Abstract
In the midst of a plethora of nanomaterial-based drug delivery platforms, liposomes have been predominantly successful with several products conceded into clinical applications. Liposomes are well-established and effective drug delivery systems, extensively used in cancer therapy including breast cancer. In this chapter nanoliposomes designed with the breast cancer targeting feature and drug release triggering functions are emphasized. The chapter also highlights the recent advances in the nanoliposomes-based therapeutic system for breast cancer, including gene and theranostic delivery perspectives. The challenges associated with the development of liposome-based products for future clinical settings are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Subsequent to skin cancer, breast cancer (BC) is the most common cancer diagnosed in women in the United States. Breast cancer can occur in both men and women, but it’s far more common in women. BC is also one of the leading causes of cancer-related deaths. Every year, one million women are diagnosed with breast cancer, with approximately one new case diagnosed every 18 seconds (Bray et al. 2018). It has been estimated that the breast carcinomas cases could rise to 2.50 million by 2025, with 768,646 people dying as a result of this disease. An in-depth study of BC pathophysiology at the molecular level confirms the heterogeneous nature of this carcinoma. If detected in its early stage, then BC can be cured. The metastatic progression is the most challenging aspect in the alleviation of BC. In the current scenario, the therapeutic intervention has been drastically improved as reflected in increased patient survival rates. However, an advanced form of breast cancer that has spread to other organs could not be cured with current chemotherapeutics. BC is usually characterized by uninhibited and excessive cell growth that affects other nearby healthy tissues and organs (Nosrati et al. 2018). Nonetheless, breast cancer alleviation is very difficult to deal with despite the tremendous efforts and development in this field. Until now, chemotherapy, in addition to surgery, has been the foremost strategy to treat BC. There are serious concerns with most of the chemotherapeutic drugs such as they exert a toxic effect on normal cells and tissues (Zhu et al. 2016). In addition, multidrug resistance caused by chemotherapeutics drugs could lead to failure of treatment upon the recurrence of carcinomas (Lu et al. 2016). At the present, therapeutic intervention of BC lays stress upon biologically directed therapies, personalized treatment, and de-escalation of chemotherapy. Although the 5-year survival rate of advanced or metastasized BC is low (28%), the primary goal of targeted therapy is to prolong survival, control symptoms, and reduce cytotoxic drug toxicity, thereby improving the quality of life of BC patients (Harbeck et al. 2019).
Studies at the molecular level revealed that mutated genes’ expression substantially contributes to the development and progression of BC (Yang et al. 2018). Therefore, gene therapy is a promising approach that can revolutionize the BC treatment paradigm (Cardoso et al. 2012). Nevertheless, gene therapy is also very challenging owing to the issue of the safety and efficient delivery of therapeutic genes or gene-regulating products into the nucleus of mammalian cells.
Various drug and gene delivery systems, including viral and nonviral vectors, are being explored currently (Huo et al. 2017; Li et al. 2019; Maggio et al. 2020). Liposomes (ZununiVahed et al. 2017), polymeric (Zuris et al. 2015; Chen et al. 2017), and inorganic nanomaterials (Ma et al. 2015) are examples of nonviral vectors. The clinical application of viral vectors is hampered due to safety concerns and cargo size limitations. On the other hand, liposomes are well-established potential nanocarriers for drug/gene delivery with high loading capacity, ease of preparation, and excellent physiological compatibility (Zylberberg et al. 2017, Zuris et al. 2015; Chen et al. 2017).
Liposomes are established and absolute nanomaterials for drug/gene delivery (Pattni et al. 2015; Zylberberg et al. 2017). The predominant merits of liposomes are high loading capacity, convenient preparation, and excellent biocompatibility (Zuris et al. 2015; Wang et al. 2017a, b, c). Liposomes are chiefly composed of phospholipid molecules having hydrophobic tails and hydrophilic heads, forming the amphiphilic vesicle structures in aqueous solutions. Structurally, liposomes are alienated into small unilamellar vesicles (~ 100 nm) and large unilamellar vesicles (200–800 nm) with a single bilayer, and multilamellar vesicles (500–5000 nm) containing multiple bilayers. Owing to their amphipathic nature, liposomes can successfully encapsulate both hydrophilic and hydrophobic compounds (Olusanya et al. 2018a, b; Yang et al. 2021).
The mitigation of adverse effects of anticancer drugs for the patients can be accomplished via targeted liposomes (Federman and Denny 2010). The liposomes’ surface can be adapted by apt ligands to target the specific receptors of BC or its microenvironment to attain selective delivery. Triggering is one more approach that allows to control the local dose of the drug and, for example, initiate the drug release at a certain time point after accumulation of a required dose, depending upon the sensitivity of the tumor (Moussa et al. 2015). Nonetheless, liposomes represent a promising nanoparticle-based delivery system in cancer therapy including breast cancer.
In this chapter, we first discuss characteristics of breast cancer followed by recent achievements in liposome-based drug delivery for therapeutic intervention in breast cancer. The key challenges that need to be addressed to improve the utility of liposomes in clinical settings are also discussed.
10.2 Breast Cancer Characteristics and Novel Targets
The molecular mechanisms involved in BC pathophysiology, invasion, and metastasis are explicitly studied (Allred et al. 2001). The profound understanding of molecular events in BC resulted in novel targeted therapies. The different targets and receptors expressed on breast cancer cells along with their endogenous ligands and drugs that bind to them are summarized in Table 10.1.
Nonhormonal targets regulate the process of cell progression and mobility, cellular communications between cancer cells, and the microenvironment of the tumor. Hormonal targets primarily regulate cell differentiation. Steroid receptors and nonsteroid receptors are among the possible hormone targets. Signal transduction mediators, cell-cycle mediators, and angiogenesis mediators are the three categories of nonhormonal targets. There are five different types of steroid hormone receptors that have been implicated in the pathophysiology of breast cancer. Estrogen receptors (ERs), progesterone receptors (PRs), androgen receptors (ARs), glucocorticoid receptors (GRs), and mineralocorticoid receptors (MCRs) are some of them. The importance of estrogen receptors and progesterone receptors in breast cancer upregulation is well established. Vitamin D’s biological actions are mediated by the Vitamin D receptor (VDR) (Haussler et al. 1998), which plays a key role in the regulation of cell growth and differentiation. The VDRs are ligand-dependent nuclear receptors that regulate gene expression. In about 80–90% of breast cancer cases, VDR expression has been identified (Colston et al. 1989). Cellular retinol-binding proteins control the actions of every member of this family (Mangelsdorf et al. 1995). During breast carcinogenesis, RAR loss has been observed (Ariga et al. 2000). Thyroid hormones (tri-iodothyronine (T3) and the prohormone thyroxin) have also been studied in the context of breast cancer. T3 receptors are abundant in normal mammary epithelial cells, but how T3 acts on mammary epithelial cells at the cellular or molecular level is still unknown. The nuclear receptor superfamily includes three members (Tontonoz et al. 1994). PPARγ are transcription factors that belong to the nuclear receptor superfamily (Selliti et al. 1983). PPARγ is found primarily in adipose tissue and is expressed in both primary and metastatic breast cancers. Changes in gene expression occur when PPARγ is activated in breast cancer cells (Mueller et al. 1998). Natural prostaglandin (PGJ2) and synthetic antidiabetic thiazolidinediones are PPARγ ligands (Schwartz 1997). Hence, PPARγ serves as an interesting target for the prevention and treatment of breast cancer.
