Abstract
Fourier Transform Infrared (FTIR) spectroscopy is a method of infrared absorption spectra and emanation of a solid, fluid, or gaseous material. It gathers and digitizes the interferogram followed by Fourier transformation and displays the spectrum. Infrared spectra obtained from the FTIR spectrometer lie in the mid-infrared region of wave number 4000–666 cm−1. This technique is widely applied to foods, forensics, the environment, and pharmaceuticals. It is a rapid (i.e., less time-consuming), nondestructive, operator-friendly, and precise method. A highly accurate fingerprint of the material being analyzed is provided based on its chemical and physical state. FTIR in combination with other chemometric techniques has been successfully applied to analyze food products and their constituents, such as milk, meat, oil, fruits, and different carbohydrates. The determination of product quality and authenticity is also a major concern among food manufacturers and consumers. Authentication of different food products like honey, milk, oil, and tea is carried out successfully through the provision of the highly accurate fingerprint of the material under analysis. Single bonds are detected in the high wavenumber region of 2500–4000 cm−1, double bonds in region 1500–2000 cm−1, and triple bonds in the middle region of 2000–2500 cm−1. This technique possesses a huge scope in the food industry and it is being immensely flourished worldwide over the past decade.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The Infrared spectroscopic technique employs spectra in near-infrared and far-infrared regions for the quantitative and qualitative analysis of food products and other commodities (Rohman & Che Man, 2012a). A vast range of spectra which falls within the region of 0.7–1000 μm can be applied to get useful information about food constituents but for chemical analysis mid-infrared region 2.5–25 μm (4000–400 cm−1) is the most attractive. This information gave birth to the idea of commercial Fourier Transform Infrared (FTIR) Spectroscopy in the 1970s since then the science of FTIR has advanced in infrared spectroscopy (Doyle, 1992). However, the basic principle remained the same in earlier and modern models of the equipment that makes use of an infrared source to irradiate the sample. The absorption of these radiations excites the molecules into vibrational motion by the deposition of energy (Duygu et al., 2009). The interferogram collected is translated into a spectrum through a mathematical model called Fourier transformation Modern FTIR systems are equipped with chemometric software that increases the application of this equipment to obtain useful information on the qualitative and quantitative parameters (Rahman, 2012; Rohman & Che Man, 2012a). Several elements like a non-homogenous sample, temperature, refractive index, nonlinear detector, and reflection losses may affect FTIR results. These elements can be minimized by frequent calibration checks, using more samples for calibration, using peak areas rather than heights, and maintaining the absorbance between 0.2 and 0.7 absorbance units (Van de Voort & Ismail, 1991). A wide range of food and non-food items can be analyzed using the FTIR technique. At the industrial and research level, it applies to four major areas, i.e., identification of compounds, elucidation of structures, quantitative analysis of species, and measurement of molecular properties (Gaffney et al., 2002). Valuable information can be achieved about carbohydrates, lipids, proteins, moisture, and other components in food products using this technique (Sivakesava & Irudayaraj, 2000; Van de Voort & Ismail, 1991). Furthermore, this technique has a great potential to evaluate adulteration in different food products including jams, purees, oils, coffee, meat, and milk products (Syahariza et al., 2005). Recent research in this decade elucidated its potential as a reliable technique for the identification of microorganisms, especially food spoilage and food pathogenic bacteria down to the strain level (Duygu et al., 2009; Wenning et al., 2014). Some researchers showed its application in the pharmaceutical industry, astronomical measurements, and waste management (Baravkar et al., 2011; Smidt & Schwanninger, 2005). All of these applications depicted the perspective of FTIR spectroscopy as a fast, efficient, free of chemicals, and relatively low-cost technique, which requires no or less sample preparation and small sample size (Triebel et al., 2007; Vongsvivut et al., 2014). As compared to other conventional instruments, FTIR reproduces better, has high energy throughput, and has better accuracy from the laser source (Baravkar et al., 2011; Syahariza et al., 2005). This technique is equally good for aqueous, powder, or dehydrated samples (Duygu et al., 2009). Major advantages associated with this technique are fast, reliable, environmentally friendly, and has no waste generation (Vongsvivut et al., 2012). The modern models of FTIR also offer improved signal-to-noise ratio, detection of all frequencies simultaneously, wavelength accuracy, and superior spectral resolution (Sivakesava & Irudayaraj, 2000; Van de Voort & Ismail, 1991).
Today in the modern era, a large variety of analytical techniques are available for food analysis in research laboratories and industry. The stakeholders are always interested in accuracy and precision while making use of analytical techniques for the assessment of quality, nutritive value determination, and detect adulteration (Franca & Oliveira, 2011). For this purpose, they make use of the traditional high-performance liquid chromatography, mass spectroscopy, gas chromatography, atomic absorption spectroscopy, X-ray fluorescence spectroscopy, polymerase chain reaction (PCR), and gas chromatography-mass spectrometry. However, these techniques may have certain limitations (i.e., the maintenance costs, the time required for measurement, and sampling difficulty) and these limitations justify to develop of new rapid and efficient analysis techniques (Cifuentes, 2012; Franca & Oliveira, 2011; Kizil et al., 2002; Sezer et al., 2017). These conventional analysis techniques may be replaced by more efficient techniques like near-infrared spectroscopy, laser-induced breakdown spectroscopy, Raman spectroscopy, and mid-Fourier transform infrared spectroscopy (Sezer et al., 2017). Among all these techniques FTIR spectroscopy has turned out to be a powerful tool for analyzing foods and their components (Kačuráková & Wilson, 2001).
Food product authentication is a major concern in today’s world for food processors, consumers, and regulatory bodies (Černá et al., 2003; Syahariza et al., 2005). For a long time, various methods of authenticating food are applicable worldwide, such as gas chromatography, high-pressure liquid chromatography, DNA-based methods (PCR), ELISA, nuclear magnetic resonance, differential scanning calorimetry, specific natural isotope fractionation (SNIF), electronic nose x-ray diffraction, mass spectrometry, fluorescence spectroscopy, and visible spectroscopy (Gouilleux et al., 2018). Most of the conventional authentication methods are time-consuming and expensive. There is always a need for rapid, accurate, and efficient methods for food authentication. Raman spectroscopy, laser-induced breakdown spectroscopy, near-infrared spectroscopy, and FTIR spectroscopy have a great potential to achieve desired objectives for the future industry and regulatory authorities to make decisions on the authentication of foods. All types of food products (e.g., milk, meat, oils, and nuts) and ingredients are efficiently analyzed and authenticated through these techniques (Grassi et al., 2018; Sezer et al., 2017; Yang et al., 2005; Zhang et al., 2017).
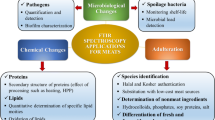
The first part of this chapter presents the advantages, basic principles, and operation of the FTIR technique. The second part of this chapter explains the application of the FTIR technique in analyzing and authenticating different foods and their constituents. It also reviews the analysis of bacteria involved in food and authentication of halal products. The gathered information can be beneficial for the food industry, researchers, students, data scientists, academics, and food business personnel.
2 Fourier Transform Infrared Spectroscopy (FTIR)
Early infrared spectrometers are referred to as dispersive instruments as they use monochromators to scatter radiations into their spectral constituents while modern infrared spectrometers make use of interferometers for the distribution of source radiations (Gaffney et al., 2002). Modern Fourier transforms infrared spectrophotometers provide a much rapid infrared spectrum as compared to traditional spectrophotometers (Hilal et al., 2017). A resolution of 4 cm−1 can be obtained at mid-infrared spectrum on dispersive spectroscopy in 15 min while the modern Fourier transform infrared spectrometer can get the same spectra in just 1 s (Griffiths & De Haseth, 2007). The FTIR spectra reflect the molecular structure as well as the molecular environment. In the FTIR technique, infrared radiations from the infrared source irradiate the sample and the absorption of these radiations deposit the quanta of energy into vibrational modes hence stimulating the vibrational motions. Thus, the exposure of a molecule to the radiations emitted by the infrared source absorbs only at frequencies that correspond to its mode of vibration in the electromagnetic spectrum region between visible and microwaves (Duygu et al., 2009). A flow diagram of the FTIR system is shown in Fig. 1.
2.1 Principle
Fourier transform spectroscopy records the radiant power data as a function of time. The interferometer changes the electromagnetic radiation frequency to slow the oscillating signal. A Computer then mathematically separates the sum of slower oscillating signals into individual oscillation signals and further computes the fluctuations in the frequencies of the radiations observed followed by a continuous recording of this data. Fourier transform is a mathematical method that converts the time domain spectrum into frequency domain spectrum (Baravkar et al., 2011). The transformation of the interferogram is carried out by instrument software. The intensity of infrared radiations before and after passing through the sample is calculated to measure the infrared spectrum. Moreover, the spectrum is plotted with Y-axis units as absorbance/transmittance and the X-axis as wavenumber units. FTIR absorbance spectrum follows Beer’s law which relates the absorbance with the concentration as shown in the equation below:
where Aλ is Absorbance, l is path length, ε is Absorptivity, c is concentration
However, the transmittance is not directly proportional to concentration and is shown in the below equation:
where T is transmittance, IS is intensity of the beam after passing sample, IR is intensity of the beam before passing sample.
2.2 Equipment and Their Components
2.2.1 Infrared [IR] Source
Generally, infrared sources are hot body type, which is made up of a strong material warmed by an electric current. The power of the radiant and the wavenumber of peak emission increases as the temperature rises. Common infrared sources used are silicon carbide rods commonly known as Globar, Nernst glowers, and tungsten glowers. Most of the equipment working in the mid-infrared region make use of Globar in the form of a silicon carbide heated rod, with 2–5 cm in length and 0.5–1 cm in width. It usually operates at a temperature of 1300 K. As an electric current passes through the silicon carbide rods (infrared source), it becomes hot thus emitting a huge amount of infrared radiation. Nernst glowers are quite different compared to globar and are made of refractory oxide mixtures such as cerium, zirconium, and thorium and they can achieve more temperature than Globar although they are not capable of producing infrared radiations above 2000 cm−1. The range of temperature a Nernst glower can achieve is around 1500–2000 K but it falls off quickly above 2000 cm−1. Thus, these glowers are a good source for spectral regions below 2000 cm−1 although they are not effective for the whole mid-infrared region. Quartz-tungsten-halogen (QTH) lamp contains additional inert gas and halogen forming tungsten oxy-halide extending the shelf life of the lamp (Gaffney et al., 2002). Nernst glower is shown in Fig. 2.
2.2.2 Interferometer
The Interferometer was first invented by Albert Abraham Michelson and it was also known as the Michelson interferometer. The Interferometer is the heart of any FTIR spectrometer. It consists of a beam splitter, a compensating plate, and two mirrors (stationary & movable) (Doyle, 1992). Two beams reflect from the mirrors back to the beam splitter where they are reunited, focused, and sent to the detector. The path difference because the two traveling beams create a phase difference between them. If the paths of the two traveling beams are the same then the signal will be maximum with both beams being in phase while the path difference between two beams can generate the smallest signal at the detector causing destructive interference (Larkin, 2017). Entrance apertures of >1 cm in diameter are used in the interferometers while monochromators need narrow slits for achieving good spectral resolution (Griffiths & De Haseth, 2007).
2.2.3 Beam Splitter
A Beam splitter is composed of a different material transmitting half of the radiations while reflecting the remaining half. The infrared radiations from the infrared source after striking the beam splitter are converted into two beams. One beam gets transmitted to the fixed mirror through the beam splitter while the second beam reflects the movable mirror through a beam splitter. Both of these mirrors reflect the radiations to the beam splitter where the two beams interfere to get an interferogram (Baravkar et al., 2011).
2.2.4 Mirrors
A moving mirror is an extremely reactive flat surface allowing the mirror to move with high speed. The mirror moves after every millisecond and a few millimeters away from the beam splitter. The fixed mirror is also a highly reflective flat surface (Larkin, 2017). Ideally, 50% of the light is refracted towards a fixed mirror while 50% is transmitted towards the moving mirror. Light reflects from the mirrors to the beam splitter and focused on the sample. Moreover, if the distance from the splitter to the fixed mirror is not the same as the distance from the splitter to the moveable mirror then there will be constructive and destructive interference depending upon the distance between the two mirrors.
2.2.5 Laser
Helium-Neon laser is employed by many instruments as a calibration standard of internal wavelength. It makes a record of optical path differences in the interferometer precisely (Larkin, 2017). The precise constant velocity of the moving mirror is timed through the use of accurate laser wavelength. The laser beam intensity in the interferometer is measured at two points. Due to the enhancement and cancellation of He-Ne beam paths, the intensity of the moving mirror rise and fall at these two points. The relative phase of the sine wave tells the direction of the moving mirror while the number of fringes in the wave tells how far the mirror has moved (Willey, 1976).
