Abstract
Artemisia annua L. is an annual, herbaceous, aromatic medicinal plant belonging to the family Asteraceae. It is mentioned in traditional Chinese medicine as a cure for different diseases like fever, hemorrhoid, and malaria. It is native to the mild and temperate climate of Asia but has been naturalized to other countries outside Asia as well. After the discovery of its antimalarial potential by Prof. Tu Youyou in 1972, the World Health Organization has recommended it as an antimalarial. The most common ethnobotanical usage of this plant involves the use of whole plant decoction for the treatment of cold, malaria, and cough. The whole flowering plant is known to be antipyretic, antihelminth, antispasmodic, antiseptic, and antimalarial. The antimalarial activity of this plant is due to artemisinin, a sesquiterpene lactone containing an endoperoxide moiety that acts as a key pharmacore. Artemisinin forms an important part of combinatorial treatment therapy recommended for the treatment of malaria. Artemisinin and its derivatives like artesunate have also been reported to have potent anticancer properties as well. Besides artemisinin, certain other phytochemicals reported in this plant, particularly flavonoids, have been found to have medicinal properties. They have been reported to synergize the activity of artemisinin and its derivatives against malaria. Considering the immense medicinal properties of this plant, immense research is being carried out throughout the world to isolate and characterize the different phytochemicals present in this plant. This chapter comprises the information about the general biology, distribution, and phytochemical composition of A. annua, updated information about its medicinal properties and health benefits, and an overview of its safety and toxicity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Medicinal plants have been used in the treatment and prevention of numerous diseases since time immemorial. At the beginning of civilization, they were important components of medication whereas nowadays they are instrumental in the manufacturing of drugs. A medicinal plant can be defined as a plant that, in one or more of its organs, contains substances that act as precursors for the synthesis of certain drugs or has therapeutic value. There are certain medicinal plants that have been scientifically proven to have medicinal and therapeutic properties, whereas some plants are regarded as medicinal and used in traditional medicines but have not been subjected to thorough scientific studies to prove their efficacy. Due to the proven effectiveness and safety claims, the consumption of medicinal plants is showing a steep rise (Perez Gutierrez and Baez 2009).
Asteraceae is regarded as one of the largest families of flowering plants which has a cosmopolitan distribution, as its plants are found all over the world, including Antarctica where it is most probably anthropogenically mediated (Smith and Richardson 2011). This family of flowering plants consists of about 13 subfamilies, 1620 genera, and around 23,600 accepted species. It comprises about 10% of total flowering plants and is rivaled only by Orchidaceae in terms of total species. The most distinguishing and diagnostic characteristics of the Asteraceae family are the presence of capitulum inflorescence, inferior ovary, and anthers united in a tube (Fornara 2014).
Artemisia annua L. (“sweet wormwood,” “annual wormwood,” “Qinghao”; 2n = 36), belonging to Asteraceae family of flowering plants, is annual, aromatic, herbaceous, and glabrous or sparsely hairy, with an upright brownish colored stem. It naturally grows up to a height of 1 meter, but under cultivated conditions, it may reach up to a height of 2 meters. It usually consists of a single stem with alternate branches and deeply dissected leaves. The inflorescence consists of small capitula arranged in loose panicles with bisexual disc florets at the center and pistillate ray florets at the margins. The pollens are smooth and tricolpate, a typical characteristic of anemophilous species (Stix 1960) whereas the ovary is unilocular and inferior. The glandular secretory trichomes which are the sites of biosynthesis of antimalarial compound artemisinin (Wani et al. 2022), and nonglandular T-shaped trichomes are present on stem, leaves, and inflorescences (Ferreira and Janick 1996) which are easily visible by scanning electron microscopy (Fig. 2.1).
The plant is native to the mild and temperate climate of Asia, most probably China, and has been found as native to Myanmar, Japan, Korea, Northern India, Southern Siberia, and Vietnam; however, it has become naturalized in many countries including some areas of North America (Desrosiers and Weathers 2016). Its cultivation is carried out on a large scale in countries such as China, Tanzania, and Kenya, with small-scale cultivation being done in India and certain countries of South America and South Europe (WHO 2006).
2.2 Traditional Uses
Artemisia annua has a long history of being used in traditional Chinese medicine as an anti-malarial and antipyretic, dating back to over 2000 years. China has a long history of its cultivation with its remains found in Shengjindian cemetery (about 2400–2000BP based on 14C dating), Xinjiang, China. This archaeological discovery gives an idea about its use in ancient China. As this plant is highly aromatic, the most probable reason for its use in the tombs in cemeteries would have been to eliminate the unpleasant odor of the dead (Liu et al. 2013; Sadiq et al. 2014). The essential oils extracted from this plant have also been used in the perfume industry. A treatise with a description of 224 medicines and their preparation methods was excavated in 1973 and named “Prescriptions for 52 diseases.” It is regarded as one of the oldest sources of knowledge about Chinese pharmaceutics (Unschuld 1986). It described A. annua (qinghao) as a prescription for the treatment of hemorrhoids (Riddle 2010). Its utility as an herbal remedy against malaria was first described in A Handbook of Prescriptions for Emergency by Hong Ge. The described recipes were in the form of infusion, drink, powder, wine, and pills. A. annua decoction is also mentioned in the “General Medical Collection of Royal Benevolence” written during the Song Dynasty (Ekiert et al. 2021). Its preparation was recommended for paroxysmal malarial fever in the book Compendium of Materia Medica by Shizhen Li. In Chinese literature, its scientific name Artemisia annua was given in the twentieth century in the publication First Chinese Pharmacopoeia in 1930 (Riddle 2010; Ekiert et al. 2021). However, this plant was not given any attention in western herbal medicine until the start of the twentieth century.
Based on the traditional Chinese medicinal records, A. annua has a long-established tradition of being used in the treatment of diseases. In traditional medicine, all the parts of this plant and its extracts are used in the treatment of diseases like fever, jaundice, and dysentery (Ekiert et al. 2021). Its usage in China dates back to over 2000 years when it was used against tuberculosis, and fever caused by malaria and summer heat (WHO 2006; https://www.herbsociety.org/file_download/inline/d52eae8c-be89-497d-94b3-7fc8da4105f1). A. annua is also used in the treatment of hemorrhoids, wounds, and infections caused by protozoan species belonging to different genera like Leishmania, Plasmodium, Schistosoma, and Acanthamoeba (Alesaeidi and Miraj 2016). It is regarded as an important ethnomedicinal herb, and its most common method of usage is in the form of whole plant decoction, against cold, cough, and malaria. The powder made from its dried leaves has also been used in treating diarrhea, whereas the entire plant is effective as an antiseptic, antipyretic, anthelminthic, antispasmodic, and stimulant (Nigam et al. 2019). The crushed plants of A. annua have been used in the preparation of liniment whereas its tincture has been used in the treatment of nervous diseases (Sadiq et al. 2014). In African countries, the tea infusion made from it has been used to treat malaria. Furthermore, A. annua has also been associated with increased longevity, hair growth, and brightening of eyes (Hsu 2006). Qinghao has also been described as a food supplement in some of the records (Hsu 2009). Presently, research on this plant is mainly focused on its efficacy against malaria, cancer, and as an antioxidant (Willcox 2009; Ferreira et al. 2010; Wright et al. 2010).
2.3 Phytochemical Composition of A. annua
After the discovery of artemisinin, intensive research has been going on with A. annua to evaluate its phytochemical composition. It is a type with variable chemical composition, and a vast variety of phytochemicals have been identified (Marinas et al. 2015). The chemical composition of the plant also depends upon the climate in which it’s growing (Hwang et al. 2016). Various chemical compounds that have been identified in A. annua include essential oil with monoterpenes and sesquiterpenes, coumarins, flavonoids, phenolic acids (Willcox et al. 2004), fatty acids, phytosterols, saponins, tannins, sesquiterpene lactones, and polyalkenes (Ashok and Upadhyaya 2013).
In A. annua the essential oils consist of both volatile and nonvolatile components. The main volatile constituents of essential oils which have been identified include camphene, camphene hydrate, β-camphene, 1-camphor, alpha-pinene, β-pinene, isoartemisia ketone, artemisia ketone, artemisia alcohol, cuminal, germacrene D, 1,8-cineole, camphor, betacaryophyllene, and myrcene (WHO 2006; Ferreira and Janick 2009). The major nonvolatile ingredients include flavonoids, coumarins, sesquiterpenoids, proteins like β-glucosidase, β-galactosidase, and steroids such as β-sitosterol, and stigmasterol (WHO 2006; Cafferata et al. 2010; Brown 2010).