Signal transduction moderators perform as a messenger to control the cell cycle and communication between the cell’s intracellular and extracellular compartments (Aaronson 1991). The constituents of signal transduction could be categorized into cell surface receptors, growth factors, and intracellular signaling pathways.
Breast cancer has also been linked to members of the human EGFR family, particularly HER1 and HER2 (Bacus et al. 1994). HER2 is established as a promising therapeutic target for metastatic breast cancer as its activation results in neo-angiogenesis (Cobleigh et al. 1999; Viloria Petit et al. 1997).
The C-kit receptor tyrosine kinase expresses structural similarities with the macrophage colony-stimulating factor and the PDGF receptor (PDGFR) (Qiu et al. 1988). The appearance of c-Kit is critical for the maintenance of normal hematopoiesis as well as several other functions. Several studies have shown that c-Kit is expressed in both malignant and benign breast epithelia (Matsuda et al. 1993; DiPaola et al. 1997). Cancers of mesenchymal origin like human breast carcinoma have been linked to the PDGFR (Lokker et al. 2002; Bhardwaj et al. 1996). Ras is a membrane-bound GTP/GDP-binding (G) protein that converts signals from the cell surface to the nucleus (Valencia et al. 1991). Breast cancer has also been shown to regulate the expression of Rho C and RAC1. Rho C expression may also be a determinant of recurrence in axillary negative node breast cancer in patients, even in lesions just under 1 cm in length (Kleer et al. 2002).
The ability to regulate cell proliferation and apoptosis is crucial for the growth and development of cancer cells. Cyclins and CDKs have been linked to cell-cycle control (Sherr and Roberts 1999). TNF and the TNF-receptor family are two components of apoptotic cell death. Cell-cycle control and apoptosis are impossible without p53 working properly. The activation of p53 is essential for cells to respond effectively to stress stimuli like DNA damage and hypoxia (Giaccia and Kastan 1998). Growth arrest, senescence, or apoptosis are examples of the response (Asker et al. 1999). There is a link between p53 abnormalities and more aggressive tumors, early metastasis, and lower overall survival (Pharoah et al. 1999).
Angiogenesis is the formation of blood vessel networks to transport nutrients and oxygen to the tumor cells, as well as waste products. Many proangiogenic and antiangiogenic factors are involved in angiogenesis that can be used as molecular targets in the treatment of breast cancer. (Carmeliet 2000). Vascular endothelium cells are activated by angiogenesis factors such as b-FGF and VEGF, which secrete and activate MMPs and plasminogen activators. There are several factors involved in the expression of VEGF, a vascular endothelium cell-specific mitogen, which tends to increase tumor growth and angiogenesis. When VEGF binds to its receptor proteins, recognized as VEGF receptors 1 (Flt-1) and 2 (KDR/fek-1), signaling pathways that control cellular functions involved in new blood vessel formation are initiated (Ullrich and Schlessinger 1990). Small molecules with tyrosine kinase inhibitory activity or neutralizing antibodies (rhuMab-VEGF) are explored in the testing of BC for therapeutic VEGF targeting. Several proteinases, including MMPs, serine, and cysteine proteinases, are critical in the progression of cancer (Fox et al. 2001). High levels of uPA in the primary tumor have been found to have prognostic significance, including axillary node-negative breast cancer (Malmstrom et al. 2001). It has been discovered that uPA system antagonists impede tumorigenesis (Rabbani and Gladu 2002). In the integrin family, there are transmembrane subunit pairs and that is chosen from at least 16a and 8a subunits to form more than 20 heterodimeric receptors on the cell surface. Integrins a3 1 and a6 4 have been linked to the invasion and spread of mammary carcinoma (Mercurio et al. 2001). When it comes to cancer metastasis and invasion, specific modulators may be used to target members of the interleukin (IL) family, particularly a6 4 (and possibly a3 1). Another intriguing component of the angiogenesis process involves prostaglandins, which appear to be involved in cell proliferation, migration, and the ability to form new vessels (Rozic et al. 2001).
10.3 Different Types of Nanoliposomes-Based Drug Delivery in Breast Cancer
Liposomes represent an ideal approach with desirable characteristics prerequisite for targeted delivery of anticancer agents in BC therapeutic intervention. Examples of commercially available liposomes include Doxil® (PEGylated liposomal doxorubicin; Centocor Ortho Biotech Inc., USA), DaunoXome® (non-PEGylated liposomal daunorubicin; Diatos, France), and Myocet® (non-PEGylated liposomal doxorubicin; Sopherion Therapeutics, USA) (Misra et al. 2010). Liposomes can efficiently incorporate both lipophilic and hydrophilic compounds within the lipid bilayer and aqueous core phase, respectively. Liposomes possess good biocompatibility and a high drug loading capacity. Liposomes can be easily customized to achieve desirable attributes such as prolong blood circulation time and receptor-mediated site-specific dissemination (Wang et al. 2017a, b, c).
Recently, a plethora of research studies are being carried out to explore different aspects of liposomal-based therapeutic delivery to cancer cells. Functionalized liposomes are being explicitly explored at present to reap the benefits of biochemical and physiological differences between normal and cancerous tissue. The most promising strategy in tumor therapy is active targeting combined with a conjunction of other strategies, such as the stimuli-responsive targeting approach. Liposomes are transplanted with a variety of targeting ligands including peptides, dendrimers, and monoclonal antibodies employing apt surface engineering technology. Surface functionalization has played a remarkable role in the development of a liposomal delivery system for efficient targeting, endocytosis, and producing an optimal therapeutic response. Additionally, other advantages of liposomes include avoiding lysosomal degradation, high accumulation at the tumor site, and stimuli-responsive drug release at the desired location. Moreover, simultaneous diagnostic and therapeutic functions can be incorporated in liposomes (Durymanov et al. 2015). A typical structure of liposomes with their advantages is shown in Fig. 10.1.
Conventional Liposomes
It is constituted of phospholipid bilayers (Fig. 10.1) and, when injected intravenously, are prone to be engulfed by the reticuloendothelial system (RES), resulting in short circulation times.
They are destroyed by phagocytes because opsonin recognizes them as foreign substances.
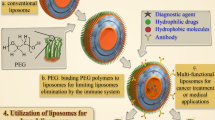
To enhance circulation time, the outer layer of the liposomes was coated with a hydrophilic polymer, namely polyethylene glycol (PEG), and termed as PEGylated or stealth liposomes (Hatakeyama et al. 2013). It improved the electrostatic repulsive between the liposomes and serum components. Stealth liposomes prevent opsonization and hence, extended circulation half-life. Caelyx® (liposomal doxorubicin; Merck & Co., Whitehouse Station, New Jersey, USA) is an indicator of the effective use of stealth liposomes in the chemotherapeutic agent. Figure 10.2 highlights the basic characteristic attributes of conventional and novel surface-engineered targeted liposomes.