2.2.6 Detectors
Infrared [IR] detectors compute the strength of infrared radiations from an infrared source converting them into electrical signals which are further processed by generating spectra. Photosensitivity, detectivity, and noise equivalent power are the major parameters determining the performance of a detector. Detectivity is defined as the photosensitivity per unit area of the detector. Infrared detectors are either thermal or photonic types. Thermal detectors use infrared radiations as heat and detect the temperature changes of absorbing material. Thermal detectors are mostly used in the MIR region but these are equally good in the NIR region. The most common of these is the pyroelectric detector providing good linearity over a wide spectral region. Photodetectors use infrared radiations as light and are more sensitive as compared to thermal detectors. These detectors are exclusively made to compute the distinctive interferogram signal coming from the interferometer (Gaffney et al., 2002).
2.2.7 Computer System and Software
Information received from the detector in the form of a digitized signal is forwarded to a computer where the signal is transformed into Fourier series. It converts it into final infrared spectra which are used by the user for further elucidation and operation (Larkin, 2017). The Fourier transformation of an interferogram using a computer is a rapid way to obtain the composite spectrum of the source, instrument, and sample. It simultaneously measures the wavelengths rather than sequential measurement (Doyle, 1992).
2.2.8 Background Spectrum
A background spectral measurement is a procedure performed where the beam run without the sample. This measurement can be matched with the sample in the beam to determine the % transmittance. This technique removes all the instrumental characteristics of a spectrum originating as noise. Hence, all the spectral features obtained while running the sample can be purely due to the sample. Computer software subtracts the background spectrum from the sample spectrum using the Fourier transform technique to generate a reliable spectrum originating solely from the sample (Hilal et al., 2017).
2.2.9 Parameters Optimization
Fourier transform infrared spectrometers are furnished with powerful hardware and software to get accurate and precise results for a specific sample. For the data collection, certain processing parameters are set by these software packages which should be adjusted for a specific sample and application. The parameters are the spectral resolution, several scans, choosing the mirror velocity, digital filtering, and the application of phase correction to the interferogram (Gaffney et al., 2002).
2.3 Operation of FTIR
FTIR spectroscopy comprises an infrared radiation source, an interferometer (fixed and moving mirror, a beam splitter, and laser), the sample chamber, and a detector (Fig. 1). It uses a Michelson interferometer to analyze the infrared radiations passing through a sample. The infrared radiations are made accurately parallel by the mirror and the infrared beam is divided at the beam splitter. The stationary mirror passes half of the beams while the moving mirror refracts the other half. After reflecting from the moveable and stationary mirror, the beams reunite at the beam splitter and the radiation is focused on the detector (Baravkar et al., 2011). The path differences between stationary and movable mirrors bring back together the beams experiencing a constructive and destructive interference called an interferogram (Hilal et al., 2017; Van de Voort & Ismail, 1991). The radiations only from the two mirrors can arise in phase at the beam splitter, cell, and detector when the distance between the beam splitter and stationary mirror is the same as that of the beam splitter and moving mirror. With the changing position of the moving mirror remoteness between mirror and splitter also changes hence the radiations of the stable wavelength reach in phase only to the cell and detector. With the change in the distance, the wavelength of the radiation beams either arrive in phase or out of phase. By controlling the mirror motion rate a series of simultaneous signals arrive at the detector which measures the interferogram signal for all frequencies and carries the information to the computer (Baravkar et al., 2011; Hilal et al., 2017).
A beam is superimposed to provide a background for the instrumental operation. After the subtraction of the background spectrum from the sample spectrum through Fourier transformation software, the desired spectrum is obtained (Hilal et al., 2017). A Computer using a Fourier transform method with mathematical algorithms converts the output into a plot of absorption/transmission against wavenumber (Munajad et al., 2018; Schmitt & Flemming, 1998). It usually takes 2 min to record a plot starting with the sample insertion (Baravkar et al., 2011).
2.4 Modeling Techniques Used for FTIR
Different modeling techniques are used to analyze and authenticate food products using FTIR. FTIR is considered powerful as a quantitative tool because of its ability to carry out multicomponent analysis. Most of the FTIR multicomponent analysis procedures are based on K and P matrix methods. They use the known concentration of mixtures and compute the concentration of an unidentified blend. Analytical methods based on spectroscopy possess some limitations when it comes to similar or overlapping bands of some compounds which led to the use of chemometric techniques like principal component analysis, partial least square regression, and discriminant analysis (Franca & Oliveira, 2011). Several studies show that FTIR-based methods can be applied in the food industry to detect and authenticate products successfully.
PLS (Partial Least Square) is one of the modeling techniques commonly used in combination with FTIR. It is a form of factor analysis. It is very useful in studying the complete spectrum of the mixture and determining the spectrums of interest through correlation (Van de Voort, 1992). PLS does not need to establish a direct relationship between absorbance and concentration at specified frequencies, it is rather developed by compacting the spectral data for training set into mathematical spectra. When the spectrum of an unidentified sample is analyzed the spectrum is renovated by PLS from loading spectra and their volumes of each are then used to forecast the unidentified concentration. It possesses the advantages of both the CLS and Inverse Least Square but does not possess the limitations they have (Ismail et al., 1997).
One of the most crucial modeling techniques used for the analysis and authentication of food products based on inverse calibration and data reduction is principal component analysis (PCA). It is a type of factor analysis in which the spectral and concentration data is incorporated into the model in one step. It condenses the multidimensional data into major dominant components while conserving the applicable variation. The natural groups are recognized by PCA with the first principal component showing the major variation followed by the second and third components. Moreover, score plots in PCA are used to look for similarities or differentiate the compounds (Davis & Mauer, 2010). Likewise, the closer the samples more similarities they possess. CLS (Classical Least Square) is another such multivariate least square technique used in FTIR spectroscopy. A linear arrangement of single constituent reference spectra of separate composites is tailored to the measured spectrum such that the sum of squares of residuals at each frequency is minimized (Bacsik et al., 2004). Quantitative results are provided by the amount of each absorber required for the best fit. However, this method does possess certain limitations as well.
Hierarchical cluster analysis (HCA) is also used to detect similarities between the spectra of samples. It recognizes them based on the aggression algorithms and distance between spectra. Furthermore, discriminant analysis (DA) is another statistical tool to categorize substances into clusters. It determines the similarity of values from an unidentified sample to an identified sample. Moreover, soft independent modeling by class analogy (SIMCA) is another model used for the spectral organization of data and is mainly applied in the classification of bacteria. The sample is first analyzed through PCA before SIMCA modeling so that the important components are retained. It requires a training data set comprising samples with a set of classes and qualities (Davis & Mauer, 2010).
3 Food Analysis Using FTIR Spectroscopy
Proximate analysis of food is the most common analytical requirement of the food industry whether it is used for processed products, raw materials, or finished products. Proteins, fats, carbohydrates, moisture, and the other minor constituents, like vitamins and minerals are the core of food systems and these constituents contribute to the obtained spectrum. The distinctive absorption bands are related to these main constituents. To determine the carbohydrates using FTIR, the presence of hydroxyl (–OH) bonds elucidate their molecular structure, whereas for proteins presence of amide I and amide II bands explain useful information. The presence of fat bands depicting ester linkage and C-H stretch at specific wavelengths provide fundamental information (Van de Voort & Ismail, 1991).
The infrared spectrum obtained from the FTIR spectrometer lies usually in the mid-infrared region between 4000–666 cm−1. The transition energies which correspond to the changes in vibrational energy states of most functional groups within the food lie in the mid-infrared region, i.e., 4000–400 cm−1. The absorption band appearance in this region can be applied to check the presence of the particular functional groups in the molecule. Single bonds (O–H, C–H, N–H) can be detected in the high wavenumber region of 2500–4000 cm−1, double bonds are detected in the wavenumber region 1500–2000 cm−1 while triple bonds can be detected in the middle wavenumber region 2000–2500 cm−1 (Hilal et al., 2017).
3.1 Carbohydrates
Natural polysaccharides or food additives polysaccharides like pectin, starch, carrageenan, and galactan are well distinguished through FTIR in the region of 1200–800 cm−1. These polysaccharides constitute the plant cell wall along with proteins, lignin, and inorganic compounds (Kyomugasho et al., 2015). The shape of the spectrum of a polysaccharide is mainly determined by the composition of polysaccharides and their side-chain components. The information about the major polysaccharides obtained from the FTIR spectrum appears in the region of 1200–800 cm−1 (Kacurakova et al., 2000). The anomeric region, i.e., 950–750 cm−1 is the most commonly used analysis region in carbohydrates providing vital information about α and β anomers of polysaccharide monomers. The conformers (alpha and beta) of mannose, glucose, and galactose are illustrated using bands at 870–840 and 890 cm−1 (Kačuráková & Wilson, 2001). Moreover, the detection of these polysaccharides may be affected by their water absorption but the studies show that though the spectra are affected by hydration but still no information about these polysaccharides was lost (Čopíková et al., 2006).
The effect of additives (agar, alginate, lecithin, and glycerol) on the potato puree prepared from commercial powder is also studied through FTIR spectroscopy. The presence of various starch-based food additives in processing brings variable characteristics to the product and can be analyzed through the FTIR technique. In this regard, spectra may be recorded in the region of 349–4000 cm−1, the obtained peaks depict how these starch-based food additives affect water holding capacity by interacting with the hydroxyl group and hydrogen bonds. Similar investigations can be confirmed for other food additives such as glycerol and lecithin that may induce a greater effect on the structure because of their small molecules (Dankar et al., 2018). This technique is also valuable to detect other structural groups of exopolysaccharides (EPS) that is added to food products as food additives. A good example is the development of milk and yogurt-based products by the use of EPS-producing cultures in these products. All the cocultured exopolysaccharides in these products show peaks in a range of 3395.1–589.1 cm−1. The stretching vibration of the –OH group showed a band at 3395.1 cm−1 while the band at 2924.2 cm−1 indicates the C–H stretching vibration. The carbohydrates presence is depicted by the presence of broad stretches of C–O–C, C–O at 1000–1200 cm−1 (Ahmed, Wang, Anjum, Ahmad, & Khan, 2013).
3.1.1 Starch
Starch is present in plants as α-(1 → 4) linked glucan. The gelation and retrogradation are the two major factors that affect the nature and characteristics of these starches during the processing of food. It is possible to study these changes in characteristics through FTIR. The characteristics of infrared bands for starch gels can be detected at 1046 and 1019 cm−1. The intensity and shape changes of peaks between 995 and 1020 cm−1 indicate the onset of retrogradation. The stretching region (1300–800 cm−1) of C–C and C–O bonds are very sensitive to retrogradation. The retrogradation rates vary considerably for both amylose and amylopectin (Kačuráková & Wilson, 2001). Irradiated starches are also characterized and classified through this technique (Kizil et al., 2002). Moreover, the abundance of resistant starches in refrigerated tortillas is because of the changes in the starch structure imposed by increased crystallinity. The bands appear at 1047 cm−1 and disappear at 1022 cm−1 which depict the retrogradation of starch. The band at 1047 cm−1 is because of the weakening intermolecular hydrogen bonds. The peaks observed show that the storage of tortillas has a great effect on the resistant starch formation (Flores-Morales et al., 2012). FTIR spectroscopy is also helpful in elucidating structural and conformational information of protein in conjunction with polysaccharides in bread and other baking products (Sivam et al., 2013).
3.1.2 Pectin
The most extensively studied polysaccharides through infrared spectroscopy are the pectin. These constituents are available mainly in the cell walls of higher plants. Pectins are made up of (1 → 4) linked α-D-galacturonan and residues of (1 → 2)-α-L-rhamnopyranoseyl (Kacurakova et al., 2000). FTIR read the functional groups of pectin and its derivatives in the region of 1900–1500 cm−1. Pectate interacts with the potassium and magnesium ions even when there is no gel formation. Cadmium, copper, zinc, and lead form complexes with pectinates at 59% esterification while lead and copper form complexes even with 93% esterified pectinate (Kačuráková & Wilson, 2001). Esterification also reduces the negative charges and ion-binding sites. The complexes of ions are merely constant in the zones of the gel joint. The gels are formed by calcium (Ca+2) and strontium (Sr+2) with pectate and pectinates having 23.8% esterification. The spectra show a complex formation of nickel, zinc, copper, and lead with pectinates up to 59.1% esterification. In prominently esterified pectinate, i.e., 93.4%, copper (Cu+2) to a greater extent and lead and zinc to a lesser extent interact strongly with the pectinate chain. Copper complex even forms a gel when carboxylate-free groups are just 6% because of their high stability (Wellner et al., 1998). Moreover, this technique is also used to analyze the degree of methyl esterification in pectin thus providing information about how the setting characteristics of pectin (Kyomugasho et al., 2015).
3.1.3 Cellulose
Cellulose is a β-(1 → 4) linked glucan present in plants, algae, and bacteria. The identification of the types of cellulose, the degree of crystallinity, and the effect of the source on the crystallinity are studied by the Fourier transform infrared spectroscopy. FTIR data about celluloses from various sources indicate that the intensity of the bands is limited while the infrared band position is the same in the bacterial cellulose and cotton linter cellulose (Kačuráková & Wilson, 2001). Hemicellulose polysaccharides are often composed of xylans, glucomannans, galactoglucomannans, and xyloglucans. Among these molecules β-(1 → 6), (1 → 3) linked galactan bands appear in the range of 1078–1072 cm−1, β-(1 → 4) linked mannans in 1066–1064 cm−1, xyloglucan and β-glucan at 1041 cm−1, arabinan at 1039 cm−1, rhamnose at 1043 cm−1 while galactose showed the strongest band at 1070 cm−1, mannose at 1070 cm−1, and glucose at 1035 cm−1. The position of these distinctive bands identifies the structures and composition of polysaccharides (Kacurakova et al., 2000).
