical trials for vaccines compare outcomes (such as how many people get sick) between people who are vaccinated and people who are not. Because COVID-19 continues to be widespread, the vaccine clinical trials have been conducted more quickly than if the disease was less common. Results from these trials have shown that COVID-19 vaccines are effective, especially against severe illness, hospitalization, and death.
19.5.2.1 Phase I
Phase I trials are short-term studies in which the vaccine is given to a small number of volunteers typically 30–100, to evaluate its safety, confirm its ability to induce an immune response, and determine the right dosage and preferred route of administration to achieve the effective immune response. Generally, in this phase, vaccines are tested in young, healthy adult volunteers who are at low risk of acquiring natural infection (WHO Technical Report 2004; European Medicines Agency, Committee for Medicinal Products for Human Use 2005; Hudgens et al. 2004).
19.5.2.2 Phase II
After the successful completion of Phase I trials, the vaccine is then given to several hundred volunteers to further evaluate its safety and ability to generate an immune response. Participants in this phase have the same characteristics (such as age, sex) and include the responses of such individuals who are at risk of acquiring the infection. There are usually multiple trials in this phase to assess immune response in various age groups, gender, and different formulations of the vaccine. Phase II trials usually include a comparator group that did not get the vaccine to determine whether the changes in the vaccinated group are attributed to the vaccine or have happened by chance (WHO Technical Report 2004; Hudgens et al. 2004; Farrington and Miller 2001).
19.5.2.3 Phase III
In Phase III trials, the vaccine is given to thousands of volunteers. These trials should be randomized, double-blinded, and placebo-controlled (the placebo may be a saline solution, a vaccine for another disease or some other substance). Phase III trials are typically designed to evaluate vaccine efficacy and its safety in large number of people (WHO Technical Report 2004; European Medicines Agency, Committee for Medicinal Products for Human Use 2005; Farrington and Miller 2001).
After the successful completion of Phase III trials, a biologics license application is filed with regulatory agencies (e.g., the United States Food and Drug Administration (FDA) or the European Medicines Agency). The licensing process can take another 1–2 years, especially if additional data are requested. Importantly, because it is very expensive, the overall process of vaccine development is slowed by economic risk assessment at every step. Vaccine development progresses through these stages only if the developer is convinced that the data are promising that the risk of failure is relatively low and that there is (still) a market for the vaccine.
In various cases, the production process of vaccines was simply adapted from the preexisting vaccines or vaccine applicants, and in certain cases, preclinical and toxicology data from related vaccines could be used. As a result, the first clinical trial of a vaccine candidate for SARS-CoV-2 began in March 2020 (NCT04283461). Trials were designed such that clinical phases are overlapping, and trial starts are staggered, with initial Phase I/II trials followed by rapid progression to Phase III trials after interim analysis of the Phase I/II data. Currently, several manufacturers have already started the commercial production of vaccines at risk without any results from Phase III trials. Although the licensure pathways are not yet completely clear, it is possible that reviews could be expedited and that vaccines could even be approved through an emergency use authorization. The FDA has released a guidance document for the development and license of SARS-CoV-2 vaccines, which as well as providing additional details states that an efficacy of at least 50% will be required (FDA 2020). It is very important to point out that moving forward at financial risk is the main factor that has enabled the accelerated development of SARS-CoV-2 vaccine candidates, and no corners have been or should be cut in terms of safety evaluation. Although vaccine development is moving forward at an unparalleled speed, there are still many open questions. It is likely that two doses of a vaccine will be required, with booster doses potentially necessary at later time points. In this case, at least 16 billion doses will be needed to meet the global demand. Many of the vaccines that are described below are being developed by entities that have never brought a vaccine to market or use technologies that have never resulted in a licensed vaccine. Therefore, unforeseen issues with scaling could cause delays. It is also not yet clear whether bottlenecks will occur in the availability of syringes or glass vials. Finally, for certain vaccine candidates against SARS-CoV and MERS-CoV, vaccine-enhanced disease was reported in some animal models. For SARS-CoV-2 vaccine candidates, there have so far been no signals of enhanced disease in animal models or in humans; however, such a safety signal would certainly derail the development of a vaccine candidate and would negatively affect vaccine development in general (Fig. 19.3) (Krammer 2020).