Surface-engineered Liposomes
A diverse range of therapeutics, together with bioactives, have demonstrated potential anticancer efficacy via multiple mechanisms, including angiogenesis, tumor growth, invasion, and metastasis suppression. Despite their potential antitumor activity, they showed limited applications in cancer therapy (Liu et al. 2020; Feng et al. 2017). Perhaps the key reason is that their low hydrophobicity results in poor cellular uptake and devastated physicochemical stability. Furthermore, in animals, several bioactive constituents undergo a series of reactions that transform them into water-soluble metabolites, which are then excreted in urine and bile, and some drugs are also excreted in their original form. Because of their hydrophilic nature, they generally show low serum protein binding and are rapidly cleared up by the reticuloendothelial system (RES) (Zhan et al. 2014). Novel liposomal formulations are being developed that are surface-modified with a low molecular weight lipid conjugate and can be used in place of PEG. The formulations had very good stability characteristics in ion- and protein-rich mediums. Adsorption of proteins to the liposomal surface did not affect the cellular interaction. The limitation of PEGylation includes its potential to cause impaired cellular interactions, allergic reactions to be triggered, and an increase in IgM production after repeated dosing.
Multifunctional Liposomes
The multifunctional liposomes transport therapeutic molecules with excellent targeting and imaging properties. Liposomes with a single functionality face numerous challenges, and multifunctional liposomes have the potential to solve these problems. The formulation of nanoscale liposomes with a wide range of functions was made possible by combining surface-functionalization and modification techniques. Liposomes with two ligands, such as two peptides (Yuan et al. 2015), two ligands, and two anticancer drugs (Zhang et al. 2017), targeting ligand and an imaging agent (Erdogan and Torchilin 2017; Portnoy et al. 2011; Al-Jamal et al. 2008), have been reported by various studies (Fig. 10.3).
Liposomes and characteristics of its different types: Conventional liposomes are made of phospholipids (a); PEGylated/stealth liposomes contain a layer of polyethylene glycol (PEG) at the surface of liposomes (b); targeted liposomes contain a specific targeting ligand to target a cancer site (c); and multifunctional such as theranostic liposomes, which can be used for diagnosis and treatment of breast tumors (d). (Reproduced from Riaz et al. 2018)
Liposomes containing iron-oxide or metal have therapeutic and imaging properties in one package. The imaging in core and ligand on the liposome surface of a multifunctional carrier are decorated. HER-2 overexpressing breast cancer was imaged using an immunoliposome-encapsulated nitroxide sensor developed by Burks and coworkers (Burks et al. 2010). These multifunctional liposomes have been shown to preferentially generate an intracellular EPR signal in the cells overexpressed with HER-2. High nitroxide concentrations resulted in greatly reduced EPR spectral transmissions and endocytosis of liposomes produced by a cell-activated contrast-generating technique.
Stimuli-responsive Liposomes
The slow release of drugs from liposomes could be attributed to passive diffusion as the main mechanism of release from them. Stimuli-responsive liposomes can modulate the release rate of encapsulated drugs. Liposomes do not release their contents unless structural changes are induced by an endogenous or exogenous stimulus. These liposomes provide rapid release of drugs at the desired target sites and reduce the risk of the emergence of MDR tumors. Endogenous stimuli include pathological changes in the target cancer tissues, such as reduced pH (Karanth and Murthy 2007), overexpression of specific enzymes, and abundance of reducing agents (Deshpande et al. 2013).
Temperature-sensitive liposomes
Encapsulated bioactives are released near the liposome’s phase transition temperature, where the lipid membrane transitions from its gel to liquid crystal phase (Yatvin et al. 1978). Lipid bilayer transient pores are created by the incorporation of lysolipids into the liposomal membrane. These transient pores allow the rapid release of the encapsulants. Hyperthermia is the medical term for heating a tumor to a temperature that is slightly higher than normal (40–43 °C). Hyperthermia increases liposome uptake by two to three times (Leopold et al. 1992). Celsion Corporation has developed a thermoresponsive liposomal formulation of DOX, i.e., ThermoDox® for the treatment of various cancers, including breast cancer. ThermoDox®, when given intravenously and combined with hyperthermia, exhibited a potentially inhibiting effect on tumor growth.
Enzyme-Responsive Liposomes
Several enzyme concentrations are elevated in several pathological conditions, including cancer. Enzyme-responsive liposomes were developed using this approach (de la Rica et al. 2012; Yan and Boyd 2007). Secreted phospholipase A2 (sPLA2) levels increase substantially of various inflammatory conditions, atherosclerosis, and cancer. Prostate, breast, and pancreatic cancer exhibited elevated levels of sPLA2 (Dennis et al. 2011; Dong et al. 2006; Graff et al. 2001). Hence, sPLA2 responsive liposomes loading Doxorubicin were formulated with 1,2-distearyl-sn-glycero-3-phosphocholine (DSPC), 1,2-distearyl-sn-glycero-3-phosphoethanolamine (DSPE), cholesterol, and DSPE-PEG. The developed enzyme responsive liposomes exhibited a higher (2.5 times) decrease in tumor growth than conventional liposomes in a mouse model of prostate cancer. MMPs play a significant role in tumor growth, invasion, and metastasis (Curran and Murray 2000; Chambers and Matrisian 1997). Zhu et al. (2012) described enzyme MMP responsive liposomes comprising two kinds of components: TATp-PEG −1,2-dioctadecanoyl-sn-glycero-3-phosphoethanolamine and mAb 2C5-PEG-MMP2 cleavable peptide-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine to target hepatocellular tumor; PEG was attached to the phospholipid DOPE via an MMP-2 cleavable peptide linker. These MMP-sensitive peptides act as a linkage between the lipids and the polymer. The conjugated liposomes with polymer prevent liposome uptake. The peptide is cleaved when it comes into contact with MMPs at the target site, resulting in the dissociation of the polymer and finally uptake of liposomes. Liposomes’ galactose ligand is protected from cellular uptake by the PEG group, which forms a barrier on their surface. MMP-2 overexpression in HCC, on the other hand, cleaves the linker and releases PEG. The exposed galactose ligand then binds to HCC cells’ asialoglycoprotein receptors. Gal-PEG-DOPE (0.5 percent) liposome uptake was significantly enhanced in the presence of over 5 mcg/mL hMMP-2 in HepG2 cells, according to Terada et al. (Terada et al. 2006). An elevated level of a serine protease known as urokinase plasminogen activator (uPA) is reported in several human cancers, including breast, colon, bladder, and ovarian (Liu et al. 2001). Tumor angiogenesis, progression, and metastasis are aided by uPA (Duffy 2002). In peptides containing the consensus sequence Ser-Gly-Arg-Ser-Ala, the enzyme cleaves the Arg-Ser bond. Liposomes encapsulating uPA-cleavable peptides could thus discharge their encapsulated payload while they come into contact with uPA at tumor sites. It was discovered by Basel et al. that uPA-responsive liposomes in a hyperosmotic medium can be stabilized with a copolymer cage on their surface (Basel et al. 2011).
