Abstract
The heart and the liver are closely related organs in health and disease. Hemodynamic swings in liver transplantation (LT) surgery impose extreme stress on the cardiovascular system. It is hardly surprising that cardiovascular complications following liver transplant are the third leading cause of mortality, the first two being infection and multiorgan failure [1]. In fact, cardiac dysfunction in cirrhosis may contribute to 50% mortality [2]. Coronary artery disease (CAD) progresses with age, and as the median age of transplant candidates is progressively increasing with improvement in antiviral and overall medical therapy, the percentage of chronic liver disease (CLD) patients with significant cardiovascular disease too is rising. There is a 16.2% incidence of severe CAD (>70% stenosis) in patients with cirrhosis, and 13.3% of them are asymptomatic, despite angiographically evident severe CAD [3].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
The heart and the liver are closely related organs in health and disease. Hemodynamic swings in liver transplantation (LT) surgery impose extreme stress on the cardiovascular system. It is hardly surprising that cardiovascular complications following liver transplant are the third leading cause of mortality, the first two being infection and multiorgan failure [1]. In fact, cardiac dysfunction in cirrhosis may contribute to 50% mortality [2]. Coronary artery disease (CAD) progresses with age, and as the median age of transplant candidates is progressively increasing with improvement in antiviral and overall medical therapy, the percentage of chronic liver disease (CLD) patients with significant cardiovascular disease too is rising. There is a 16.2% incidence of severe CAD (>70% stenosis) in patients with cirrhosis, and 13.3% of them are asymptomatic, despite angiographically evident severe CAD [3].
Though cardiovascular disease rises with age, liver disease itself may contribute to higher cardiovascular risk. Further the spectrum of cardiac disease in cirrhosis may range from diseases that affect both the heart and the liver to cardiac diseases unique to cirrhosis (Table 10.1).
Earlier liver disease was considered to confer protection from CAD, but this has been challenged by recent research [4], and liver disease patients are now considered to be at an equivalent or increased risk of cardiovascular disease. Many of the common symptoms of CAD like exertional dyspnea may be missed because of the restricted mobility of liver disease patients. Similarly elevated BP is infrequent in advanced liver diseases because of decreased systemic vascular resistance (SVR). Furthermore, reduced cholesterol synthesis in diseased liver states [5] coupled with cardio-protective protection of estrogens seen in ESLD was considered to reduce the cardiovascular diseases burden. Thus, many patients with advanced cardiovascular disease are likely to be missed on routine examination unless actively screened for the same.
Even after the diagnosis of cardiac disease is established the preoperative optimization of patients with stenotic lesions presents a challenge to the transplant team. Revascularization strategies with stenting necessitate antiplatelet therapy which enhance bleeding risk. On the other hand, surgical revascularization involving coronary artery bypass grafting (CABG) in advanced liver disease may carry unacceptable risk. Intraoperative management too would be a challenge and includes advanced cardiac monitoring to maintain stable hemodynamic parameters. Reperfusion injury may be graver in patients with compromised cardiovascular systems. Many patients decompensate in the postoperative period as the SVR increases to normal levels, and cardiovascular mortality is of significant concern in the postoperative period. A clear understanding of the pathophysiology, a protocol for screening, and perioperative optimization of these patients may improve the outcome in this fragile group of patients.
1 Hemodynamic Changes in Patients with Cirrhosis
The cardiovascular system is hyperdynamic in patients with portal hypertension secondary to cirrhosis [6]. The mean arterial pressure (MAP) and SVR are lower, whereas the cardiac output (CO) and heart rate are increased. However, there is blunted ventricular inotropic and chronotropic response to stressful stimuli like surgery, bleeding, or vasoactive drug administration [7], making these patients extremely frail candidates for liver transplantation. Decreased clearance of gut-derived or locally produced humoral factors like endogenous cannabinoids and nitric oxide (NO) has been implicated as the possible mechanism behind peripheral vasodilatation leading to reduced vascular resistance [8].
