Abstract
The most common indication for admission in NICU is respiratory distress. Bronchoscopy is an important tool in the process of diagnosis of most neonatal respiratory disorders such as airway and lung pathologies, and to determine the cause of difficulty to wean neonates. Fiberoptic Bronchoscopy (FOB) is gradually replacing rigid bronchoscopy in neonates. Bronchoscopy can be done under general anesthesia with a tracheal tube in situ. For dynamic airway evaluation, it needs to be done in a spontaneously breathing baby, under mild to moderate sedation. This poses a challenge in neonates, because of the airway invasiveness, underlying pathological indication, and procedure-related complications. However, evidence shows that when done systematically by experienced hands, it is safe in neonates.
In this chapter we will discuss the indications, contraindications, limitations, technique, equipment, pre-procedural preparation, and sedation techniques for FOB in neonates.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Respiratory disorders are the commonest indication of admission in the NICU and are responsible for increased morbidity and mortality in the neonate. Bronchoscopy is an useful adjunct to diagnose and treat many respiratory and airway-related illnesses and pathologies.
In earlier times, until the introduction of the fiberoptic technology, rigid bronchoscopy was performed by the surgeons in small babies. Because of the wall thickness of the rigid scope the internal diameter was much smaller compared to the FOB, making clear detailed visualization a problem. It not only required smaller bronchoscope, but also general anesthesia with muscle relaxants, thereby making it more invasive and high risk in the neonates. It is now replaced by FOB, except in centers where appropriate size fiberoptic bronchoscope or expertise is not available.
Fiberoptic bronchoscopy (FOB) has been in clinical practice in adults since 1967. Robert Wood pioneered FOB in children in 1978, but it was in 1990s that it was first performed in neonates, but with limited use restricted mainly to the quaternary NICU setups. Technological advancement and better understanding of the neonatal anatomy and physiology have opened new doors in neonatal anesthesia and intensive care. The neonatal fiberoptic bronchoscopes are gradually being made available to many centers now, and the anesthesiologists are beginning to gain expertise and experience in the procedure.
2 Fiberoptic Bronchoscopy in Neonates
It was only in the last two decades that neonatal FOB has been truly possible, and in India mostly in the last 5 years. Limitations to its routine use are nonavailability of appropriately sized scopes, and/or the patient (neonate) was deemed to be too sick to undergo airway-related non-therapeutic procedure. It was mostly performed by either the otorhinolaryngologist, pulmonologist, or the neonatologist. Many of these centers have an airway team including all the above, and a pediatric surgeon, who would take a combined decision regarding the indication, best person, and place (NICU or operation theater) to perform the procedure. Anesthesiologists were usually not a part of the team. It is only in the last decade that anesthesiologists have gained expertise in performing FOB in children as well as neonates. Pediatric anesthesiologists have an edge over other members of the airway team, by virtue of their training in sedation in sick children as well as advance airway management.
3 Indications for Neonatal FOB
In the neonates, indications are both diagnostic and therapeutic (Table 39.1). The diagnostic indications are more common than the therapeutic usefulness [7, 8]. Sachdeva et al. found it particularly useful in diagnosis of persistent or unexplained airway and lung abnormalities and obstruction [9]. Failure or difficult to wean from mechanical ventilation is quite common in NICU patients, often due to ventilator-associated pneumonias (VAP) or ventilator-related lung disease . Bronchoalveolar lavage (BAL) through the bronchoscope provides sampling, considered as the best marker of lung infection. The in-house NICU bugs are known to be the commonest cause of nosocomial infection. BAL helps in making the right choice of antibiotics, and negative results save the babies from unnecessary medications. Better ventilator strategies can be planned to prevent lung injury [10]. Atelectasis due to airway blockade by the tracheal or bronchial secretions is another reason for weaning troubles. Bronchoscopy can be very useful for airway toilet without disrupting the ventilation in sick babies [11]. Vijayasekaran et al. observed that of 84 neonates in whom FOB was performed, in 57% neonates it helped in the modification of diagnosis [7].