pH-sensitive Liposomes
Because of lactic acid production and ATP hydrolysis, the pH around the tumor cells is lower than healthy tissue (Gerweck and Seetharaman 1996). The endosomal or lysosomal partitions of tumor cells have low pH, which can be utilized to construct pH-responsive liposomal delivery models. The pH-sensitive liposomes systems are fabricated for extracellular or endosomal/lysosomal triggered release. The pH-responsive liposomes have been optimized so that they can transform at various stages between pH 7.4 and 5.0 to efficiently deliver anticancer agents at target sites. If pH is low because of pathological conditions like inflammation, infection, or malignancy, the pH-sensitive liposomes become soft and leaky (Torchilin et al. 1993). The pH-sensitive liposomes are intricately fabricated to circumvent the obstacles such as recognition and endocytosis by the RES, so that they could efficiently release their anticancer agents in the endosome and partially into the cytosol (Budker et al. 1996). Antitumor drugs, antigens, antisense oligonucleotides, and plasmid DNA have been delivered in vitro cytoplasmically using these methods (Legendre et al. 1992). Liposomal systems encoded with specific ligands can bind to and be taken up by cells, allowing for pH-mediated intracellular drug delivery (Harel and Kato 2007). Accumulation in low pH compartments, such as endosomes and lysosomes, can be utilized to trigger drug release after internalization in tumor cells. Karve and associates reported pH-sensitive immunoliposomes encapsulating DOX for site-specific delivery (Karve et al. 2009). The study demonstrated that pH-sensitive liposomes prevent the growth of cancer cells in a much better way than simple immunoliposomes, and hence, could potentially enhance the therapeutic prospect via liposomal chemotherapy.
10.4 Advances in Nanoliposomes-Based Drug Delivery for Therapeutic Intervention in Breast Cancer
Cationic liposomes are being investigated as a possible efficient method for gene delivery. The complex formed between encapsulated plasmids DNA (Kaneda et al. 1989) and cationic lipids of liposomes is termed as lipoplex (Alino et al. 1996; Crystal 1995). The lipoplex can be fused with the plasma or endosome membrane to transport into cells (Sharma and Sharma 1997). The lipid composition, lipid/DNA ratio, and particle size of the liposome–DNA complex greatly impact their transfection efficiency (Zou et al. 2000). The limitation of cationic liposomes is their poor specificity. This limitation can be addressed by modifying cationic liposomes with cell-specific targeting moieties. For direct cytosolic delivery of liposomes, several cell-penetrating peptides have been implicated. For breast cancer gene therapy, antiangiogenesis strategies involve nonviral vectors (Feldman and Libutti 2000). Angiostatin and endostatin gene therapy is one way to deliver angiogenic polypeptide inhibitors. Previously, liposomes complexed with plasmids encoding angiostatin (PCI-Angio) or endostatin (PCIEndo) exhibited successful inhibition of angiogenesis in BC cells (Chen et al. 1999). Such liposomal delivery of antiangiogenic genes could represent promising therapeutic prospects. The wild-type p53 gene was also included in a liposome–plasmid complex used to treat naked mice injected with breast carcinoma cells (Lesoon-Wood et al. 1995). Xu et al. investigated a cationic immunoliposome tagged with single-chain antibody Fv fragment (scFv) for systemic p53 tumor suppressor gene therapy for treating BC (Xu et al. 2001). The scFv-tagged immunolipoplexes significantly improved transfection efficiency and extended the animals’ survival time. However, the scFv-targeted immunoplex’s expression was found to be low. A new expression strategy for anti-TfRscFv was reported by Xu and colleagues (Xu et al. 2002). In this, the scFv was covalently coupled to the liposome via a cysteine at the 3′ end of the protein and a maleimide group on the liposome. According to the findings, the immunological activity and targeting ability of the scFv were not affected by this conjugation. The scFv-cys-targeted tumor cells with a cationic liposome–DNA complex (lipoplex) markedly improved transfection efficiencies in BC models. BC cells overexpress the human HER-2/neu oncogene, and research has shown that the E1A gene acts as a tumor inhibitor gene by reducing HER-2/neu transcription. tgDCC-E1A, a stable E1A lipid complex, was developed by Yoo and coworkers using cationic liposomes and plasmid DNA encoding E1A (Yoo et al. 2001). In accordance with the results of preliminary tests carried out on animals as well as on humans, researchers moved forward with clinical trials to see if delivering the E1A gene via liposomes would be safe and would have an effect on tumor response when administered to cancer patients with an advanced form of BCs. Recent advancements in the use of liposomes as a diagnostic tool underscore the use of imaging techniques and the recognition of diverse molecular targets. There are numerous medical imaging techniques that make use of liposomes as nanomedicines. Some of these methodologies encompass ultrasound, nuclear imaging, fluorescence, and magnetic resonance.
Fluorescence imaging is the most commonly used diagnostic tool. Imagining the position of the biomolecule, enzyme activity as well as gene expression is also possible in living cells or tissues. Functionalized quantum dot–liposome (f-QD-L) was developed by encompassing quantum dots by PEG (QD) for cancer diagnosis (Chen et al. 2000). There has been a lot of interest in NIR fluorescence imaging because of the low photon absorption and scattering (Min et al. 2014). Rare-earth-doped nanoparticles are one material under consideration for use in NIR fluorescence (Eliseeva and Bunzli 2010; Bunzli 2010). Using a liposome-nanoparticle hybrid, Soga and coworkers found that it has a strong NIR fluorescence component.
Radiofrequency pulses are commonly used in MRI for whole-body imaging (Sun et al. 2008). Liposomes can encapsulate a wide range of contrast agents like fluorophores, enabling efficient implementation and controlled release of probes for better image analysis (Kamaly et al. 2009; Soenen et al. 2011). Studies have been reported in which liposomes were concomitantly loaded with the MRI contrast agent Gd-DTPA and doxorubicin (DOX) (Tagami et al. 2011). The simultaneous delivery of Gd-DTPA and DOX allows controlled drug release in a tumor’s microenvironment where localized heating could potentially trigger the release of a drug. Another paramagnetic MRI contrast agent is ferrimagnetic iron oxide (FMIO) nanoparticles, which have been used in the preparation of liposomal MRI probes (Mikhaylov et al. 2011). An external magnetic field was used to target the liposomal FMIO nanoparticle cluster at tumors and the tumor microenvironment.