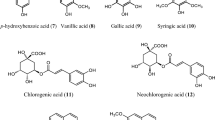
The phenolic constituents of A. annua consist of phenolic acids, coumarins, flavones, flavonols, and certain miscellaneous compounds. The phenolic acids include coumaric acid, chlorogenic acid, rosmarinic acid, and quinic acid. Coumarins reported in A. annua include coumarin, scopolin, aesculetin, scopoletin, and iso-fraxidin. Flavones identified in A. annua include luteolin-7-methyl ether, apigenin, acacetin, luteolin, chrysin, cynaroside, chrysoeriol, cirsilineol, cirsimaritin, cirsiliol, and eupatorin. A large number of flavonols have been identified in this plant which includes eupatin, casticin, quercimeritin, artemetin, chrysosplenol C, retusin, mikanin, syringetin, tetra-methoxyflavone, mearnsetin-glucoside, quercetin, kaempferol-6-methoxy glucoside, rhamnetin, chrysosplenol D, astragalin, axillarin, kaempferol, tamarixetin, myricetin, laricitrin, gossypetin-3,-dimethyl ether, mearnsetin, quercetin-3-methyl ether, quercetin-3-glucoside, rutin, isorhamnetin, chrysosplenetin, and isokaempferide (Shatar et al. 2003; Rajeswara Rao et al. 2014; Shahrajabian et al. 2020). The chemical structures of some of the major phenolic compounds found in A. annua have been shown in Fig. 2.2.
The majority of the research carried out on A. annua revolves around artemisinin and its derivatives like dihydroartemisinin, artemether, arteether, and artesunate due to their antimalarial potential, with less attention given to other chemical constituents like flavonoids. The qualitative profile of the A. annua plants as determined by Baraldi et al. (2008) has shown the presence of different flavonoids in leaves and flowers like artemetin, eupatin, chrysoplenetin, and casticin (Mesa et al. 2015). Among these flavonoids, casticin and chrysoplenetin exist in the form of an inseparable mixture, and their content is highest as compared to other flavonoids. Using HPLC and NMR analysis, chrysoplenol-D, camphor, 5-hydroxy-3,6,7-trimethoxy-2-(4′-methoxyphenyl)-4H-chromen-4-one), and 2,4-dihydroxy-6-methoxy-acetophenone have also been reported to be present in A. annua along with other flavonoids (Kontogianni et al. 2020). Flavonoids have been found to synergize the effect of artemisinin with luteolin showing both antimalarial and antioxidant properties (Ferreira et al. 2005).
Phytosterols are the secondary metabolites present in plants either in free form or can be found esterified with fatty acids or with phenolic acids, with glycosylated sterols also showing their presence in very minute quantities (Ostlund Jr 2007). The most prevalent phytosterols are stigmasterol, campesterol, and β-sitosterol (Fig. 2.3). Ergosterol, which is found in considerable amounts in algae, fungi, and lichens, shows very little or no presence in higher plants. Phytosterols have immunomodulatory and anti-inflammatory properties (Bouic 2002). The β-sitosterol, stigmasterol, campesterol, and ergosterol content per 100-gram dry weight of aerial parts of A. annua is about 119.570, 119.538, 17.528, and 0.79 mg, respectively (Ivanescu et al. 2013). The total sterol content in A. annua is around 250 mg per 100 g of dry matter.
Among all the phytochemicals present in this plant, sesquiterpene lactones are the most important. The most vital sesquiterpene lactone in this plant is the antimalarial compound artemisinin which is synthesized in the glandular secretory trichomes present in the aerial parts of the plant and characterized by the presence of an endoperoxide bridge as shown in Fig. 2.4 (Aftab et al. 2014; Salehi et al. 2018; Wani et al. 2021). The artemisinin content in the plant is usually low and lies in the range of 0.01–1.50% dry weight (Wani et al. 2021). The credit for the discovery of this lifesaving antimalarial compound goes to Prof. Tu Youyou, a pharmaceutical chemist and a malariologist who discovered it in 1972 by analyzing Chinese herb recipes having antimalarial activities under the project 523 (Su and Miller 2015). She discovered a proper method to isolate the active antimalarial ingredients (ethyl extract) and found that it could inhibit monkey and rodent malaria with 100% efficacy. After that, a clinical trial of the plant extracts was conducted on 21 patients, and a 95–100% success rate was achieved. For this discovery, Prof. Tu Youyou was awarded Nobel Prize in Physiology or Medicine in 2015 (Efferth et al. 2015). Artemisinin-based combination therapies have been endorsed by WHO for the treatment of uncomplicated malaria. These therapies consist of artemisinin or its derivatives in combination with a partner drug like mefloquine (WHO 2015). Various semisynthetic derivatives are also produced from artemisinin which include dihydroartemisinin, artemether, arteether, and artesunate which act as potent antimalarials (Fig. 2.4). Various other sesquiterpenes found in artemisinin include artemisinic acid, epoxyarteannuinic acid, artemisinol, and artemisinin isomers like artemisinin I, II, III, IV, and V (WHO 2006; Das 2012).
Chemical structure of artemisinin and its derivatives: The endoperoxide bridge (C – O – O – C) shown in red is common to artemisinin and its derivatives and acts as the key pharmacore. The modifications at C10 position are highlighted in pink, which are unique to each of these derivatives and determine their solubility and some of their pharmacokinetic properties
2.4 Pharmacological Activities
2.4.1 Antimalarial Activity
The most important constituent of A. annua responsible for its antimalarial properties is artemisinin. However, certain other chemical constituents from this plant have been reported to have a synergistic impact on its antimalarial potential. The importance of other chemical constituents of A. annua has also increased due to resistance shown by Plasmodium against monotherapy which has already emerged more than 25 years ago. Artemisinin is now prescribed in combination with certain other drugs in a practice called Artemisinin-based combination therapies (ACTs). A number of studies have shown the effectiveness of using the whole plant in the form of infusion, powder, or tablets against malaria (El Hadji Omar et al. 2013). This is further confirmed by a study in which a 40 mg dry weight of plant powder (containing nearly 600 μg Artemisinin) equivalent to 24 mg artemisinin/kg live body weight was effective in controlling the infection caused by Plasmodium chabaudi in rodents as compared to an equivalent dose of pure artemisinin (Elfawal et al. 2012). The increased efficacy of whole plant extracts is most probably due to the increased artemisinin bioavailability (40-fold) in the mice blood (Elfawal et al. 2012).
The effects of A. annua tea on malaria-causing P. falciparum has also been studied under in vitro conditions. The herbal tea extract from the plant was tested against chloroquine-sensitive D10 and chloroquine-resistant W2 strains of this parasite (De Donno et al. 2012). From the experimental results, it was found that herbal tea extract had a dose-dependent inhibitory effect on this parasite. Drug-free parasitized and unparasitized erythrocytes were used as blank and positive control. The impact of the herbal tea was three times greater than that of artemisinin alone which implies that there could be a synergistic impact of other constituents like flavonoids on its anti-plasmodial activity (Elford et al. 1987). The presence of other amphiphilic constituents like glucosides, flavonoids, or saponins may also improve its solubility in water. So, the presence of different active ingredients against malaria makes A. annua a natural artemisinin combination therapy.
Mouton et al. (2013) conducted an in vitro study to test the claims of synergism enhancing the antimalarial activity of artemisinin using non-polar extracts and tea infusions. Contrary to some earlier studies like De Donno et al. (2012), they reported that the IC50 of artemisinin in nonpolar extracts and tea infusions did not show any significant difference as compared to the IC50 of pure artemisinin. Pure artemisinin was used as a positive control. They also tested the tea infusions from A. afra against P. falciparum, which may be regarded as a negative control as A. afra contains negligible/does not contain artemisinin but shows the presence of other chemical constituents as reported in A. annua (Liu et al. 2010). The infusions of A. afra did not show any activity against the Plasmodium parasite (Mouton et al. 2013).
The aqueous extracts of A. annua and A. sieberi have also been found to have an inhibitory impact on β-hematin formation (under in vitro conditions), which is a synthetic analogue of hemozoin formed by Plasmodium parasites for its protection against excess ferriprotoporphyrin (IX) accumulation (Akkawi et al. 2014). In this study 2-mercaptopyrimidine (1 mg/mL) and chloroquine (0.1 mg/mL) were dissolved in ultrapure water, and both were used as positive controls, whereas ultrapure water was used as a negative control. They demonstrated that A. annua leaf infusions prepared in salt water (0.5 g salt/150 mL H2O) had greater efficacy in inhibiting β-hematin formation than those prepared in distilled water. However, these extracts had a decreased activity as time progressed so using dried leaf powder may be a better option in vivo.
The antimalarial action of hydroalcoholic and aqueous extracts of A. annua was studied in vitro against P. falciparum and in vivo in P. berghei NK 173-infected mice, with artemisinin (98%) used as a positive control for in vitro analysis, and 140 mg/kg/day for in vivo analysis (Zime-Diawara et al. 2015). Under in vitro conditions, the activity of the abovementioned extracts was similar to that of pure artemisinin at the same dosage. Moreover, the in vivo studies conducted on mice revealed that the aqueous extracts (artemisinin content of 20 mg/kg) have the same efficacy as compared to pure artemisinin (140 mg/kg dosage). Furthermore, the hydroalcoholic extract of A. annua (artemisinin content of 20 mg/kg) revealed the best results in comparison to aqueous extracts. The difference in the activity of aqueous and hydroalcoholic extracts is probably due to their composition. The comparison of TLC chromatograms of these two extracts revealed that hydro alcoholic extracts contain more sesquiterpenes and extra flavonoids as compared to aqueous extracts (Zime-Diawara et al. 2015). The increased activity of hydroalcoholic extracts against plasmodium might be potentiated by the presence of these extra flavonoids (Elford et al. 1987) and sesquiterpenes.