The incompetence of the cardiovascular system in coping with the physiological stresses has been termed as “cirrhotic cardiomyopathy [9].” It is a distinct entity and the clinical features comprise blunted systolic and diastolic contractile response to stress, accompanied with signs of ventricular hypertrophy/chamber dilatation and electrophysiological abnormalities. Altered membrane fluidity, impaired beta-adrenergic receptor signaling pathway, and over-activity of NO, carbon monoxide, and endocannabinoid pathways have been implicated in the pathogenesis of cirrhotic cardiomyopathy [10].
CO decreases, and BP and SVR start rising after liver transplantation as liver functions normalize. This may be partly attributable to immune-suppressants like cyclosporin [1]. Many cardiac patients may not be able to cope with the rapidly changing milieu leading to decompensation and heart failure.
2 Preoperative Cardiac Evaluation of Liver Transplant Candidates
The preoperative assessment starts with clinical history and physical examination and includes some basic diagnostic screening tests. More specific tests are indicated on the basis of the clinical profile and preliminary cardiac screening.
History and physical examination—The patient is asked about any specific symptoms of breathlessness on exertion, angina, past history of hypertension or CAD, relevant family history, and effort tolerance which should be objectively documented as METs to allow for detection of any deterioration in clinical status in subsequent visits. Exercise capacity may be severely compromised because of severe ascites, poor nutrition, or lack of motivation necessitating other diagnostic modalities to assess the functional status of the heart. In addition, the presence of some risk factors warrants further investigations (Table 10.2). Diabetes is a significant independent risk factor for CAD in these patients [3, 11, 12].
Lipid profile should be done for all patients apart from routine laboratory and biochemical profile.
Other important routine diagnostic screening tests include:
-
1.
Electrocardiogram could help to detect any ischemic changes due to long-standing CAD, arrhythmias due to electrolyte disturbances, or signs of right heart dysfunction like right axis deviation or right ventricular strain pattern. Left ventricular hypertrophy, left axis deviation, or left bundle branch block may also be detected on routine 12-lead ECG. It is important to note any QT-interval prolongation, and a prolonged QTc >440 msec is associated with a significantly reduced survival [13].
-
2.
Echocardiography is invaluable in the diagnostic screening of liver transplant recipients [14] as it can detect structural and functional abnormalities, which cannot be detected on ECG. It can indicate LV dysfunction, valvular defects, left ventricular outflow defects (LVOTO), right ventricle (RV) dysfunction, elevated pulmonary artery pressure (PAP), and pericardial fluid or intracardiac shunt. In fact Garg et al. [15] have suggested that even a mildly reduced left ventricular function should raise the suspicion of a cardiomyopathy. An additional advantage is that Bubble ECHO can be simultaneously performed, and delayed passage of bubbles to the left side may indicate hepatopulmonary syndrome. However, a transthoracic echocardiogram (TTE) does not detect the presence or severity of CAD for which further screening is recommended.
-
3.
Stress tests—Stress echocardiogram is a family of examinations in which 2-D echocardiographic monitoring is undertaken before, during, and after cardiovascular stress. Bedsides TTE provides the functional status of the heart at rest, but liver transplant is a major procedure, and these tests help to assess the functioning of the heart under stress provided in the form of exercise or pharmacological agents. Though exercise stress testing has the advantage of evaluating exercise capacity too, most patients with advanced cirrhosis have markedly reduced motivation and mobility and are unlikely to achieve target heart rate. This makes pharmacological testing with dobutamine necessary—Dobutamine Stress Echocardiogram (DSE). Stress increases myocardial oxygen demand leading to imbalance in the supply-demand ratio. This leads to myocardial thickening and/or impaired motility which can be detected on the echocardiogram. Further testing may be warranted on the basis of stress echocardiogram reports necessitating medical therapy or intervention procedures for optimizing the heart before undertaking transplant surgery.
Varying reports on the predictive ability of DSE have been quoted in literature. While some authors have reported a high sensitivity [16] and a high negative predictive value (86–100%) [17, 18], a recent review by Hogan et al. [14] has suggested that overall DSE has a poor sensitivity for ruling out CAD, but is reliable at predicting post-LT CV events. They attributed this discordance to the fact that 19–50% of patients did not achieve the target heart rate, which is 85% of the maximal predicted. Many patients of cirrhosis are on β-blocker therapy or suffer from chronotropic incompetence and consequently may not reach their target heart rate [19]. Thus, the test may be inconclusive in 26–56% patients. Recent protocols suggest that β-blockers may be safely withdrawn without rebound hypertension or variceal bleeding [20]. However, DSE may require early termination in 28% of patients due to chest pain, arrhythmias, or marked changes in blood pressure [14].