19.6 Large-Scale Production of Vaccine
19.6.1 Manufacturing Steps
The most difficult, time-consuming, and resource-intensive aspect of vaccine development is to scale up the production of a vaccine from small amounts to commercial levels. For scientists, it is easy to work readily with vaccine in 1–10 L bioreactors in a bench-level laboratory. But, transferring the technology from laboratory to the pilot scale of 50–100 L volumes is not simple. It requires a close check on the behavior of the microorganisms, biochemical and physiological interaction, and the rate of yield to ensure that the product is equivalent to that developed in the laboratory. Hence, production of vaccine on large scale (550 liters or more) by well-established pharmaceutical firms is a quite challenging process. For example, the recent scale-up of a Haemophilus influenzae type b conjugate vaccine (Hib-CV) and a Hib-CV-diphtheria and tetanus toxoids and pertussis vaccine (DTP) combination was more difficult than anticipated (Siber et al. 1992). Siber et al. reported that many manufacturers of single-component Hib-CV noted reductions in the immunogenicities of their vaccines that appeared to coincide with the scale-up process itself (Siber et al. 1992). In these recent cases, sophisticated physical and biochemical characterizations of the vaccines and animal testing did not predict the reduced immunogenicity.
Considering viral vector vaccines as an example, once the required concentration of vaccine has been grown and extracted from the cells, to achieve a large volume of pure vaccine a series of key steps take place: filtration, membrane chromatography, and ultrafiltration.
Filtration—like sieving—removes unwanted residual products. This is achieved using special membranes with pores.
Membrane chromatography allows the vaccine to bind to a surface to ensure only the product that is needed is left.
Ultrafiltration “buffers” the vaccine to control how acidic or alkaline it is.
To ensure that the vaccine is readily acceptable and serves the purpose, there should be zero chances of errors happening. There are three main factors taken into consideration, while mass-producing any vaccine includes production of antigens and other biochemical compounds, adding adjuvants and enzymes for support, using DNA and mRNA technologies to develop a vaccine.
The shortcomings in vaccine supplies have inevitably led to a deflection of blame with vaccine manufacturers in the firing line. Questions over vaccine prices, manufacturing capacity, and the destination of supplies have beset the world’s largest vaccine manufacturer. As the drug substance in production progresses to become the drug product that can be put into suitable containers and used, “fill and finish” takes place to transport the vaccine in multidose vials packaged into cartons. Virologists and pharmaceutical experts have said that the Indian government involved more companies in manufacturing to cope up with current COVID vaccine shortages.
19.6.2 Quality Control
Many methods of quality testing are used throughout the manufacturing and production process. These are designed to ensure high quality by the following:
-
Checking that the vaccine carries the correct instructions to the body.
-
Assessing vaccine purity.
-
Measuring vaccine concentration to ensure the amount of vaccine required is present.
The next crucial step is to ensure consistency and quality of vaccine manufacture by pharmaceutical companies. It includes various key processes like testing of raw materials, conducting quality control tests throughout vaccine production, site visits and virtual tours by central personnel, electronic monitoring to ensure careful control of temperature for storage and transport and use of virtual technology to provide real-time technical support, and coaching for production teams on site. By putting these types of processes in place, vaccine production and manufacturing can progress rapidly without compromising safety, quality, or effectiveness.
19.6.3 Packaging
Once the vaccine has been made in bulk quantities, it is bottled in glass vials and then carefully packaged for safe cold storage and transport.
Vaccine packaging must be able to withstand extreme temperatures, as well as the risks involved in being transported globally. Therefore, vaccine vials are most commonly made from glass, as it is durable and able to maintain its integrity in extreme temperatures.