Protein, pectin, lignin, and phenolics may be distinctively detected in the complex samples of xylan. The presence of both phenolic and proteins in a sample might overlap in the region 1700–1500 cm−1 but their band intensities vary. Xylan structures are different depending upon the source. Xylan from cereal is a dietary fiber that is often used as gum. They are composed of β-(1 → 4)-D-xylopyranosyl residues while their branches are made up of L-arabinose and D-glucuronic acid. Xylan is bound to lignin through its uronic acid constituent. Xylan with (1 → 4) spectrum is dominated by the band at 1047 cm−1 while in xylan (1 → 3) two overlapping bands exist at 1066 and 1030 cm−1 (Kačuráková et al., 1999). The (1 → 3) linkage in xylans affects the infrared spectral pattern while the gelling mechanism of xylan is greatly affected by the position and type of glycosidic linkage. Hydration may relate to the existence or non-existence of 1150 and 1000 cm−1 band in infrared spectra in xylooligosaccharides and Xylo-polysaccharides (Kačuráková et al., 1998).
3.1.4 Dextran and Pullulan
Dextrans are produced by L. mesenteroides, Lactobacilli, and Weisella species while the dextran produced by L. mesenteroides and Weissellacibaria has 95% α-(1 → 6) and 5% α-(1 → 3) linkage (Ahmed & Ahmad, 2017). Pullulan and dextrans are used in food, the cosmetic, and pharmaceutical industry. The mobility of the dextran chain increase because of the presence of (1 → 6) glycosidic linkage but pullulan consists of both (1 → 4) and (1 → 6) linkages which differentiate both. Highly merged bands are observed in the spectral region (1175–975 cm−1) of irradiated dextran and pullulan. The band at 1150 cm−1 is assigned to the exocyclic stretching –CO vibrations which were previously assigned to –COC. The varying frequency position of this band is because of the different repeating units and the OH group orientation of different polysaccharides. The dextran spectra contain a less distinct 1080 cm−1 band as compared to pullulan. The band 1024 cm−1 in pullulan appears in the spectra of dextran at 1018 cm−1 while the band at 996 cm−1 is not present in the dextran spectra. Hence, these bands differentiate the pullulan and dextran based on their presence or absence (Shingel, 2002).
3.1.5 Alginate
Phycocolloids are the polysaccharides present in seaweeds and are used as thickeners in the food industry. Brown seaweeds’ major structural polysaccharide is alginate while that of red seaweed is carrageenan (Pereira et al., 2009). Alginic acid is a heteropolysaccharide present in brown seaweeds. Alginic acid is made up of β-D-mannuronic acid and α-L-guluronic acid (Papageorgiou et al., 2010). In brown seaweed alginate, the value of mannuronic acid is higher than guluronic acid blocks. The quality of raw seaweed can be checked nondestructively and rapidly through this technique (Gómez-Ordóñez & Rupérez, 2011). Bands around 700 and 800 cm−1 are assigned to the guluronic and mannuronic acids while the wavenumber ranging from 3000 to 3600 cm−1 shows the stretching O–H bonds while 2920–2850 cm−1 shows the stretching of aliphatic C–H. Furthermore, enzyme tannase is entrapped in the alginate beads it can be helpful in the controlled release of enzymes or in improving tea and juice quality (Larosa et al., 2018).
3.1.6 Guar Gum, Chitosan, and Agar
Guar gum has a wide application in the food industry as a stabilizer and thickener. It is derived from cluster bean and is composed of β-1 → 4-linked mannose and α-1 → 6-linked galactose units. Partly hydrolyzed guar gum has gained consideration as water-soluble dietary fibers and its production is controlled through mannase and pectinase enzymes. There is no alteration in the functional group of partially hydrolyzed guar gum compared with the native gum. Sharp absorption bands around 1648 cm−1 also indicate the increased solubility of semi-hydrolyzed guar gum (Mudgil et al., 2012). Chitin is also a polysaccharide widely present in nature and its films are promising due to its good antimicrobial and barrier properties. The effect of moisture on the neutralization of amorphous chitosan films shows that chitosan does not present bands in the range of 2500–1750 cm−1. The shift of the amide band to 1640 cm−1 and the vibration of carboxylate ions at 1560 and 1410 cm−1 distinguish the spectrum of chitosan acetate. Moreover, at the water activity of 0.44 and 0.55, the spontaneous neutralization of acetate chitosan films takes place which is an alternative neutralization method without film damage. These films also maintain their original form at aw = 0.11 (Mauricio-Sánchez et al., 2018). Agars and crystalline cellulose structure are also studied through FTIR spectroscopy which shows that the agar and its derivatives show characteristic bands in the region between 800 and 700 cm−1 (Kačuráková & Wilson, 2001).
3.1.7 β-Glucan
FTIR is a valuable tool used to study the glycans and carboxymethyl glucan hence obtaining fingerprints of different bonds (Ahmed & Ahmad, 2017). The stretching (ʋ) vibrations of carboxylate anion in carboxymethyl glucan are observed at 1600 and 1421 cm−1 (Kačuráková & Wilson, 2001). Along with barley and oat, other plants such as rye, mushrooms, apple pomace, and tomato fibers are also rich in β-glucan (Ahmad & Khalid, 2018). FTIR also provides structural information of β-glucans thus a valuable technique to distinguish between yeast and fungi β-glucans (Ahmad & Anjum, 2010). The importance of oat cereal and its use as healthy human food throughout the world is well known (Ahmad et al., 2010). Similarly, dietary fibers from barley such as β-glucan reduce the glycemic and cholesterol responses and can be used as a functional or nutraceutical ingredient in food products (Ahmad et al., 2009; Ahmad, Munir, et al., 2012). It has also got emulsification, gelation, and stabilizing properties (Grumezescu, 2016). The chemical characterization revealed that barley β-glucan is composed of β-[1-4]-linked glucose units (Din et al., 2009). If the sequence of (1 → 4)-linkage is longer then the glucan is less soluble (Ahmad, Anjum, et al., 2012). Most of the beta-glucan portion lies in the endosperm cell wall while its quantity and extraction are influenced by the cultivars. Dehulled barley and oats contain 3–7% β-glucan while wheat contains <0.5%. Seventy-five percent is present in the cell wall while 25% is in the aleurone layer. Beta-glucan extracted from the endosperm of barley also improves the quality parameters of bread. FTIR technique serves as an important tool to identify β-glucan in various products and even distinguish the sources of these glucan molecules based on the spectra obtained from the FTIR technique. (Ahmad et al., 2008). Similarly, β-glucan extracted from the oat grains is incorporated into different products. The comparison between oat and barley β-glucan gum pellets indicates that the absorption patterns are almost similar but band intensities vary. β-glucan from different sources show different bands at different frequencies (Table 1).
Carbohydrates in β-glucan gum pellets show a peak at 1040 cm−1 for C–O bond, 2940 cm−1 for C–H stretching, and 3400 cm−1 for –OH stretching (Fig. 3). The stretching of C–O in the region of 1200–950 cm−1 indicate the carbohydrate nature of β-glucan. Band in the region 1000–1200 cm−1 depicts polysaccharide nature while the region 1500–1700 cm−1 depicts residual proteins attached with β-glucan molecules (Ahmad & Anjum, 2010). The absorption peak at 1156–1165 cm−1 depicts the linearity in the structure of β-D-glucan (Ahmad et al., 2010). Bands at 1156, 1076, and 894 cm−1 characterize the (1 → 3) and (1 → 4)-linked β -D-glucan while the absence of a band at 834 cm−1 region shows that there are no alpha linkages present in the samples (Ahmad & Anjum, 2010). The stretching of protein groups (CN and NH) give bands at 1566 and 1658 cm−1 (Ahmad et al., 2010). Absorption peaks for lipids appeared in the 2840–3000 cm−1 region while the major peak at 2918–2924 cm−1 is because of the CH2 stretching. The bands in the region of 1640 and 3415 cm−1 indicate the presence of water. The water spectra (1600–1650 cm−1) overlap with the protein spectra. A major portion of β-glucan gum pellets is composed of carbohydrates with some fat, protein, and water impurities (Ahmad & Anjum, 2010).
3.2 Meat and Meat Products
The information about the quality of meat throughout the production process is beneficial both for the consumers and manufacturers. FTIR together with chemometric methods quickly calculates the bacterial count from the surface of meat while it also quantifies the pork in beef meatballs. It also studies the collagen and gelatin from skin and bones along with the denaturation process of myofibrillar and connective tissues. It shows that with the increase in temperature α-helix structure decreases while β-sheet structure increases while these changes are more noticeable in myofibrils (Damez & Clerjon, 2013). As the freezing and thawing of chicken greatly affect its quality by forming crystals, protein degradation, and lipid oxidation hence it is important to differentiate fresh meat from frozen one. Based on this information, the FTIR technique can now predict the storage conditions of the meat products at which these are kept during storage. Hence, the frozen minced meat when analyzed using FTIR, revealed the differences in the refrigerated and frozen samples at 1660–1628 cm−1. The amide I band indicates the alterations in the backbone of protein and the pattern of hydrogen bonding. The use of improved pattern recognizing algorithms could improve the performance of FTIR spectroscopy in identifying frozen and thawed meat (Grunert et al., 2016).
The composition of meat fat is the major determining factor of meat quality. Mid-infrared Fourier transform spectroscopy productively forecast fatty acids in the beef, chicken, and pork fats. As per the regulation of the European Union (EU), it is compulsory to specify the components of saturated fats along with total fat content. FTIR in combination with chemometric methods quantifies the saturated and unsaturated fats efficiently (Lucarini et al., 2018). Fish are rich in omega-3 EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid). The quality of fish meat is affected by the change in the lipid and protein portions. Fatty acid composition, total fat, and nutritional parameters of fish are studied by FTIR in combination with partial least square where the strong bands at 3360 cm−1 are assigned to the OH stretching of water, 3270 cm−1 to N–H stretching of proteins, 1550 cm−1 to the bending and stretching of N–H and C–N protein respectively and 1652 cm−1 to C=O stretching of proteins while weak bands at 2958 cm−1 are assigned to methyl asymmetric stretching of lipids, 1239 cm−1 to asymmetric stretching of phospholipids, and 1124 cm−1 to the C–O stretching vibrations of ester (Hernández-Martínez et al., 2013).
3.3 Fruits and Vegetables
Natural polysaccharides or food additives polysaccharides like pectin, starch, and galactan are well distinguished through FTIR in the region of 1200–800 cm−1. Mid-infrared Fourier transform spectroscopy in combination with multivariate analysis study the contents of capsicum. Capsicum has an antioxidant activity while capsaicinoids possess an antibacterial effect. The absorption peak 3600–3000 cm−1 are assigned to hydroxyl stretching of water while peaks at 2950–2800 cm−1 were allocated to C–H stretching of methylene and methyl groups. The peak at 1750–1710 cm−1 is allocated to the stretching of the carbonyl group while the peak at 1650 cm−1 to amide and OH stretching. The bands at 1200–950 cm−1 represented the stretching of C–O and C–C (Domínguez-Martínez et al., 2014). Tomato cultivars are also classified and determined through ATR-FTIR based on sugar and acid along with the prediction of its quality parameters (Bobelyn et al., 2006). The absorbance peaks of tomato sample at 3359 and 1677 cm−1 are the same as that of other fruits and vegetables while the major difference is in the regions of 1500–1000 and 3300–2750 cm−1 which depict the presence of sugars and organic acids. The absorption range, i.e., 1153–904 cm−1 shows the stretching of C–O and C–C along with O–C–H, C–C–H, and C–O–H bend in the region of 1474–1199 cm−1 are indicators for the presence of carbohydrates. The revealing C–H bending at 1583 cm−1 relates to the presence of organic acids. Soluble solids are depicted by the peaks 1067, 1082, and 1593 cm−1 while the presence of glucose and fructose in tomatoes is depicted at 1034 and 1067 cm−1. Absorption peaks at 1718, 1593, 1369, and 1227 cm−1 are strong indicators of acidity and reveal the presence of citric acid (Ścibisz et al., 2011).
Apple wax, vitamin C in mixtures, and polymethoxylated flavones in orange peel oil are also analyzed through this technique. Polymethoxylated flavones are anticancer and anti-inflammatory. The low intensive C=O vibration at 1724 cm−1 depicts the low amount of non-PMF constituents (Bernal et al., 2011). In apricot slurries, the absorption zones 3700–2800 and 1800–1470 cm−1 are assigned to water while sugars and organic acids are present in the zone (1500–900 cm−1). Band in 1500–1200 cm−1 region depicts the deformation of –CH2, C–C–H, and H–C–O while bands in the range 1200–950 cm−1 show the C–C and C–O stretching (Bureau et al., 2009). Moreover, the contents of bioactive compounds in kiwi fruit are also studied through FTIR showing that kiwi fruits are composed of different components such as polyphenols, tannins, ascorbic acid, phenolic, and flavonoids. The wavelength numbers (866, 1026, 1237, 1451, 1542, and 1619 cm−1) are shown by the gallic acid while catechin spectra at wavelength numbers (831, 1040, 1112, 1144, 1285, 1478, 1512, and 1611 cm−1) are assigned to –CH alkenes, COH alcohols, CO alcohols, CH alkane, C=C aromatic ring, and C=C alkenes (Park et al., 2011).