-
4.
Nuclear myocardial perfusion imaging is done with the administration of vasodilator drugs (such as adenosine or dipyridamole), but is not reliable in cirrhosis as they are already maximally vasodilated due to low SVR and further vasodilatation may not be a possibility.
-
5.
Functional testing—Perioperative risk is increased in patients who are unable to perform at least 4 METs of work. Current UK guidelines consider NAFLD patients at low risk if they are able to climb 2 flights of stairs which is the equivalent of 4 METs [14].
Cardiopulmonary exercise testing (CPET)—Maximum aerobic capacity measure as VO2 max correlates with maximal fitness and is usually not possible in severe disease. Only 32% of ESLD patients achieve it, but ventilatory threshold or anaerobic threshold (AT) can be achieved in 90% of these patients. It is the physiological point at which oxygen supply is inadequate and muscles switch over to anaerobic glycolysis, and indicates cardiopulmonary reserve. The remainder patients can be motivated or functional reserves built up to achieve AT. Patients with cirrhosis have a reduced aerobic capacity and researchers from the UK reported that preoperative CPET is a specific predictor of 90-day survival following liver transplantation. In a study involving 182 patients followed over a 3-year period, the mean anaerobic threshold (AT) was significantly higher in survivors compared to nonsurvivors and AT <9.0 mL/minute/kg was associated with reduced 90-day survival [21].
Though this is not routinely followed in transplant centers over the world Dr. James Findlay from the Mayo Clinic in Rochester, Minnesota, commented that CPET could be a promising modality in assessing risk-benefit ratio and merits further evaluation. He further advocated simpler tests like the 6-minute walk test (6MWT). Carey et al. [22] reported that each 100-m increase in the 6MWT was significantly associated with increased survival, with 6MWT < 250 m being associated with an increased risk of death on the waiting list. They observed that the 6MWT is significantly reduced in patients awaiting LT and is inversely correlated with the native MELD score.
-
6.
Cardiac MRI provides detailed structural and functional evaluation of heart as well as its tissue characterization. It is useful in early recognition of cirrhotic cardiomyopathy and aids in evaluating cardiac iron overload in hemochromatosis. Gadolinium-enhanced cardiac MRI is used to detect cardiac involvement in amyloidosis. MRI is also used to evaluate the presence of myocardial scars and viable myocardium.
-
7.
Right cardiac catheterization should be performed to characterize the pressure-resistance relationship in the pulmonary artery whenever there is clinical suspicion of pulmonary HTN or porto-pulmonary hypertension as indicated by elevated Right Ventricular Systolic Pressure >50 mmHg on routine echocardiography.
-
8.
Cardiac computed tomography angiography may be done in patients with unclear or inconclusive stress test results. It is noninvasive, hence carries lower bleeding risks in coagulopathic ESLD patients. However, its utility is limited to patients with low to intermediate risk of CAD, and it cannot replace conventional coronary angiography in symptomatic patients with high probability of CAD. It is more sensitive but less specific than conventional angiography. However, contrast-induced nephropathy is a problem in patients with ESLD who have renal dysfunction. Candidates suitable for CT angiography should have normal renal function, nontachycardiac regular cardiac rhythm, normal body habitus, and the ability to lie still and perform breath holding maneuvers. The last may be problematic in patients with severe ascites.
-
9.
Coronary artery calcium score (CACS) may be a useful noninvasive test in patients with liver disease as it measures the calcium deposits within coronary vasculature by CT [23, 24].
It is gaining popularity as an adjunct surveillance tool for screening CAD, but the study samples have been small, and are not considered conclusive.
Higher CACS suggests a greater degree of coronary artery stenosis but the score has limited predictive value as a single screening study for CAD. Patients with CACS >100 are five times more likely to have ischemic events than with CACS<100 (Table 10.3).