19.6.4 Storage
Most of the vaccines require refrigerated storage at between 2 °C and 8 °C because at too hot or too cold temperature, it becomes less effective or even inactive. However, some vaccines require temperatures as cold as −20 °C, but some of the newer vaccines need to be kept ultracold at −70 °C. Hence, specialized medical refrigerators are required for these precious products.
19.6.5 Shipping
Vaccines are shipped in the destination country by using specialized equipment that does not compromise the integrity of the product. From the warehouse, portable iceboxes are used to transport vaccines to regional centers or the place outside of the regional center, where they are stored in refrigerators. Nowadays, some portable devices are used that can keep vaccines at their cold temperature for several days without needing electricity.
Once vaccines start being administered, national authorities and WHO constantly monitor for and establish the severity of any possible adverse side effects and responses from people who have received the vaccine. The safety of the vaccine is paramount, with regular assessments and postapproval clinical studies to report on its safety and effectiveness.
19.7 Key Challenges to Scale Up Vaccine Production
Typically, a good workforce and more than 200 individual components are required for the large-scale production of a vaccine. The components include glass vials, filters, syringes, tubing, disposable bags, and stabilizing agents. These components are often manufactured in different countries, and hence, the travel restriction on people and shortage of supply of any of the components may result in break of supply chain of the vaccine.
Another key challenge is to develop an affordable vaccine for low- and middle-income countries (LMICs). Several companies, such as AstraZeneca and Johnson & Johnson, which rely on public sector investments, have claimed to sell their vaccine globally at a low cost during the pandemic to improve accessibility. Indian manufacturers have stated that they have the capacity to meet the country’s future needs for COVID-19 vaccines. The Indian government has taken urgent measures to expand the country’s vaccine manufacturing capacity and has also developed an efficient digital system to address and monitor all the aspects of vaccine administration (Kumar et al. 2021). Serum Institute of India (SII), Pune, has signed agreements with a few manufacturers such as Oxford–AstraZeneca, Codagenix, and Novavax. It is now producing at a large scale, the Oxford–AstraZeneca adenovirus vector-based vaccine AZD1222 (which goes under the name “Covishield” in India), and it has stockpiled about 50 million doses (Voysey et al. 2021). Covaxin™ is an inactivated virus vaccine, developed in Vero cells. The inactivated virus is combined with Alhydroxiquim-II (Algel-IMDG), chemosorbed imidazoquinoline onto aluminum hydroxide gel, as an adjuvant to boost immune response and longer-lasting immunity (Kumar et al. 2021).
19.8 Overcoming Key Challenges Related to Vaccine Scale-Up
According to a special report published in Nature, researchers explain that to accelerate vaccine production, collaboration with multiple supply partners and analytical testing sites is essential in different countries. Recently, Martin Friede, head of vaccine development at the WHO, stated that WHO has recognized many organizations around the world which provide matchmaking services by connecting distributors of vaccine components and major manufacturing companies. For example, AstraZeneca has collaborated with multiple manufacturing companies across the world where each and every process is governed and technically guided by the company to support each stage of production.
In many organizations, across multiple industries, data sharing remains a key issue which can lead to the generation of data silos especially in pharmaceutical companies. Besides clinical trial data on vaccine candidates, reports on monoclonal antibodies and epidemiological modeling studies should be shared widely among biopharmaceutical companies in advanced countries as well as in LMICs (low- and middle-income countries). Therefore, pharmaceutical industry is taking steps toward solving the problem by “connecting” siloed data. Further, multiple technology transfer hub is established by WHO and its partners to scale up the vaccine production capabilities of LMICs (low- and middle-income countries).
Another key area that can help in the large-scale manufacturing of vaccine is optimization and quality checking. Environmental conditions, such as heat, light, and radiation, can affect the quality and purity of vaccines. Different technologies like polymerase chain reaction (PCR), high-performance liquid chromatography (HPLC), anion exchange chromatography (AEX), and affinity chromatography are being used to evaluate vaccine quality. To ensure stability, shelf life, and safety, researchers will continue to assess and refine storage and handling conditions, at each phase of the vaccine production. In the current scenario where production rate is extremely high, quality and stability testing are being carried out side by side with the manufacturing process.