More recently a study was conducted using whole leaf extracts from artemisinin-producing homozygous chi1–1 (flavonoid lacking) and heterozygous chi1–1 (flavonoid containing) Artemis hybrids of A. annua (Czechowski et al. 2019). Moreover, the extracts from RNAi lines impaired in amorpha-4,11-diene synthase gene expression and cyp71av1–1 mutants, both of which are impaired in artemisinin biosynthesis were also tested. Based on their observations, they reported that flavonoids do not add to the anti-plasmodial activity beyond that provided by artemisinin under in vitro conditions.
However, the findings from different studies need to be checked by taking into account the time period for which the plant has been stored, the phase of the life cycle of the parasite tested, and some constituents of the tea infusion may become active after metabolization (Mouton et al. 2013). To detect the components, present in A. annua extracts which synergize the anti-plasmodial potential of artemisinin through regulation of its metabolism, a study was carried out by Cai et al. (2017). The extracts from dried plants were eluted using different concentrations of methanol (3%, 50%, and 85%). The pharmacokinetic profiles of artemisinin and monohydroxylated artemisinin (major phase 1 metabolite) were studied in rats after a single oral dose of artemisinin in each A. annua extract. Chief components isolated from the methanol extracts were assessed for their enzyme inhibition, and only arteannuin B was found to have a repressive effect on CYP3A4. In order to test the synergism between artemisinin and the other component, mice infected with P. yoelii were used, and the pharmacokinetic study was carried out. They reported synergism between artemisinin and arteannuin B which was related to increased artemisinin exposure due to enzyme inhibition (Cai et al. 2017).
2.4.2 Anticancer Activity
Various semisynthetic derivatives of artemisinin like artesunate (Fig. 2.4) have been found to have anticancer properties. Various studies (both in vitro and in vivo) have revealed that artemisinin-type drugs have anticancer activities (Efferth 2017). The anticancer properties of artemisinin and its various derivatives like artesunate, dihydroartemisinin, artemether, and arteether include:
-
Reactive oxygen species and nitric oxide-mediated oxidative stress.
-
DNA damage and repair.
-
Cell death by necrosis, apoptosis, ferroptosis, autophagy, oncosis, etc.
-
Inhibition of tumor-related signaling pathways like Wnt/β-catenin pathway; signal transducers like MYC/MAX, NF-κB, mTOR, AP-1, etc.; and angiogenesis.
The cytotoxicity of artemisinin and its different derivatives toward cancer cells has been described in a number of studies carried out in 1990s like Woerdenbag et al. (1993). Various studies have been conducted to test these claims, and vast pieces of evidence are suggesting that artemisinin-type compounds may inhibit tumor cells in vitro. Endoperoxide bridge, which is a characteristic feature of artemisinin has been found to be essential for the anticancer activity of these compounds, as artemisinin-type compounds without it have shown inactivity towards cancer inhibition (Beekman et al. 1998). The antitumor activity of these compounds has been confirmed by in vitro as well as in vivo studies (Efferth 2005; Seo et al. 2015; Subedi et al. 2016), with some studies involving human xenograft tumors transplanted on nude mice (Hou et al. 2008; Chen et al. 2009; Zhang et al. 2012; Tong et al. 2016). However, these athymic (lacking or deteriorated thymus) mice characterized by a lack of body hair have an inhibited immune system with reduced number of T cells. Keeping this disadvantage in mind, some researchers have also used syngeneic models in which rodent tumors are transplanted on rats or mice (Efferth 2017). The activity of artemisinin-type compounds against syngeneic and xenograft tumor models further validated the claims of anticancer activity of these compounds. The anticancer activity of these compounds has been even seen against orthopedically transplanted tumors.
The endoperoxide-bridge in artemisinin (Fig. 2.2) is important for its bioactivity as it produces ROS after its cleavage which results in oxidative stress. The generation of oxidative stress due to ROS by artemisinin-type drugs has been confirmed by a number of studies involving hematopoietic, epithelial, or mesenchymal cell lines from diverse tumor origins (Efferth and Oesch 2004; Du et al. 2010; Zhu et al. 2014; Gerhardt et al. 2015; Jia et al. 2016). The efficacy of ROS-mediated antitumor activity of such drugs was supported by many studies in which certain prooxidants (e.g., vitamin C) amplified artemisinin-mediated cytotoxicity and antioxidants (e.g. vitamin E) decreased the rate of tumor cell death (Efferth and Volm 2005; Noori et al. 2014; Papanikolaou et al. 2014; Beccafico et al. 2015; Lemke et al. 2016).
Artemisinin and its derivatives like artesunate have been reported to have a genotoxic effect as they induce breaks in DNA in a dose-dependent manner as observed by single-cell gel electrophoresis (Li et al. 2008). These findings were further supported by a dose-dependent buildup of γ-H2AX, a histone protein produced upon double-stranded DNA breaks (Li et al. 2008). Polymerase β-deficient cells, cells deficient in nonhomologous end joining (ku80 inactive) and homologous recombination (BRCA2 and XRCC2 inactive), have been found more sensitive to artesunate as compared to wild type cells as these cells lack the required DNA repair pathways (Li et al. 2008). So artesunate induces DNA breaks which makes it a therapeutic compound against cancer cells. A number of other studies have also revealed this anticancer activity of artesunate and other related compounds (Berdelle et al. 2011; Alcântara et al. 2013; Park et al. 2015).
ROS-mediated oxidative stress and DNA damage affect cell division, integrity, and replication which in turn causes cell cycle arrest (usually at G1 or G2 checkpoints) and ultimately cell death. Various artesunate type drugs have also been found to have this impact as observed in a number of studies (Hou et al. 2008; Li et al. 2009; Tin et al. 2012; Zhao et al. 2013; Tran et al. 2014; Lu et al. 2014; Islam and Mohammad 2020)). Other than cell cycle arrest, oxidative stress and DNA damage may also induce apoptosis as has been seen in cancer cells using artesunate, artemisinin, artemether, and arteether (Lai and Singh 1995; Disbrow et al. 2005; Hou et al. 2008; Zhang et al. 2016). Depending upon the cell model, artesunate can induce mitochondrial, intrinsic, or extrinsic FAS-receptor-mediated apoptosis (Efferth et al. 2007; Sieber et al. 2009) with enhanced Fas/CD95 expression, cessation of the mitochondrial membrane potential, release of cytochrome C, poly-(ADP-ribose) polymerase cleavage, and caspase 3/9 activation (Efferth 2017). Cells transfected with BCL2 gene (encodes a mitochondrial membrane protein that blocks programmed cell death) have been found to be more resistant to artesunate (Efferth et al. 2003). Various artemisinin-type drugs have also been found to induce caspase-independent non-apoptotic cell death-like autophagy (Zhou et al. 2013; Beccafico et al. 2015; Chen et al. 2015). Such drugs have also been found to induce other types of cell death-like necrosis, necroptosis, oncosis, anoikis, and iron-dependent ferroptosis (Du et al. 2010; Dixon et al. 2012; Zhou et al. 2013; Dixon et al. 2014; Zhu et al. 2021). Artemisinin derivatives have also been reported to have anti-angiogenic potential as tested in Zebrafish as reviewed by Wei and Liu (2017).
Artemisinin-related drugs have been shown to affect different signaling pathways in cancer cells as reviewed by Efferth (2017). These drugs were found to inhibit Wnt/β-catenin signaling, BCR/ABL signaling, as well as epidermal growth factor receptors. They also inactivated various transcription factors related to cancer like MYC/MYX, NF-κB, mTOR, CREB, etc. Artesunate has also been found to inhibit metastasis and invasion by targeting certain extracellular proteases (Rasheed et al. 2010). So artesunate may be used as an anti-invasive and anti-metastatic agent and can thus be added to the cancer treatment approaches.
2.4.3 Immunosuppressive Activity
Artemisia annua extracts obtained with hot water as mentioned in traditional Chinese medicine have been extensively used for treating different autoimmune diseases such as systemic lupus –erythematosus and rheumatoid arthritis (Zhao et al. 1998). In order to test the immunosuppressive activity of A. annua extracts, Zhang and Sun (2009) used the ethanol extracts of these plants on mice splenocyte proliferation in vitro and specific antibody and cellular immune responses in the ovalbumin-immunized mice. The immunized mice were given ethanol extracts of 0.25, 0.5, and 1.0 mg and cyclosporin A (positive drug) at a single dose of 0.1 mg in 0.2 mL of saline solution at intervals of 7 days. The ethanol extracts of these plants showed a significant inhibitory activity on splenocyte proliferation induced by lipopolysaccharides and concanavalin A under in vitro conditions. The plant extracts also showed inhibitory activity against concanavalin A, lipopolysaccharides, and ovalbumin-induced splenocyte proliferation in ovalbumin immunized mice. These extracts also reduced the ovalbumin-specific antibodies like IgG, IgG1, and IgG2b, with a significant reduction at 1.0 mg (Zhang and Sun 2009). These findings show that A. annua has immunosuppressive properties and could be used as immunosuppressants. The immunosuppressive effect of artemisinin has also been found to be effective against IgA nephropathy, an autoimmune kidney disease, with combination treatment of artemisinin and hydroxychloroquine giving better results as compared to their individual application in rats. The combination treatment decreased the deposition of complement 3 and IgA immune complexes (Bai et al. 2019).