-
10.
Conventional cardiac angiography continues being the gold standard for diagnosing CAD. It is an invasive technique but has the advantage that both diagnosis and treatment can be accomplished in a single session, thus avoiding the need for repeated contrast exposure in liver patients with vulnerable or damaged kidneys. Bleeding remains a significant risk, and procoagulant cover may be indicated during the procedure. Trans-radial approach may minimize bleeding complications in ESLD patients and may be indicated in suitable patients.
Although active screening for cardiac diseases is desirable a brief summary of common cardiac diseases associated with cirrhosis would further aid in the understanding and management of these patients.
3 Systemic Disease That Affect Both Heart and Liver
Alcoholic cirrhosis is seen in chronic alcoholics. It is characterized by nonischemic dilated cardiomyopathy. There is myocardial fibrosis, disruption of myofibrillary structure, and an increased risk for CAD. Ejection fraction is decreased and there is increased propensity for supraventricular arrhythmias.
Hemochromatosis is characterized by dilated or restrictive cardiomyopathy, cardiac arrhythmias, and heart failure. Cardiac MRI is especially useful in diagnosis.
Nonalcoholic fatty liver disease (NAFLD) is often associated with metabolic syndrome and atherosclerotic plaques, and fatty liver is a strong predictor for CAD [25].
Amyloidosis—Restrictive cardiomyopathy and arrhythmias are seen along with small vessel CAD.
Cirrhotic cardiomyopathy has a 40–50% incidence in cirrhotic patients [14] and is characterized by an abnormal cardiac response during transplant. There is a 3.3–7% incidence of severe left heart failure after transplant which carries a 45% mortality. Cirrhotic cardiomyopathy is defined as cardiac dysfunction in patients suffering from cirrhosis characterized by impaired contractile responsiveness to physical and pharmacological stress and/or altered diastolic relaxation with associated electrophysiological abnormalities in absence of other known cardiac disease. The pathogenesis of cirrhotic cardiomyopathy involves autonomic dysfunction, cardio-depressant substances, and abnormal plasma membrane fluidity.
There is reduced systolic as well as diastolic function. Systolic dysfunction may be revealed as reduced cardiovascular response to stress testing even though the resting cardiac output is high. Diastolic dysfunction is indicated by fibrosis, myocardial hypertrophy, and subendothelial edema leading to reduced compliance and relaxation [26]. Diagnosis involves diastolic dysfunction (E/A ratio < 1) on 2D-ECHO and systolic dysfunction (chronotropic incompetence) on Stress ECHO.
Electrophysiological abnormalities include prolonged QT interval, and the QT interval corrected for heart rate (QTc) is more than 440 ms. In cirrhosis, gender difference in QT interval length is abolished. There is a higher chance of torsades de pointes and other rhythm disturbances. Electromechanical dissociation may be seen and there could be failure to recruit the whole myocardium during contraction.
Pericardial effusion—Fluid retention during ESLD may cause pericardial effusion which may further lead to cardiac tamponade. It can be diagnosed by complete bedside examination but preoperative screening with transthoracic ECHO (TTE) is invaluable, though the sensitivity may be reduced in the setting of porto-pulmonary hypertension. Treatment with pericardiocentesis or a pericardial window may be indicated.
Heart failure—Preoperative assessment of cardiac function with TTE could rule out heart failure. This pretransplant heart failure may resolve or worsen postoperatively in this cohort of patients. Perioperative medical therapy for heart failure may help to optimize cardiac function.
Left ventricular outflow tract obstruction (LVOTO)—Left ventricular hypertrophy (LVH) with hyperdynamic systolic function in ESLD may result in LVOTO. These patients exhibit poor tolerance to hemodynamic stresses encountered in transplantation. LVOTO may be functional secondary to high flow state or mechanical secondary to septal hypertrophy. The risk of intraoperative hypotension is increased if LVOTO exceeds 36 mmHg [27]. Intraoperative strategies to minimize LVOTO include avoiding tachycardia, minimizing preload reduction, and limiting inotropic agents. TEE-guided volume replacement may be useful [28,29,30]. Alcohol septal ablation may be indicated in cases of symptomatic heart failure with underlying hypertrophic obstructive cardiomyopathy (HOCM) [31].