In the process of meeting the unprecedented demand for COVID-19 vaccines, manufacturers whole over the world have had the opportunity to innovate and streamline vaccine production on a large scale. Such optimization may yield rich dividends to the manufacturing companies in the future.
19.9 Business Plan for Novel Corona Vaccine
With promising COVID-19 vaccines from companies like Pfizer, Moderna, and Johnson & Johnson now being distributed across the USA, business owners might want to start thinking about how their operations could change. The vaccines, which were approved for emergency use by the government in December 2020, will likely have far-reaching impacts on both employees and customers, so it is important to get planning.
The most important point for business is to start thinking how their employees align with the government’s latest recommendations for receiving the vaccine first. Just because vaccines are available, it does not mean every eligible individual will be lining up to receive their doses. Employers have a unique role to play in encouraging widespread vaccination and dispelling any hesitancy among their workforce. To substantially reduce morbidity and mortality from COVID-19, an efficacious and safe vaccine must be delivered swiftly and broadly to the public as soon as it is available. However, the mere availability of a vaccine is insufficient to guarantee broad immunological protection; the vaccine must also be acceptable to both the health community and general public (Schafer et al. 2021). Vaccine hesitancy is a major barrier to vaccine uptake and the achievement of herd immunity, which is required to protect the most vulnerable populations. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population (Sanche et al. 2020).
Given that certain individuals will be ineligible for COVID-19 vaccination due to age, immune compromise, and other preexisting medical conditions, a vaccine refusal rate greater than 10% could significantly impede attainment of this goal. Recent surveys, that included 493 and 2200 individuals, suggest only 3 in 4 people would get vaccinated if a COVID-19 vaccine were available, and only 30% would want to receive the vaccine soon after it becomes available (Trujillo and Motta 2020). Confidence in vaccines lies along a spectrum, and individuals who have hesitation about routine childhood vaccines have expressed various concerns (Edwards and Hackell 2016). In their report on vaccine hesitancy, Edwards and Hackell identified 3 broad categories of parents’ concerns regarding childhood vaccines: (1) the necessity of vaccines, (2) vaccine safety, and (3) freedom of choice (Edwards and Hackell 2016). This viewpoint describes these categories of concerns with regard to a future COVID-19 vaccine and presents suggestions to enhance the likelihood of rapid, widespread vaccine uptake in the United States.
Frontline healthcare workers will play a central role in encouraging COVID-19 vaccination. Many studies have found that physicians are the most important influencers of vaccine decision making (Edwards and Hackell 2016). Thus, strong physician recommendations can bolster public and individual support for a COVID-19 vaccine. Physicians who share personal anecdotes about being immunized and immunizing their family members are effective in encouraging vaccine uptake in vaccine-hesitant families (Kempe et al. 2015). As such, achieving a high vaccination coverage level of healthcare workers early on not only ensures an adequate workforce to treat infected patients, but also allows medical authority figures to share their positive vaccination experiences with patients. While most studies have focused on the role of physicians, the influence of nurses and allied health professionals on patients’ vaccination attitudes and beliefs also is important. Healthcare workers are exposed to the same media stories as the general public and may be subjected to the same cognitive biases that can lead to excessive reliance on anecdotal evidence and false conclusions. Ensuring that all individuals who interface with patients in the clinical setting are confident about the safety and effectiveness of a future COVID-19 vaccine is critical for presenting a unified message of strong vaccination support from the medical community.
19.10 Conclusion
In India, free vaccination against COVID-19 has begun on January 16, 2021, and the government is advising all of its citizens to be immunized and is expected to be the largest vaccination program in the world. India, which has a robust vaccine development program, not only plans for domestic manufacture of COVID-19 vaccine but also plans for the distribution in countries that cannot afford to buy vaccines. While obtaining the vaccine is the first requirement, distribution and vaccination of the huge Indian population present a significant logistic challenge. The purpose of this perspective was to highlight the overall crux of the vaccine development and vaccination strategies that were implemented during a pandemic in a densely populated country. This report can be viewed as a baseline document for future pandemic preparedness and to effectively tailor and refine the strategies that will help the population at large. These data analyses can hold the keys to the future effective public health management of COVID-19. India’s experience in immunization for COVID-19 offers tips for strategy preparation, not only for countries with similar economic strength and health facilities but also for the world at large.