3.4 Dairy and Dairy Products
UHT-treated milk endures significant changes in the physiochemical properties during processing and storage leading towards sedimentation or gelation. These changes being high in skim and whole milk at high temperature are affiliated with the protein-fat and protein-protein interaction. Milk proteins exhibit three absorption bands in the IR spectrum, i.e., amide I (1600–1695 cm−1), amide II (1520–1560 cm−1), and amide III (1230–1300 cm−1) while the carbohydrates appear in the range of 800–1300 cm−1. Moreover, water bands predominate around 1600 cm−1 and 3000 cm−1. An increase in the sedimentation of milk is associated with the increasing intermolecular β-sheets. The presence of lipid absorbance in regions of 3000–2800 cm−1 and 1800–1700 cm−1 indicate the participation of protein-lipid interactions in sedimentation. Water bands predominated in the region 3500–3000 cm−1 and 1730–1600 cm−1 while the changes in the carbohydrates and protein are less intensive (Grewal et al., 2017). The microwave treatment of β-lactoglobulin in D2O solution shows that the increasing temperature increases the protein aggregation more as compared to conventional heated solutions. The unfolding of β-lactoglobulin is also enhanced at 70–90 °C (Gomaa et al., 2013). Cholesterol in milk products is also determined in the region of 3200–2800 cm−1 in nearly 5 min. Vibrations in the region 2940–2962 cm−1 and 2872–2875 cm−1 are associated with the stretching of the CH bond in the methyl group. The peaks in region 3100–2990 cm−1 are related to the stretching of C-H bonds in cyclic alkanes (Paradkar & Irudayaraj, 2002).
Fourier transform infrared spectroscopy is also used to analyze the fresh milk over the range of 5000–900 cm−1 to predict the yield of cheese. The region 1582–930 cm−1 consists of peaks for carbohydrates and organic acids. It predicts the major nutritional contents and their retention in the cheese (Ferragina et al., 2013). Ripening of cheese is a complex procedure including events like metabolism of lipid, protein, lactose, citrate, and lactate. The analysis of water-soluble fractions shows a high amount of amino acids like leucine, glutamic acid, asparagine, etc., while the changes are more intense in the range of 1800–900 cm−1 (Subramanian et al., 2011). Italian cheeses are also classified based on their ripening time and their technique of manufacturing. Major differences in the cheese spectra are observed in the regions 3700–3000 and 1700–1000 cm−1 while it also inspects the bands of fats, lactose, lactic acid, and proteins in the milk products (Lerma-García, Gori, et al., 2010).
The study of chemical composition, fatty acid profile, and trans-fat of margarine show that the major variations are in the region 3690–2942 cm−1 due to OH stretching and cis-trans double bonds while the region 2911–2876 cm−1 is associated with the vibration of methyl asymmetrical stretching. Different parameters of margarine composition are obtained in 10 min while the same analysis takes hours during conventional analysis while the concentration of adulterants such as sodium bicarbonate, sodium citrate, and cheese whey in milk is also measured through FTIR. It is an effective technique to monitor adulterants in dairy products possessing a low cost of operation and higher analytical performance (Cassoli et al., 2011; Hernández-Martínez et al., 2010).
3.5 Oil and Fats
Physicochemical properties of corn and mustard oil to assess their compositional quality, as well as the effect of frying on various characteristics of oil, can be best studied through FTIR. This technique is also effective in the elucidation of changes in oils during repeating frying operation and shed light on oxidative and thermal reactions which alter the physiochemical, nutritional, and sensory properties. Though all these changes in the fried oil depend upon the frying conditions, oil quality, and can also be detected through conventional chemical analysis FTIR provides a rapid solution to get the overall quality of frying oils. As fats and oils are simple molecular systems and mainly possess an uncomplicated spectrum between 800 and 3500 cm−1 (Ismail et al., 1997). In oil samples at room temperature, the specific band at 3473 cm−1 is associated with stretching of O-H, stretching of C-H is revealed at 3006 cm−1 and bands between 2854 and 2925 cm−1 appear due to double bond vibration. At boiling point, the band 1745.5 cm−1 is assigned to double bond stretching while 1654–1658 cm−1 band to C=C stretching. The fresh and used oils show similar spectra though the heights in specific bands indicate the difference in quality (Zahir et al., 2017). Whereas, the oil absorption by the starchy fried products is also a huge concern for the consumers as it affects the health and its content in fried starchy products are determined through ATR-FTIR spectroscopy showing that the huge amount of oil is absorbed by the starch but the major quantity of it is located near the surface. Soybean oil exhibits –CH3 characteristics in the range 2995–2886 cm−1 while ester group at 1793–1693 cm−1 (Chen et al., 2018). Moreover, the analysis of the peanut butter and mayonnaise show that the fat and water peaks are quite prominent in mayonnaise while the water peak in the peanut butter spectrum is missing which may be because of less water content (van de Voort et al., 1993).
Virgin coconut oil has a variable effect on HDL cholesterol, total cholesterol, LDL, and triglycerides levels. It differentiates from olive oil in the frequency region of 1111 and 962 cm−1 while with palm oil at 1120–1105 and 965–960 cm−1. The virgin coconut oil if mixed with palm and olive oil show bands in the region of frequency 1120–1105 and 965–960 cm−1 is effectively analyzed through FTIR spectroscopy in combination with ATR and PLS (Rohman et al., 2010). Whereas, the polyunsaturated fatty acids are quantitatively analyzed in oils showing the band at 3013 cm−1 which represent the unsaturated fatty acids while bands in the range of 3000–2800 cm−1 attribute to the C–H stretching. Strong peaks at 1145 and 1098 cm−1 are assigned to bonds found in esters and ethers (Vongsvivut et al., 2014). Likewise, the analysis of cinnamon oil shows a peak at 1727 cm−1 which is assigned to the aldehyde of saturated fat while peaks at 1679 and 1626 cm−1 represent the stretching of aldehyde carbonyl C O. Peak at 1573 cm−1 represent aromatic ring C C, 1450 cm−1 for alcohol COH, 1248 cm−1 to aromatic acid ester C O C. The volatile components of oil display high diversity and complexity (Li et al., 2013).
Oils from different marine sources including marine microorganisms are rich in n-3 polyunsaturated fatty acids (eicosapentaenoic acid and docosahexaenoic acid). The bands at 3013 cm−1 are allocated to C–H stretching of cis-alkene which represent unsaturated fatty acids while bands in region 3000–2800 cm−1 represent the C–H stretching of methyl and methylene pillars of lipids. The peak at 1743 cm−1 is because of the C=O stretching of ester groups from lipids while the intense bands at 720 cm−1 attribute to methylene deformation in cis-substituted olefins (Vongsvivut et al., 2014). Moreover, encapsulated fish oil is also analyzed through FTIR spectroscopy revealing bands at 3290 cm−1 assigned to O–H stretching, C–H stretching at 3015 cm−1 and C–H stretching of methyl and methylene pillars of lipids at 3000–2800 cm−1. Overlapping bands at 1460–1370 cm−1 represent the deformed modes of methyl and methylene groups from proteins as well as lipids. The band intensity tends to increase at 2923, 1744, 1146 cm−1 with the increase in oil quantity (Vongsvivut et al., 2012).
3.6 Limitations of FTIR
One of the greatest powers of FTIR is its capability to acquire spectra from a wide range of solids. However, at times certain solids and liquids need sample preparation to acquire a spectrum of good quality (Franca & Oliveira, 2011). For instance, one of the common methods of sample preparation for solids is to grind them into powder form and then dissolve them in the solid medium which in most cases is KBr. Moreover, the liquid transmission needs an easier sample preparation as compared to solids though both the medium of samples can face unavoidable reproducibility concerns. However, this problem has been somewhat minimized recently through Attenuated Total Reflectance (ATR) and will get better over time because of the invention of new supporting methods and techniques. Moreover, FTIR is more applied to determine the change in the molecular structure during processing and treatments. It is a challenge to use it to determine the actual compound present in the sample (e.g., how much sugar). However, it can be done by using standards that are tough to find and calibrate.
In FTIR, the results can also be misinterpreted because of the overlapping spectra produced by complex samples especially a mixture of bacteria. Therefore, in certain cases a bacterial purification or separation step becomes necessary. This overlapping can be resolved by the Fourier deconvolution as it increases the peak height and reduced the bandwidth. Moreover, it may well need hard data gathering, calibration, and skill in chemometric analysis of spectra. Furthermore, few protocols are being developed which could help one understand and apply this technique better. Hence, reproducible, rapid, and accurate methods need to be developed to make the process smoother. Also, for the analysis of a compound in IR spectroscopy dipole moment is required thus the compounds having no dipole moment will not absorb IR rays and would not be analyzed. Likewise, during the analysis of a mixture of compounds two compounds may absorb IR rays at the same wavelength which causes the masking of peaks. All these limitations can be reduced by developing more and more protocols considering the major limitations. FTIR is more applied to determine the change in the molecular structure during processing, source, and treatments. It is a challenge to use it to determine the actual compound present in the sample (e.g., how much sugar). However, it could be done by using standards, which are not easy to find and calibrate.
3.7 Comparison with Other Similar Methods
Food is a complex system that makes food analysis an intricate process. Various techniques are being used to analyze food while the choice of an analytical technique depends upon the type of analysis and the properties of food to be analyzed. Ultraviolet and visible spectroscopy is one of the commonly used techniques to analyze food. Ultraviolet wavelength ranges from 200–350 nm while visible wavelength range from 350 to 700 nm. The sample solution used must be clear and uniform while the sample solution is normally water or an aqueous buffer. Moreover, for good results, the cuvette should be quartz or silicate glass. Different anthocyanins, flavonoids, phenolic, and carotenoid content in food can be measured through this technique (Tiwari et al., 2013). Another analytical technique used in food analysis is fluorescence spectroscopy but it is more sensitive and uses a different optical system than UV. Moreover, two-wavelength selectors are required so they can study the conformational changes in protein as well as the content of vitamins (Lakowicz, 2013). Both these techniques differ from FTIR as UV spectroscopy uses a different range of wavelength and source while it is mainly a quantitative technique whereas FTIR is qualitative as well as a quantitative technique. Moreover, the limitations of fluorescence include loss of photostability and not all molecules being fluorescent which is not an issue when it comes to FTIR.
Atomic spectroscopy is another spectroscopy technique used mainly for the measurement of trace amounts of minerals in food which limits its functioning to elements. On the other hand, it requires more samples, is less sensitive, and can analyze only solutions. Furthermore, Raman spectroscopy is a vibrational technique that complements infrared spectroscopy specially FTIR. It is mainly used for the analysis of organic compounds. It can also measure the changes in chemical bonding, e.g., when a substrate is added to an enzyme (Li-Chan et al., 2010). As compared to the infrared it possesses weak water scattering hence a low quantity of organic molecules in an aqueous solution is allowed.
Nuclear magnetic resonance spectroscopy (NMR) studies a wide range of food constituents and provides their structural data. It can analyze samples in solution as well as solid state. Insufficiently concentrated samples, low sensitivity, difficulty in handling mixtures, and magnetic field drift are some of the limitations of this technique. Additionally, the X-ray diffraction technique is also used to identify the structures of carbohydrates, lipids, and proteins. X-ray fluorescence detects the small number of metal ions in food. All these and some more techniques can be used for the analysis of different food components and products. Moreover, they are all different from each other in one way or the other and possess certain limitations. What makes FTIR different from these techniques is its properties such as rapid, little or no sample preparation, nondestructive, sensitive, multiple sample environment, qualitative as well as quantitative and mechanical simplicity. Moreover, it can be modified according to the sample and is internally calibrated. Additionally, different variety of food components and products can be analyzed using FTIR such as butter, sugar, milk, meat, oils, and juices which makes it superior to the other methods.
3.8 Bacteria in Food Industry
The composition of bacterial cells is analyzed by FTIR spectroscopy. In combination with different chemometrics, it is possible to detect, classify, quantify, and differentiate the bacterial cultures in food (Davis & Mauer, 2010). Bacteria are often used in the food industry as starter cultures but they can also cause food spoilage and food poisoning. FTIR identifies these strains by comparing the obtained identification spectra with the reference spectra (Wenning et al., 2014). For the identification of bacteria five main regions of absorbance in infrared spectra should be analyzed. The fatty acid region is 2800–3000 cm−1, proteins and peptides (1500–1700 cm−1), a mixed region of peptides, phosphate compounds and fatty acid (1200–1500 cm−1), carbohydrates (900–1200 cm−1), and 700–900 cm−1 is called as fingerprint region which contains absorbances that are the attributes to specific bacteria. This technique is also used to differentiate between the fresh, semi-fresh, and spoiled beef fillets. The comparison provides information about the biochemical changes during spoilage. The peak at 1640 and 1550 cm−1 represent the moisture and amide respectively while peaks at 1460, 1240, and 1175 cm−1 represent the fat presence (Argyri et al., 2010). Raw meat deteriorates rapidly even if stored under refrigeration. The major peak is observed at 1639 cm−1 indicating the presence of water while the peak at 1550 cm−1 represents the absorbance of amide II. The wavenumbers from 1408 to 1370 and 1320–1305 cm−1 are linked to the amides and amines and are strongly associated with the spoilage of meat (Vasconcelos et al., 2014).