Medical ultrasound, which is defined as sound waves with frequencies greater than 20,000 Hz, is another commonly used noninvasive diagnostic imaging technique. (Cheng et al. 2010) Ultrasound imaging is performed by directing ultrasound pulses into tissue and measuring the echoes caused by the tissue at various reflection angles. Contrast agents for ultrasound imaging, like MRI, have the ability to label specific tissue types or tumors. Acoustic liposomes (ALs), which contain perfluoropropane gas, can be used as ultrasound imaging probes (Deckers and Moonen 2010). For passive tumor tissue localization, high-permeability/high-retention acoustic liposomes can be used because of their small diameter (100 nm) and high retention effect (90% retention). With the help of high-frequency ultrasound (HF-US), acoustic liposomes can be used to test both drug delivery efficiency and antitumor efficacy (Ferrara et al. 2009). Using HF-US imaging, a cisplatin-loaded AL was evaluated for its antitumor effects (Kodama et al. 2011). Nuclear imaging is another noninvasive imaging technique that uses small molecule radioactive tracers (Srivatsan and Chen 2014). PET (positron emission tomography) works by detecting gamma rays emitted from the destruction of positrons of radioactive materials. SPECT (single-photon emission computed tomography) is able to detect gamma rays directly emitted from isotopes such as 99mTc (Rahmim and Zaidi 2008). Various research studies have been reported indicating that liposomes can encapsulate radionuclide tracers inside their aqueous compartment or within a chemically engineered lipid bilayer (Henriksen et al. 2015; Ogawa et al. 2014; Seo et al. 2008; Petersen et al. 2011). Ferrara and colleagues designed temperature-sensitive liposomes using a combination of PET and fluorescence imaging (Paoli et al. 2010). They used 18F- or 64Cu-labeled lipids in liposome preparations, which embody the fluorophore Alexa Fluor 750 as a hydrophilic model drug. The tumor fluorescence images correlate well with PET and ex vivo fluorescence images when using a stable liposome formulation. Hence, a combination of PET and optical imaging techniques could be very beneficial for alleviating tumors.
188Re-labeled DSPC-based liposomal doxorubicin was fabricated and evaluated their targeting efficiency and antitumor effects in a C26murine tumor model by SPECT imaging (Chang et al. 2010). In in vivo micro, SPECT/CT imaging was used to assess the liposomes tumor targeting by measuring their biodistribution and pharmacokinetics. The bimodal radiochemotherapeutic 188Re-liposome-DOX showed greater tumor inhibition and a longer median survival time than either single-functionalized 188Re-liposomes or liposome-DOX.
10.5 Challenges in Translation of Nanoliposomes-Based Drug Delivery in Clinical Settings
The advancements in the development of liposome delivery systems are progressing at a fast pace, in light of the demand for the new stratagems for breast cancer therapy. However, a well-built understanding or road map on the design of the new liposome formulation for BC is lacking somewhere. Selection of the targeting and triggering modalities depends on the molecular subtypes of the tumor and the ongoing conventional treatments. Although the conventional liposome-encapsulated chemotherapeutic drug-based formulation is being frequently used in the clinical practice in BC treatment, still there are countless obstacles in the clinical implementation of these novel and advance versions of liposome formulations (Yang et al. 2021). With triggerable liposomes, the triggering mechanisms need to be further explored while designing such liposome formulations. For example, the selection of the phospholipid component for light-triggered liposomes needs to be in accordance with desired photo-induced mechanisms. In a photochemical pathway, for instance, photooxidative reaction, unsaturated phospholipids should be used in the formulation (Yang et al. 2021). Furthermore, active ingredients employed in the triggerable liposome formulation should be optimized to weigh up their benefits and risks to healthy tissues (Yang et al. 2021). From the perspective of the clinical applications, the far-reaching development of liposome technology will ultimately benefit BC patients. Recent studies confirmed that various liposome constructs loaded with drugs can reduce the intensities of cardiotoxicity, address drug resistance, and improve the overall drug release profile. Modification of the liposome surface with targeting ligands offers surplus opportunities for designing site-specific therapy and curtailing the nonspecific effect of conventional chemotherapeutic drugs. The new genera liposomes with triggering features also allow efficient control of payload release and thus substantially augmenting the therapeutic outcomes for BC patients. Advance forms of liposome formulations could magnify the assortment of drug delivery options for the treatments of BC by efficaciously addressing the critical problems of drug toxicity and limited therapeutic effects.
10.6 Conclusion
The liposomes represent a promising approach as efficient and targeted delivery of anticancer agents to various tumor sites in BC alleviation. The advantages of encapsulating the drug in the nanoliposomal carrier are improved solubilization of the drug, increased half-life, prolonged circulation, selective accessibility of the drug to the target site, and substantially overcoming of multidrug resistance. The advanced and modified versions of liposomes as discussed in this chapter curtail the highly desirable characteristic features of tumor cell recognition and internalizing that could contribute substantially to the therapeutic intervention of BC. Precise molecular targeting can be accomplished with these smart generations of nanoliposomes, besides enhanced pharmacokinetic and biodistribution of anticancer agents. The integrated approaches such as theranostic liposomes or antibody-targeted ones can be efficiently employed for targeting small molecule drugs as well as biological agents with anticancer activity and can provide a new landscape in the liposomes-based BC therapeutic front. Earlier, PEGylated liposomes have addressed critical pharmacological concerns accompanied with the conventional drug delivery system, such as disruption by blood lipoproteins, uptake by RES, and rapid clearance from blood circulation. Now PEGylated liposomes are approved by regulatory authorities and have successfully reached the market. However, the clinical success of PEGylated liposomes is hampered by some grave limitations, such as a lack of tumor cell specificity. Therefore, to increase the target specificity and the amount of released therapeutic agent at the tumor site, stimuli-sensitive liposomes and multifunctional carriers such as theranostic liposomes have been designed. The increasing complexity of advanced versions of liposomal formulation needs rigorous in vitro and in vivo preclinical studies for their translation to the clinics. Certainly, recent research studies demonstrate that new generation liposomes could be a viable anticancer therapeutic tool in the treatment of BC.