Rigid bronchoscopy is still in use for all the above-mentioned indications in the neonates in centers where FOB is not available. However, its role over FOB is more in infants and children mainly for foreign body removal, massive hemoptysis, large tumor, etc.
4 Technique of FOB
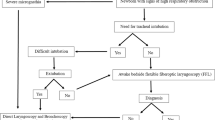
FOB can be performed through an endotracheal tube (ET) or without directly through the mouth or nose. For evaluation of the airway dynamics, basic requirement is retention of spontaneous respiration, as in an awake or mildly sedated neonate. It is easier to diagnose upper airway anomalies such as airway malacias , edema , stenosis , vocal cord palsy , or paralysis when FOB is performed without the ET. Supraglottic airway (e.g., LMA , i-gel ) also provides a good conduit for the bronchoscope. Preoxygenation and supplemental O2 during the procedure are beneficial. Neonates usually do not tolerate longer duration of hypoventilation , and desaturate fast, therefore the procedure must be done quickly. Essential intraprocedural monitoring includes SpO2 , ECG , NIBP , and EtCO2 .
5 Fiberoptic Bronchoscope
Neonates require smaller diameter bronchoscopes , appropriate for their airway anatomy. Most neonates would need a 2.8 mm (outer diameter) bronchoscope, which can be maneuvered through a 3.5 mm (internal diameter) ET, with assisted ventilation, and 4.0 mm ET for FOB with spontaneous breathing. It has a 1.2 mm suction channel for airway secretions and BAL sampling. The distal tip has a maneuverability range of 180° and slight rotation of the shaft of the scope can help in moving the tip in and out of this range. The smallest size is 2.2 mm. It can just pass through a 2.5 mm ET. It has no suction channel and can be used only for diagnostic purpose. It is useful in premature neonates. However, this size may not be available in every center. Table 39.2 shows the details of available bronchoscopes and gives a guide to choose the right device.
The bronchoscope must be lubricated before insertion into the ET. The internal diameter of the ET should be at least 1 mm more than the outer diameter of the scope for easy maneuverability and prevention of ET blockage and inadvertent PEEP .
‘ Spray as you go’ technique with lignocaine can be used, and care being taken not to exceed safe dose.
After its use, the bronchoscope should be cleaned and disinfected as per manufacturers’ recommendations. It is best stored in a hanging position in a designated cabinet and transported in its prescribed container. It is a delicate and expensive equipment and should be handled with care to prevent breakage of internal fiberoptic fibers.
For diagnostic purposes, FOB is better performed without an ET and with spontaneous breathing. However, in neonates, on mechanical ventilation, only brief period of apnea may be tolerated.
Fiberoptic bronchoscopes do not have any O2port and oxygen should be given separately by other suitable methods. The suction port of the elbow-shaped connector is commonly used with anesthesia circuit and support of ventilation.
6 Contraindications
There is no absolute contraindication for FOB, as most often it is indicated in sick neonates, suffering from respiratory, cardiovascular, or neurological illnesses. Therefore, the procedure should be done with due diligence and care to maintain oxygenation during and after FOB.
7 Limitation and Complications
FOB in neonates has some limitations.
-
The smallest bronchoscope 2.2 mm does not have a suction channel; this difficulty in visualization is due to presence of secretions.
-
BAL cannot be done without a channel.
-
Even larger scopes do not allow forceps for biopsy or foreign body retrieval (though usually not required in neonates).
-
The use of FOB for difficult intubation is limited by lack of expertise and shorter procedure time (neonates do not tolerate apnea or long periods of compromised ventilation).
-
The scope needs to be withdrawn if SpO2 falls, and reintroduced, when optimized.
-
Arrythmias , laryngospasm , and bronchospasm are known during or after the procedure.
-
There are reports of pneumothorax , bleeding , or other mechanical trauma also [11].
Carefully planned and systematically performed bronchoscopy can be safely done in neonates [1, 7].
8 FOB and the Role of the Anesthetist
FOB can be performed in an awake neonate, as practiced by many caregivers in the NICU. However, as an anesthetist my personal experience entails safe and smooth procedure with mild to moderate sedation with retention and maintenance of spontaneous breathing.