2.4.4 Antimicrobial Activity
Artemisia annua is known to produce various secondary metabolites which have antimicrobial properties. The antimicrobial potential of artemisinin isolated from in vitro grown plantlets was tested by Appalasamy et al. (2014) against three gram-positive bacteria Bacillus thuringiensis, Bacillus subtilis, and Staphylococcus aureus; two gram-negative bacteria Salmonella sp. and Escherichia coli; and Candida albicans. They used streptomycin and acetonitrile as positive and negative control, respectively. They reported that artemisinin and a precursor extracted from the in vitro grown plantlets showed inhibitory activity against gram-positive and gram-negative bacteria but not against C. albicans. The antimicrobial action was found to be similar to that of streptomycin and the minimum inhibitory concentration was found to be 0.09 mg/mL (Appalasamy et al. 2014). The chloroform, alcohol, and water extracts of A. annua have been found to be effective in the treatment of acanthamoebiasis, caused by Acanthamoeba sp. (Derda et al. 2016). These extracts were tested against pathogenic Acanthamoeba castellanii (309 strain) and Acanthamoeba sp. (Ac32 strain). Based on the results, they found that the extracts have inhibitory activity against Acanthamoeba both in vitro and in vivo. These extracts also prolonged the survival of infected animals. These results indicate A. annua extracts could be used in treating acanthamoebiasis either singly or in combination with other antibiotics.
The extracts from A. annua and A. afra have been found to have strong bactericidal activity against Mycobacterium tuberculosis (Martini et al. 2020). The minimum inhibitory concentration for pure artemisinin was 75 μg/mL. Whereas for A. annua it was the extract from 4.81 mg of dried leaves per mL media, which resulted in 39 μg/mL of artemisinin. The bactericidal activity of these extracts is stronger than pure artemisinin which hints at a possible synergism between artemisinin and other compounds in eradicating M. tuberculosis. These extracts also show bacteriostatic activity against M. abscessus (a nontuberculous mycobacterium that usually affects patients who are immunocompromised) but were not bactericidal (Martini et al. 2020). These results give a strong indication that A. annua extracts could be used in combination with other antibiotics for the treatment of tuberculosis and prevention of M. abscessus infections.
The antimicrobial activity of methanol, water, ethanol, or acetone extracts of A. annua has also been reported against certain periodontopathic microbes like Prevotella intermedia, Fusobacterium nucleatum subsp. polymorphum, F. nucleatum subsp. animalis, and Aggregatibacter actinomycetemcomitans (Kim et al. 2015). Considering the high lipophilicity of A. annua essential oils, oil in water type Pickering A. annua essential oil nanoemulsions with 20 nm Stober silica nanoparticles as the stabilizing agent were tested on mature Candida biofilms (Das et al. 2020). These emulsions showed significantly higher activity as compared to ethanol extracts and Tween 80 stabilized emulsion. These emulsions resulted in peroxide and superoxide related oxidative stress which might be the reason for the antimicrobial activity of A. annua essential oil. The nanoliposome-incorporated essential oils from A. annua have shown inhibitory activity against Candida species with C. norvegensis showing the most susceptibility (Risaliti et al. 2020). The A. annua silver nanoparticles of various concentrations (10, 20, 30, and 50 μg/mL) were tested against four microbes Klebsiella pneumonia, S. aureus, E. coli, and B. subtilis, with levofloxacin (30 μg/disc) used as a positive control (Adoni et al. 2020). These nanoparticles were found to have free radicle scavenging activity and moderate activity against gram-positive and gram-negative bacteria as compared to standard antibiotics.
2.4.5 Anti-parasitic Activity
A number of compounds isolated from A. annua have been found to have activity against Leishmania species (intracellular protozoans) which are known to cause leishmaniasis. The n-hexane extracts of its seeds and leaves have been found to cause apoptosis in intracellular amastigotes of L. donovani (studied by ex vivo macrophage-amastigote model), and it did not show cytotoxic effects on mammalian macrophages (Islamuddin et al. 2012). When these extracts were tested on infected mice, a significant decrease in splenic and hepatic parasitic load, along with reduced spleen weight was observed. Moreover, the essential oils of A. annua leaves have shown significant antileishmanial activity against intracellular amastigotes of L. donovani under in vitro conditions (Islamuddin et al. 2014). It did not show any cytotoxicity on murine macrophages. The intraperitoneal administration at a dosage of 200 mg/kg body weight to infected BALB/c mice (immunodeficient inbred strain) decreased the parasitic load by around 90% in spleen and liver. As seen in earlier cases, no toxicity was observed, as supported by the normal levels of the serum enzymes. However, a complete cure of visceral leishmaniasis with A. annua n-hexane extracts or essential oils has not been observed in these in vivo studies. However, these in vivo studies did not show any complete cure for cutaneous leishmaniasis. Mesa et al. (2017) tested the antileishmanial activity of A. annua leaf powder containing gelatin capsules (totum). These capsules showed moderate activity against amastigotes of L. (Viannia) panamensis, and no cytotoxicity or genotoxicity was reported in macrophages (U-937) and human lymphocytes in vitro. The infected hamsters given A. annua capsules (500 mg/kg/day) for 30 days were cured, with 83.3% success rate. The administration of these capsules (30 g) to two patients with uncomplicated cutaneous leishmaniasis also gave positive results (Mesa et al. 2017).
A study was conducted on Neospora canum, a protozoal parasite that infects a variety of mammals and causes abortion in cattle (MR 2009; Das 2012). N. caninum tachyzoites were made to infect the cultured host cells (Vero cells or mouse peritoneal macrophages) and then supplemented with artemisinin at 20, 10, 1, 0.1, and 0.01 μg/mL. At 10 or 20 μg/mL artemisinin for 11 days, all microscopic foci of N. caninum were eradicated. The same result was obtained when artemisinin was administered at 1 μg/mL for 14 days. Artemether has been reported to be effective against the larval stages of Schistosoma mansoni as seen in hamsters and mice. If these organisms are administered with artemether during the first month of infection, then schistosomiasis (bilharzia) does not develop (Das 2012).
2.4.6 Anti-SARS-CoV-2 Activity
The global pandemic of Covid-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused mayhem in the whole world, and the death toll has crossed the five million mark in November 2021 as per the data provided by John Hopkins University (https://www.bbc.com/news/world-59119731). Despite the intense efforts to distribute various registered vaccines against Covid-19 like Moderna, Pfizer/BioNTech, and J & J vaccines, except Remdesivir no other orally deliverable drug is available currently. The emergence of a new variant B.1.1.529 on 26 November 2021, named omicron by WHO may also pose a serious challenge in controlling its transmissibility (https://www.who.int/news/item/28-11-2021-update-on-omicron). Artemisinin-type compounds have been reported to have antiviral activity (Efferth 2018). The extracts from A. annua plants have shown activity against SARS-CoV-1 which is responsible for the SARS outbreak of 2002–2004 (Li et al. 2005). These observations suggest that the extracts of A. annua could be effective against SARS-CoV-2. Artemisinins and the extracts from A. annua decrease inflammatory cytokines like IL-6 and TNF-α in vivo (Shi et al. 2015; Hunt et al. 2015). These effector molecules can be tricky during the “cytokine storm” suffered by many patients suffering from SARS-CoV-2 (Schett et al. 2020). Fibrosis has also been reported in persons affected by SARS-CoV-2 which can cause damage to different organs, and artemisinin has been found to dampen fibrosis (Larson et al. 2019; Lechowicz et al. 2020). Recently, a study conducted by Cao et al. (2020) found that a number of compounds related to artemisinin act against SARS-CoV-2, with Arteannuin B showing the highest activity against SARS-CoV-2; dihydroartemisinin and artesunate also showed activity against it. These results indicate the potential of A. annua extracts, particularly artemisinin in the treatment of Covid-19, which needs further analysis and research for the development of suitable drugs.
2.5 Clinical Studies Involving Artemisinin-Related Compounds in the Treatment of Malaria, Schistosomiasis, Leishmaniasis, and Certain Cancers
Based on the multiple research results of in vitro and in vivo studies, it seems justified to say that the antimalarial activity of A. annua is not solely due to artemisinin; it seems its activity is potentiated by the presence of other constituents in the plant as well (Fouda 2010; De Donno et al. 2012). In a clinical trial conducted by Mueller et al. (2000), five patients suffering from malaria were treated with A. annua tea, and a rapid reduction of parasitemia was observed in these patients. They conducted another trial with a larger group of 48 malaria patients and 92% of patients showed disappearance of parasitemia within 4 days. These results justify further research for the elucidation of antimalarial properties of A. annua preparations.