Structural heart diseases—Atrial septal defect (ASD) and patent foramen ovale (PFO) may be associated with a prevalence similar to that of the general population, and do not preclude transplant [26]. Long-term ASD leads to altered pulmonary vascular resistance which may precipitate right heart failure in the postoperative period. PFO has a prevalence of 4% and is associated with paradoxical embolism, though it is not a contraindication for transplantation. Extra care should be taken to prevent thrombus formation and air entry into the venous system during surgery. Further studies are needed to determine the potential role of percutaneous PFO closure in LT candidates.
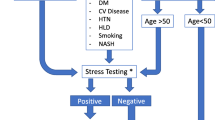
A stepwise approach to diagnosing liver disease is described in Fig. 10.1 [14].
Optimization of cardiac issues—Screening measures may detect cardiac disease which needs to be optimized for successful outcome in LT.
4 Management of Stenotic CAD
Medical management—Patients with mild to moderate obstructive diseases should be aggressively managed medically. There are no separate guidelines for patients with cirrhosis and the general principles of risk reduction are followed. Abstinence from tobacco and lifestyle modifications as applicable are followed. Hb1Ac levels should be aimed at less than 7 gm% as pretransplant diabetes carries significant risk. LDL levels should be kept below 100 mg% with lipid lowering drugs, and close monitoring of liver function tests is desirable as they may be hepatotoxic. Statins may have the dual advantage of lowering portal pressure in addition to their cardiovascular benefits [14]. Aspirin cannot be administered usually as there may be marked thrombocytopenia. β-blockers, ACE inhibitors, and aldosterone antagonists may be administered in the peri-transplant period. β-blockers attenuate both the sympathetic and neuro-endocrine response to stress. Perioperative β-blockers may be protective for death and improve perioperative cardiac outcome [32]. Among the β-blockers carvedilol may be preferred as it reduces splanchnic blood flow and porto-collateral resistance, thus reducing portal hypertension [33]. ACE-i and aldosterone antagonists may require dose reduction in the setting of renal dysfunction but are particularly indicated in patients who have suffered a myocardial infarction and have left ventricular dysfunction [34].
Preoperative revascularization strategies are indicated in patients with more severe obstruction to minimize the perioperative risk burden. There is a lack of consensus regarding the criteria of obstructive CAD in prospective liver transplant patients that need intervention. While traditional CAD is defined as >70% coronary stenosis or > 50% left main stenosis, experts have indicated that the threshold may be lowered in this cohort of patients considering the major hemodynamic swings integral to the surgery [4]. The choice for revascularization depends on the type and extent of occlusion. Most cardiologists favor percutaneous intervention (PCI) as the preferred therapy as it is less invasive and is successful in 93–94% of patients with ESLD [35,36,37].
Percutaneous transluminal coronary angioplasty (PTCA) either alone or with stenting is adequate in the majority of patients, and Coronary Artery Bypass Grafting (CABG) is reserved for the special group of patients whose anatomy is not favorable for PCI. However, PCI has its own risks in these patients with compromised renal function and coagulopathy. Carey et al. [3] observed a 5.5% rate of transient renal impairment in patients undergoing PCI secondary to contrast exposure. In addition, the antiplatelet medications increase the bleeding tendency. The new glycoprotein IIb/IIIa medications and even thienopyridine clopidogrel have not been adequately studied in ESLD patients. Bare Metal Stents are preferred as they need shorter dual antiplatelet therapy (DAPT) and may be switched over to aspirin alone after 1 month [38, 39]. This would expedite surgery in critical cases and minimize inherent bleeding risk. Drug eluting stents (DES) mandate longer DAPT and may delay transplant. Stopping DAPT before the recommended period carries a significant risk of stent thrombosis.
In the small subgroup of patients who cannot be treated by PCI techniques CABG may be considered; however, the risk of cardiac surgery in cirrhotic patients may be unacceptably high, and the one-year mortality in Child B & C has been reported between 45% and 80% [14]. CABG may only be indicated in the small subgroup of patients with anatomically unfavorable lesions which preclude stent placement, thereby denying the candidate a liver transplant.