8.1 Pre-Procedure Assessment
It is important to do a thorough assessment of the neonate before scheduling a bronchoscopy. The important concerns include
-
Prematurity and low birth weight or both,
-
Challenging or difficult airway ,
-
Frequent apneic episodes (poor respiratory reserve or on controlled ventilation - note the peak/plateau pressures, FiO2, ABG if any),
-
Hemodynamic instability (baby on inotropic support),
-
Cardiac anomalies if any, and
-
Sepsis .
Neonatal respiratory reserves are poor, have reduced functional residual capacity , are obligate nose breathers , and may not breathe well if the bronchoscope occludes one of the nostrils. Oral route may be used whenever nasal route is difficult.
8.2 Pre-Procedure Instructions
-
It is mandatory to have an adequately fasted baby for the procedure.
-
It is better to have an orogastric tube placed (to avoid gastric inflation during O2 insufflation).
-
Written informed consent from parents separately for sedation and bronchoscopy.
8.3 Pre-Procedure Preparations
-
It is wise to perform FOB in a neonate in a safe and familiar environment.
-
Unless the baby is too sick to be shifted from the NICU, it would be preferred to do the procedure in the operating room, with monitor settings suitable for a neonate.
-
FOB should only be performed by someone experienced in FOB and well-versed with neonatal anatomy.
-
There should be a separate anesthetist to administer sedation.
-
A technical assistant, familiar with the equipment and briefed about the procedure, is vital.
8.4 Before Use, Bronchoscope Should be Checked for the Following
-
Sterility - bronchoscopes are soaked in enzymatic detergent for 15–20 min, cleaned with 2% glutaraldehyde, and then wiped with 70% alcohol from the outside.
-
Integrity – there should be no external damage.
-
Function - the scope should be checked for adequate vision (white balance must be done) and maneuverability. The range of movement of the tip and the direction of rotation should be checked prior to insertion. The suction channel if present must also be checked.
8.5 Other Important Preparations
-
The airway cart with appropriately sized devices such as nasal probes , mask, circuit, oral/nasal/pharyngeal airway , LMA (size 1), ET , laryngoscope , etc. should be ready.
-
The anesthesia machine should be checked and set on neonatal mode. All standard monitoring including appropriate size SpO2 probe, NIBP cuff, ECG leads, EtCO2, and temperature probe should be ready.
-
A good intravenous access should be ensured. Intravenous fluids with or without glucose, warmed adequately, may be given when required.
-
Drugs for sedation and resuscitation must be loaded in dilution as per weight of the baby (midazolam , propofol , ketamine , glycopyrrolate , atropine , adrenaline , hydrocortisone , and any other if indicated).
-
Diluted pediatric nasal decongestant drops can be instilled in the nostrils 5 min before the procedure if nasal route is being used.
8.6 FOB under Sedation
-
After placing all monitors, checking patency of IV access , preoxygenation must be done using face mask or nasal prongs.
-
Mild to moderate sedation can be given slowly over 5–10 min using midazolam , propofol , and/or ketamine in carefully titrated doses. Overzealous sedation may lead to apnea and therefore should be avoided. The dose to be used will depend upon the age, weight, post-conceptual maturity, and general condition of the baby.
-
Strict monitoring should be done all through the procedure. The EtCO2 sampling line can be manually held next to the nostrils to get a trace on the monitor (in intubated baby it would be part of the circuit). It may not show an ideal trace, but can give a fair estimate to guide the level of sedation (a visual chest rise is always reassuring).
-
Supplemental O2 can be given via a feeding tube through the other nostril (or both nostrils if bronchoscope is inserted orally). A rapidly falling SpO2 may progress to hypoxic bradycardia , and therefore should be immediately conveyed to the person performing FOB, who may then decide to withdraw the scope and reinsert once the baby stabilizes.
-
Anticholinergic premedication (glycopyrrolate ) can be given to reduce the secretions.
-
The baby may move a little during the procedure, but excessive movements or struggle should not be allowed (prevent trauma).