References
Aaronson SA (1991) Growth factors and cancer. Science 254:1146–1153
Alino SF, Bobadilla M, Crespo J, Lejarreta M (1996) Human a1-antitrypsin gene transfer to in vivo mouse hepatocytes. Hum Gene Ther 7:531–536
Al-Jamal W, Al-Jamal K, Bomans P, Frederik P, Kostarelos K (2008) Functionalized-quantum-dot-liposome hybrids as multimodal nanoparticles for cancer. Small 4(9):1406–1415
Allred DC, Mohsin S, K, Fuqua S.A. (2001) Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer 8:47–61
Ariga N, Moriya T, Suzuki T, Kimura M, Ohuchi N, Sasano H (2000) Retinoic acid receptor and retinoid X receptor in ductal carcinoma in situ and intraductal proliferative lesions of the human breast. Jap J Cancer Res 91:1169–1176
Asker C, Wiman KG, Selivanova G (1999) p53-induced apoptosis as a safeguard against cancer. Biochem Biophys Res Comm 265:1–6
Bacus SS, Zelnick CR, Plowman G, Yarden Y (1994) Expression of the erbB-2family of growth factor receptors and their ligands in breast cancers. Implication for tumor biology and clinical behavior. Am J Clin Pathol 102:S13–S24
Basel MT, Shrestha TB, Troyer DL, Bossmann SH (2011) Protease-sensitive, polymer-caged liposomes: a method for making highly targeted liposomes using triggered release. ACS Nano 5:2162–2175
Bhardwaj B, Klassen J, Cossette N et al (1996) Localization of platelet-derived growth factor a receptor expression in the periepithelial stroma of human breast carcinoma. Clin Cancer Res 2:773–782
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Budker V, Gurevich V, Hagstrom JE, Bortzov F, Wolff JA (1996) pH sensitive, cationic liposomes: a new synthetic virus-like vector. Nat Biotechnol 14:760–764
Bunzli JCG (2010) Lanthanide luminescence for biomedical analyses and imaging. Chem Rev 110:2729–2755
Burks SR, Macedo LF, Barth ED et al (2010) Anti-HER2 immunoliposomes for selective delivery of electron paramagnetic resonance imaging probes to HER2-overexpressing breast tumor cells. Breast Cancer Res Treat 124:121–131
Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, Group E.G.W (2012) Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatmentand follow-up. Ann Oncol 23(7):11–19
Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nature Med 6:389–395
Chambers AF, Matrisian LM (1997) Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 89:1260–1270
Chang YJ, Chang CH, Yu CY, Chang TJ, Chen LC, Chen MH et al (2010) Therapeutic efficacy and micro SPECT/CT imaging of re-188-DXR-liposome in a C26 murine colon carcinoma solid tumor model. Nucl Med Biol 37:95–104
Chen J, Wu W, Tahir SK et al (2000) Down-regulation of survivin by antisense oligonucleotides increases apoptosis, inhibits cytokines and anchorage-independent growth. Neoplasia 2000(2):235–241
Chen QR, Kuma D, Stass SA, Mixson AJ (1999) Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice. Cancer Res 59:3308–3312
Chen WJ, Deng W, Goldys EM (2017) Light-triggerableliposomes for enhanced endolysosomal escape and gene silencingin PC12 cells. Mol Ther-Nucl Acids 7:366–377
Cheng HD, Shan J, Ju W, Guo YH, Zhang L (2010) Automated breast cancer detection and classification using ultrasound images: A survey. Pattern Recogn 43:299–317
Cobleigh MA, Vogel CL, Tripathy D et al (1999) Multinational study of the efficacy and safety of humanized anti-HER2antibody in women who have HER2-overexpressing breast cancer that has progressed after therapy for metastatic disease. J Clin Oncol 17:2639–2648
Colston KW, Berger U, Coombes RC (1989) Possible role for vitamin D in controlling breast cancer cell proliferation. Lancet 28:188–191
Crystal RG (1995) Transfer of genes to humans: early lesions and obstacles to success. Science 270:404–410
Curran S, Murray GI (2000) Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer 36:1621–1630
de la Rica R, Aili D, Stevens MM (2012) Enzyme-responsive nanoparticles for drug release and diagnostics. Adv Drug Deliv Rev 64:967–978
Deckers R, Moonen CTW (2010) Ultrasound triggered, image guided, local drug delivery. J Control Release 148:25–33
Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G (2011) Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 111:6130–6185
Deshpande P, Biswas S, Torchilin V (2013) Current trends in the use of liposomes for tumor targeting. Nanomedicine 8(9):1509–1528
DiPaola RS, Kuczynski W, Onodera K et al (1997) Evidence for a functional kit receptor in melanoma, breast, and lung carcinoma cells. Cancer Gene Ther 4:176–182
Dong Q, Patel M, Scott KF, Graham GG, Russell PJ, Sved P (2006) Oncogenic action of phospholipase A2 in prostate cancer. Cancer Lett 240:9–16
Duffy MJ (2002) Urokinase-type plasminogen activator: a potent marker of metastatic potential in human cancers. Biochem Soc Trans 30:207–210
Durymanov MO, Rosenkranz AA, Sobolev AS (2015) Current approaches for improving intratumoralaccumulation and distribution of nanomedicines. Theranostics 5:1007–1020
Eliseeva SV, Bunzli JCG (2010) Lanthanide luminescence for functional materials and bio-sciences. Chem Soc Rev 39:189–227
Erdogan S, Torchilin VP (2017) Gadolinium-loaded polychelating polymer-containing tumor-targeted liposomes. In: Methods in molecular biology (Clifton, N.J.), vol 1522. Springer, Berlin, Germany, pp 179–192
Federman N, Denny CT (2010) Targeting liposomes toward novel pediatric anticancer therapeutics. Pediatr Res 67(5):514–519
Feldman AL, Libutti SK (2000) Progress in antiangiogenic gene therapy. Cancer 89:1181–1194
Feng T, Wei Y, Lee R, Zhao L (2017) Liposomal curcumin and its application in cancer. Int J Nanomedicine 12:6027–6044
Ferrara KW, Borden MA, Zhang H (2009) Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery. Acc Chem Res 42:881–892
Fox SB, Taylor M, Grondahl-Hansen J, Kakolyris S, Gatter KC, Harris AL (2001) Plasminogen activator inhibitor-1 as a measure of vascular remodeling in breast cancer. J Pathol 195:236–243
Gerweck LE, Seetharaman K (1996) Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res 56:1194–1198
Giaccia AJ, Kastan MB (1998) The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev 12:2973–2983
Graff JR, Konicek BW, Deddens JA, Chedid M, Hurst BM, Colligan B, Neubauer BL, Carter HW, Carter JH (2001) Clin Cancer Res 7:3857–3861
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F (2019) Breast cancer Nat Rev Dis Primers 5:66
Harel YE, Kato Y (2007) Intracellular delivery of nanocarriers for cancer therapy. Curr Nanosci 3:329–338
Hatakeyama H, Akita H, Harashima H (2013) The polyethylene glycol dilemma: advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol Pharm Bull 36:892–899
Haussler MR, Whitfield GK, Haussler CA et al (1998) The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Min Res 13:325–349