Metabolites formed by the different microorganisms cause the spoilage of meat. Salmonella, Pseudomonas, and Campylobacter species are a major threat to meat quality which requires a rapid detection method to identify and control them. The biochemical changes in the meat are effectively measured by FTIR spectroscopy which enhances microbial spoilage detection. The most intensive peaks in fresh meat are of amide bands at 1640 and 1550 cm−1 suggesting the decrease in protein content during spoilage. These peaks start to increase after 16 h because of the production of more free amino acids by aerobic microorganisms (Ellis et al., 2002). Likewise, beef is a widely consumed food and is also very susceptible to microbial spoilage. FTIR spectroscopy allows the rapid detection of microbial spoilage in fresh meat. The absorption band at 1513 cm−1 represents C–N stretching and N–H bending while 1413 cm−1 refers to C–H stretch, 1382 cm−1 to NO2, and 1112 cm−1 to C–N stretch (Ellis et al., 2004).
Escherichia coli and Pseudomonas in the bottled drinking water are also identified through Fourier transform infrared spectroscopy using multivariate analysis. The presence of these coliforms in drinking water indicates insufficient treatment or posttreatment impurity. The comparison of inoculated and non-inoculated drinking water showed that this technique also discriminates against bacteria based on the differences in biochemical and phenotypic features (Al-Qadiri, Al-Holy, et al., 2006). It is also applicable in the characterization of different microorganisms, characterization of E. coli and Alicyclobacillus strains in the juice while it also detects the effect of chlorine on bacterial injury in water. The absorption bands vary from bacteria to bacteria and product to product (Table 2). Listeria monocytogenes is also a major threat to the safety of food products because they can grow in a refrigeration environment. Different lactic acid bacteria produce bacteriocins which are antibacterial peptides hence hindering the growth of other bacteria. The susceptibility of L. monocytogenes to these bacteriocins is also evaluated through FTIR (Oust et al., 2006).
Milk contains a high amount of water and exists at about a neutral pH range (6.5–6.7) which makes it more prone to microbial attack. Temperature between 2 °C and 30 °C is also ideal for microbial growth. FTIR technique rapidly quantifies the microbial load in pasteurized milk (Nicolaou & Goodacre, 2008). This technique also discloses the entrance of Staphylococcus aureus in dairy production through milk contamination caused by cow infections (Kümmel et al., 2016). The fortune of smear starters during the cheese ripening studied through FTIR shows that the starter cultures do not outcompete the residential bacteria which may be due to the starter culture application, competitiveness of resident microorganisms, or both these factors (Goerges et al., 2008).
Lactic acid bacteria are a group of microorganisms that highly relate to fermented food and human health. They form lactic acid by fermenting carbohydrates. Lactic acid bacteria include genera like Lactococcus, Pediococcus, Leuconostoc, Streptococcus, Bifidobacterium and Lactobacillus, etc. It is difficult to identify the LAB because of their rich and closely related species. Hence, FTIR is an effective technique to identify and characterize these bacterial strains rapidly in the spectral region of 1450–700 cm−1 (Wenning et al., 2010). Moreover, the effect of exopolysaccharide-producing strains and non-producers is also studied through FTIR. Exopolysaccharides are the macromolecules produced by different microbes. Lactobacillus kefiranofaciens is a unique lactic acid bacterium, which produce kefiran. Different cocultures improve product quality and its nutritional value. The strong peaks at 1647.31 cm−1 represent the polysaccharides while peaks at 3395.07, 2924, 1558.07, 1245, and 1061 cm−1 represent sugar residues, stretching of C H in sugar ring, N H bending, sulfate groups, and C O stretching, respectively. This study shows that the coculturing technique effectively produces exopolysaccharides along with protein and sulfur-containing substances (Ahmed, Wang, Anjum, Ahmad, Ahmad, & Raza, 2013).
Polyhydroxyalkanoates (PHA) are synthesized by the prokaryotic microorganisms while polyhydroxy butyrate (PHB) is the common PHA having application at the industrial level. It is an alternative to plastics hence its industrial fermentation is under focus. FTIR is applied to quantitatively investigate the polyhydroxy butyrate content of bacterial cells through the combined use of the Partial least square (PLS) technique. The bands at 1738–1728 cm−1 are due to ester carbonyl stretch, at 1450 cm−1 due to methyl deformation, and 1654 cm−1 show the amide carbonyl stretching. The study shows the amorphous nature of polyhydroxy butyrate while it also predicts the degree of PHB crystallinity (Kansiz et al., 2000). Furthermore, the strains of yeast are also identified through this technique and it identifies the S. cerevisiae strains with 92% success while that of D. hansenii with 91% (Wenning et al., 2002). This technique in combination with PLS utilizes the spectral range of 2000–1000 cm−1 to determine the microbial quality of minimally processed pomegranate arils (Adiani et al., 2018). Microorganisms in the biofilms are also studied efficiently within the region 4000–500 cm−1 because it holds characteristic bands for microbial characterization (Schmitt & Flemming, 1998). The FTIR study of the fermentation of shrimp shells to produce chitin and chitosan through the Bacillus subtilis starter cultures show that this bacteria is an efficient starter culture (Sini et al., 2007).
Microorganisms are also identified and differentiated through FTIR spectroscopic imaging which is a novel method having received great consideration. It possesses high molecular sensitivity in combination with spatial resolution down to the micrometer level while it also examines samples under native conditions and applies to a wide range of products (Steiner & Koch, 2009). This imaging technique also diagnoses pathogenic bacteria rapidly and cost-effectively. Different bacteria are accurately identified through the investigation of the levels of taxonomic resolution. The strains from the same species are successfully differentiated through FTIR microspectroscopic imaging (Lasch et al., 2018). It also measures the micro-colonies reducing the incubation time and accelerating the identification process. Moreover, the old colonies are better identified through conventional spectroscopy as compared to microspectroscopy (Wenning & Scherer, 2013). Furthermore, different wheat cultivars are also analyzed through this technique while the images ranging from bran to the endosperm center are obtained showing delicate differences between the cell walls of different varieties (Barron et al., 2005).
3.9 Comparison with Other Methods
Bacteria are present in large numbers in food products among which some are beneficial while some are harmful to humans. Hence, it becomes critical to analyze them in different products. Various analytical techniques are being used to analyze the bacteria for a long time. However, with each passing year, new techniques are being developed which are more efficient than the previous ones. Culturing, enumeration, and isolation of colonies for further identification is the basis of the traditional way to detect and identify the bacteria in food (Hakovirta, 2008). Culture media technique has been a traditional choice for either fresh or processed food. The microscopic colony count technique is a simple method to morphologically identify and count bacteria at initial levels. However, it has certain disadvantages as both viable and nonviable cells are enumerated while some cells might not get stained properly. Plate count is another traditional method of bacterial identification in which samples are blended, diluted, plated, and incubated at an appropriate temperature. It identifies the colony-forming units in food products. Dye reduction is another traditional technique used to detect the microbes present in food. The dye reduction time is inversely proportional to the number of microbes present in the food sample. However, this technique is not efficient for food samples that contain reductive enzymes.
Some other comparatively older bacterial identification techniques are flow cytometry, ATP bioluminescence assay, epifluorescent filter technique, ELISA, ELFA, and PCR. However, these techniques are more rapid than purely traditional techniques. All these techniques are used to enumerate bacteria in various food items such as milk, wine, water, and juices (Van Alfen, 2014). Moreover, the traditional techniques require a huge amount of time, glassware, laboratory work, and culture media preparation. Likewise, these techniques are rather insensitive, nonspecific visual appearance, and are limited to certain bacteria with known growth requirements. On the other hand, FTIR is less time-consuming, reliable, economic, more sensitive, no glassware required, less laboratory work, and no media preparation (Davis & Mauer, 2010; Vongsvivut et al., 2012). Moreover, it is more applied to detect the changes at the molecular level which makes it more efficient than the other traditional techniques. It can analyze a huge variety of bacteria present in different food products.
4 Food Authentication Using FTIR Spectroscopy
Adulteration is the addition of any non-authentic substance or removal of authentic substance without the consumer knowledge for economic gain (Zhao et al., 2014). Authentication is the process that verifies the compliance of the food with its label description. Fraud cases (68%) in 2016 were reported in different products with variable fat content (meat 27%, fish 13%, fats 11%, milk 10%, nuts 4%, and animal by-products 3%). In the USA, 1500 lbs of chicken were found contaminated with nitrofurazone (a veterinary drug). In China and India, milk, edible oils, meat, nuts, etc., are highly susceptible to adulterations (Valdés et al., 2018).
4.1 Oils and Fat Authentication
Fats and oils are simple molecular systems and mainly possess uncomplicated spectrum between 800 and 3500 cm−1 (Ismail et al., 1997). Olive oil is available in diversified forms as extra-virgin oil (EVO), virgin, refined, and pomace (de la Mata et al., 2012). Extra-virgin olive oil is adulterated with canola, hazelnut and sunflower oil as the cost of EVO is high as compared to others hence more profit is gained through adulteration (Maggio et al., 2010). Olive oil being costly is often adulterated with less expensive oils like palm oil. Both the oils possess differences at frequency 1402 and 1030 cm−1 (Rohman & Che Man, 2010). Products of olive oil contain a high amount of oleic acid and are adulterated with cheap oils such as canola and palm oil. They possess similar spectra though both the oils show a slight difference at 3005 cm−1 (Rohman et al., 2014). Moreover, sesame oil present in olive oil can be judged using frequencies 1207–1018, 1517–1222, and 3050–2927 cm−1. The presence of high amounts of stearic and linoleic acids in these oils increase with the increase in the amount of sesame oil and can be detected using the FTIR technique (Rohman & Che Man, 2012b).
FTIR coupled with the multivariate analysis is used to classify vegetable oils according to their origin (Lerma-García, Ramis-Ramos, et al., 2010). Major peaks at 2937, 2856, 1749, 1454, and 1166 cm−1 represent CH stretching, CH bending, and CO stretching. These play a vital role in the discriminant analysis (Yang et al., 2005). Vegetable oils are also adulterated with used frying oil showing less visible absorption bands in the region 4000–1650 cm−1 while additional bands in the range below 1650 cm−1 during FTIR study (Zhang et al., 2012). Different vegetable oils such as corn oil, canola oil, soybean, and sunflower oil are adulterated with lard whereas lard and vegetable oils show minor differences at 1117 and 1097 cm−1 corresponding to CH bending and deformation. An increase in the lard concentration will increase the peak height at 1097 cm−1 (Rohman et al., 2011).
Virgin coconut oil is often adulterated with cheap canola oil. This adulteration can be detected in the region of 3007 and 1654 cm−1. In region 1120–1090 cm−1 canola exhibited two peaks as compared to coconut oil (Che Man & Rohman, 2013). Avocado oil is a newcomer in the oils market and is costly hence adulterated with different oils to gain profit and deceive the consumer. FTIR in combination with the SIMCA model rapidly identify and quantify avocado oil and its adulterants (Quiñones-Islas et al., 2013). Furthermore, cod liver oil offer many nutritional advantages as it contains EPA and DHA. It is also adulterated with lard which is determined through FTIR in region 1500–1030 cm−1 (Rohman & Che Man, 2009).
4.2 Honey Authentication Using FTIR
Honey is composed of fructose, glucose, maltose, sucrose, water, minerals, and high sugars. It is often adulterated with corn syrup, cane sugar, jaggery, and beet sugar but this adulteration is difficult to determine because of the variation in the origin, species, and environment (Mishra et al., 2010). This adulteration affects both the consumers and authentic honey producers hence the development of reliable and quantitative techniques for detection is important (Li et al., 2017). Adulteration of honey with inverted cane sugar is determined through ATR-FTIR which provides a benefit over traditional techniques like wet chemistry, enzymatic technique, and chromatographic procedures which are time-consuming, destructive, and costly (Irudayaraj et al., 2003). FTIR spectroscopy coupled with PLS is expected to replace the traditional, time to consume, and high-cost analysis of honey (Wang et al., 2010). Sugars are observed in the range of 800–1500 cm−1. As the adulterated honey presents similar spectra of different components so principal component regression and partial least squares are applied to develop models for effective prediction of different variables. The bands in region 1470–1149 cm−1 represent bending C–O–H, O–C–H, and C–C–H while the bands at 1028 and 1053 cm−1 are the most intensive ones representing C–O and C–C stretching (Rios-Corripio et al., 2012).