References
Bhatraju PK et al (2020) Covid-19 in critically ill patients in the Seattle region-case series. N Engl J Med 382(21):2012–2022
Boopathi S et al (2020) Novel coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn:1–10
Buchholz UJ et al (2004) Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci U S A 101(26):9804–9809
Chen N et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513
ClinicalTrials.gov (2020) Identifier: NCT03721718. Evaluate the safety, tolerability and immunogenicity study of GLS-5300 in healthy volunteers 2020. https://clinicaltrials.gov/ct2/show/NCT03721718. Accessed 25 May 2020
Edwards KM, Hackell JM (2016) Countering vaccine hesitancy. Pediatrics 138(3):e20162146. https://doi.org/10.1542/peds.2016-2146. PMID: 27573088
European Medicines Agency, Committee for Medicinal Products for Human Use (2005) Guideline on Clinical Evaluation of New Vaccines (EMEA/CHMP/VWP/164653/2005), pp 1–19
Farrington CP, Miller E (2001) Vaccine trials. Mol Biotechnol 17:43–58
Feng L et al (2020) An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat Commun 11(1):4207
Folegatti PM et al (2020) Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis 20(7):816–826
Giacomelli A et al (2020) Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis 71(15):889–890
Global Polio Eradication Initiative (2020) The use of oral polio vaccine (OPV) to prevent SARS-CoV2. http://polioeradication.org/wp-content/uploads/2020/03/Use-of-OPV-and-COVID-20200421.pdf
Guan WJ et al (2020a) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720
Guan W-J et al (2020b) Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J 55(5):2000547
Huang C et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506
Hudgens MG et al (2004) Endpoints in vaccine trials. Stat Methods Med Res 13:89–114
Kaabi et al (2021) Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 326(1):35–45
Kempe A et al (2015) Physician response to parental requests to spread out the recommended vaccine schedule. Pediatrics 135(4):666–677. https://doi.org/10.1542/peds.2014-3474. PMID: 25733753; PMCID: PMC6046639
Kennedy SB et al (2017) Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 377(15):1438–1447
Koch T et al (2020) Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open label, phase 1 trial. Lancet Infect Dis 20(7):827–838
Koirala A et al (2020) Vaccines for COVID-19: the current state of play. Paediatr Respir Rev 35:43–49. https://doi.org/10.1016/j.prrv.2020.06.010
Krammer F (2020) SARS-CoV-2 vaccines in development. Nature 586:516–527. https://doi.org/10.1038/s41586-020-2798-3
Kumar VM et al (2021) Strategy for COVID-19 vaccination in India: the country with the second highest population and number of cases. Vaccine 6:60. https://doi.org/10.1038/s41541-021-00327-2
Kyriakidis NC, Lopez-Cortes A, Gonzalez EV, Grimaldos AB, Prado EO (2021) SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines 6(1):28. https://doi.org/10.1038/s41541-021-00292-w
Lauer SA et al (2020) The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 172(9):577–582
Lin J et al (2007) Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther 12(7):1107–1113
Lundstrom K, Aljabali AAA (2021) COVID-19 in 2021. Viruses 13(10):2098. https://doi.org/10.3390/v13102098
Martin JE et al (2008) A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a phase I clinical trial. Vaccine 26(50):6338–6343
Modjarrad K et al (2019) Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis 19(9):1013–1022
Okamura S, Ebina H (2021) Could live attenuated vaccines better control COVID-19? Vaccine 39:5719–5726
Padron-Regalado E (2020) Vaccines for SARS-CoV-2: lessons from other coronavirus strains. Infect Dis Ther 9:255–274
Pan L et al (2020) Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol 115(5):766–773
Peera H (2020) SARS-Cov-2 sequencing data: the devil is in the genomic detail. American Society for Microbiology, Washington, DC
Pulido MR et al (2010) RNA immunization can protect mice against foot-and-mouth disease virus. Antiviral Res 85(3):556–568