Newton et al. (2003) conducted a clinical trial in Thailand for 113 clinically severe malaria patients infected by P. falciparum. They reported that artesunate has a rate of mortality (12%) as compared to quinine (22%). During the trial, only a few patients (12%) treated with artesunate became hypoglycemic than quinine-administered patients (28%).
An in vivo study was conducted in Katanga province in the Democratic Republic of Congo (Tchandema and Lutgen 2016). Powdered leaf capsules of A. annua from two different geographical locations (Luxembourg and from Burundi) and A. afra were fed to patients in doses of 15 g, 7.5 g, and 7.5 g, respectively. Despite relatively low doses, all the patients were fever free within 2 days. Around 85%, 76%, and 40% of patients were free from parasites after 7 days which were fed with capsules of A. annua from Luxembourg, Burundi, and A. afra, respectively (Tchandema and Lutgen 2016).
As compressed leaf tablets of A. annua have shown antimalarial potential as seen in rodents, a study was carried out by Daddy et al. (2017) to test the efficacy of dried leaf A. annua in patients suffering from severe malaria and non-responsive to artemisinin combination therapy and i.v. artesunate. These patients were administered twice daily with 0.5 g dried leaf A. annua per os for 5 days, and the total delivered dose of artemisinin was 55 mg. All these patients who were resistant to artemisinin combination therapy were cured by these compressed leaf tablets.
Schistosomiasis and malaria coinfection has been commonly reported (Moriyasu et al. 2018). In 2015, a clinical trial was conducted to test the tea infusions of A. annua and A. afra in the treatment of schistosomiasis as compared to praziquantel which is the currently accepted drug for the treatment of Schistosomiasis (Munyangi et al. 2018). The patients who were given Artemisia infusions were cleared of S. mansoni eggs quickly (14 days) as checked in their fecal smears. The patients who were given praziquantel took more time for the complete eradication of S. mansoni eggs and experienced more adverse effects as compared to Artemisia-treated patients (Munyangi et al. 2018). These results received a published critique from Argemi et al. (2019) to which a rebuttal was then published by Cornet-Vernet et al. (2019).
As discussed in Sect. 2.4.5 above, Mesa et al. (2017) tested the antileishmanial activity of A. annua leaf powder containing gelatin capsules which gave encouraging results in vitro and in vivo (hamster) systems. Looking at these results, they also treated human males with powdered A. annua capsules containing about 0.1% artemisinin. Both of these patients were administered a total A. annua of 30 g per patient over 20 days. At the end of the trial, the ulcers had shrunk by 20–35%, and complete closure of the ulcers was reported around 45 days after the treatment ended. During the course of this whole trial, no negative effects were reported in these patients, indicating the safety of using A. annua leaf powder in the treatment of leishmaniasis (Mesa et al. 2017). But before that large human trials need to be conducted to fully validate these results.
As artemisinin and its derivatives like dihydroartemisinin and artesunate have shown anticancer activities under in vitro and in vivo conditions as discussed above in Sect. 2.4.2. Various clinical trials involving artemisinin and its derivatives have also been conducted to test the efficacy of these compounds in cancer treatment. A clinical trial was conducted involving cisplatin and vinorelbine with or without artesunate injections of 120 mg for a period of 8 days for the treatment of advanced non-small cell lung cancer (Zhang et al. 2008). The control rate of the trial group was 88.2% which was higher as compared to the control rate of 72.7% in the control group. The progression time of the artesunate-treated patients was 24 weeks as compared to 20 weeks for the control group. The longer time to progression of patients treated with artesunate shows the significance of artesunate in the treatment. Moreover, the patients administered with artesunate did not show any increased toxicity (Zhang et al. 2008).
In St. Georg’s (University of London, London, UK), Krishna et al. (2015) conducted a randomized, double-blind, placebo-controlled clinical trial of oral artesunate therapy for colorectal cancer. Before the surgery, 12 patients were given 200 mg artesunate orally for 14 days, and 11 patients were given placebo. Out of these patients, 9 artesunate and 11 placebo administered patients completed the trial. The results showed that apoptotic cell fractions of more than 7% were observed in 67% of artesunate-administered patients as compared to only 55% of placebo-treated patients. The incidence of refractory tumors was also more in case placebo administered patients (6 in number) as compared to artesunate administered patients (1 only). The results of this study were promising, and it may be concluded that artesunate shows good anticancer activity and requires further studies so that its anticancer potential may be fully explored.
2.6 Safety and Toxicity
A. annua is botanically accepted as a safe plant. It is listed in the Handbook of Phytochemical Constituents of GRAS (Generally Regarded As Safe) herbs and other economic plants by James A. Duke and even has listed dosages of 30 g of dry leaf per day which is much higher than the daily dosage prescribed for malaria treatment (Duke 1992).
The dried leaves of A. annua are widely proposed to be used as a medicinal herb malarial treatment. For treatment of malaria and certain other diseases, it has been used in a number of ways like oral administration of capsules of dried leaf powder, compressed dried leaf tablets, and tea infusion. The encapsulation of dried leaves has not shown any significant decrease in the absorption of artemisinin or flavonoids present in it but a few food items such as peanut butter may decrease its absorption (Desrosiers and Weathers 2016). The plant is generally intended for consumption by adults and children that are infected with malaria.
A study was conducted by Abolaji et al. (2013) to evaluate the effect of ethanol extracts of A. annua in pregnant Wistar rats which were given 100, 200, and 300 mg/kg body weights of ethanol extracts of A. annua leaves. Based on the biochemical and hematological studies, they reported that the extracts did not result in hematotoxicity, hepatotoxicity, and hyperlipidemia. However, at 300 mg/kg dose, 31% malformed fetuses and 21% nonviable fetuses were observed. Embryotoxicity has also been reported in rats due to consumption of certain artemisinin derivatives like dihydroartemisinin, artemether, arteether, and artesunate during the organogenesis period, with extended oral dosage (12 days or more) resulting in embryotoxicity in monkeys as well (Clark 2009). These results raise concerns about the usage of these compounds and plant extracts for treatment during the sensitive period of pregnancy in women. These results indicate that although the consumption of leaf extracts may not cause hematotoxicity, hepatotoxicity, and hyperlipidemia, care should be taken during pregnancy due to the possible risk of embryotoxicity due to its consumption beyond the therapeutic dose (Abolaji et al. 2013).
In another study carried out on male Wistar rats, it was found that these extracts may serve as antidiabetic agents, and do not cause hematotoxicity, hepatotoxicity, and testicular toxicity; however, there may be a possible risk of atherosclerosis due to cholesterol buildup as observed in this study (Eteng et al. 2013). So, persons suffering from atherosclerosis should take immense care while using the extracts of A. annua.
The genus Artemisia has a GRAS status with the United States Food and Drug Administration (FDA), as long as the final product is thujone-free. Thujone is a monoterpene found in certain Artemisia species, e.g., A. absinthium (Fig. 2.5). At high dosages, thujone acts as a neurotoxin that may cause unconsciousness, convulsions, and death in humans (Cobb 1922). However, A. annua has been found to be thujone free and therefore the utilization of A. annua is generally considered safe (Tzenkova et al. 2010).
Based on the studies conducted till date, there is not much risk in consuming dried A. annua either as a supplement or as part of any other treatment with the exception of the possible presence of heavy metals at concentrations higher than recommended by the FDA. Furthermore, immense care should be taken during its consumption by pregnant women and atherosclerosis patients, and it should not be taken above the therapeutic doses. The issue of excess heavy metal accumulation can be solved by growing these plants in soil and water that does not contain enough of these metals.
2.7 Conclusion
A. annua is an ethnomedicinally important plant, with its medicinal use well established in Chinese pharmacopoeias. It has also obtained a vital place among plant-based advanced therapeutics, particularly against malaria, for which it has undoubtedly become a good hope for treatment. It is a rich source of numerous biologically active constituents, particularly artemisinin. It has the characteristic therapeutic potential against malaria, and besides being antimalarial, it has various other biological activities such as antibacterial, antitumor, and immunosuppressive activities. Nowadays, a lot of research is going on to investigate its anti-cancer and anti-viral activities, particularly against SARS-CoV-2. The elucidation of the mechanism of action of artemisinin, its various derivatives, and different flavonoids against various diseases has become the major area of interest among researchers. The extremely low toxicity associated with A. annua is another reason for its wide usage against malaria and certain other diseases. Therefore, A. annua is a great option that can be extensively explored for the development of new drugs.