4.3 Milk and Milk Products
Melamine consumption through adulterated milk in China during the year 2008 posed adverse effects on the consumers and the economy. Mid-infrared spectroscopy is effective in melamine detection coupled with the partial least square method (Domingo et al., 2014). Melamine is a synthetic nitrogenous chemical used to produce synthetic resins. It is added to foods to deceive the consumer about the protein content of the product but is a harmful chemical if over-consumed. The band at 806 cm−1 indicates the bending of the triazine ring in melamine while this band is not present in milk. The exclusive fingerprint of each compound is detected in the fingerprint region, i.e., 1300–910 cm−1 (Jawaid et al., 2013).
Milk proteins exhibit three absorption bands in the IR spectrum, i.e., amide I (1600–1695 cm−1), amide II (1520–1560 cm−1), and amide III (1230–1300 cm−1). Milk consumption has increased in Asia over the years hence to meet the supply-demand vendors adulterate cow milk with soymilk. Soymilk contains the same proteins as cow milk excluding sulfur-containing amino acids. FTIR coupled with the chemometric analysis detects the presence of soymilk in cow milk. In soy milk amide I, amide II, and amide III are present in a high amount as compared to cow milk. Soy milk also depicts stronger absorption in the region 1639–1613 cm−1 because of the occurrence of secondary structures (Jaiswal et al., 2015). Adulteration with sodium bicarbonate, cheese whey, and sodium citrate in raw milk while chicken fat in butter is also determined through FTIR. Butter consists of more than 500 fatty acids including saturated, monounsaturated, and polyunsaturated fatty acids. The absorption band at 966 cm−1 is the marker band for determining the trans-fat associated with –HC=CH. The band at 3006 cm−1 indicates the high amount of unsaturated fats as possessed by the chicken fat particularly linoleic acid (Cassoli et al., 2011; Nurrulhidayah et al., 2013).
4.4 FTIR Authentication in Other Products
One of the most popular drinks throughout the world is tea while its color is very important for its market price and quality. Talcum powder is often added to the tea to hide the quality defects. FTIR spectroscopy discriminates the pure and adulterated tea samples showing absorbance peaks at wavenumbers 1401, 1617, and 3117 cm−1 are similar while peaks at 1016, 1182, 1249, 1340, 1631, and 2296 cm−1 distinguish talcum adulterated tea samples from pure tea sample (Li et al., 2016). The adulteration of sibutramine in green tea, green coffee, and herbal tea is also determined through this rapid technique. Sibutramine is a serotonin-norepinephrine reuptake inhibitor often added to herbal diets and supplements to enhance weight loss. The bands observed in regions 2698 and 2700–2600 cm−1 are specifically used for sibutramine detection (Cebi et al., 2017). FTIR also finds its application in the detection of adulterated chocolate with lard when coupled with ATR. The region of 4000–650 cm−1 is used to predict the lard and chocolate components. The band at 2924 cm−1 represents methylene asymmetrical stretching while the band at 2853 cm−1 represents methylene symmetrical stretching (Che Man et al., 2005).
4.5 Comparison with Other Methods
There has been a rise in the demand for food authentication because of the increasing awareness in the public considering food safety. There are different traditional methods to authenticate food being used such as sensory analysis (taste, color, and smell) and physiochemical testing. Moreover, in past few years certain other methods have also been developed such as chromatographic techniques, isotopic techniques, PCR, ELISA, mass spectrometry, and elemental profiling (Danezis et al., 2016). Physiochemical and organoleptic testing methods are comparatively simple and less expensive though they are often time-consuming and require different equipment and glassware. Gas chromatography is rapid, require small sample, and is sensitive; however, it is limited to volatile samples and some samples require extensive preparation which is not the case with FTIR spectroscopy. Moreover, ELISA can test a large number of samples, reproducible, and cost-effective; however, in some cases, it may be destructive to certain samples.
Polymerase chain reaction (PCR) is highly specific, sensitive, easy to use, and reproducible while on the other hand, it may at times give false-positive. Furthermore, mass spectrometry is fast and requires a small sample size though it does not directly give the structural information and nonvolatile compounds are difficult to handle. HPLC is another technique used to analyze food products. It is efficient, automated with less contamination however it is a costly technique, less sensitive, and complex which makes it less appealing when it comes to analyzing food products regularly. All these techniques aside, FTIR has its pros and cons. It is rapid, highly sensitive, high resolution, less expensive, provides rich information, and reproducible (Davis & Mauer, 2010; Vongsvivut et al., 2012). However, the limitations of FTIR include the inability to detect atoms or monoatomic ions, aqueous solutions are hard to analyze (i.e., water is a strong infrared absorber), inability to recognize molecules comprised of duplicate atoms, and complex blends give complex spectra.
5 Conclusion and Future Progress
Fourier transform infrared spectroscopy is a fast, nondestructive, and reliable technique requiring minimal or no sample preparation as compared to other techniques. It has the potential to analyze and authenticate a wide range of food products at macro and micro levels, though more research work is required to develop new protocols for the further acceptance of the technique in the scientific community. The use of FTIR imaging capacity enables us to examine the samples at the microscopic level and hence increase its application in the discipline of microbiology. Using this instrument, we can now analyze complex substances with ease and efficiency. Furthermore, it could be of great choice in the field of food science, food analysis, and product authentication with the further development of new protocols. Overall, it is a dynamic technique to keep a check on the quality and quantity of products and their constituents. Fourier transform infrared spectroscopy despite being an efficient tool to analyze and authenticate products has certain limitations in the application of this technique. Little protocols are being developed which could have helped one understand and apply this technique better. Reproducible, rapid, and accurate methods need to be developed to make the process smoother. Furthermore, the hardware and software of the technique should be improved to make it more cost-effective.
References
Adiani, V., Gupta, S., Ambolikar, R., & Variyar, P. S. (2018). Development of rapid method to assess microbial quality of minimally processed pomegranate arils using FTIR. Sensors and Actuators B: Chemical, 260, 800–807.
Ahmad, A., & Anjum, F. M. (2010). Persepctive of βglucan: Extraction and utilization. VDM Verlag.
Ahmad, A., Anjum, F. M., Zahoor, T., Chatha, Z. A., & Nawaz, H. (2008). Effect of barley β-glucan on sensory characteristics of bread. Pakistan Journal of Agricultural Sciences, 45, 88–94.
Ahmad, A., Munir, B., Abrar, M., Bashir, S., Adnan, M., & Tabassum, T. (2012). Perspective of β-glucan as functional ingredient for food industry. Journal of Nutrition & Food Sciences, 2, 133.
Ahmad, A., Anjum, F. M., Zahoor, T., Nawaz, H., & Ahmed, Z. (2010). Extraction and characterization of β-d-glucan from oat for industrial utilization. International Journal of Biological Macromolecules, 46, 304–309.
Ahmad, A., Anjum, F. M., Zahoor, T., Nawaz, H., & Dilshad, S. M. R. (2012). Beta glucan: A valuable functional ingredient in foods. Critical Reviews in Food Science and Nutrition, 52, 201–212.
Ahmad, A., Anjum, F. M., Zahoor, T., Nawaz, H., & Din, A. (2009). Physicochemical and functional properties of barley β-glucan as affected by different extraction procedures. International Journal of Food Science & Technology, 44, 181–187.
Ahmad, A., & Khalid, N. (2018). Dietary fibers in modern food production: A special perspective with β-glucans. In A. Grumezescu & A. M. Holban (Eds.), Biopolymers for food design (pp. 123–154). Elsevier.
Ahmed, Z., & Ahmad, A. (2017). Biopolymer produced by the lactic acid bacteria: Production and practical application. In A. M. Grumezescu & A. M. Holban (Eds.), Microbial production of food ingredients and additives (pp. 217–257). Elsevier.
Ahmed, Z., Wang, Y., Anjum, N., Ahmad, A., & Khan, S. T. (2013). Characterization of exopolysaccharide produced by lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir–part II. Food Hydrocolloids, 30, 343–350.
Ahmed, Z., Wang, Y., Anjum, N., Ahmad, H., Ahmad, A., & Raza, M. (2013). Characterization of new exopolysaccharides produced by coculturing of L. kefiranofaciens with yoghurt strains. International Journal of Biological Macromolecules, 59, 377–383.
Al-Qadiri, H. M., Al-Alami, N. I., Al-Holy, M. A., & Rasco, B. A. (2008). Using Fourier transform infrared (FT-IR) absorbance spectroscopy and multivariate analysis to study the effect of chlorine-induced bacterial injury in water. Journal of Agricultural and Food Chemistry, 56, 8992–8997.
Al-Qadiri, H. M., Al-Holy, M. A., Lin, M., Alami, N. I., Cavinato, A. G., & Rasco, B. A. (2006). Rapid detection and identification of Pseudomonas aeruginosa and Escherichia coli as pure and mixed cultures in bottled drinking water using Fourier transform infrared spectroscopy and multivariate analysis. Journal of Agricultural and Food Chemistry, 54, 5749–5754.
Al-Qadiri, H. M., Lin, M., Cavinato, A. G., & Rasco, B. A. (2006). Fourier transform infrared spectroscopy, detection and identification of Escherichia coli O157: H7 and Alicyclobacillus strains in apple juice. International Journal of Food Microbiology, 111, 73–80.
Argyri, A. A., Panagou, E. Z., Tarantilis, P. A., Polysiou, M., & Nychas, G.-J. E. (2010). Rapid qualitative and quantitative detection of beef fillets spoilage based on Fourier transform infrared spectroscopy data and artificial neural networks. Sensors and Actuators B: Chemical, 145, 146–154.
Bacsik, Z., Mink, J., & Keresztury, G. (2004). FTIR spectroscopy of the atmosphere. I. Principles and methods. Applied Spectroscopy Reviews, 39, 295–363.
Baravkar, A. A., Kale, R. N., & Sawant, S. D. (2011). FT-IR spectroscopy: Principle, technique and mathematics. International Journal of Pharma and Bio Sciences, 2, 513–519.
Barron, C., Parker, M. L., Mills, E. N. C., Rouau, X., & Wilson, R. H. (2005). FTIR imaging of wheat endosperm cell walls in situ reveals compositional and architectural heterogeneity related to grain hardness. Planta, 220, 667–677.
Bernal, J., Mendiola, J. A., Ibáñez, E., & Cifuentes, A. (2011). Advanced analysis of nutraceuticals. Journal of Pharmaceutical and Biomedical Analysis, 55, 758–774.
Bobelyn, E., Hertog, M. L. A. T. M., Schenk, A., Vermeir, S., Lammertyn, J., Nicolaï, B. M., & Beullens, K. (2006). Systems to characterise internal quality of fruit and vegetables. IV International Conference on Managing Quality in Chains―The Integrated View on Fruits and Vegetables Quality, 712, 59–66.
Bureau, S., Ruiz, D., Reich, M., Gouble, B., Bertrand, D., Audergon, J.-M., & Renard, C. M. G. C. (2009). Application of ATR-FTIR for a rapid and simultaneous determination of sugars and organic acids in apricot fruit. Food Chemistry, 115, 1133–1140.
Cassoli, L. D., Sartori, B., & Machado, P. F. (2011). The use of the Fourier transform infrared spectroscopy to determine adulterants in raw milk. Revista Brasileira de Zootecnia, 40, 2591–2596.
Cebi, N., Yilmaz, M. T., & Sagdic, O. (2017). A rapid ATR-FTIR spectroscopic method for detection of sibutramine adulteration in tea and coffee based on hierarchical cluster and principal component analyses. Food Chemistry, 229, 517–526.
Černá, M., Barros, A. S., Nunes, A., Rocha, S. M., Delgadillo, I., Čopı́ková, J., & Coimbra, M. A. (2003). Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydrate Polymers, 51, 383–389.
Chang, Y. J., Lee, S., Yoo, M. A., & Lee, H. G. (2006). Structural and biological characterization of sulfated-derivatized oat β-glucan. Journal of Agricultural and Food Chemistry, 54, 3815–3818.
Che Man, Y. B., Syahariza, Z. A., Mirghani, M. E. S., Jinap, S., & Bakar, J. (2005). Analysis of potential lard adulteration in chocolate and chocolate products using Fourier transform infrared spectroscopy. Food Chemistry, 90, 815–819.
Che Man, Y. B., & Rohman, A. (2013). Analysis of canola oil in virgin coconut oil using FTIR spectroscopy and chemometrics. Journal of Food and Pharmaceutical Sciences, 1, 5–9.
Chen, L., Tian, Y., Sun, B., Cai, C., Ma, R., & Jin, Z. (2018). Measurement and characterization of external oil in the fried waxy maize starch granules using ATR-FTIR and XRD. Food Chemistry, 242, 131–138.
Cifuentes, A. (2012). Food analysis: Present, future, and foodomics. ISRN Analytical Chemistry, 2012, 801607.
Čopíková, J., Barros, A. S., Šmídová, I., Černá, M., Teixeira, D. H., Delgadillo, I., Synytsya, A., & Coimbra, M. A. (2006). Influence of hydration of food additive polysaccharides on FT-IR spectra distinction. Carbohydrate Polymers, 63, 355–359.