Another important decision is regarding the timing of CABG—whether it should precede the transplant, follow the transplant, or be done alongside the transplant as a combined procedure. CABG before LT may be attempted in moderate ESLD, but there is a risk of postoperative liver decompensation and an urgent transplant may be needed in this period. Moreover, there is risk of acute liver decompensation intraoperatively which may hamper operative repair of CAD. CABG may be attempted after liver transplant if the cardiac disease is not deemed to be very severe and would permit the patient to tolerate the surgery without any major adverse cardiac event. An added theoretical advantage of CABG after LT would be that normal liver function would reduce bleeding during CABG, but the majority of CAD patients with significant occlusion would not be able to tolerate a transplant surgery without adequate optimization of cardiac function. With advancement in surgical and anesthetic techniques a few case reports of combined CABG and liver transplant have been reported, although the decision is not to be taken lightly [40, 41]. Major complications included renal failure, massive blood loss, myocardial dysfunction, wound infection, and even one intraoperative death. Lebbinck et al. [42] used Offpump Coronary Artery Bypass Graft (OPCAB) sequential procedure in a patient with Child C and described it as a “Good option for those in need of LT and coronary revascularization.” In the author’s institute of current affiliation 5 combined CABG LT procedures have been conducted in the last decade with 100% 1 year graft and patient survival. All patients were managed off pump and had a pretransplant EF > 45%. OPCAB reduces bleeding as the equilibrium between procoagulant and anticoagulant activity is less disturbed. Moreover, there is lower inflammation, lower cardiac stunning, and reduced volume shifts. Besides these anesthetic modifications, organ quality is also important in combined procedure as graft dysfunction could predispose to increased bleeding and cardiac tamponade.
It needs to be emphasized that the decision for combined CABG-LT is not to be taken lightly and should be a multidisciplinary opinion. Main indication for combined procedure is the presence of significant, high-risk coronary lesions with preserved left ventricular function and very advanced liver disease.
Cirrhotic Cardiomyopathy—ESLD may be associated with cirrhotic cardiomyopathy and circulating inflammatory mediators with inherent cardio-depressant properties. There may be reduced sensitivity to beta-agonists, and such a heart may decompensate in the postoperative period, as preload reduces; and afterload normalizes with improved SVR.
No specific treatment can yet be recommended but caution is advised with respect to procedures that may stress the heart. Balance of myocardial oxygen supply and demand is critical in the perioperative period. Patients with heart failure should be treated following general guidelines.
An institution-specific protocol for cardiac screening is imperative for ruling out major cardiovascular diseases, which may otherwise be missed. Noninvasive tests should be a part of all screening processes, which may dictate further invasive tests [26]. The anesthesiologist must optimize the cardiac status before proceeding with the transplant. Revascularization by PCI is indicated in obstructive CAD, and stents which require minimal dual antiplatelet therapy are preferred. The risk of cirrhotic cardiomyopathy must always be kept in mind, and usually manifests in the postoperative period. Meticulous volume and hemodynamic management in the perioperative period and close invasive monitoring may minimize the risk.
A thorough understanding of the cardiovascular physiology in advanced liver disease combined with an awareness of diseases unique to this subset of population is necessary for successful optimization and perioperative management.
Key Points
-
Cardiovascular physiology is considerably altered in liver disease.
-
Cardiovascular diseases are a leading cause of post-transplant mortality.
-
Active screening for cardiovascular disease should be a part of pretransplant workup.
-
Revascularization with stenting is the preferred treatment modality in occlusive CAD.
-
Stents which require minimal period of dual antiplatelet therapy are preferred.
-
Cirrhotic cardiomyopathy is a distinct entity, and these patients may decompensate in the postoperative period.
-
Close invasive monitoring with rapid response to hemodynamic swings may improve perioperative outcome.
References
Burroughs AK, et al. 3-month and 12-month mortality after first liver transplant in adults in Europe: predictive models for outcome. Lancet. 2006;367(9506):225–32. https://doi.org/10.1016/s0140-6736(06)68033-1.
Plotkin J, et al. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Hepatol Res. 1997;7(2):136–7. https://doi.org/10.1016/s0928-4346(97)85773-8.