References
Abolaji AO, Eteng MU, Ebong PE, Brisibe EA, Dar A, Kabir N, Choudhary MI (2013) A safety assessment of the antimalarial herb Artemisia annua during pregnancy in Wistar rats. Phytother Res 27(5):647–654
Adoni M, Yadam M, Gaddam SA, Rayalacheruvu U, Kotakadi VS (2020) Antimicrobial, antioxidant, and dye degradation properties of biosynthesized silver nanoparticles from Artemisia annua L. Lett Appl NanoBioSci 10(1):1981–1992
Aftab T, Ferreira JF, Khan MMA, Naeem M (eds) (2014) Artemisia annua-pharmacology and biotechnology. Springer, Berlin
Akkawi M, Jaber S, Abu-Remeleh Q, Engeu OP, Lutgen P (2014) Investigations of Artemisia annua and Artemisia sieberi water extracts inhibitory effects on β-hematin formation. Med Aromat Plants 3(150):2167-0412
Alcântara DDFÁ, Ribeiro HF, Cardoso PCDS, Araújo TMT, Burbano RR, Guimarães AC, de Oliveira Bahia M (2013) In vitro evaluation of the cytotoxic and genotoxic effects of artemether, an antimalarial drug, in a gastric cancer cell line (PG100). J Appl Toxicol 33(2):151–156
Alesaeidi S, Miraj S (2016) A systematic review of anti-malarial properties, immunosuppressive properties, anti-inflammatory properties, and anti-cancer properties of Artemisia annua. Electron Physician 8(10):3150
Appalasamy S, Lo KY, Ch’ng SJ, Nornadia K, Othman AS, Chan LK (2014) Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of Artemisia annua L. BioMed Res Int
Argemi X, Hansmann Y, Gaudart J, Gillibert A, Caumes E, Jauréguiberry S, Meyer N (2019) Comment on “Effect of Artemisia annua and Artemisia afra tea infusions on schistosomiasis in a large clinical trial”. Phytomedicine 62:152804
Ashok PK, Upadhyaya K (2013) Preliminary phytochemical screening and physico-chemical parameters of Artemisia absinthium and Artemisia annua. J Pharmacogn Phytochem 1(6):229
Bai L, Li H, Li J, Song J, Zhou Y, Liu B, Zhou J (2019) Immunosuppressive effect of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via regulating the differentiation of CD4+ T cell subsets in rats. Int Immunopharmacol 70:313–323
Baraldi R, Isacchi B, Predieri S, Marconi G, Vincieri FF, Bilia AR (2008) Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochem Syst Ecol 36(5–6):340–348
Beccafico S, Morozzi G, Marchetti MC, Riccardi C, Sidoni A, Donato R, Sorci G (2015) Artesunate induces ROS-and p38 MAPK-mediated apoptosis and counteracts tumor growth in vivo in embryonal rhabdomyosarcoma cells. Carcinogenesis 36(9):1071–1083
Beekman AC, Wierenga PK, Woerdenbag HJ, Van Uden W, Pras N, Konings AW, Wikström HV (1998) Artemisinin-derived sesquiterpene lactones as potential antitumour compounds: cytotoxic action against bone marrow and tumor cells. Planta Med 64(07):615–619
Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B (2011) Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol Cancer Ther 10(12):2224–2233
Bouic PJ (2002) Sterols and sterolins: new drugs for the immune system? Drug Discov Today 7(14):775–778
Brown GD (2010) The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules 15(11):7603–7698
Cafferata LF, Gatti WO, Mijailosky S (2010) Secondary gaseous metabolites analyses of wild Artemisia annua L. Mol Med Chem 21:48–52
Cai TY, Zhang YR, Ji JB, Xing J (2017) Investigation of the component in Artemisia annua L. leading to enhanced antiplasmodial potency of artemisinin via regulation of its metabolism. J Ethnopharmacol 207:86–91
Cao R, Hu H, Li Y, Wang X, Xu M, Liu J, Zhang H, Yan Y, Zhao L, Li W, Zhang T (2020) Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect Dis 6:2524–2531
Chen H, Sun B, Pan S, Jiang H, Sun X (2009) Dihydroartemisinin inhibits growth of pancreatic cancer cells in vitro and in vivo. Anti-Cancer Drugs 20(2):131–140
Chen SS, Hu W, Wang Z, Lou XE, Zhou HJ (2015) p8 attenuates the apoptosis induced by dihydroartemisinin in cancer cells through promoting autophagy. Cancer Biol Ther 16(5):770–779
Clark RL (2009) Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester. Reprod Toxicol 28(3):285–296
Cobb S (1922) A case of epilepsy with a general discussion of the pathology. Med Clin N Am 5:1403–1420
Cornet-Vernet L, Munyangi J, Chen L, Towler M, Weathers P (2019) Response to Argemi et al. (2019). Phytomedicine 62:152943
Czechowski T, Rinaldi MA, Famodimu MT, Van Veelen M, Larson TR, Winzer T, Graham IA (2019) Flavonoid versus artemisinin anti-malarial activity in Artemisia annua whole-leaf extracts. Front Plant Sci 10:984
Daddy NB, Kalisya LM, Bagire PG, Watt RL, Towler MJ, Weathers PJ (2017) Artemisia annua dried leaf tablets treated malaria resistant to ACT and iv artesunate. Phytomedicine 32:37–40
Das S (2012) Artemisia annua (Qinghao): a pharmacological review. Int J Pharm Sci Res 3(12):4573–4577
Das S, Vörös-Horváth B, Bencsik T, Micalizzi G, Mondello L, Horváth G, Széchenyi A (2020) Antimicrobial activity of different Artemisia essential oil formulations. Molecules 25(10):2390
De Donno A, Grassi T, Idolo A, Guido M, Papadia P, Caccioppola A, Fanizzi FP (2012) First-time comparison of the in vitro antimalarial activity of Artemisia annua herbal tea and artemisinin. Trans R Soc Trop Med Hyg 106(11):696–700
Derda M, Hadaś E, Cholewiński M, Skrzypczak Ł, Grzondziel A, Wojtkowiak-Giera A (2016) Artemisia annua L. as a plant with potential use in the treatment of acanthamoebiasis. Parasitol Res 115(4):1635–1639
Desrosiers MR, Weathers PJ (2016) Effect of leaf digestion and artemisinin solubility for use in oral consumption of dried Artemisia annua leaves to treat malaria. J Ethnopharmacol 190:313–318. https://doi.org/10.1016/j.jep.2016.06.041
Disbrow GL, Baege AC, Kierpiec KA, Yuan H, Centeno JA, Thibodeaux CA, Schlegel R (2005) Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res 65(23):10854–10861
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Stockwell BR (2014) Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3:e02523
Du JH, Zhang HD, Ma ZJ, Ji KM (2010) Artesunate induces oncosis-like cell death in vitro and has antitumor activity against pancreatic cancer xenografts in vivo. Cancer Chemother Pharmacol 65(5):895–902
Duke JA (1992) Handbook of phytochemical constituent grass, herbs and other economic plants. CRC press, London
Efferth T (2005) Mechanistic perspectives for 1, 2, 4-trioxanes in anti-cancer therapy. Drug Resist Updat 8(1–2):85–97
Efferth T (2017) From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. In: Seminars in cancer biology, vol 46. Academic Press, London, pp 65–83
Efferth T (2018) Beyond malaria: the inhibition of viruses by artemisinin-type compounds. Biotechnol Adv 36:1730–1737
Efferth T, Oesch F (2004) Oxidative stress response of tumor cells: microarray-based comparison between artemisinins and anthracyclines. Biochem Pharmacol 68(1):3–10
Efferth T, Volm M (2005) Glutathione-related enzymes contribute to resistance of tumor cells and low toxicity in normal organs to artesunate. In Vivo 19(1):225–232
Efferth T, Briehl MM, Tome ME (2003) Role of antioxidant genes for the activity of artesunate against tumor cells. Int J Oncol 23(4):1231–1235
Efferth T, Giaisi M, Merling A, Krammer PH, Li-Weber M (2007) Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS One 2(8):e693
Efferth T, Zacchino S, Georgiev MI, Liu L, Wagner H, Panossian A (2015) Nobel Prize for artemisinin brings phytotherapy into the spotlight. Phytomedicine 22(13):A1–A3
Ekiert H, Świątkowska J, Klin P, Rzepiela A, Szopa A (2021) Artemisia annua–importance in traditional medicine and current state of knowledge on the chemistry, biological activity and possible applications. Planta Med 87(8):584–599
El Hadji Omar GP, Mouhamadou D, Bineta DA, Sadikh BA, Mare DD, Ambroise A, Ousmane S (2013) Tea Artemisia annua inhibits Plasmodium falciparum isolates collected in Pikine, Senegal. Afr J Biochem Res 7(7):107–112
Elfawal MA, Towler MJ, Reich NG, Golenbock D, Weathers PJ, Rich SM (2012) Dried whole plant Artemisia annua as an antimalarial therapy. PLoS One 7(12):e52746
Elford BC, Roberts MF, Phillipson JD, Wilson RJ (1987) Potentiation of the antimalarial activity of qinghaosu by methoxylated flavones. Trans R Soc Trop Med Hyg 81(3):434–436