Henriksen JR, Petersen AL, Hansen AE, Frankaer CG, Harris P, Elema DR et al (2015) Remote loading of 64Cu2+ into liposomes without the use of ion transport enhancers. ACS Appl Mater Interfaces 7:22796–22806
Huo WY, Zhao GN, Yin JG, Ouyang X, Wang YN, Yang CH, Wang BJ, Dong PX, Wang ZX, Watari H, Chaum E, Pfeffer LM, Yue JM (2017) Lentiviral CRISPR/Cas9 vector mediated miR-21gene editing inhibits the epithelial to mesenchymal transition inovarian cancer cells. J Cancer 8(1):57–64
Kamaly N, Kalber T, Thanou M, Bell JD, Miller AD (2009) Folate receptor targeted bimodal liposomes for tumor magnetic resonance imaging. Bioconjug Chem 20:648–655
Kaneda Y, Iwai K, Uchida T (1989) Increased expression of DNA cointroduced with nuclear protein in adult rat liver. Science 243:375–378
Karanth H, Murthy R (2007) pH-sensitive liposomes-principle and application in cancer therapy. J Pharm Pharmacol 59(4):469–483
Karve S, Alaouie A, Zhou Y, Rotolo J, Sofou S (2009) The use of pH-triggered leaky heterogeneities on rigid lipid bilayers to improve intracellular trafficking and therapeutic potential of targeted liposomal immunochemotherapy. Biomaterials 30:6055–6064
Kleer CG, van Golen KL, Zhang Y, Wu ZF, Rubin MA, Merajver SD (2002) Characterization of RhoC expression in benign and malignant breast disease: a potential new marker for small breast carcinomas with metastatic ability. Am J Pathol 160:579–584
Kodama T, Tomita N, Yagishita Y, Horie S, Funamoto K, Hayase T et al (2011) Volumetric and Angiogenic evaluation of antitumor effects with acoustic liposome and high-frequency ultrasound. Cancer Res 71:6957–6964
Legendre JY, Szoka FC Jr (1992) Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes. Pharm Res 9:1235–1242
Leopold KA, Dewhirst MW, Samulski TV et al (1992) Relationship among tumor temperature, treatment time, and histopathological outcome using preoperative hyperthermia with radiation in soft tissue sarcomas. Int J Radiat Oncol Biol Phys 22:989–998
Lesoon-Wood LA, Kim WH, Kleinman HK, Weintraub BD, Mixson AJ (1995) Systemic gene therapy with p53 reduces growth and metastases of a malignant human breast cancer in nude mice. Hum Gene Ther 6:395–405
Li A, Lee CM, Hurley AE, Jarrett KE, De Giorgi M, Lu WQ, Balderrama KS, Doerfler AM, Deshmukh H, Ray A, Bao G, Lagor WR (2019) A self-deleting AAV-CRISPR system forin vivo genome editing. MolTher-Meth Clin D 12:111–122
Liu S, Bugge TH, Leppla SH (2001) Targeting of tumor cells by cell surface urokinase plasminogen activator-dependent anthrax toxin. J Biol Chem 276:17976–17984
Liu Y, Xie X, Hou X, Shen J, Shi J, Chen H, He Y, Wang Z, Feng N (2020) Functional oral nanoparticles for delivering silibinin and cryptotanshinone against breast cancer lung metastasis. J Nanobiotechnology 18(1):83
Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA (2002) Platelet-derived growth factor (PDGF) autocrine signalling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res 62:3729–3735
Lu C, Shieh G, Wang C, Su B, Su Y, Chen Y et al (2016) Chemotherapeutics-induced Oct4 expression contributes to drug resistance and tumor recurrence in bladder cancer. Oncotarget 8(19):30844–30858
Ma PA, Xiao HH, Li CX, Dai YL, Cheng ZY, Hou ZY, Lin J (2015) Inorganic nanocarriers for platinum drug delivery. Mater Today 18(10):554–564
Maggio I, Zittersteijn HA, Wang Q, Liu J, Janssen JM, Ojeda IT, van der Maarel SM, Lankester AC, Hoeben RC, Goncalves MAFV (2020) Integrating gene delivery and gene-editing technologiesby adenoviral vector transfer of optimized CRISPRCas9components. Gene Ther 27:209–225
Malmstrom P, Bendahl PO, Boisen P, Brunner N, Idvall I, Ferno M (2001) S-phase fraction and urokinase plasminogen activator are better markers for distant recurrences than Nottingham prognostic index and histologic grade in a prospective study of premenopausal lymph node-negative breast cancer. J Clin Oncol 19:2010–2019
Mangelsdorf DJ, Thummel C, Beato M et al (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839
Matsuda R, Takahashi T, Nakamura S et al (1993) Expression of the c-kit protein in human solid tumors and in corresponding fetal and adult normal tissues. Am J Pathol 142:339–346
Mercurio AM, Bachelder RE, Chung J et al (2001) Integrin laminin receptors and breast carcinoma progression. J Mammary Gland Biol Neoplasia 6:299–309
Mikhaylov G, Mikac U, Magaeva AA, Itin VI, Naiden EP, Psakhye I et al (2011) Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat Nanotechnol 6:594–602
Min YZ, Li JM, Liu F, Padmanabhan P, Yeow EKL, Xing BG (2014) Recent advance of biological molecular imaging based on lanthanide-doped Upconversion-luminescent nanomaterials. Nano 4:129–154
Misra R, Acharya S, Sahoo S (2010) Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov Today 15(19–20):842–850
Moussa HG, Martins AM, Husseini GA (2015) Review on triggered liposomal drug delivery with a focus on ultrasound. Curr Cancer Drug Tar 15(4):282–313
Mueller EF, Sarraf P, Tontonoz P et al (1998) Terminal differentiation of human breast cancer through PPAR. Mol Cell 1:465–470
Nosrati H, Salehiabar M, Manjili H, Danafar H, Davaran S (2018) Preparation of magnetic albumin nanoparticles via a simple and one-pot desolvation and co-precipitation method for medical and pharmaceutical applications. Int J Biol Macromol 108:909–915
Ogawa M, Umeda IO, Kosugi M, Kawai A, Hamaya Y, Takashima M et al (2014) Development of in-111-labeled liposomes for vulnerable atherosclerotic plaque imaging. J Nucl Med 55:115–120
Olusanya TOB, Haj Ahmad RR, Ibegbu DM, Smith JR, Elkordy AA (2018a) Liposomal drug delivery systems and anticancer drugs. Molecules Molecules 23(4):907
Olusanya TOB, Ahmad RRH, Ibegbu DM, Smith JR, Elkordy AA (2018b) Liposomal drug delivery systems and anticancer drugs. Molecules 23(4):907
Paoli EE, Kruse DE, Seo JW, Zhang H, Kheirolomoom A, Watson KD et al (2010) An optical and micro PET assessment of thermally-sensitive liposome biodistribution in the Met-1 tumor model: importance of formulation. J Control Release 143:13–22
Pattni BS, Chupin VV, Torchilin VP (2015) New developments in liposomal drug delivery. Chem Rev 115(19):10938–10966
Petersen AL, Binderup T, Rasmussen P, Henriksen JR, Elema DR, Kjaer A et al (2011) 64Cu loaded liposomes as positron emission tomography imaging agents. Biomaterials 32:2334–2341
Pharoah PDP, Day NE, Caldas C (1999) Somatic mutations in the p53 gene and prognosis in breast cancer: a meta-analysis. Br J Cancer 80:1968–1973
Portnoy E, Lecht S, Lazarovici P, Danino D, Magdassi S (2011) Cetuximab-labeled liposomes containing near-infrared probe for in vivo imaging. Nanomedicine 7(4):480–488