Damez, J.-L., & Clerjon, S. (2013). Quantifying and predicting meat and meat products quality attributes using electromagnetic waves: An overview. Meat Science, 95, 879–896.
Danezis, G. P., Tsagkaris, A. S., Camin, F., Brusic, V., & Georgiou, C. A. (2016). Food authentication: Techniques, trends & emerging approaches. Trends in Analytical Chemistry, 85, 123–132.
Dankar, I., Haddarah, A., EL Omar, F., Pujolà, M., & Sepulcre, F. (2018). Characterization of food additive-potato starch complexes by FTIR and X-ray diffraction. Food Chemistry, 260, 7–12.
Davis, R., & Mauer, L. J. (2010). Fourier transform infrared (FT-IR) spectroscopy: A rapid tool for detection and analysis of foodborne pathogenic bacteria. In A. Mendez-Vilasa (Ed.), Current research, technology, education topics in applied microbiology, and microbial biotechnology (Vol. 2, pp. 1582–1594). Formatex Research Center.
de la Mata, P., Dominguez-Vidal, A., Bosque-Sendra, J. M., Ruiz-Medina, A., Cuadros-Rodríguez, L., & Ayora-Cañada, M. J. (2012). Olive oil assessment in edible oil blends by means of ATR-FTIR and chemometrics. Food Control, 23, 449–455.
Din, A., Anjum, F. M., Zahoor, T., & Nawaz, H. (2009). Extraction and utilization of barley β-glucan for the preparation of functional beverage. International Journal of Agriculture and Biology, 11, 737–740.
Domingo, E., Tirelli, A. A., Nunes, C. A., Guerreiro, M. C., & Pinto, S. M. (2014). Melamine detection in milk using vibrational spectroscopy and chemometrics analysis: A review. Food Research International, 60, 131–139.
Domínguez-Martínez, I., Meza-Márquez, O. G., Osorio-Revilla, G., Proal-Nájera, J., & Gallardo-Velázquez, T. (2014). Determination of capsaicin, ascorbic acid, total phenolic compounds and antioxidant activity of Capsicum annuum L. var. serrano by mid infrared spectroscopy (mid-FTIR) and chemometric analysis. Journal of Korean Society for Applied Biological Chemistry, 57, 133–142.
Doyle, W. M. (1992). Principles and applications of Fourier transform infrared (FTIR) process analysis. Process Control and Quality, 2, 11–41.
Duygu, D. Y., Baykal, T., Açikgöz, İ., & Yildiz, K. (2009). Fourier transform infrared (FT-IR) spectroscopy for biological studies. Gazi University Journal of Science, 22, 117–121.
Ellis, D. I., Broadhurst, D., & Goodacre, R. (2004). Rapid and quantitative detection of the microbial spoilage of beef by Fourier transform infrared spectroscopy and machine learning. Analytica Chimica Acta, 514, 193–201.
Ellis, D. I., Broadhurst, D., Kell, D. B., Rowland, J. J., & Goodacre, R. (2002). Rapid and quantitative detection of the microbial spoilage of meat by Fourier transform infrared spectroscopy and machine learning. Applied and Environmental Microbiology, 68, 2822–2828.
Ferragina, A., Cipolat-Gotet, C., Cecchinato, A., & Bittante, G. (2013). The use of Fourier-transform infrared spectroscopy to predict cheese yield and nutrient recovery or whey loss traits from unprocessed bovine milk samples. Journal of Dairy Science, 96, 7980–7990.
Flores-Morales, A., Jiménez-Estrada, M., & Mora-Escobedo, R. (2012). Determination of the structural changes by FT-IR, Raman, and CP/MAS 13C NMR spectroscopy on retrograded starch of maize tortillas. Carbohydrate Polymers, 87, 61–68.
Franca, A. S., & Oliveira, L. S. (2011). Potential uses of fourier transform infrared spectroscopy (FTIR) in food processing and engineering. In B. C. Siegler (Ed.), Food engineering (pp. 211–257). Nova Science Publishers Inc..
Gaffney, Jeffrey S, Nancy A Marley, and Darin E Jones. 2002. Fourier transform infrared (FTIR) spectroscopy. In Characterization of materials (pp. 1–33). Wiley.
Goerges, S., Mounier, J., Rea, M. C., Gelsomino, R., Heise, V., Beduhn, R., Cogan, T. M., Vancanneyt, M., & Scherer, S. (2008). Commercial ripening starter microorganisms inoculated into cheese milk do not successfully establish themselves in the resident microbial ripening consortia of a south German red smear cheese. Applied and Environmental Microbiology, 74, 2210–2217.
Gomaa, A. I., Sedman, J., & Ismail, A. A. (2013). An investigation of the effect of microwave treatment on the structure and unfolding pathways of β-lactoglobulin using FTIR spectroscopy with the application of two-dimensional correlation spectroscopy (2D-COS). Vibrational Spectroscopy, 65, 101–109.
Gómez-Ordóñez, E., & Rupérez, P. (2011). FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocolloids, 25, 1514–1520.
Gouilleux, B., Marchand, J., Charrier, B., Remaud, G. S., & Giraudeau, P. (2018). High-throughput authentication of edible oils with benchtop ultrafast 2D NMR. Food Chemistry, 244, 153–158.
Grassi, S., Casiraghi, E., & Alamprese, C. (2018). Handheld NIR device: A non-targeted approach to assess authenticity of fish fillets and patties. Food Chemistry, 243, 382–388.
Grewal, M. K., Chandrapala, J., Donkor, O., Apostolopoulos, V., Stojanovska, L., & Vasiljevic, T. (2017). Fourier transform infrared spectroscopy analysis of physicochemical changes in UHT milk during accelerated storage. International Dairy Journal, 66, 99–107.
Griffiths, P. R., & De Haseth, J. A. (2007). Fourier transform infrared spectrometry. Wiley.
Grumezescu, A. (2016). Nutraceuticals. Academic Press.
Grunert, T., Stephan, R., Ehling-Schulz, M., & Johler, S. (2016). Fourier transform infrared spectroscopy enables rapid differentiation of fresh and frozen/thawed chicken. Food Control, 60, 361–364.
Hakovirta, J. (2008). Modern techniques in detection, identification and quantification of bacteria and peptides from foods. University of Helsinki.
Hernández-Martínez, M., Gallardo-Velázquez, T., & Osorio-Revilla, G. (2010). Rapid characterization and identification of fatty acids in margarines using horizontal attenuate total reflectance Fourier transform infrared spectroscopy (HATR-FTIR). European Food Research and Technology, 231, 321–329.
Hernández-Martínez, M., Gallardo-Velázquez, T., Osorio-Revilla, G., Almaraz-Abarca, N., Ponce-Mendoza, A., & Vásquez-Murrieta, M. S. (2013). Prediction of total fat, fatty acid composition and nutritional parameters in fish fillets using MID-FTIR spectroscopy and chemometrics. LWT- Food Science and Technology, 52, 12–20.
Hilal, N., Ismail, A. F., Matsuura, T., & Oatley-Radcliffe, D. (2017). Membrane characterization. Elsevier.
Irudayaraj, J., Xu, R., & Tewari, J. (2003). Rapid determination of invert cane sugar adulteration in honey using FTIR spectroscopy and multivariate analysis. Journal of Food Science, 68, 2040–2045.
Irudayaraj, J., Yang, H., & Sakhamuri, S. (2002). Differentiation and detection of microorganisms using Fourier transform infrared photoacoustic spectroscopy. Journal of Molecular Structure, 606, 181–188.
Ismail, A. A., van de Voort, F. R., & Sedman, J. (1997). Fourier transform infrared spectroscopy: principles and applications. Techniques and Instrumentation in Analytical Chemistry, 18, 93–139.
Jaiswal, P., Jha, S. N., Borah, A., Gautam, A., Grewal, M. K., & Jindal, G. (2015). Detection and quantification of soymilk in cow–buffalo milk using attenuated total reflectance Fourier transform infrared spectroscopy (ATR–FTIR). Food Chemistry, 168, 41–47.
Jawaid, S., Talpur, F. N., Sherazi, S. T. H., Nizamani, S. M., & Khaskheli, A. A. (2013). Rapid detection of melamine adulteration in dairy milk by SB-ATR–Fourier transform infrared spectroscopy. Food Chemistry, 141, 3066–3071.
Kacurakova, M., Capek, P., Sasinkova, V., Wellner, N., & Ebringerova, A. (2000). FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydrate Polymers, 43, 195–203.
Kačuráková, M., Wellner, N., Ebringerová, A., Hromádková, Z., Wilson, R. H., & Belton, P. S. (1999). Characterisation of xylan-type polysaccharides and associated cell wall components by FT-IR and FT-Raman spectroscopies. Food Hydrocolloids, 13, 35–41.
Kačuráková, M., & Wilson, R. H. (2001). Developments in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydrate Polymers, 44, 291–303.
Kačuráková, M., Belton, P. S., Wilson, R. H., Hirsch, J., & Ebringerová, A. (1998). Hydration properties of xylan-type structures: An FTIR study of xylooligosaccharides. Journal of the Science of Food and Agriculture, 77, 38–44.
Kansiz, M., Billman-Jacobe, H., & McNaughton, D. (2000). Quantitative determination of the biodegradable polymer poly (β-hydroxybutyrate) in a recombinant Escherichia coli strain by use of mid-infrared spectroscopy and multivariative statistics. Applied and Environmental Microbiology, 66, 3415–3420.
Kizil, R., Irudayaraj, J., & Seetharaman, K. (2002). Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. Journal of Agricultural and Food Chemistry, 50, 3912–3918.
Kümmel, J., Stessl, B., Gonano, M., Walcher, G., Bereuter, O., Fricker, M., Grunert, T., Wagner, M., & Ehling-Schulz, M. (2016). Staphylococcus aureus entrance into the dairy chain: Tracking S. aureus from dairy cow to cheese. Frontiers in Microbiology, 7, 1603.
Kyomugasho, C., Christiaens, S., Shpigelman, A., Van Loey, A. M., & Hendrickx, M. E. (2015). FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit-and vegetable-based matrices. Food Chemistry, 176, 82–90.
Lakowicz, J. R. (2013). Principles of fluorescence spectroscopy. Springer Science & Business Media.
Larkin, P. (2017). Infrared and Raman spectroscopy: Principles and spectral interpretation. Elsevier.
Larosa, C., Salerno, M., de Lima, J. S., Meri, R. M., da Silva, M. F., de Carvalho, L. B., & Converti, A. (2018). Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. International Journal of Biological Macromolecules, 115, 900–906.
Lasch, P., Stämmler, M., Zhang, M., Baranska, M., Bosch, A., & Majzner, K. (2018). FT-IR hyperspectral imaging and artificial neural network analysis for identification of pathogenic bacteria. Analytical Chemistry, 90, 8896–8904.
Lerma-García, M. J., Gori, A., Cerretani, L., Simó-Alfonso, E. F., & Caboni, M. F. (2010). Classification of pecorino cheeses produced in Italy according to their ripening time and manufacturing technique using Fourier transform infrared spectroscopy. Journal of Dairy Science, 93, 4490–4496.
Lerma-García, M. J., Ramis-Ramos, G., Herrero-Martínez, J. M., & Simó-Alfonso, E. F. (2010). Authentication of extra virgin olive oils by Fourier-transform infrared spectroscopy. Food Chemistry, 118, 78–83.
Li, S., Zhang, X., Yang, S., Donglin, S., Ma, Q., Wen, R., & Li, J. (2017). Qualitative and quantitative detection of honey adulterated with high-fructose corn syrup and maltose syrup by using near-infrared spectroscopy. Food Chemistry, 218, 231–236.
Li, X., Zhang, Y., & He, Y. (2016). Rapid detection of talcum powder in tea using FT-IR spectroscopy coupled with chemometrics. Scientific Reports, 6, 30313.
Li, Y.-q., Kong, D.-x., & Wu, H. (2013). Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Industrial Crops and Products, 41, 269–278.
Li-Chan, E., Chalmers, J. M., & Griffiths, P. R. (2010). Applications of vibrational spectroscopy in food science. Wiley.
Lin, M., Al-Holy, M., Al-Qadiri, H., Kang, D.-H., Cavinato, A. G., Huang, Y., & Rasco, B. A. (2004). Discrimination of intact and injured listeria monocytogenes by Fourier transform infrared spectroscopy and principal component analysis. Journal of Agricultural and Food Chemistry, 52, 5769–5772.
Lucarini, M., Durazzo, A., Sánchez del Pulgar, J., Gabrielli, P., & Lombardi-Boccia, G. (2018). Determination of fatty acid content in meat and meat products: The FTIR-ATR approach. Food Chemistry, 267, 223–230.
Maggio, R. M., Cerretani, L., Chiavaro, E., Kaufman, T. S., & Bendini, A. (2010). A novel chemometric strategy for the estimation of extra virgin olive oil adulteration with edible oils. Food Control, 21, 890–895.
Mauricio-Sánchez, R. A., Ricardo, S., Gabriel Luna-Bárcenas, J., & Mendoza-Galván, A. (2018). FTIR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vibrational Spectroscopy, 94, 1–6.