-
The sedation can be given as small boluses, although it is better to run a low dose continuous infusion. Ketamine can be used as a rescue drug during the procedure.
-
0.5% lignocaine should be loaded in a syringe for instillation through the suction channel of the scope (total not more than the 3 mg/kg).
Some clinicians do the bronchoscopy in awake babies. It helps in assessment of airway dynamics, but can be traumatic and unethical (excessive physical restraint).
8.7 The Procedure
The neck and shoulder of the baby can be supported and raised by a roll kept underneath for better extension (head being larger in neonates). Most studies show FOB in neonates via nasal route, but the risk of trauma and bleeding is more unless an ultrathin scope is used. If the size of the bronchoscope is relatively bigger for the nostril, then oral route is preferred (mouth guard can be used though not mandatory).
In my personal experience, I have done most bronchoscopies through the mouth, slightly more difficult to maneuver but easier to insufflate O2 nasally, and without trauma. The bronchoscope can be inserted through an ET/LMA/tracheostomy tube, but this restricts movement of the scope and does not allow visualization of the larynx and trachea. Besides, their placement requires deeper sedation which in turn may lead to loss of spontaneous breathing (and airway dynamics as in laryngo /tracheomalacia). It is best to perform FOB without the ET, provided sedation is administered safely. If baby is already intubated, ET can be removed for the procedure and replaced afterwards.
The scope should be well-lubricated (special lubricant spray available for the bronchoscope or common jelly available in the OT-apply and gently wipe off) and advanced gently under direct vision on the external video monitor. No force should be applied to push it in any direction. Reinsert the scope when not able to progress or any resistance is felt.
The vocal cords are visualized, and the movement of the cords observed. The baby may move when the scope crosses the glottis, and therefore additional bolus of sedation or spraying of lignocaine is desirable at this step.
8.8 Pathologies Visualized
-
Laryngomalacia is seen as a curled-up epiglottis.
-
Tracheomalacia as collapsing of the tracheal wall.
-
Laryngeal web as a cover of mucosa between the vocal cords.
-
Laryngeal perforation .
-
Congenital laryngeal stenosis .
-
Tracheal and bronchial granulomas .
-
TEF is seen as an opening in the tracheal wall.
-
Complete laryngotracheoesophageal cleft .
-
Tracheobronchitis .
-
Subglottic stenosis is seen as a narrowed opening often not allowing the scope to advance further (these may be an indication for elective tracheostomy).
-
The mucus plugs can be easily seen and sucked out (with saline irrigation) to relieve any blockage of bronchi.
In case of difficult airway, an ET may be mounted over the bronchoscope and advanced through the glottis. The scope should be withdrawn slowly while seeing all the structures again on the way out. The baby should be oxygenated well, monitored, and observed for any complications after the procedure in the recovery room. High pressures should never be used for suctioning in neonates.
9 Practical Trouble-Shooters
-
The availability of appropriate sized bronchoscope is very important. Do not pass bigger scopes through the nose or trachea or the tracheal tube.
-
Deep sedation can often lead to hypoventilation or complete apnea. It is very important to maintain a good balance between level of sedation and maintenance of adequate spontaneous breathing.
-
The scope should be withdrawn, and ventilation assisted as soon as the baby begins to desaturate.
-
Post-procedure nebulization may be required in sick babies using saline, steroids, or adrenaline.
-
Neonatal FOB should never be done without proper planning and expertise. An extra hand is always useful.
-
The presence of secretions can be troublesome in some cases. Increase the depth of sedation, use anticholinergic premedication and/or do a continuous oral suction/suction through the bronchoscope (the button has to be pressed in a sustained manner for effective suction).