Eteng MU, Abolaji AO, Ebong PE, Brisibe EA, Dar A, Kabir N, Iqbal Choudhary M (2013) Biochemical and haematological evaluation of repeated dose exposure of male Wistar rats to an ethanolic extract of Artemisia annua. Phytother Res 27(4):602–609
Ferreira JF, Janick J (1996) Distribution of artemisinin in Artemisia annua. In: Janick J (ed) Progress in new crops. ASHS Press, Arlington, VA, pp 579–584
Ferreira J, Janick J (2009) Annual wormwood (Artemisia annua L.). New crop FactSHEET. Purdue University
Ferreira JF, Laughlin JC, Delabays N, de Magalhães PM (2005) Cultivation and genetics of Artemisia annua L. for increased production of the antimalarial artemisinin. Plant Genet Resour 3(2):206–229
Ferreira JFS, Luthria DL, Sasaki T, Heyerick A (2010) Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 15:3135–3170
Fornara F (2014) The molecular genetics of floral transition and flower development. Elsevier, London
Fouda E (2010) Etude clinique sur l’efficacité thérapeutique de l’Artemisia annua sur l’accès palustre simple. District de santé de la Cité Verte, Yaoundé, Personal Communication
Gerhardt T, Jones R, Park J, Lu R, Chan HW, Fang Q, Lai H (2015) Effects of antioxidants and pro-oxidants on cytotoxicity of dihydroartemisinin to Molt-4 human leukemia cells. Anticancer Res 35(4):1867–1871
Hou J, Wang D, Zhang R, Wang H (2008) Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res 14(17):5519–5530
Hsu E (2006) The history of qing hao in the Chinese materia medica. Trans R Soc Trop Med Hyg 100:505–508
Hsu E (2009) Diverse biologies and experiential continuities: did the ancient Chinese know that qinghao had anti-malarial properties? Can Bull Med Hist 26:203–213
Hunt S, Yoshida M, Davis CE, Greenhill NS, Davis PF (2015) An extract of the medicinal plant Artemisia annua modulates production of inflammatory markers in activated neutrophils. J Inflamm Res 8:9–14
Hwang DI, Won KJ, Kim DY, Yoon SW, Park JH, Kim B, Lee HM (2016) Anti-adipocyte differentiation activity and chemical composition of essential oil from Artemisia annua. Nat Prod Commun 11(4):539–542
Islam S, Mohammad F (2020) Triacontanol as a dynamic growth regulator for plants under diverse environmental conditions. Physiol Mol Biol Plants 26(5):871–883
Islamuddin M, Farooque A, Dwarakanath BS, Sahal D, Afrin F (2012) Extracts of Artemisia annua leaves and seeds mediate programmed cell death in Leishmania donovani. J Med Microbiol 61(12):1709–1718
Islamuddin M, Chouhan G, Want MY, Tyagi M, Abdin MZ, Sahal D, Afrin F (2014) Leishmanicidal activities of Artemisia annua leaf essential oil against visceral Leishmaniasis. Front Microbiol 5:626
Ivanescu B, Vlase L, Corciova A (2013, November) Importance of phytosterols and their determination in herbal medicines. In: In 2013 E-health and bioengineering conference (EHB). IEEE, pp 1–4
Jia J, Qin Y, Zhang L, Guo C, Wang Y, Yue X, Qian J (2016) Artemisinin inhibits gallbladder cancer cell lines through triggering cell cycle arrest and apoptosis. Mol Med Rep 13(5):4461–4468
Kim WS, Choi WJ, Lee S, Kim WJ, Lee DC, Sohn UD et al (2015) Anti-inflammatory, antioxidant and antimicrobial effects of artemisinin extracts from Artemisia annua L. Korean J Physiol Pharmacol 19(1):21
Kontogianni VG, Primikyri A, Sakka M, Gerothanassis IP (2020) Simultaneous determination of artemisinin and its analogs and flavonoids in Artemisia annua crude extracts with the use of NMR spectroscopy. Magn Reson Chem 58(3):232–244
Krishna S, Ganapathi S, Ster IC, Saeed ME, Cowan M, Finlayson C, Kumar D (2015) A randomised, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine 2(1):82–90
Lai H, Singh NP (1995) Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett 91(1):41–46
Larson SA, Dolivo DM, Dominko T (2019) Artesunate inhibits myofibroblast formation via induction of apoptosis and antagonism of pro-fibrotic gene expression in human dermal fibroblasts. Cell Biol Int 43(11):1317–1322
Lechowicz K, Drożdżal S, Machaj F, Rosik J, Szostak B, Zegan-Barańska M, Biernawska J, Dabrowski W, Rotter I, Kotfis K (2020) COVID-19: the potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J Clin Med 9(6):1917
Lemke D, Pledl HW, Zorn M, Jugold M (2016) Slowing down glioblastoma progression in mice by running or the anti-malarial drug dihydroartemisinin? Induction of oxidative stress in murine glioblastoma therapy. Oncotarget 7(35):56713
Li SY, Chen C, Zhang HQ, Guo HY, Wang H, Wang L, Zhang X, Hua SN, Yu J, Xiao PG, Li RS (2005) Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir Res 67(1):18–23
Li PC, Lam E, Roos WP, Zdzienicka MZ, Kaina B, Efferth T (2008) Artesunate derived from traditional Chinese medicine induces DNA damage and repair. Cancer Res 68(11):4347–4351
Li S, Xue F, Cheng Z, Yang X, Wang S, Geng F, Pan L (2009) Effect of artesunate on inhibiting proliferation and inducing apoptosis of SP2/0 myeloma cells through affecting NFκB p65. Int J Hematol 90(4):513–521
Liu NQ, Cao M, Frédérich M, Choi YH, Verpoorte R, van der Kooy F (2010) Metabolomic investigation of the ethnopharmacological use of Artemisia afra with NMR spectroscopy and multivariate data analysis. J Ethnopharmacol 128(1):230–235
Liu H, Tian X, Zhang Y, Wang C, Jiang H (2013) The discovery of Artemisia annua L. in the Shengjindian cemetery, Xinjiang, China and its implications for early uses of traditional Chinese herbal medicine qinghao. J Ethnopharmacol 146(1):278–286
Lu M, Sun L, Zhou J, Yang J (2014) Dihydroartemisinin induces apoptosis in colorectal cancer cells through the mitochondria-dependent pathway. Tumor Biol 35(6):5307–5314
Marinas IC, Oprea E, Chifiriuc MC, Badea IA, Buleandra M, Lazar V (2015) Chemical composition and antipathogenic activity of Artemisia annua essential oil from Romania. Chem Biodivers 12(10):1554–1564
Martini MC, Zhang T, Williams JT, Abramovitch RB, Weathers PJ, Shell SS (2020) Artemisia annua and Artemisia afra extracts exhibit strong bactericidal activity against Mycobacterium tuberculosis. J Ethnopharmacol 262:113191
Mesa LE, Lutgen P, Velez ID, Segura AM, Robledo SM (2015) Artemisia annua L., potential source of molecules with pharmacological activity in human diseases. Am J Phytomed Clin Ther 3(5):436–450
Mesa LE, Vasquez D, Lutgen P, Vélez ID, Restrepo AM, Ortiz I, Robledo SM (2017) In vitro and in vivo antileishmanial activity of Artemisia annua L. leaf powder and its potential usefulness in the treatment of uncomplicated cutaneous leishmaniasis in humans. Rev Soc Bras Med Trop 50:52–60
Moriyasu T, Nakamura R, Deloer S, Senba M, Kubo M, Inoue M, Hamano S (2018) Schistosoma mansoni infection suppresses the growth of Plasmodium yoelii parasites in the liver and reduces gametocyte infectivity to mosquitoes. PLoS Negl Trop Dis 12(1):e0006197
Mouton J, Jansen O, Frédérich M, van der Kooy F (2013) Is artemisinin the only antiplasmodial compound in the Artemisia annua tea infusion? An in vitro study. Planta Med 79(06):468–470
MR VR (2009) Chemical composition and antimicrobial activity of the essential oil of Artemisia annua L. from Iran. Pharm Res 1(1)
Mueller MS, Karhagomba IB, Hirt HM, Wemakor E (2000) The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: agricultural, chemical and clinical aspects. J Ethnopharmacol 73(3):487–493
Munyangi J, Cornet-Vernet L, Idumbo M, Lu C, Lutgen P, Perronne C, Weathers P (2018) Effect of Artemisia annua and Artemisia afra tea infusions on schistosomiasis in a large clinical trial. Phytomedicine 51:233
Newton PN, Angus BJ, Chierakul W, Dondorp A, Ruangveerayuth R, Silamut K, White NJ (2003) Randomized comparison of artesunate and quinine in the treatment of severe falciparum malaria. Clin Infect Dis 37(1):7–16
Nigam M, Atanassova M, Mishra AP, Pezzani R, Devkota HP, Plygun S, Sharifi-Rad J (2019) Bioactive compounds and health benefits of Artemisia species. Nat Prod Commun 14(7). https://doi.org/10.1177/1934578X19850354
Noori S, Hassan ZM, Farsam V (2014) Artemisinin as a Chinese medicine, selectively induces apoptosis in pancreatic tumor cell line. Chin J Integr Med 20(8):618–623
Ostlund RE Jr (2007) Phytosterols, cholesterol absorption and healthy diets. Lipids 42(1):41–45