Mishra, S., Kamboj, U., Kaur, H., & Kapur, P. (2010). Detection of jaggery syrup in honey using near-infrared spectroscopy. International Journal of Food Sciences and Nutrition, 61, 306–315.
Mudgil, D., Barak, S., & Khatkar, B. S. (2012). X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. International Journal of Biological Macromolecules, 50, 1035–1039.
Munajad, A., Subroto, C., & Suwarno, S. (2018). Fourier transform infrared (FTIR) spectroscopy analysis of transformer paper in mineral oil-paper composite insulation under accelerated thermal aging. Energies, 11, 364.
Nicolaou, N., & Goodacre, R. (2008). Rapid and quantitative detection of the microbial spoilage in milk using Fourier transform infrared spectroscopy and chemometrics. Analyst, 133, 1424–1431.
Novák, M., Synytsya, A., Gedeon, O., Slepička, P., Procházka, V., Synytsya, A., Blahovec, J., Hejlová, A., & Čopíková, J. (2012). Yeast β (1–3),(1–6)-d-glucan films: preparation and characterization of some structural and physical properties. Carbohydrate Polymers, 87, 2496–2504.
Nurrulhidayah, A. F., Rohman, A., Amin, I., Shuhaimi, M., & Khatib, A. (2013). Analysis of chicken fat as adulterant in butter using Fourier transform infrared spectroscopy and chemometrics. Grasas y Aceites, 64, 349–355.
Oust, A., Møretrø, T., Naterstad, K., Sockalingum, G. D., Adt, I., Manfait, M., & Kohler, A. (2006). Fourier transform infrared and Raman spectroscopy for characterization of listeria monocytogenes strains. Applied and Environmental Microbiology, 72, 228–232.
Papageorgiou, S. K., Kouvelos, E. P., Favvas, E. P., Sapalidis, A. A., Romanos, G. E., & Katsaros, F. K. (2010). Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydrate Research, 345, 469–473.
Paradkar, M. M., & Irudayaraj, J. (2002). Determination of cholesterol in dairy products using infrared techniques: 1. FTIR spectroscopy. International Journal of Dairy Technology, 55, 127–132.
Park, Y.-S., Leontowicz, H., Leontowicz, M., Namiesnik, J., Suhaj, M. M. C. M. O., Cvikrová, M., Olga, M., Weisz, M., & Gorinstein, S. (2011). Comparison of the contents of bioactive compounds and the level of antioxidant activity in different kiwifruit cultivars. Journal of Food Composition and Analysis, 24, 963–970.
Pereira, L., Amado, A. M., Critchley, A. T., Van de Velde, F., & Ribeiro-Claro, P. J. A. (2009). Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocolloids, 23, 1903–1909.
Quiñones-Islas, N., Meza-Márquez, O. G., Osorio-Revilla, G., & Gallardo-Velazquez, T. (2013). Detection of adulterants in avocado oil by mid-FTIR spectroscopy and multivariate analysis. Food Research International, 51, 148–154.
Rahman, A. (2012). Application of Fourier transform infrared spectroscopy for quality control of pharmaceutical products: A review. Indonesian Journal of Pharmacy, 23, 1–8.
Rios-Corripio, M. A., Rojas-López, M., & Delgado-Macuil, R. (2012). Analysis of adulteration in honey with standard sugar solutions and syrups using attenuated total reflectance-Fourier transform infrared spectroscopy and multivariate methods. CyTA Journal of Food, 10, 119–122.
Rohman, A., & Che Man, Y. B. (2010). Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food Research International, 43, 886–892.
Rohman, A., & Che Man, Y. B. (2012a). Analysis of pig derivatives for halal authentication studies. Food Reviews International, 28, 97–112.
Rohman, A., & Che Man, Y. B. (2012b). Authentication of extra virgin olive oil from sesame oil using FTIR spectroscopy and gas chromatography. International Journal of Food Properties, 15, 1309–1318.
Rohman, A., Che Man, Y. B., Hashim, P., & Ismail, A. (2011). FTIR spectroscopy combined with chemometrics for analysis of lard adulteration in some vegetable oils Espectroscopia FTIR combinada con quimiometría para el análisis de adulteración con grasa de cerdo de aceites vegetales. CyTA Journal of Food, 9, 96–101.
Rohman, A., Che Man, Y. B., & Yusof, F. M. (2014). The use of FTIR spectroscopy and chemometrics for rapid authentication of extra virgin olive oil. Journal of the American Oil Chemists' Society, 91, 207–213.
Rohman, A., & Che Man, Y. B. (2009). Analysis of cod-liver oil adulteration using Fourier transform infrared (FTIR) spectroscopy. Journal of the American Oil Chemists' Society, 86, 1149.
Rohman, A., Che Man, Y. B., Ismail, A., & Hashim, P. (2010). Application of FTIR spectroscopy for the determination of virgin coconut oil in binary mixtures with olive oil and palm oil. Journal of the American Oil Chemists' Society, 87, 601–606.
Schmitt, J., & Flemming, H.-C. (1998). FTIR-spectroscopy in microbial and material analysis. International Biodeterioration & Biodegradation, 41, 1–11.
Ścibisz, I., Reich, M., Bureau, S., Gouble, B., Causse, M., Bertrand, D., & Renard, C. M. G. C. (2011). Mid-infrared spectroscopy as a tool for rapid determination of internal quality parameters in tomato. Food Chemistry, 125, 1390–1397.
Sezer, B., Bilge, G., & Boyaci, I. H. (2017). Capabilities and limitations of LIBS in food analysis. TrAC Trends in Analytical Chemistry, 97, 345–353.
Shin, M. S., Lee, S., Lee, K. Y., & Lee, H. G. (2005). Structural and biological characterization of aminated-derivatized oat β-glucan. Journal of Agricultural and Food Chemistry, 53, 5554–5558.
Shingel, K. I. (2002). Determination of structural peculiarities of dexran, pullulan and γ-irradiated pullulan by Fourier-transform IR spectroscopy. Carbohydrate Research, 337, 1445–1451.
Sini, T. K., Santhosh, S., & Mathew, P. T. (2007). Study on the production of chitin and chitosan from shrimp shell by using Bacillus subtilis fermentation. Carbohydrate Research, 342, 2423–2429.
Sivakesava, S., & Irudayaraj, J. (2000). Analysis of potato chips using FTIR photoacoustic spectroscopy. Journal of the Science of Food and Agriculture, 80, 1805–1810.
Sivam, A. S., Sun-Waterhouse, D., Perera, C. O., & Waterhouse, G. I. N. (2013). Application of FT-IR and Raman spectroscopy for the study of biopolymers in breads fortified with fibre and polyphenols. Food Research International, 50, 574–585.
Smidt, E., & Schwanninger, M. (2005). Characterization of waste materials using FTIR spectroscopy: Process monitoring and quality assessment. Spectroscopy Letters, 38, 247–270.
Steiner, G., & Koch, E. (2009). Trends in Fourier transform infrared spectroscopic imaging. Analytical and Bioanalytical Chemistry, 394, 671–678.
Subramanian, A., Alvarez, V. B., Harper, W. J., & Rodriguez-Saona, L. E. (2011). Monitoring amino acids, organic acids, and ripening changes in Cheddar cheese using Fourier-transform infrared spectroscopy. International Dairy Journal, 21, 434–440.
Syahariza, Z. A., Che Man, Y. B., Selamat, J., & Bakar, J. (2005). Detection of lard adulteration in cake formulation by Fourier transform infrared (FTIR) spectroscopy. Food Chemistry, 92, 365–371.
Tiwari, B. K., Brunton, N. P., & Brennan, C. (2013). Handbook of plant food phytochemicals: Sources, stability and extraction. Wiley.
Triebel, S., Sproll, C., Reusch, H., Godelmann, R., & Lachenmeier, D. W. (2007). Rapid analysis of taurine in energy drinks using amino acid analyzer and Fourier transform infrared (FTIR) spectroscopy as basis for toxicological evaluation. Amino Acids, 33, 451–457.
Valdés, A., Beltrán, A., Mellinas, C., Jiménez, A., & Garrigós, M. C. (2018). Analytical methods combined with multivariate analysis for authentication of animal and vegetable food products with high fat content. Trends in Food Science & Technology, 77, 120–130.
Van Alfen, N. K. (2014). Encyclopedia of agriculture and food systems. Elsevier.
Van de Voort, F. R. (1992). Fourier transform infrared spectroscopy applied to food analysis. Food Research International, 25, 397–403.
Van de Voort, F. R., & Ismail, A. A. (1991). Proximate analysis of foods by mid-FTIR spectroscopy. Trends in Food Science & Technology, 2, 13–17.
van de Voort, F. R., Sedman, J., & Ismail, A. A. (1993). A rapid FTIR quality-control method for determining fat and moisture in high-fat products. Food Chemistry, 48, 213–221.
Vasconcelos, H., Saraiva, C., & de Almeida, J. M. M. M. (2014). Evaluation of the spoilage of raw chicken breast fillets using Fourier Transform Infrared Spectroscopy in tandem with chemometrics. Food and Bioprocess Technology, 7, 2330–2341.
Vongsvivut, J., Heraud, P., Zhang, W., Kralovec, J. A., McNaughton, D., & Barrow, C. J. (2012). Quantitative determination of fatty acid compositions in micro-encapsulated fish-oil supplements using Fourier transform infrared (FTIR) spectroscopy. Food Chemistry, 135, 603–609.
Vongsvivut, J., Miller, M. R., McNaughton, D., Heraud, P., & Barrow, C. J. (2014). Rapid discrimination and determination of polyunsaturated fatty acid composition in marine oils by FTIR spectroscopy and multivariate data analysis. Food and Bioprocess Technology, 7, 2410–2422.
Wang, J., Kliks, M. M., Jun, S., Jackson, M., & Li, Q. X. (2010). Rapid analysis of glucose, fructose, sucrose, and maltose in honeys from different geographic regions using Fourier transform infrared spectroscopy and multivariate analysis. Journal of Food Science, 75, C208–CC14.
Wellner, N., Kačuráková, M., Malovı́ková, A., Wilson, R. H., & Belton, P. S. (1998). FT-IR study of pectate and pectinate gels formed by divalent cations. Carbohydrate Research, 308, 123–131.
Wenning, M., Breitenwieser, F., Konrad, R., Huber, I., Busch, U., & Scherer, S. (2014). Identification and differentiation of food-related bacteria: A comparison of FTIR spectroscopy and MALDI-TOF mass spectrometry. Journal of Microbiological Methods, 103, 44–52.
Wenning, M., Büchl, N. R., & Scherer, S. (2010). Species and strain identification of lactic acid bacteria using FTIR spectroscopy and artificial neural networks. Journal of Biophotonics, 3, 493–505.
Wenning, M., & Scherer, S. (2013). Identification of microorganisms by FTIR spectroscopy: Perspectives and limitations of the method. Applied Microbiology and Biotechnology, 97, 7111–7120.
Wenning, M., Seiler, H., & Scherer, S. (2002). Fourier-transform infrared microspectroscopy, a novel and rapid tool for identification of yeasts. Applied and Environmental Microbiology, 68, 4717–4721.
Willey, R. R. (1976). Fourier transform infrared spectrophotometer for transmittance and diffuse reflectance measurements. Applied Spectroscopy, 30, 593–601.
Yang, H., Irudayaraj, J., & Paradkar, M. M. (2005). Discriminant analysis of edible oils and fats by FTIR, FT-NIR and FT-Raman spectroscopy. Food Chemistry, 93, 25–32.
Yu, C., & Irudayaraj, J. (2005). Spectroscopic characterization of microorganisms by Fourier transform infrared microspectroscopy. Biopolymers, 77, 368–377.
Zahir, E., Saeed, R., Hameed, M. A., & Yousuf, A. (2017). Study of physicochemical properties of edible oil and evaluation of frying oil quality by Fourier transform-infrared (FT-IR) Spectroscopy. Arabian Journal of Chemistry, 10, S3870–S3S76.
Zhang, L., Yuan, Z., Li, P., Wang, X., Mao, J., Zhang, Q., & Chundi, H. (2017). Targeted multivariate adulteration detection based on fatty acid profiles and Monte Carlo one-class partial least squares. Chemometrics and Intelligent Laboratory Systems, 169, 94–99.
Zhang, Q., Liu, C., Sun, Z., Hu, X., Shen, Q., & Wu, J. (2012). Authentication of edible vegetable oils adulterated with used frying oil by Fourier Transform Infrared Spectroscopy. Food Chemistry, 132, 1607–1613.
Zhao, M., Downey, G., & O’Donnell, C. P. (2014). Detection of adulteration in fresh and frozen beefburger products by beef offal using mid-infrared ATR spectroscopy and multivariate data analysis. Meat Science, 96, 1003–1011.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ahmad, A., Ayub, H. (2022). Fourier Transform Infrared Spectroscopy (FTIR) Technique for Food Analysis and Authentication. In: Pathare, P.B., Rahman, M.S. (eds) Nondestructive Quality Assessment Techniques for Fresh Fruits and Vegetables . Springer, Singapore. https://doi.org/10.1007/978-981-19-5422-1_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-5422-1_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5421-4
Online ISBN: 978-981-19-5422-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)