Qiu F, Ray P, Brown K et al (1988) Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family–oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J 7:1003–1011
Rabbani SA, Gladu J (2002) Urokinase receptor antibody can reduce tumor volume and detect the presence of occult tumor metastases in vivo. Cancer Res 62:2390–2397
Rahmim A, Zaidi H (2008) PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun 29:193–207
Riaz MK, Riaz MA, Zhang X, Lin C, Wong KH, Chen X, Zhang G, Lu A, Yang Z (2018) Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int J Mol Sci 19(1):195
Rozic JH, Chakraborty C, Lala PK (2001) Cyclooxygenase inhibitors retard murine mammary tumor progression by reducing tumor cell migration, invasiveness and angiogenesis. Int J Cancer 93:497–506
Schwartz MA (1997) Integrins, oncogenes, and anchorage independence. J Cell Biol 139:575–578
Selliti D, Tseng Y, Latham K (1983) Nuclear thyroid hormone receptors in C3H/HeN mouse mammary glands and spontaneous tumors. Cancer Res 43:1030–1038
Seo JW, Zhang H, Kukis DL, Meares CF, Ferrara KWA (2008) Novel method to label preformed liposomes with 64Cu for positron emission tomography (PET) imaging. Bioconjug Chem 19:2577–2584
Sharma A, Sharma US (1997) Liposomes in drug delivery: progress and limitations. Int J Pharm 154:123–140
Sherr C, Roberts JM (1999) CDK inibitors: positive and negative regulators of G1-phase progression. Genes Dev 13:1501–1512
Soenen SJ, VandeVelde G, Ketkar-Atre A, Himmelreich U, De Cuyper M (2011) Magnetoliposomes as magnetic resonance imaging contrast agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol 3:197–211
Srivatsan A, Chen XY (2014) Recent advances in nanoparticle-based nuclear imaging of cancers. Emerging Applications of Molecular Imaging to Oncol 124:83–129
Sun C, Lee JSH, Zhang MQ (2008) Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliver Rev 60:1252–1265
Tagami T, Foltz WD, Ernsting MJ, Lee CM, Tannock IF, May JP et al (2011) MRI monitoring of intratumoral drug delivery and prediction of the therapeutic effect with a multifunctional thermosensitive liposome. Biomaterials 32:6570–6578
Terada T, Iwai M, Kawakami S, Yamashita F, Hashida M (2006) J Control Release 111:333–342
Tontonoz P, Hu E, Graves RA, Budavari A, Spiegelman BM (1994) mPPAR tissue-specific regulator of an adipocyte enhancer. Genes Dev 8:1224–1234
Torchilin VP, Zhou F, Huang L (1993) pH-sensitive liposomes. J Liposome Res 3:201–205
Ullrich A, Schlessinger J (1990) Signal transduction by receptors with tyrosine kinase activity. Cell 61:203–212
Valencia A, Chardin P, Wittinghofer A, Sander C (1991) The ras protein family: evolutionary tree and role of conserved amino acids. Biochemist 30:4637–4648
Viloria Petit AM, Rak J, Hung MC et al (1997) Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol 151:1523–1530
Wang HX, Li M, Lee CM, Chakraborty S, Kim HW, Bao G, Leong KW (2017a) CRISPR/Cas9-based genome editing fordisease modeling and therapy: challenges and opportunities fornonviral delivery. Chem Rev 117(15):9874–9906
Wang M, Zuris JA, Meng FT, Rees H, Sun S, Deng P, Han Y, Gao X, Pouli D, Wu Q, Georgakoudi I, Liu DR, Xu QB (2017b) Efficient delivery of genome-editing proteins using bioreduciblelipid nanoparticles. Proc Natl Acad Sci U S A 113(11):2868–2873
Wang X, Wang Q, Liu Z, Zheng X (2017c) Preparation, pharmacokinetics and tumour-suppressive activity of berberine liposomes. J Pharm Pharmacol 69(6):625–632.2
Xu L, Huang C, Huang W, Tang WH, Rait A, Yin YZ (2002) Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes. Mol Cancer Therap 1:337–346
Xu L, Tang WH, Huang CC, Alexander W, Xiang LM, Pirollo KF (2001) Systemic p53 gene therapy of cancer with immunolipoplexes targeted by anti-transferrin receptor scFv. Mol Med 7:726–738
Yan C, Boyd DD (2007) Regulation of matrix metalloproteinase gene expression. J Cell Physiol 211:19–26
Yang B, Song BP, Shankar S, Guller A, Deng W (2021 Jul) Recent advances in liposome formulations for breast cancer therapeutics. Cell Mol Life Sci 78(13):5225–5243
Yang HT, Jaeger M, Walker A, Wei D, Leiker K, Tao WT (2018) Break breast cancer addiction by CRISPR/Cas9 genome editing. J Cancer 9(2):219–231
Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R (1978) Design of liposomes forenhanced local release of drugs byhyperthermia. Science 202:1290–1293
Yoo GH, Hung MC, Lopez-Berestein G, LaFollette S, Ensley JF, Carey M (2001) Phase I trial of intratumoral liposome E1A gene therapy in patients with recurrent breast and head and neck cancer. Clin Cancer Res 7:1237–1245
Yuan D, Zong T, Gao H, He Q (2015) Cell penetrating peptide TAT and brain tumor targeting peptide T7 dual modified liposome preparation and in vitro targeting evaluation. Yao XueXueBao 50:104–110
Zhan S, Guo W, Shao Q, Fan X, Li Z, Cheng Y (2014) A pharmacokinetic and pharmacodynamic study of drug–drug interaction between ginsenoside Rg1, ginsenoside Rb1 and schizandrin after intravenous administration to rats. J Ethnopharmacol 152(2):333–339
Zhang Y, Zhai M, Chen Z, Han X, Yu F, Li Z et al (2017) Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv 24(1):1045–1055
Zhu D, Tao W, Zhang H, Liu G, Wang T, Zhang L et al (2016) Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Acta Biomater 30:144–154
Zhu L, Kate P, Torchilin VP (2012) Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano 6:3491–3498
Zou Y, Zong G, Ling YH, Perez-Soler R (2000) Development of cationic liposome formulations for intratracheal gene therapy of early lung cancer. Cancer Gene Ther 7:683–696
ZununiVahed S, Salehi R, Davaran S, Sharifi S (2017) Liposome-based drug co-delivery systems in cancer cells. Mater Sci Eng C Mater Biol Appl 71:1327–1341
Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY, Liu DR (2015) Cationic lipidmediateddelivery of proteins enables efficient protein-basedgenome editing in vitro and in vivo. Nat Biotechnol 33(1):73–80
Zylberberg C, Gaskill K, Pasley S, Matosevic S (2017) Engineeringliposomal nanoparticles for targeted gene therapy. Gene Ther 24(8):441–452
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mustafa, G., Ahmad, M.Z., Aslam, M., Garg, A., Ahmad, J. (2022). Nanoliposomal System for Breast Cancer Therapy. In: Rahman, M., H Almalki, W., Alrobaian, M., Beg, S., Alharbi, K.S. (eds) Hormone Related Cancer Mechanistic and Nanomedicines. Springer, Singapore. https://doi.org/10.1007/978-981-19-5558-7_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-5558-7_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5557-0
Online ISBN: 978-981-19-5558-7
eBook Packages: MedicineMedicine (R0)