10 Rigid Bronchoscopy in Neonates
Rigid bronchoscope has been used in the past for diagnostic purposes in the neonates. It involves a different set of equipment in smaller size for the neonates, preterms, and small for age babies. It is best done under deep sedation/general anesthesia with good muscle relaxation. The ET needs to be removed to give way to the rigid bronchoscope. These small rigid bronchoscopes do not have side port for O2 and ventilation. This means that bronchoscopy is done in the apnea time, and therefore a limiting factor in sick neonates. Additionally, O2 can only be insufflated through nasal prongs/feeding tube/any other device. Once the bronchoscope is removed, the ET is replaced until recovery and when extubation is possible. Standard monitoring is mandatory during the procedure. Rigid bronchoscopy is gradually fading because the FOB method is better, faster, less invasive, with minimal morbidity. Moreover, the rigid scope cannot be pushed beyond the carina as opposed to fiberoptic that can be manoeuvred into the bronchioles.
11 Conclusion
In the last few years, fiberoptic bronchoscopy has been successfully performed, for diagnostic and therapeutic indications, largely replacing rigid bronchoscopy in neonates. This has been possible because of the availability of smaller sized bronchoscopes and better monitoring and ventilating facility. It has helped in diagnosing dynamic airway abnormalities and lung pathologies. Its role is vital in determining the cause in difficult to wean neonates in intensive care units. It is found to be a safe when performed systematically in experienced hands. Thorough knowledge of neonatal anatomy, physiology, pharmacology, and experience in handling pediatric bronchoscopes is vital. Good planning, expertise, and assistance are the key to success. However, it should only be performed in centers where there is facility to undertake urgent thoracotomy and lung surgery in the neonate, in case required.
References
Lindahl H, Rintala R, Malinen L, Leijala M, Sairanen H. Bronchoscopy during the first month of life. J Pediatr Surg. 1992;27(5):548–50.
Pereira KD, Weinstock YE. Bronchoscopy assisted neonatal tracheostomy (BANT): a new technique. Int J Pediatr Otorhinolaryngol. 2007;71:211–5.
Atzori P, Iacobelli BD, Bottero S, et al. Preoperative tracheobronchoscopy in newborns with esophageal atresia: does it matter? J Pediatr Surg. 2006;41:1054–7.
Parolini F, Boroni G, Stefini S, Agapiti C, Bazzana T, et al. Role of preoperative tracheobronchoscopy in newborns with esophageal atresia: a review. World J Gastrointest Endosc. 2014;6:482–7.
Pietsch JB, Nagaraj HS, Groff DB, Yacoub UA, Roberts JL. Necrotizing tracheobronchitis: a new indication for emergency bronchoscopy in the neonate. J Pediatr Surg. 1985;20:391–3.
Deanovic D, Gerber AC, Dodge-Khatami A, Dillier CM, Meuli M, Weiss M. Tracheoscopy assisted repair of tracheo-esophageal fistula (TARTEF): a 10-year experience. Paediatr Anaesth. 2007;17:557–62.
Vijayasekaran D, Kalpana S, Ramachandran P, Nedunchelian K. Indications and outcome of flexible bronchoscopy in neonates. Indian J Pediatr. 2012;79(9):1181–4.
Soni A, Badatya S, Modi M, Saluja S. Neonatal bronchoscopy—a review. Curr Med Res Pract. 2016;6:192–201.
Sachdeva A, Chhawchharia R, Gupta D, Gupta N. Flexible fiberopic bronchoscopy directed interventions in neonatal intensive care unit. Indian Pediatr. 2019;56:563–6.
Mackanjee HR, Naidoo L, Ramkaran P, Sartorius B, et al. Neonatal bronchoscopy: role in respiratory disease of the newborn—a 7-year experience. Pediatr Pulmonol. 2019;415–20
Kohelet D, Arbel E, Shinwell ES. Flexible fibreoptic bronchoscopy – a bedside technique for neonatologist. J Matern Fetal Neonat Med. 2011;24:531–5.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Goyal, R. (2023). Anesthesia for Bronchoscopy in Neonates. In: Saha, U. (eds) Clinical Anesthesia for the Newborn and the Neonate. Springer, Singapore. https://doi.org/10.1007/978-981-19-5458-0_39
Download citation
DOI: https://doi.org/10.1007/978-981-19-5458-0_39
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5457-3
Online ISBN: 978-981-19-5458-0
eBook Packages: MedicineMedicine (R0)