Carey WD, et al. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation. 1995;59(6):859–63. https://doi.org/10.1097/00007890-199503000-00010.
Keeffe B. Detection and treatment of coronary artery disease in liver transplant candidates. Liver Transpl. 2001;7(9):755–61. https://doi.org/10.1053/jlts.2001.26063.
Cicognani C. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med. 1997;157(7):792–6. https://doi.org/10.1001/archinte.157.7.792.
Groszmann RJ. Hyperdynamic circulation of liver disease 40 years later: pathophysiology and clinical consequences. Hepatology. 1994;20(5):1359–63. https://doi.org/10.1002/hep.1840200538.
Lee SS, et al. Desensitization of myocardial β-adrenergic receptors in cirrhotic rats. Hepatology. 1990;12(3):481–5. https://doi.org/10.1002/hep.1840120306.
Carter EP, et al. Regulation of heme oxygenase-1 by nitric oxide during hepatopulmonary syndrome. Am J Phys Lung Cell Mol Phys. 2002;283(2):L346–53. https://doi.org/10.1152/ajplung.00385.2001.
Moller S. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87(1):9–15. https://doi.org/10.1136/heart.87.1.9.
Rockey D. Vascular mediators in the injured liver. Hepatology. 2003;37(1):4–12. https://doi.org/10.1053/jhep.2003.50044.
Garcia-Compean D, et al. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15(3):280. https://doi.org/10.3748/wjg.15.280.
Rossetto A, et al. Cardiovascular risk factors and immunosuppressive regimen after liver transplantation. Transplant Proc. 2010;42(7):2576–8. https://doi.org/10.1016/j.transproceed.2010.05.160.
Bernardi M, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27(1):28–34. https://doi.org/10.1002/hep.510270106.
Hogan BJ, et al. Evaluation of coronary artery disease in potential liver transplant recipients. Liver Transpl. 2017;23(3):386–95. https://doi.org/10.1002/lt.24679.
Garg A, Armstrong WF. Echocardiography in liver transplant candidates. JACC Cardiovasc Imaging. 2013;6(1):105–19. https://doi.org/10.1016/j.jcmg.2012.11.002.
Geleijnse ML, et al. Methodology, feasibility, safety and diagnostic accuracy of dobutamine stress echocardiography. J Am Coll Cardiol. 1997;30(3):595–606. https://doi.org/10.1016/s0735-1097(97)00206-4.
Plotkin JS, et al. Dobutamine stress echocardiography for preoperative cardiac risk stratification in patients undergoing orthotopic liver transplantation. Liver Transpl Surg. 1998;4(4):253–7. https://doi.org/10.1002/lt.500040415.
Donovan CL, et al. Two-dimensional and dobutamine stress echocardiography in the preoperative assessment of patients with end-stage liver disease prior to orthotopic liver transplantation. Transplantation. 1996;61(8):1180–8. https://doi.org/10.1097/00007890-199604270-00011.
Findlay JY, et al. Preoperative dobutamine stress echocardiography, intraoperative events, and intraoperative myocardial injury in liver transplantation. Transplant Proc. 2005;37(5):2209–13. https://doi.org/10.1016/j.transproceed.2005.03.023.
Payancé A, et al. Lack of clinical or haemodynamic rebound after abrupt interruption of beta-blockers in patients with cirrhosis. Aliment Pharmacol Ther. 2016;43(9):966–73. https://doi.org/10.1111/apt.13577.
Prentis JM, et al. Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transpl. 2012;18(2):152–9. https://doi.org/10.1002/lt.22426.
Carey EJ, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16(12):1373–8. https://doi.org/10.1002/lt.22167.
Cassagneau P, et al. Prognostic value of preoperative coronary computed tomography angiography in patients treated by orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2012;24(5):558–62. https://doi.org/10.1097/meg.0b013e3283522df3.
Kemmer N, et al. The role of coronary calcium score in the risk assessment of liver transplant candidates. Transplant Proc. 2014;46(1):230–3. https://doi.org/10.1016/j.transproceed.2013.09.035.
Vanwagner LB, et al. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56(5):1741–50. https://doi.org/10.1002/hep.25855.