Papanikolaou X, Johnson S, Garg T, Tian E, Tytarenko R, Zhang Q, Heuck C (2014) Artesunate overcomes drug resistance in multiple myeloma by inducing mitochondrial stress and non-caspase apoptosis. Oncotarget 5(12):4118
Park J, Lai HC, Sasaki T, Singh NP (2015) DNA damage in dihydroartemisinin-resistant Molt-4 cells. Anticancer Res 35(3):1339–1343
Perez Gutierrez RM, Baez EG (2009) Cardioactive agents from plants. Mini Rev Med Chem 9:878–899. https://doi.org/10.2174/138955709788452612
Rajeswara Rao BR, Syamasundar KV, Patel RP (2014) Effect of method of distillation on the yield and chemical composition of Artemisia annua essential oil. J Essent Oil Res 26(6):486–491
Rasheed SAK, Efferth T, Asangani IA, Allgayer H (2010) First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int J Cancer 127(6):1475–1485
Riddle J (2010) Goddesses, elixirs, and witches: plants and sexuality throughout human history. Elsevier Inc., New York
Risaliti L, Pini G, Ascrizzi R, Donato R, Sacco C, Bergonzi MC, Bilia AR (2020) Artemisia annua essential oil extraction, characterization, and incorporation in nanoliposomes, smart drug delivery systems against Candida species. J Drug Delivery Sci Technol 59:101849
Sadiq A, Hayat MQ, Ashraf M (2014) Ethnopharmacology of Artemisia annua L.: A review. Artemisia Annua Pharmacol Biotechnol 9–25
Salehi M, Karimzadeh G, Naghavi MR, Badi HN, Monfared SR (2018) Expression of key genes affecting artemisinin content in five Artemisia species. Sci Rep 8(1):1–11
Schett G, Sticherling M, Neurath MF (2020) COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol 20(5):271–272
Seo EJ, Wiench B, Hamm R, Paulsen M, Zu Y, Fu Y, Efferth T (2015) Cytotoxicity of natural products and derivatives toward MCF-7 cell monolayers and cancer stem-like mammospheres. Phytomedicine 22(4):438–443
Shahrajabian MH, Wenli SUN, Cheng Q (2020) Exploring Artemisia annua L., artemisinin and its derivatives, from traditional Chinese wonder medicinal science. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 48(4):1719–1741
Shatar S, Dung NX, Karashawa D (2003) Essential oil composition of some Mongolian Artemisia species. Journal of Essential Oil Bearing Plants 6(3):203–206
Shi C, Li H, Yang Y, Hou L (2015) Anti-inflammatory and immunoregulatory functions of artemisinin and its derivatives. Mediat Inflamm 2015:435713
Sieber S, Gdynia G, Roth W, Bonavida B, Efferth T (2009) Combination treatment of malignant B cells using the anti-CD20 antibody rituximab and the anti-malarial artesunate. Int J Oncol 35(1):149–158
Smith RIL, Richardson M (2011) Fuegian plants in Antarctica: natural or anthropogenically assisted immigrants? Biol Invasions 13(1):1–5
Stix E (1960) Pollenmorphologische untersuchungen an Compositen. Grana 2(2):41–104
Su XZ, Miller LH (2015) The discovery of artemisinin and the Nobel prize in physiology or medicine. Sci China Life Sci 58:1175–1179
Subedi A, Futamura Y, Nishi M, Ryo A, Watanabe N, Osada H (2016) High-throughput screening identifies artesunate as selective inhibitor of cancer stemness: involvement of mitochondrial metabolism. Biochem Biophys Res Commun 477(4):737–742
Tchandema CK, Lutgen P (2016) In vivo trials on the therapeutic effects of encapsulated Artemisia annua and Artemisia afra. Glob J Res Anal 5(6):228–234
Tin AS, Sundar SN, Tran KQ, Park AH, Poindexter KM, Firestone GL (2012) Antiproliferative effects of artemisinin on human breast cancer cells requires the downregulated expression of the E2F1 transcription factor and loss of E2F1-target cell cycle genes. Anti-Cancer Drugs 23(4):370–379
Tong Y, Liu Y, Zheng H, Zheng L, Liu W, Wu J, Lu L (2016) Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β-catenin signaling. Oncotarget 7(21):31413
Tran KQ, Tin AS, Firestone GL (2014) Artemisinin triggers a G1 cell cycle arrest of human Ishikawa endometrial cancer cells and inhibits cyclin dependent Kinase-4 promoter activity and expression by disrupting NF-kB transcriptional signaling. Anti-Cancer Drugs 25(3):270
Tzenkova R, Kamenarska Z, Draganov A, Atanassov A (2010) Composition of Artemisia annua essential oil obtained from species growing wild in Bulgaria. Biotechnol Biotechnol Equip 24(2):1833–1835
Unschuld PU (1986) Medicine in China: a history of pharmaceutics. University of California Press, Berkeley
Wani KI, Choudhary S, Zehra A, Naeem M, Weathers P, Aftab T (2021) Enhancing artemisinin content in and delivery from Artemisia annua: a review of alternative, classical, and transgenic approaches. Planta 254(2):1–15
Wani KI, Zehra A, Choudhary S, Naeem M, Khan M, Khan R, Aftab T (2022) Exogenous strigolactone (GR24) positively regulates growth, photosynthesis, and improves glandular trichome attributes for enhanced artemisinin production in Artemisia annua. J Plant Growth Regul 1–10
Wei T, Liu J (2017) Anti-angiogenic properties of artemisinin derivatives. Int J Mol Med 40(4):972–978
Willcox M (2009) Artemisia species: from traditional medicines to modern antimalarials—and back again. J Altern Complement Med 15:101–109
Willcox M, Bodeker G, Rasoanaivo P, Addae-Kyereme J (eds) (2004) Traditional medicinal plants and malaria, vol 4. CRC Press, Boca Raton
Woerdenbag HJ, Moskal TA, Pras N, Malingré TM, El-Feraly FS, Kampinga HH, Konings AW (1993) Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J Nat Prod 56(6):849–856
World Health Organization (2006) WHO monograph on good agricultural and collection practices (GACP) for Artemisia annua L. World Health Organization, Geneva
World Health Organization (2015) Guidelines for the treatment of malaria. World Health Organization
Wright CW, Linley PA, Brun R, Wittlin S, Hsu E (2010) Ancient Chinese methods are remarkably effective for the preparation of artemisinin-rich extracts of Qing Hao with potent antimalarial activity. Molecules 15:804–812
Zhang YX, Sun HX (2009) Immunosuppressive effect of ethanol extract of Artemisia annua on specific antibody and cellular responses of mice against ovalbumin. Immunopharmacol Immunotoxicol 31(4):625–630
Zhang ZY, Yu SQ, Miao LY, Huang XY, Zhang XP, Zhu YP, Li DQ (2008) Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 6(2):134–138
Zhang CZ, Zhang H, Yun J, Chen GG, San Lai PB (2012) Dihydroartemisinin exhibits antitumor activity toward hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol 83(9):1278–1289
Zhang J, Guo L, Zhou X, Dong F, Li L, Cheng Z, Liu J (2016) Dihydroartemisinin induces endothelial cell anoikis through the activation of the JNK signaling pathway. Oncol Lett 12(3):1896–1900
Zhao HF, Zhong JX, Peng SQ, Liu SC (1998) Study of sweet wormwood compound on SLE in mice. Chin J Information Trad Chin Med 5(8):18–19
Zhao F, Wang H, Kunda P, Chen X, Liu QL, Liu T (2013) Artesunate exerts specific cytotoxicity in retinoblastoma cells via CD71. Oncol Rep 30(3):1473–1482
Zhou X, Sun WJ, Wang WM, Chen K, Zheng JH, Lu MD, Zheng ZQ (2013) Artesunate inhibits the growth of gastric cancer cells through the mechanism of promoting oncosis both in vitro and in vivo. Anti-Cancer Drugs 24(9):920–927
Zhu H, Liao SD, Shi JJ, Chang LL, Tong YG, Cao J, Lu JJ (2014) DJ-1 mediates the resistance of cancer cells to dihydroartemisinin through reactive oxygen species removal. Free Radic Biol Med 71:121–132
Zhu S, Yu Q, Huo C, Li Y, He L, Ran B et al (2021) Ferroptosis: a novel mechanism of artemisinin and its derivatives in cancer therapy. Curr Med Chem 28(2):329–345
Zime-Diawara H, Ganfon H, Gbaguidi F, Yemoa A, Bero J, Jansen O, Quetin-Leclercq J (2015) The antimalarial action of aqueous and hydro alcoholic extracts of Artemisia annua L. cultivated in Benin: in vitro and in vivo studies. J Chem Pharm Res 7(8):817–823
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Wani, K.I. et al. (2022). Artemisia annua L.: Traditional Uses, Phytochemistry, and Pharmacological Activities. In: Devkota, H.P., Aftab, T. (eds) Medicinal Plants of the Asteraceae Family. Springer, Singapore. https://doi.org/10.1007/978-981-19-6080-2_2
Download citation
DOI: https://doi.org/10.1007/978-981-19-6080-2_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-6079-6
Online ISBN: 978-981-19-6080-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)