Rauch S et al (2018) New vaccine technologies to combat outbreak situations. Front Immunol 9:1963
Recalcati S (2020) Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 34(5):e212–e213. https://doi.org/10.1111/jdv.16387
Regules JA et al (2017) A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med 376(4):330–341
Roels GL et al (2011) Vaccine development. In: Understanding modern vaccines: perspectives in vaccinology, vol 1. Elsevier, Amsterdam, pp 115–150
Sanche S et al (2020) High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis 26(7):1470–1477. https://doi.org/10.3201/eid2607.200282
Saxena S et al (2009) Induction of immune responses and protection in mice against rabies using a self-replicating RNA vaccine encoding rabies virus glycoprotein. Vet Microbiol 136(1–2):36–44
Schafer A et al (2021) Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J Exp Med 218(3):e20201993. PMID: 33211088; PMCID: PMC7673958. https://doi.org/10.1084/jem.20201993
Schiedner G et al (1998) Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat Genet 18:180–183
Siber GR et al (1992) Evaluation of bacterial polysaccharide immune globulin for the treatment or prevention of haemophilus influenzae type b and pneumococcal disease. J Infect Dis 165(1):129–133. PMID: 1588146. https://doi.org/10.1093/infdis/165-supplement_1-s129
Song Y et al (2020a) SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut 69(6):1143–1144
Song P et al (2020b) Cytokine storm induced by SARS-CoV-2. Clin Chim Acta 509:280–287
Torii S et al (2021) Establishment of a reverse genetics system for SARS-CoV-2 using circular polymerase extension reaction. Cell Rep 35(3):109014
Trujillo KL, Motta M (2020) Why are wealthier countries more vaccine skeptical? https://doi.org/10.33774/apsa-2020-bbpld-v2. Corpus ID: 226374269
VanBlargan LA et al (2018) An mRNA vaccine protects mice against multiple tick transmitted flavivirus infections. Cell Rep 25(12):3382–3392
Voysey M et al (2021) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397:99–111
Walls AC et al (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181(2):281–292
Wang D et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069
WHO (2020) Draft landscape of COVID-19 candidate vaccines. World Health Organization, Geneva. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
WHO Technical Report (2004) Annex 1: WHO guidelines on clinical evaluation of vaccines: regulatory expectations. World Health Organization, Geneva, pp 36–96
World Health Organization (2019) Preliminary results on the efficacy of rVSVZEBOV- GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: an example of integration of research into epidemic response. WHO, Geneva
World-Health-Organization (2020) Draft landscape of COVID-19 candidate vaccines. World-Health-Organization, Geneva
Yang J et al (2020) A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 586:572–577. https://doi.org/10.1038/s41586-020-2599-8(2020)
Ye C et al (2020) Rescue of SARS-CoV-2 from a single bacterial artificial chromosome. MBio 11(5):e02168–e02120
Yuan X et al (2020) Coagulopathy in elderly patients with coronavirus disease 2019. Aging Med 3(4):260–265
Zhang C et al (2019) Advances in mRNA vaccines for infectious diseases. Front Immunol 10:594
Zhou P et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273
Zou L et al (2020) SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382(12):1177–1179
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kumari, A., Rani, S. (2022). Large-Scale Production and Business Plan for Novel Corona Vaccine. In: Amaresan, N., Dharumadurai, D., Cundell, D.R. (eds) Industrial Microbiology Based Entrepreneurship. Microorganisms for Sustainability, vol 42. Springer, Singapore. https://doi.org/10.1007/978-981-19-6664-4_19
Download citation
DOI: https://doi.org/10.1007/978-981-19-6664-4_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-6663-7
Online ISBN: 978-981-19-6664-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)