Raval Z, et al. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. 2011;58(3):223–31. https://doi.org/10.1016/j.jacc.2011.03.026.
Maraj S, et al. Inducible left ventricular outflow tract gradient during dobutamine stress echocardiography: an association with intraoperative hypotension but not a contraindication to liver transplantation. Echocardiography. 2004;21(8):681–5. https://doi.org/10.1111/j.0742-2822.2004.03068.x.
Harley ID, et al. Orthotopic liver transplantation in two patients with hypertrophic obstructive cardiomyopathy. Br J Anaesth. 1996;77(5):675–7. https://doi.org/10.1093/bja/77.5.675.
Lim YC, et al. Intraoperative transesophageal echocardiography in orthotopic liver transplantation in a patient with hypertrophic cardiomyopathy. J Clin Anesth. 1995;7(3):245–9. https://doi.org/10.1016/0952-8180(94)00049-a.
Cywinski JB, et al. Dynamic left ventricular outflow tract obstruction in an orthotopic liver transplant recipient. Liver Transpl. 2005;11(6):692–5. https://doi.org/10.1002/lt.20440.
Paramesh AS, et al. Amelioration of hypertrophic cardiomyopathy using nonsurgical septal ablation in a cirrhotic patient prior to liver transplantation. Liver Transpl. 2005;11(2):236–8. https://doi.org/10.1002/lt.20327.
Safadi A, et al. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation. 2009;120(13):1189–94. https://doi.org/10.1161/circulationaha.108.847178.
Tripathi D, Hayes PC. The role of carvedilol in the management of portal hypertension. Eur J Gastroenterol Hepatol. 2010;22(8):905–11. https://doi.org/10.1097/meg.0b013e3283367a99.
Raval Z. Role of cardiovascular intervention as a bridge to liver transplantation. World J Gastroenterol. 2014;20(31):10651. https://doi.org/10.3748/wjg.v20.i31.10651.
Azarbal B, et al. Feasibility and safety of percutaneous coronary intervention in patients with end-stage liver disease referred for liver transplantation. Liver Transpl. 2011;17(7):809–13. https://doi.org/10.1002/lt.22301.
Pillarisetti J, et al. Cardiac catheterization in patients with end-stage liver disease: safety and outcomes. Catheter Cardiovasc Interv. 2010;77(1):45–8. https://doi.org/10.1002/ccd.22591.
Noureddin N, et al. Cardiac catheterization and percutaneous coronary intervention in patients with end stage liver disease. J Am Coll Cardiol. 2015;65(10):A1776. https://doi.org/10.1016/s0735-1097(15)61776-4.
Fleisher LA, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary. Circulation. 2014;130(24):2215–45. https://doi.org/10.1161/cir.0000000000000105.
Lentine KL, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates. Circulation. 2012;126(5):617–63. https://doi.org/10.1161/cir.0b013e31823eb07a.
Benedetti E, et al. Is the presence of surgically treatable coronary artery disease a contraindication to liver transplantation? Clin Transpl. 1999;13(1):59–61. https://doi.org/10.1034/j.1399-0012.1999.t01-1-130109.x.
Giakoustidis A, et al. Combined cardiac surgery and liver transplantation: three decades of worldwide result. J Gastrointestin Liver Dis. 2014;23(4):415–21. https://doi.org/10.15403/jgld.2014.1121.234.
Lebbinck H, et al. Sequential off-pump coronary artery bypass and liver transplantation. Transpl Int. 2006;19(5):432–4. https://doi.org/10.1111/j.1432-2277.2006.00298.x.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jolly, A.S., Bhalotra, S., Kumar, M. (2023). Preoperative Assessment and Optimization of Liver Transplant Patients: Cardiac Issues in Liver Disease. In: Vohra, V., Gupta, N., Jolly, A.S., Bhalotra, S. (eds) Peri-operative Anesthetic Management in Liver Transplantation. Springer, Singapore. https://doi.org/10.1007/978-981-19-6045-1_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-6045-1_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-6044-4
Online ISBN: 978-981-19-6045-1
eBook Packages: MedicineMedicine (R0)